Multiscale Simulation of the Formation of Platinum-Particles on Alumina Nanoparticles in a Spray Flame Experiment
Abstract
1. Introduction
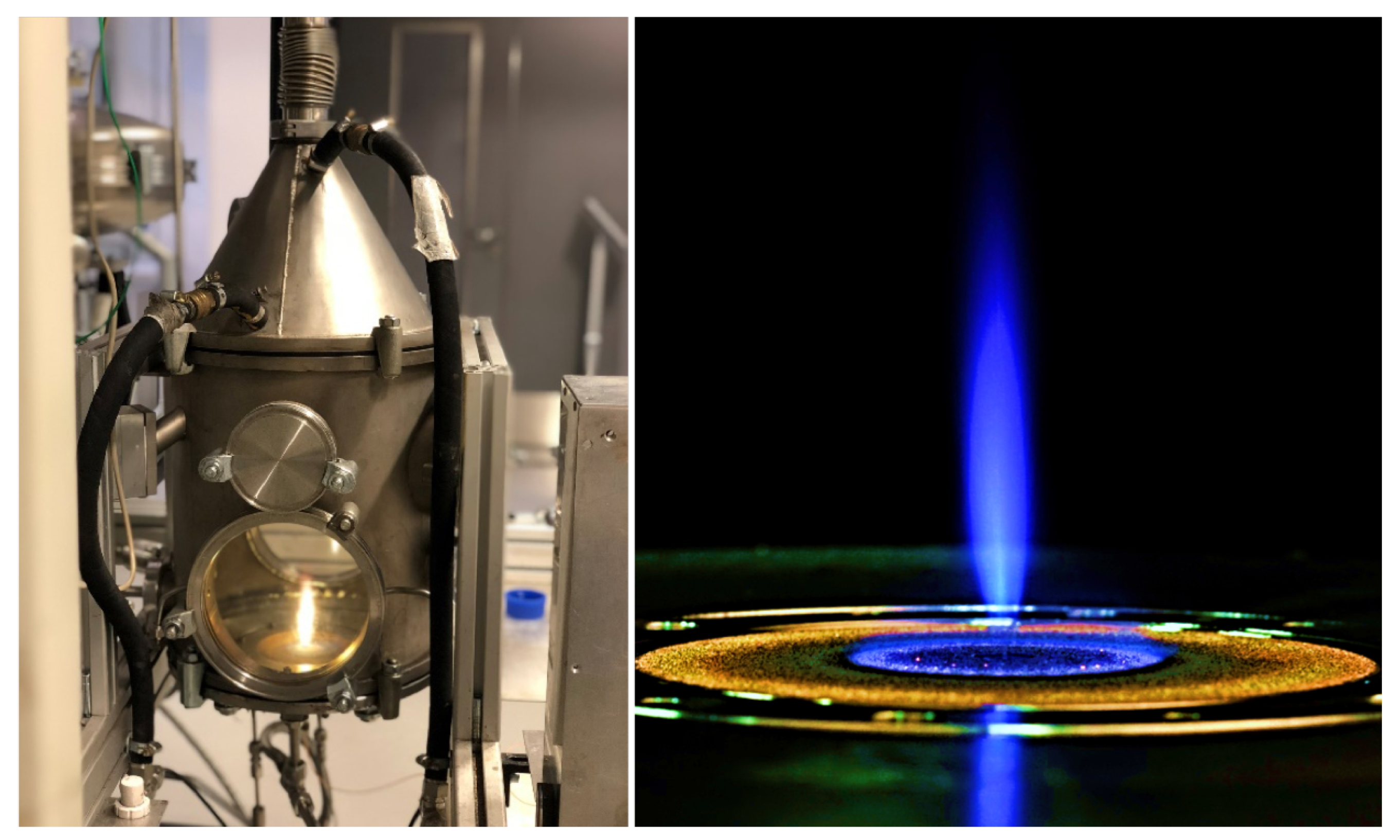
2. Experimental
3. Modeling
3.1. Reaction Kinetics and Turbulence Chemistry Interaction
3.2. Modeling Nanoparticle Dynamics
4. Simulation Results
4.1. Numerical Setup
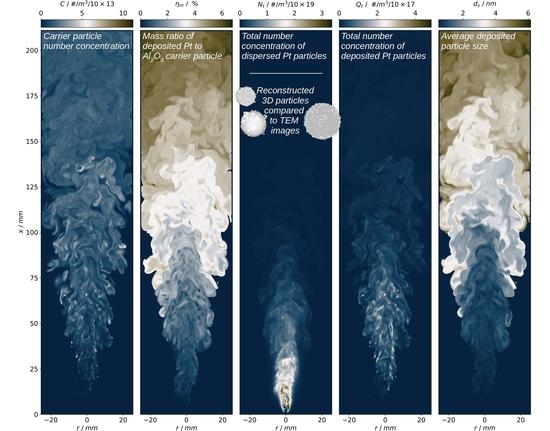
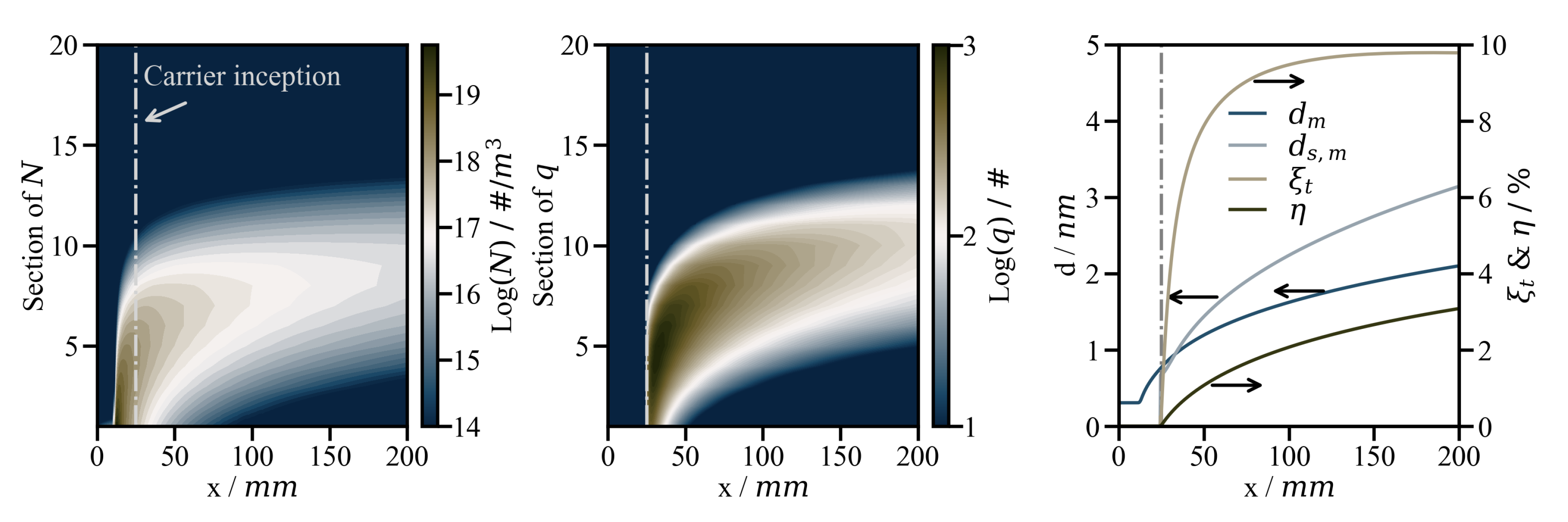
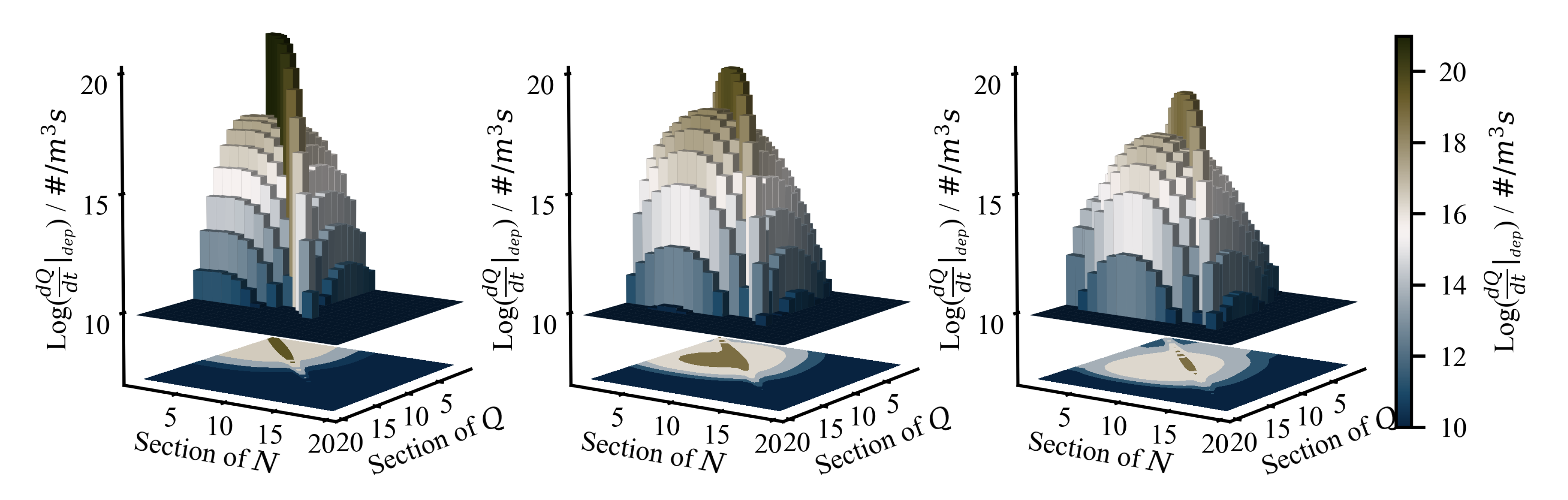
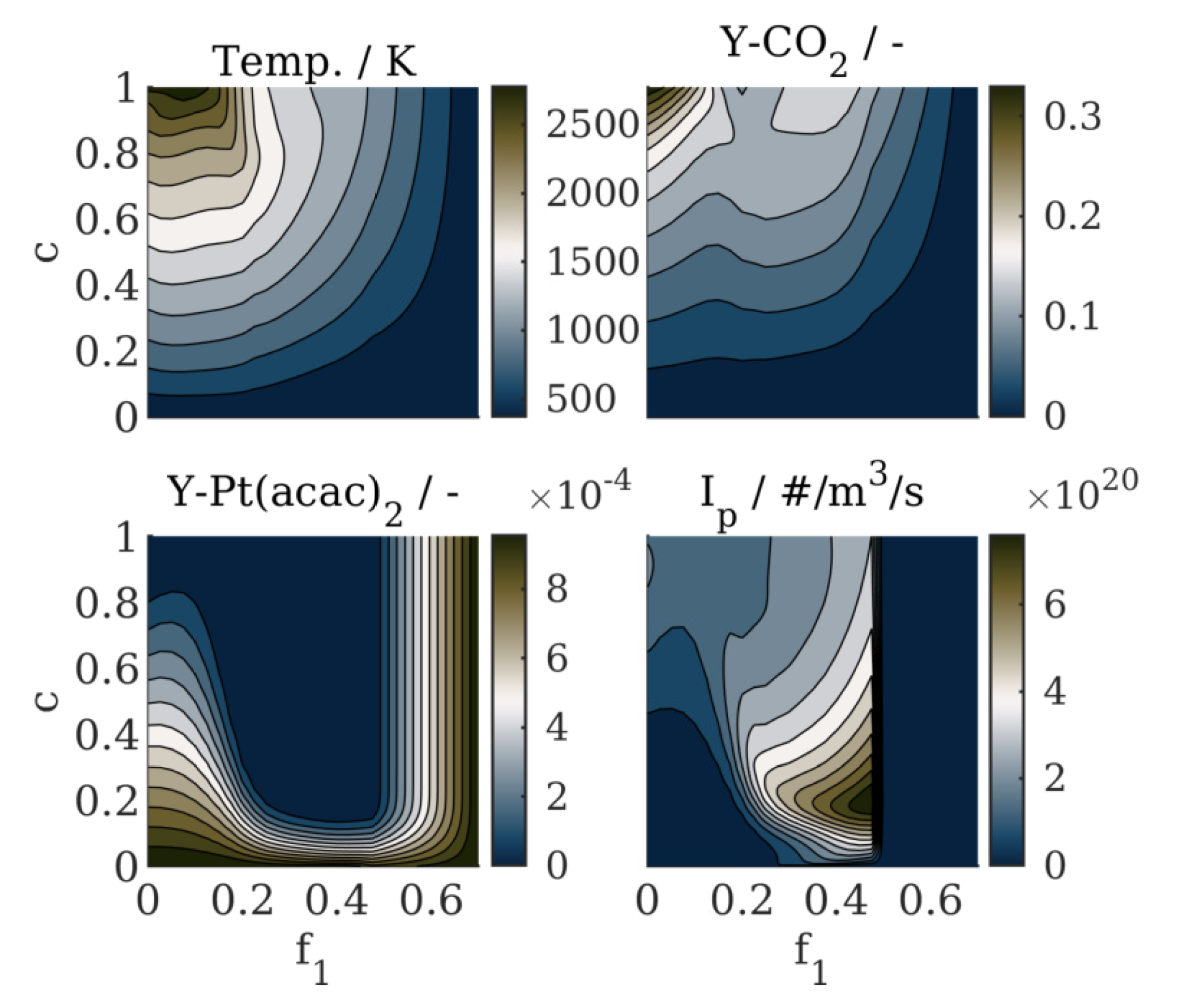
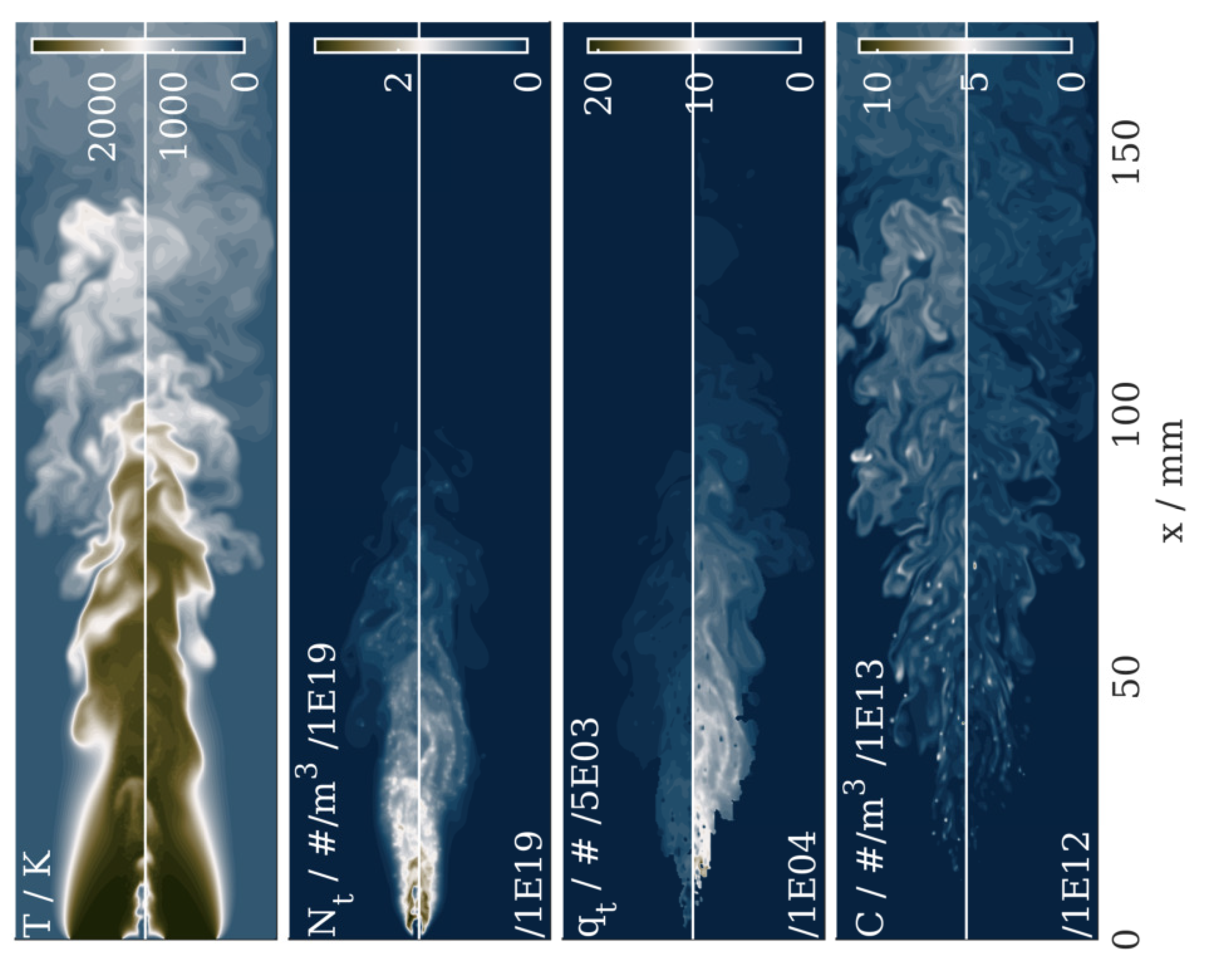
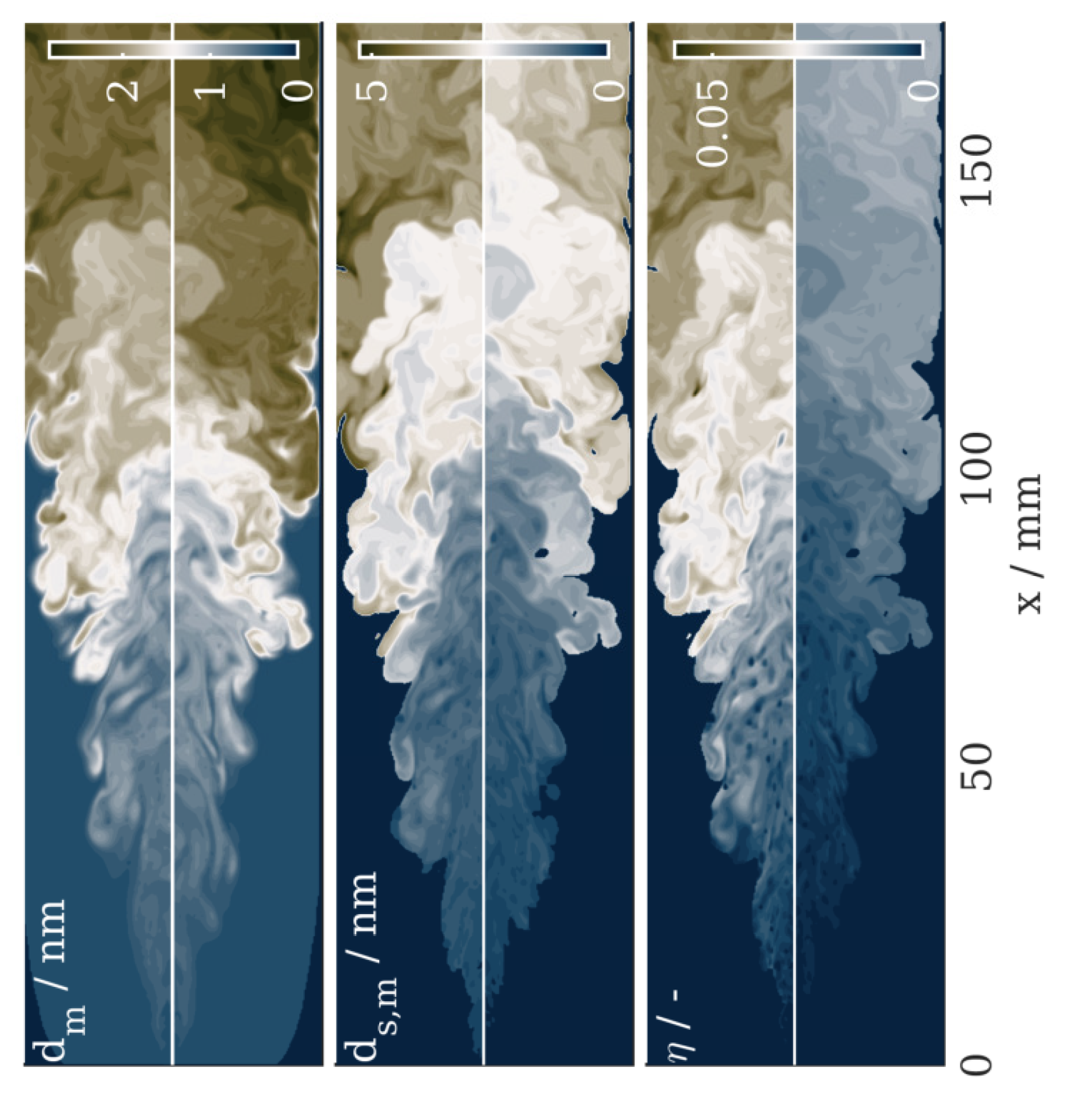
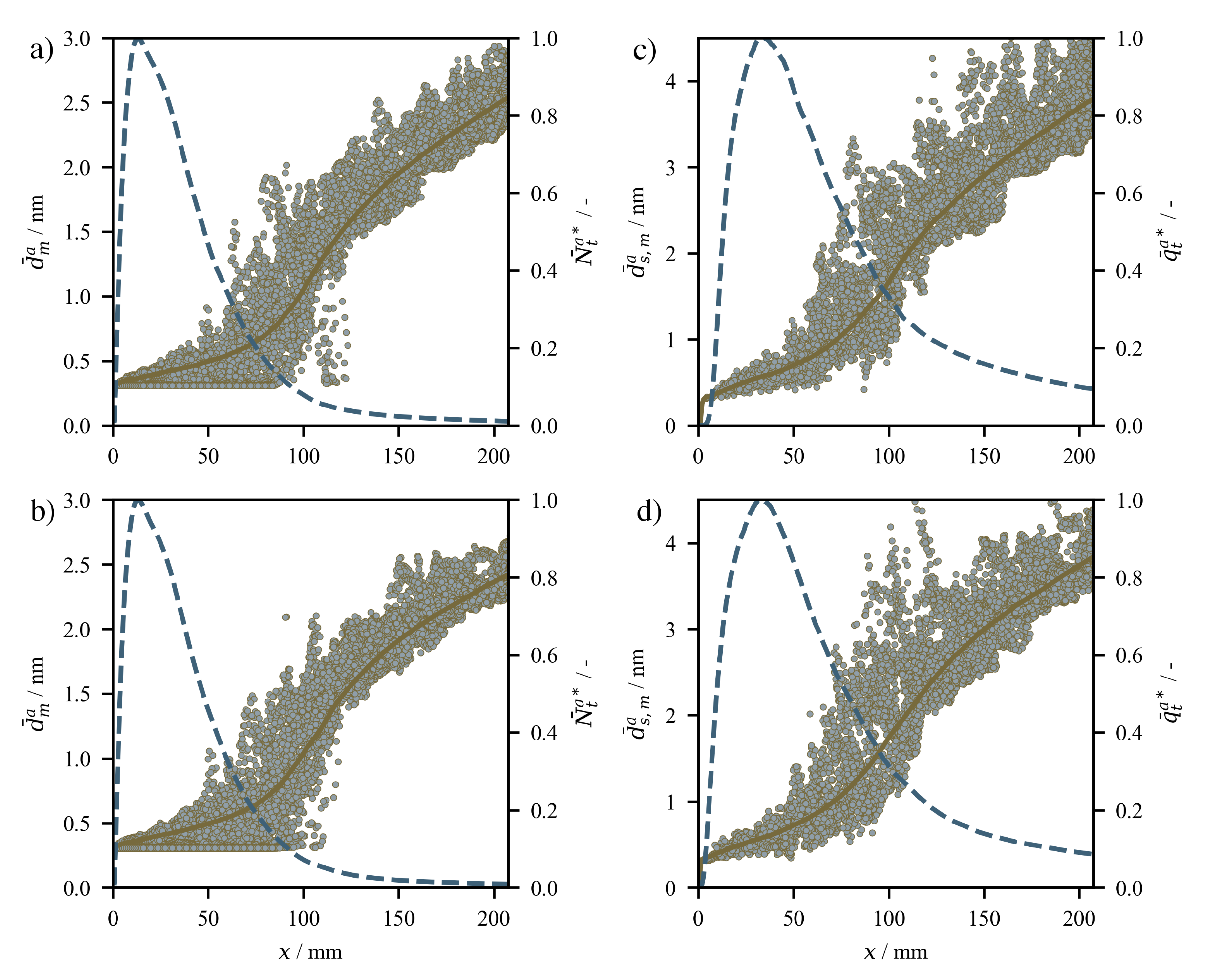
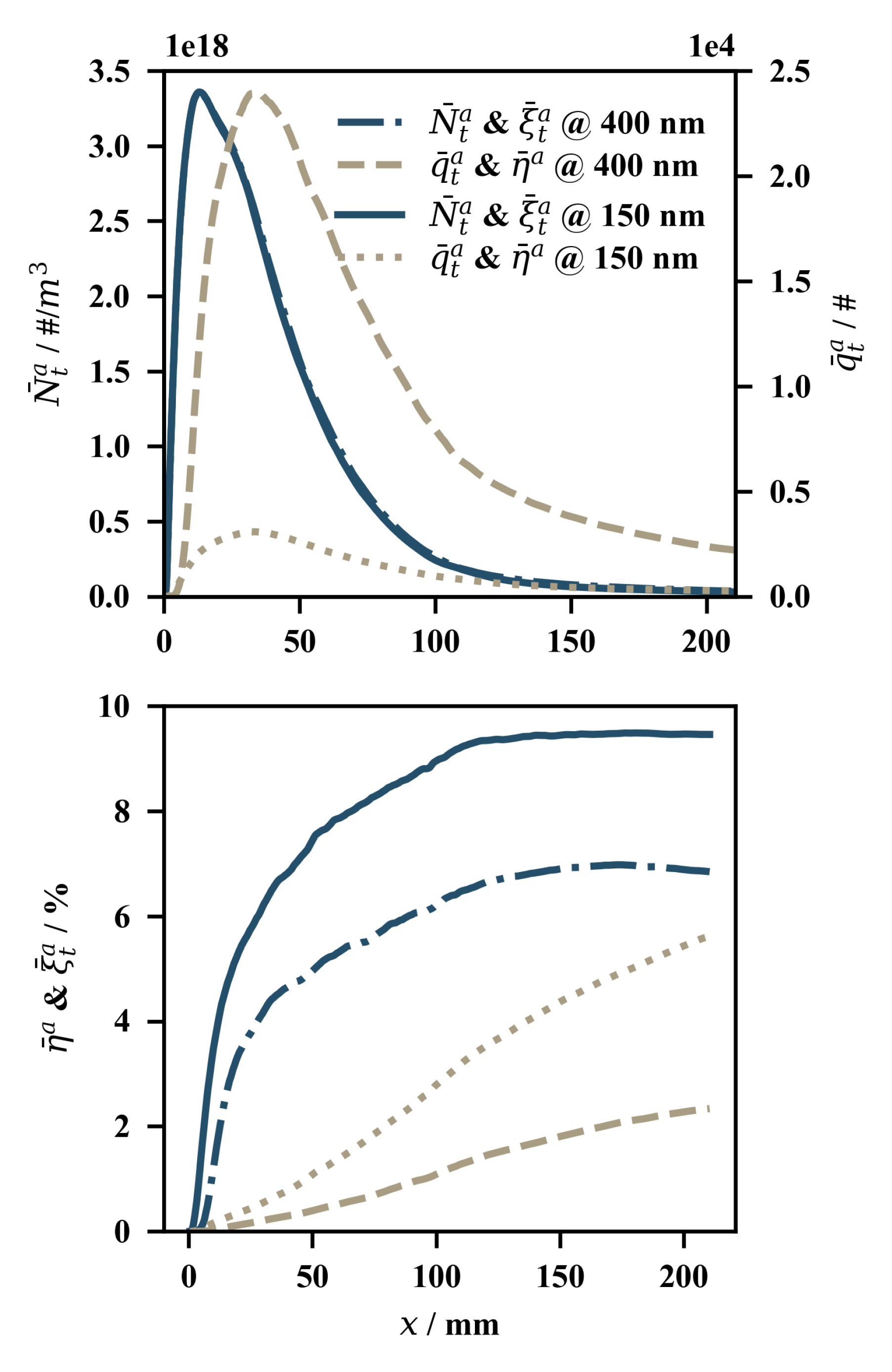
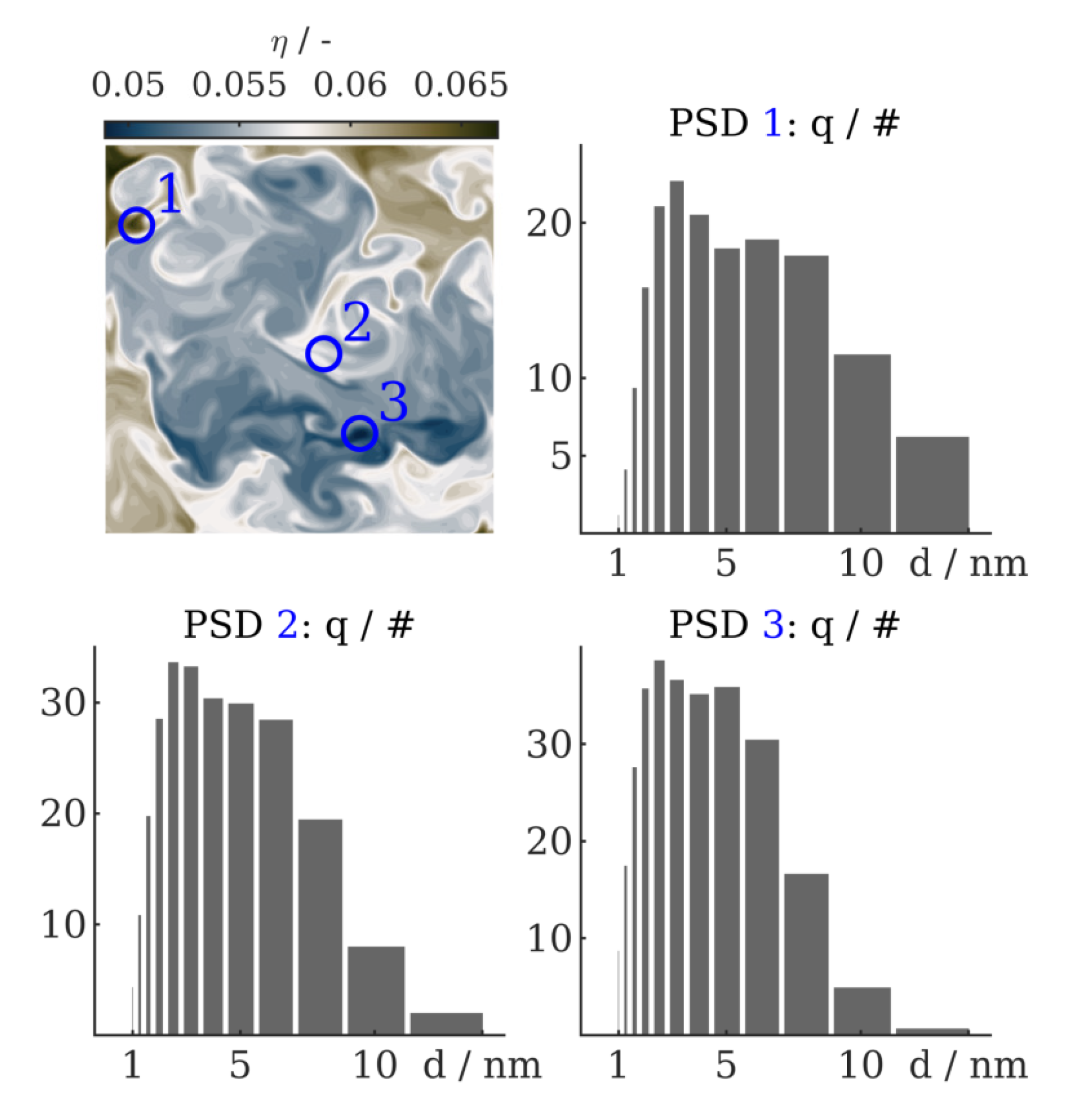
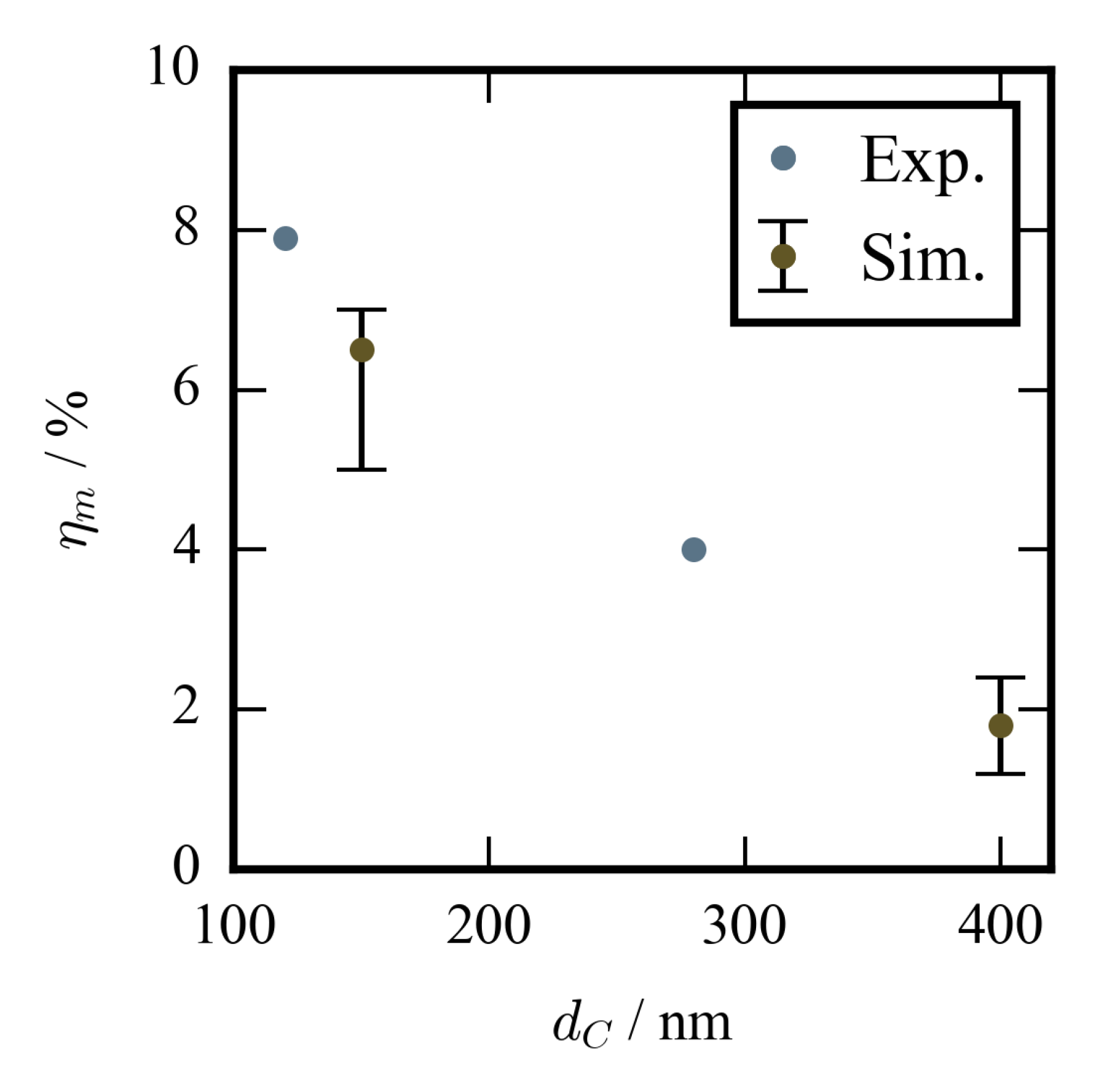
4.2. Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Verification of Model Implementation
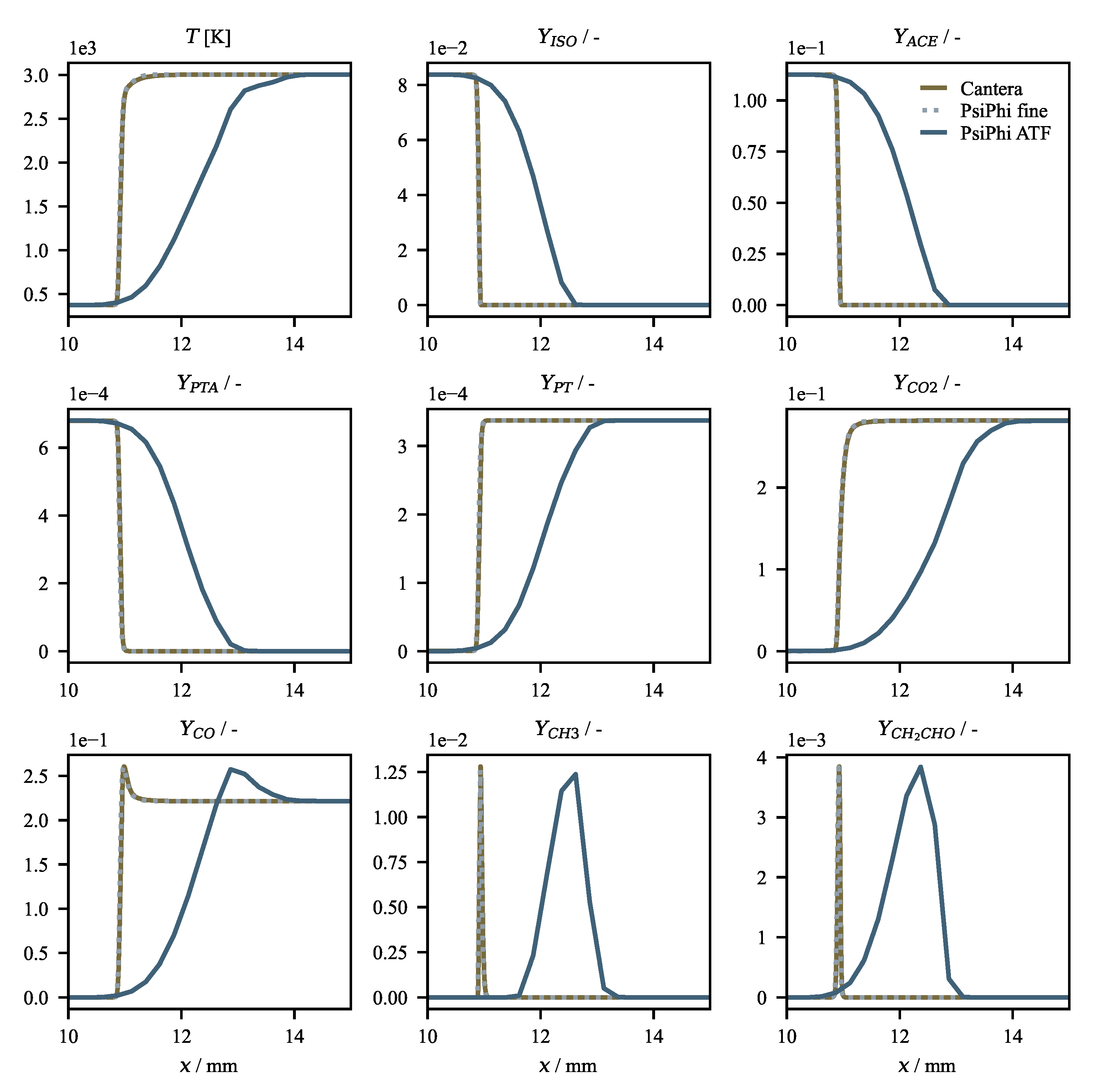
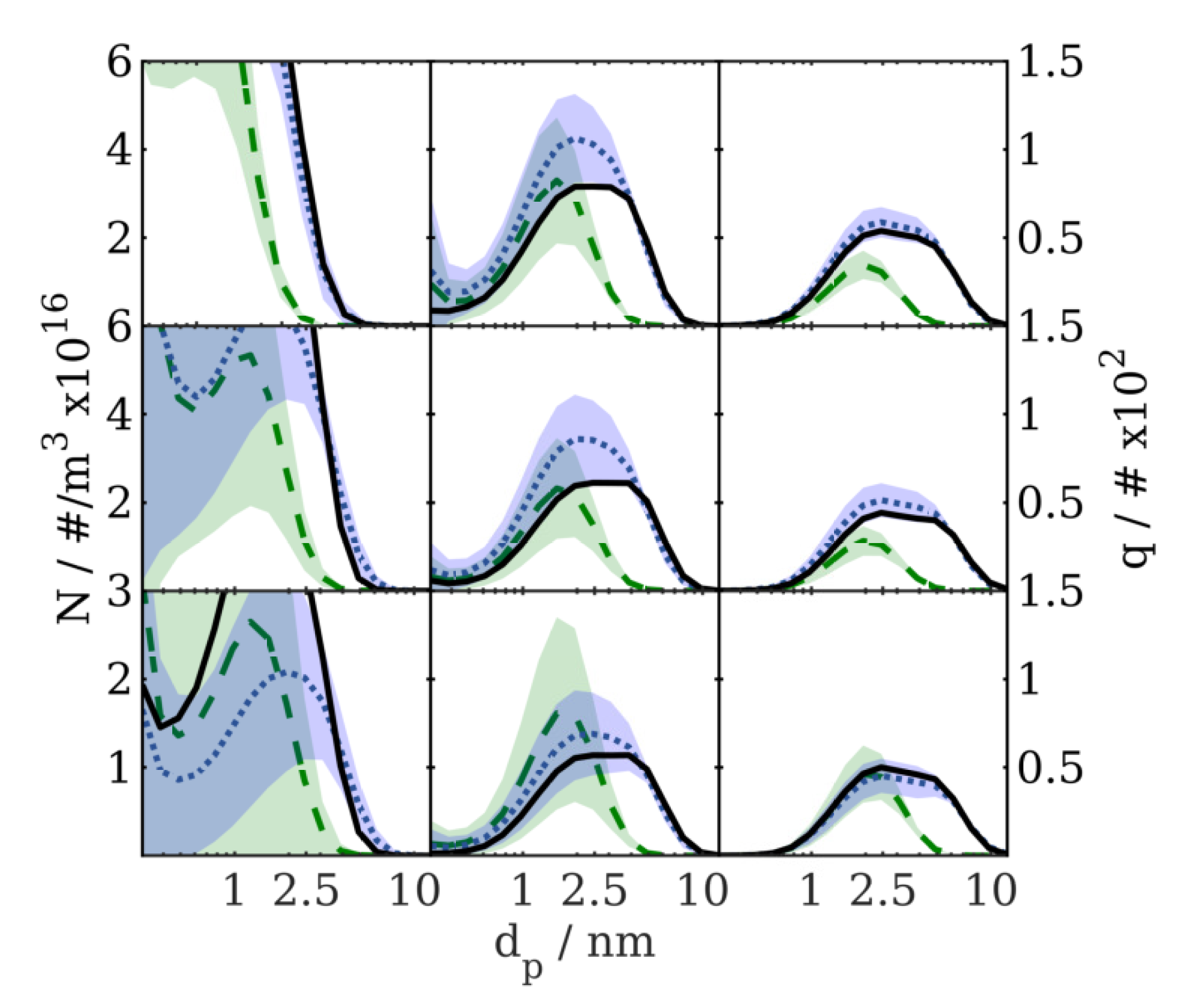
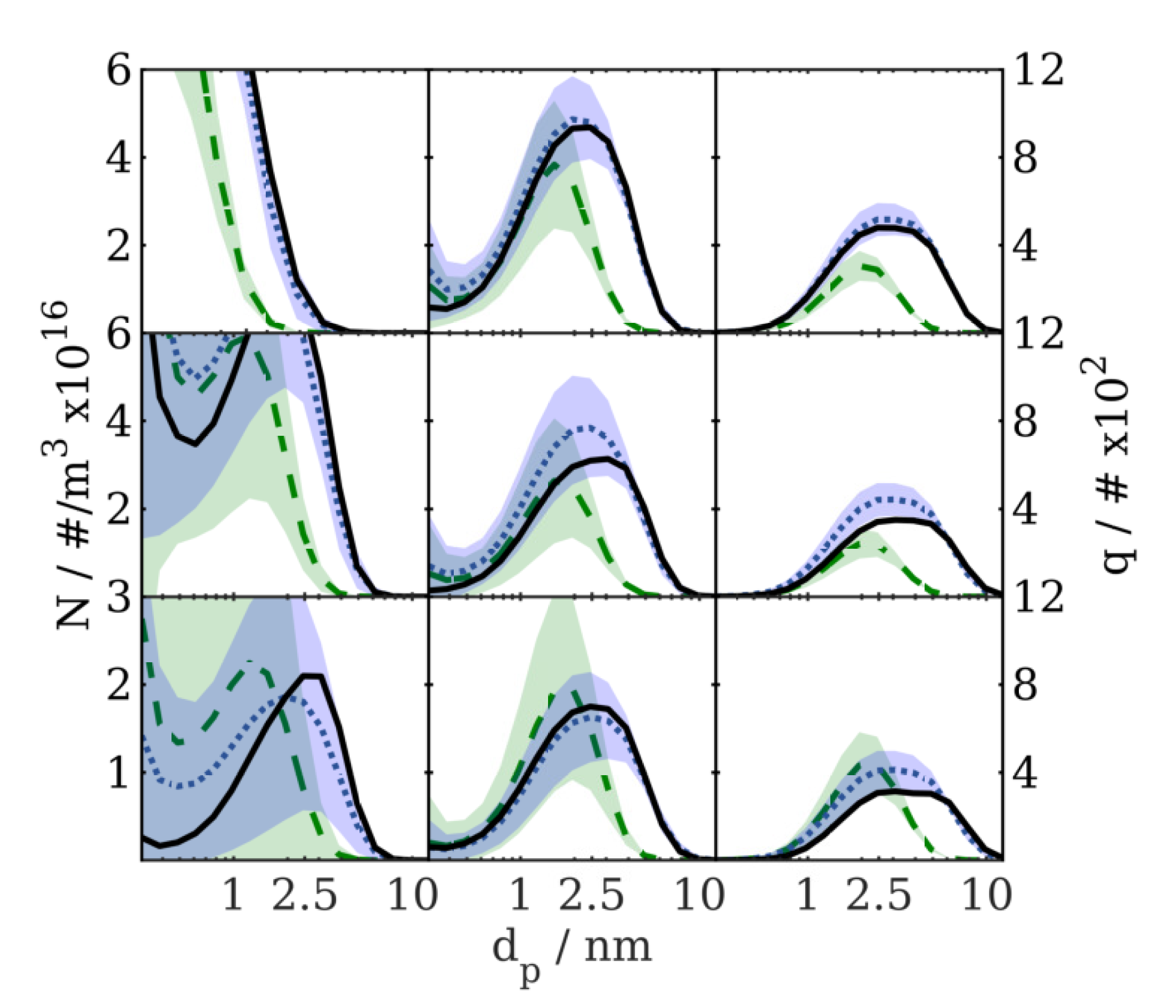
Verification Results



References
- Mädler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled synthesis of nanostructured particles by flame spray pyrolysis. J. Aerosol. Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Grossmann, H.K.; Grieb, T.; Meierhofer, F.; Hodapp, M.J.; Noriler, D.; Gröhn, A.; Meier, H.F.; Fritsching, U.; Wegner, K.; Mädler, L. Nanoscale mixing during double-flame spray synthesis of heterostructured nanoparticles. J. Nanopart. Res. 2015, 17, 174. [Google Scholar] [CrossRef]
- Kempf, A.M.; Schulz, C. SpraySyn Standardbrenner: Definition, Gesamtsimulation, Charakterisierung. Available online: https://gepris.dfg.de/gepris/projekt/375220870 (accessed on 1 October 2020).
- Schneider, F.; Suleiman, S.; Menser, J.; Borukhovich, E.; Wlokas, I.; Kempf, A.; Wiggers, H.; Schulz, C. SpraySyn—A standardized burner configuration for nanoparticle synthesis in spray flames. Rev. Sci. Instrum. 2019, 90, 085108. [Google Scholar] [CrossRef] [PubMed]
- Buesser, B.; Groehn, A.J. Multiscale Aspects of Modeling Gas-Phase Nanoparticle Synthesis. Chem. Eng. Technol. 2012, 35, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Noriler, D.; Hodapp, M.J.; Decker, R.K.; Meier, H.F.; Meierhofer, F.; Fritsching, U. Numerical simulation of flame spray pyrolysis process for nanoparticle productions: Effects of 2d and 3d approaches. In Fluids Engineering Division Summer Meeting; American Society of Mechanical Engineers: New York, NY, USA, 2014; Volume 46216, p. V01AT03A021. [Google Scholar]
- Weise, C.; Menser, J.; Kaiser, S.; Kempf, A.; Wlokas, I. Numerical investigation of the process steps in a spray flame reactor for nanoparticle synthesis. Proc. Combust. Inst. 2015, 35, 2259–2266. [Google Scholar] [CrossRef]
- Gröhn, A.J.; Pratsinis, S.E.; Sánchez-Ferrer, A.; Mezzenga, R.; Wegner, K. Scale-up of nanoparticle synthesis by flame spray pyrolysis: The high-temperature particle residence time. Ind. Eng. Chem. Res. 2014, 53, 10734–10742. [Google Scholar] [CrossRef]
- Kruis, F.E.; Kusters, K.A.; Pratsinis, S.E.; Scarlett, B. A Simple Model for the Evolution of the Characteristics of Aggregate Particles Undergoing Coagulation and Sintering. Aerosol Sci. Technol. 1993, 19, 514–526. [Google Scholar] [CrossRef]
- Rittler, A.; Deng, L.; Wlokas, I.; Kempf, A.M. Large eddy simulations of nanoparticle synthesis from flame spray pyrolysis. Proc. Combust. Inst. 2016, 36, 1077–1087. [Google Scholar] [CrossRef]
- Abdelsamie, A.; Chi, C.; Nanjaiah, M.; Skenderović, I.; Suleiman, S.; Thévenin, D. Direct Numerical Simulation of Turbulent Spray Combustion in the SpraySyn Burner: Impact of Injector Geometry. Flow Turbul. Combust. 2020, 1–17. [Google Scholar] [CrossRef]
- Vemury, S.; Pratsinis, S.E. Self-preserving size distributions of agglomerates. J. Aerosol Sci. 1995, 26, 175–185. [Google Scholar] [CrossRef]
- Vemury, S.; Pratsinis, S.E.; Kibbey, L. Electrically Controlled Flame Synthesis of Nanophase TiO2, SiO2, and SnO2 Powders. J. Mater. Res. 1997, 12, 1031–1042. [Google Scholar] [CrossRef]
- Gelbard, F.; Seinfeld, J.H. The general dynamic equation for aerosols. Theory and application to aerosol formation and growth. J. Colloid Interface Sci. 1979, 68, 363–382. [Google Scholar] [CrossRef]
- Rigopoulos, S. Population balance modelling of polydispersed particles in reactive flows. Prog. Energy Combust. Sci. 2010, 36, 412–443. [Google Scholar] [CrossRef]
- Ramkrishna, D.; Singh, M.R. Population balance modeling: Current status and future prospects. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 123–146. [Google Scholar] [CrossRef]
- Gelbard, F.; Tambour, Y.; Seinfeld, J.H. Sectional representations for simulating aerosol dynamics. J. Colloid Interface Sci. 1980, 76, 541–556. [Google Scholar] [CrossRef]
- Garrick, S.; Zachariah, M.; Lehtinen, K. Modeling and simulation of nanoparticle coagulation in high reynolds number incompressible flows. In Proceedings of the National Conference of the Combustion Institute, Oakland, CA, USA, 25–28 March 2001. [Google Scholar]
- Loeffler, J.; Das, S.; Garrick, S.C. Large Eddy Simulation of Titanium Dioxide Nanoparticle Formation and Growth in Turbulent Jets. Aerosol Sci. Technol. 2011, 45, 616–628. [Google Scholar] [CrossRef]
- Blacha, T.; Di Domenico, M.; Gerlinger, P.; Aigner, M. Soot predictions in premixed and non-premixed laminar flames using a sectional approach for PAHs and soot. Combust. Flame 2012, 159, 181–193. [Google Scholar] [CrossRef]
- Lindstedt, R.; Waldheim, B. Modeling of soot particle size distributions in premixed stagnation flow flames. Proc. Combust. Inst. 2013, 34, 1861–1868. [Google Scholar] [CrossRef]
- Rodrigues, P.; Franzelli, B.; Vicquelin, R.; Gicquel, O.; Darabiha, N. Coupling an LES approach and a soot sectional model for the study of sooting turbulent non-premixed flames. Combust. Flame 2018, 190, 477–499. [Google Scholar] [CrossRef]
- Schiener, M.; Lindstedt, R. Transported probability density function based modelling of soot particle size distributions in non-premixed turbulent jet flames. Proc. Combust. Inst. 2019, 37, 1049–1056. [Google Scholar] [CrossRef]
- Hardt, S.; Wlokas, I.; Schulz, C.; Wiggers, H. Impact of Ambient Pressure on Titania Nanoparticle Formation During Spray-Flame Synthesis. J. Nanosci. Nanotechnol. 2015, 15, 9449–9456. [Google Scholar] [CrossRef] [PubMed]
- Hamid, N.A.; Wennig, S.; Hardt, S.; Heinzel, A.; Schulz, C.; Wiggers, H. High-capacity cathodes for lithium-ion batteries from nanostructured LiFePO4 synthesized by highly-flexible and scalable flame spray pyrolysis. J. Power Sources 2012, 216, 76–83. [Google Scholar] [CrossRef]
- Van Oijen, J.A.; de Goey, L.P.H. Modelling of Premixed Laminar Flames using Flamelet-Generated Manifolds. Combust. Sci. Technol. 2000, 161, 113–137. [Google Scholar] [CrossRef]
- Peters, N. Laminar flamelet concepts in turbulent combustion. Symp. (Int.) Combust. 1988, 21, 1231–1250. [Google Scholar] [CrossRef]
- Utriainen, M.; Kröger-Laukkanen, M.; Johansson, L.S.; Niinistö, L. Studies of metallic thin film growth in an atomic layer epitaxy reactor using M(acac)2 (M=Ni, Cu, Pt) precursors. Appl. Surf. Sci. 2000, 157, 151–158. [Google Scholar] [CrossRef]
- Chaston, J.C. Reactions of Oxygen with the Platinum Metals. Platinum Met. Rev. 1965, 9, 51–56. [Google Scholar]
- Mosquera, A.; Horwat, D.; Vazquez, L.; Gutiérrez, A.; Erko, A.; Anders, A.; Andersson, J.; Endrino, J.L. Thermal decomposition and fractal properties of sputter-deposited platinum oxide thin films. J. Mater. Res. 2012, 27, 829–836. [Google Scholar] [CrossRef]
- Simonsen, S.B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Direct Observations of Oxygen-induced Platinum Nanoparticle Ripening Studied by In Situ TEM. J. Am. Chem. Soc. 2010, 132, 7968–7975. [Google Scholar] [CrossRef]
- Peev, N.S. Particle Collision Frequency and the Two-dimensional Particles Nucleation. C. R. Acad. Sci. 2013, 66, 29–34. [Google Scholar] [CrossRef]
- Pelucchi, M.; Bissoli, M.; Cavallotti, C.; Cuoci, A.; Faravelli, T.; Frassoldati, A.; Ranzi, E.; Stagni, A. Improved kinetic model of the low-temperature oxidation of n-heptane. Energy Fuels 2014, 28, 7178–7193. [Google Scholar] [CrossRef]
- Djokic, M.R.; Van Geem, K.M.; Cavallotti, C.; Frassoldati, A.; Ranzi, E.; Marin, G.B. An experimental and kinetic modeling study of cyclopentadiene pyrolysis: First growth of polycyclic aromatic hydrocarbons. Combust. Flame 2014, 161, 2739–2751. [Google Scholar] [CrossRef]
- Brönstrup, M.; Schröder, D.; Kretzschmar, I.; Schwarz, H.; Harvey, J.N. Platinum Dioxide Cation: Easy to Generate Experimentally but Difficult to Describe Theoretically. J. Am. Chem. Soc. 2001, 123, 142–147. [Google Scholar] [CrossRef]
- Van Oijen, J.A.; Bastiaans, R.J.M.; De Goey, L.P.H. Low-dimensional manifolds in direct numerical simulations of premixed turbulent flames. Proc. Combust. Inst. 2007, 31, 1377–1384. [Google Scholar] [CrossRef]
- Peters, N. Laminar diffusion flamelet models in non-premixed turbulent combustion. Prog. Energy Combust. Sci. 1984, 10, 319–339. [Google Scholar] [CrossRef]
- Peters, N. Turbulent Combustion; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Pope, S.B. Turbulent Flows; IOP Publishing: Bristol, UK, 2001. [Google Scholar]
- Nicoud, F.; Toda, H.B.; Cabrit, O.; Bose, S.; Lee, J. Using singular values to build a subgrid-scale model for large eddy simulations. Phys. Fluids 2011, 23, 085106. [Google Scholar] [CrossRef]
- Butler, T.; O’rourke, P. A numerical method for two dimensional unsteady reacting flows. Symp. (Int.) Combust. 1977, 16, 1503–1515. [Google Scholar] [CrossRef]
- Legier, J.P.; Poinsot, T.J.; Veynante, D. Dynamically thickened flame LES model for premixed and non-premixed turbulent combustion. Proc. Summer Prog. 2000, 12, 157–168. [Google Scholar]
- Proch, F.; Domingo, P.; Vervisch, L.; Kempf, A.M. Flame resolved simulation of a turbulent premixed bluff-body burner experiment. Part II: A-priori and a-posteriori investigation of sub-grid scale wrinkling closures in the context of artificially thickened flame modeling. Combust. Flame 2017, 180, 340–350. [Google Scholar] [CrossRef]
- Charlette, F.; Meneveau, C.; Veynante, D. A Power-Law Flame Wrinkling Model for LES of Premixed Turbulent Combustion Part I- Non-Dynamic Formulation and Initial Tests. Combust. Flame 2002, 131, 159–180. [Google Scholar] [CrossRef]
- Wang, G.; Boileau, M.; Veynante, D. Implementation of a dynamic thickened flame model for large eddy simulations of turbulent premixed combustion. Combust. Flame 2011, 158, 2199–2213. [Google Scholar] [CrossRef]
- Rittler, A.; Proch, F.; Kempf, A.M. LES of the Sydney piloted spray flame series with the PFGM/ATF approach and different sub-filter models. Combust. Flame 2015, 162, 1575–1598. [Google Scholar] [CrossRef]
- Berg, H.C. Random Walks in Biology; Princeton University Press: Princeton, NJ, USA, 1993. [Google Scholar]
- Friedlander, S. Smoke, Dust and Haze: Fundamentals of Aerosol Behavior; Oxford University Press: Oxford, UK, 1977. [Google Scholar]
- Pratsinis, S.E. Simultaneous nucleation, condensation, and coagulation in aerosol reactors. J. Colloid Interface Sci. 1988, 124, 416–427. [Google Scholar] [CrossRef]
- Kazakov, A.; Frenklach, M. Dynamic Modeling of Soot Particle Coagulation and Aggregation: Implementation with the Method of Moments and Application to High-Pressure Laminar Premixed Flames. Combust. Flame 1998, 114, 484–501. [Google Scholar] [CrossRef]
- Harris, S.J.; Kennedy, I.M. The Coagulation of Soot Particles with van der Waals Forces. Combust. Sci. Technol. 1988, 59, 443–454. [Google Scholar] [CrossRef]
- Menz, W.J.; Kraft, M. A new model for silicon nanoparticle synthesis. Combust. Flame 2013, 160, 947–958. [Google Scholar] [CrossRef]
- Shekar, S.; Sander, M.; Riehl, R.C.; Smith, A.J.; Braumann, A.; Kraft, M. Modelling the flame synthesis of silica nanoparticles from tetraethoxysilane. Chem. Eng. Sci. 2012, 70, 54–66. [Google Scholar] [CrossRef]
- Sander, M.; West, R.H.; Celnik, M.S.; Kraft, M. A Detailed Model for the Sintering of Polydispersed Nanoparticle Agglomerates. Aerosol Sci. Technol. 2009, 43, 978–989. [Google Scholar] [CrossRef]
- Wang, C.S.; Friedlander, S.K. The self-preserving particle size distribution for coagulation by Brownian motion: II. Small particle slip correction and simultaneous shear flow. J. Colloid Interface Sci. 1967, 24, 170–179. [Google Scholar] [CrossRef]
- Prakash, A.; Bapat, A.P.; Zachariah, M.R. A Simple Numerical Algorithm and Software for Solution of Nucleation, Surface Growth, and Coagulation Problems. Aerosol Sci. Technol. 2003, 37, 892–898. [Google Scholar] [CrossRef]
- Spicer, P.T.; Chaoul, O.; Tsantilis, S.; Pratsinis, S.E. Titania formation by TiCl4 gas phase oxidation, surface growth and coagulation. J. Aerosol Sci. 2002, 33, 17–34. [Google Scholar] [CrossRef]
- Proch, F.; Kempf, A.M. Numerical analysis of the Cambridge stratified flame series using artificial thickened flame LES with tabulated premixed flame chemistry. Combust. Flame 2014, 161, 2627–2646. [Google Scholar] [CrossRef]
- Kempf, A.M.; Geurts, B.J.; Oefelein, J.C. Error analysis of large-eddy simulation of the turbulent non-premixed sydney bluff-body flame. Combust. Flame 2011, 158, 2408–2419. [Google Scholar] [CrossRef]
- Proch, F.; Kempf, A.M. Modeling heat loss effects in the large eddy simulation of a model gas turbine combustor with premixed flamelet generated manifolds. Proc. Combust. Inst. 2015, 35, 3337–3345. [Google Scholar] [CrossRef]
- Zhou, G. Numerical Simulations of Physical Discontinuities in Single and Multi-Fluid Flows for Arbitrary Mach Numbers. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 1995. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wollny, P.; Angel, S.; Wiggers, H.; Kempf, A.M.; Wlokas, I. Multiscale Simulation of the Formation of Platinum-Particles on Alumina Nanoparticles in a Spray Flame Experiment. Fluids 2020, 5, 201. https://doi.org/10.3390/fluids5040201
Wollny P, Angel S, Wiggers H, Kempf AM, Wlokas I. Multiscale Simulation of the Formation of Platinum-Particles on Alumina Nanoparticles in a Spray Flame Experiment. Fluids. 2020; 5(4):201. https://doi.org/10.3390/fluids5040201
Chicago/Turabian StyleWollny, Patrick, Steven Angel, Hartmut Wiggers, Andreas M. Kempf, and Irenaeus Wlokas. 2020. "Multiscale Simulation of the Formation of Platinum-Particles on Alumina Nanoparticles in a Spray Flame Experiment" Fluids 5, no. 4: 201. https://doi.org/10.3390/fluids5040201
APA StyleWollny, P., Angel, S., Wiggers, H., Kempf, A. M., & Wlokas, I. (2020). Multiscale Simulation of the Formation of Platinum-Particles on Alumina Nanoparticles in a Spray Flame Experiment. Fluids, 5(4), 201. https://doi.org/10.3390/fluids5040201