Microbubble-Mediated Delivery for Cancer Therapy
Abstract
1. Introduction
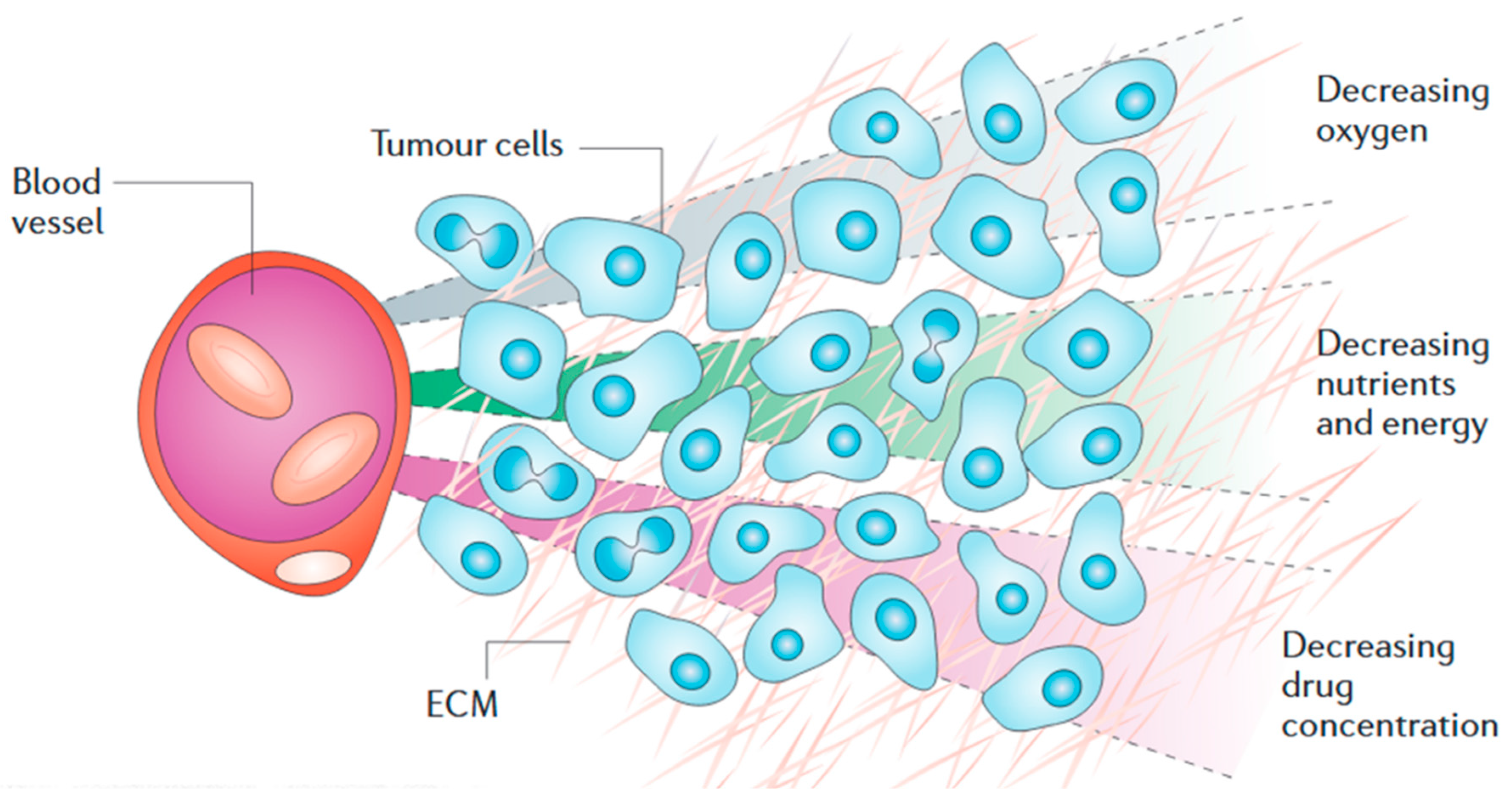
2. Solid Tumour Pathology
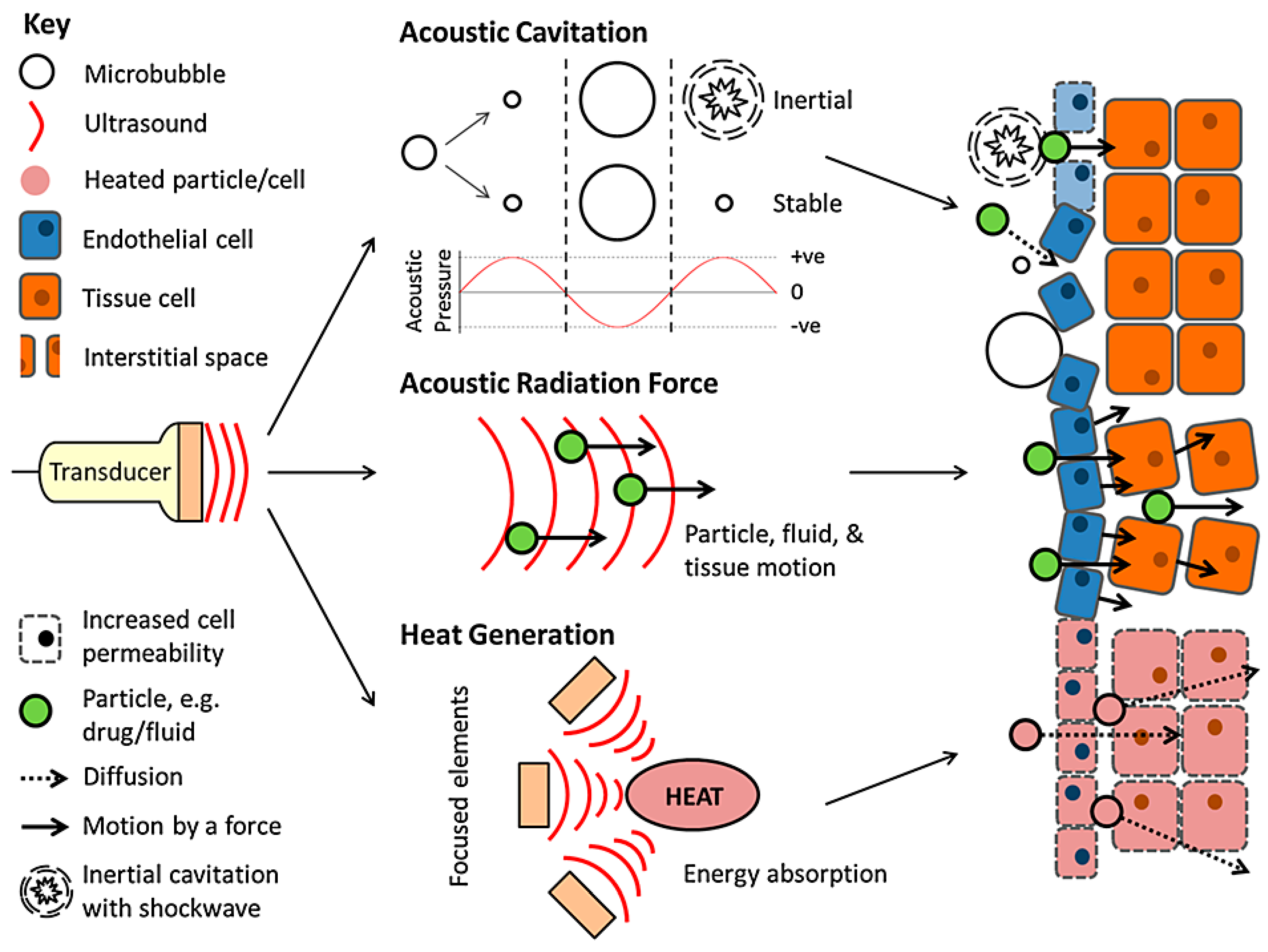
3. Ultrasound
3.1. Thermal Delivery
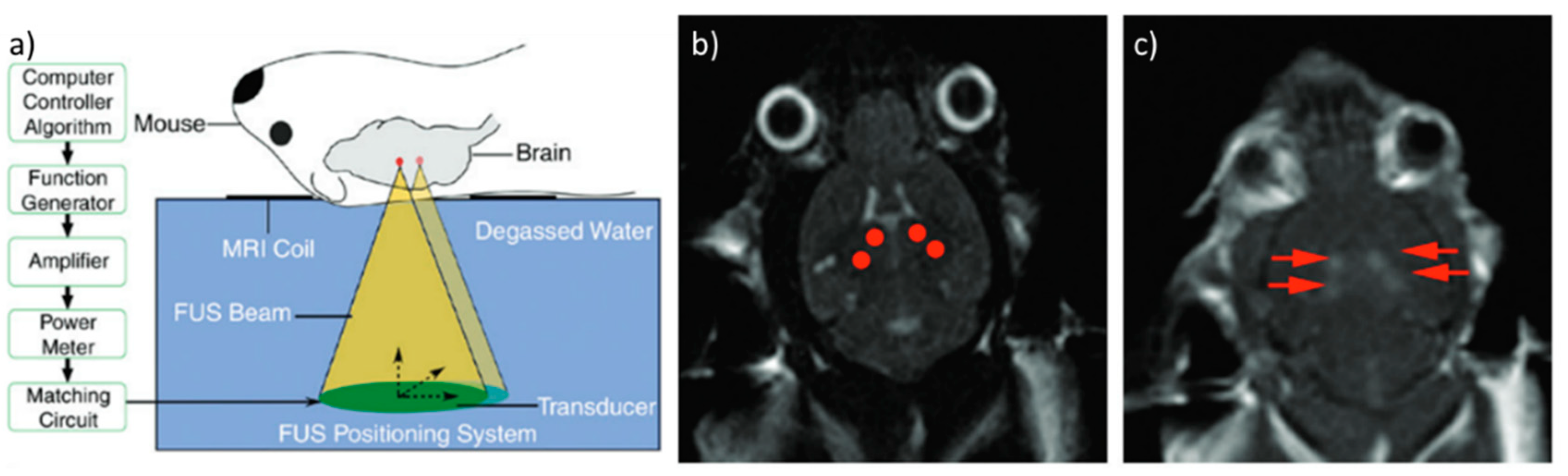
3.2. Mechanical Delivery
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). Cancer. Available online: http://www.who.int/cancer/en/ (accessed on 22 September 2018).
- Horsman, M.R.; Overgaard, J. The impact of hypoxia and its modification of the outcome of radiotherapy. J. Radiat. Res. 2016, 57, i90–i98. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.; Singh, A.; Ganta, S.; Amiji, M.M. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1784–1802. [Google Scholar] [CrossRef] [PubMed]
- Futreal, P.A.; Coin, L.; Marshall, M.; Down, T.; Hubbard, T.; Wooster, R.; Rahman, N.; Stratton, M.R. A census of human cancer genes. Nat. Rev. Cancer 2004, 4, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A comprehensive survey of ras mutations in cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to drug delivery in solid tumors. Tissue Barriers 2014, 2, e29528. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.D. Physical barriers to drug delivery in tumors. Crit. Rev. Oncol. Hematol. 1993, 14, 29–39. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.C. Tumor endothelial cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006536. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Transport of molecules in the tumor interstitium: A review. Cancer Res. 1987, 47, 3039–3051. [Google Scholar] [PubMed]
- Jain, R.K.; Baxter, L.T. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: Significance of elevated interstitial pressure. Cancer Res. 1988, 48, 7022–7032. [Google Scholar] [PubMed]
- Onozuka, H.; Tsuchihara, K.; Esumi, H. Hypoglycemic/hypoxic condition in vitro mimicking the tumor microenvironment markedly reduced the efficacy of anticancer drugs. Cancer Sci. 2011, 102, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of hifs and cell death pathways. Drug Resist. Updat. 2011, 14, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A.; Boukerroui, D. Ultrasound image segmentation: A survey. IEEE Trans. Med. Imaging 2006, 25, 987–1010. [Google Scholar] [CrossRef] [PubMed]
- Aaron, F.; Dónal, B.D.; Cardinal, H.N. Three-dimensional ultrasound imaging. Phys. Med. Biol. 2001, 46, R67. [Google Scholar]
- Jean, P.; Clement, P.; Juan Esteban, A.; Marion, I.; Mathias, F.; Jean-Luc, G.; Mickael, T.; Mathieu, P. 3D ultrafast ultrasound imaging in vivo. Phys. Med. Biol. 2014, 59, L1–L13. [Google Scholar]
- Vallancien, G.; Harouni, M.; Veillon, B.; Mombet, A.; Prapotnich, D.; Brisset, J.M.; Bougaran, J. Focused extracorporeal pyrotherapy: Feasibility study in man. J. Endourol. 1992, 6, 173–181. [Google Scholar] [CrossRef]
- Tempany, C.M.C.; Stewart, E.A.; McDannold, N.; Quade, B.J.; Jolesz, F.A.; Hynynen, K. MR imaging–guided focused ultrasound surgery of uterine leiomyomas: A feasibility study. Radiology 2003, 226, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Gedroyc, W.M.W.; Tempany, C.M.C.; Quade, B.J.; Inbar, Y.; Ehrenstein, T.; Shushan, A.; Hindley, J.T.; Goldin, R.D.; David, M.; et al. Focused ultrasound treatment of uterine fibroid tumors: Safety and feasibility of a noninvasive thermoablative technique. Am. J. Obstet. Gynecol. 2003, 189, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Koes, B.W.; Bouter, L.M.; van Mameren, H.; Essers, A.H.M.; Verstegen, G.M.J.R.; Hofhuizen, D.M.; Houben, J.P.; Knipschild, P.G. Randomised clinical trial of manipulative therapy and physiotherapy for persistent back and neck complaints: Results of one year follow up. Br. Med. J. 1992, 304, 601–605. [Google Scholar] [CrossRef]
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.B.; Ursin, H.; et al. Chapter 4 european guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006, 15, s192–s300. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Edwards, D.A.; Blankschtein, D.; Langer, R. A mechanistic study of ultrasonically-enhanced transdermal drug delivery. J. Pharm. Sci. 1995, 84, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.E.; Blankschtein, D.; Langer, R. Low-frequency sonophoresis: Application to the transdermal delivery of macromolecules and hydrophilic drugs. Expert Opin. Drug Deliv. 2010, 7, 1415–1432. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Park, H.; Seo, J.; Lee, S. Sonophoresis in transdermal drug deliverys. Ultrasonics 2014, 54, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Sauerbruch, T.; Delius, M.; Paumgartner, G.; Holl, J.; Wess, O.; Weber, W.; Hepp, W.; Brendel, W. Fragmentation of gallstones by extracorporeal shock waves. N. Engl. J. Med. 1986, 314, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Browning, R.J.; Rajkumar, V.; Pedley, R.B.; Eckersley, R.J.; Blower, P.J. Prospects for enhancement of targeted radionuclide therapy of cancer using ultrasound. J. Label. Compd. Radiopharm. 2014, 57, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.R.; Ter Haar, G.R. High intensity focused ultrasound—Potential for cancer treatment. Br. J. Radiol. 1995, 68, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K. Mrighifu: A tool for image-guided therapeutics. J. Magn. Reson. Imaging 2011, 34, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, Z.B.; Cao, Y.D.; Chen, W.Z.; Bai, J.; Zou, J.Z.; Zhu, H. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br. J. Cancer 2003, 89, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cvetkovic, D.; Ma, C.M.; Chen, L. Quantitative study of focused ultrasound enhanced doxorubicin delivery to prostate tumor in vivo with mri guidance. Med. Phys. 2012, 39, 2780–2786. [Google Scholar] [CrossRef] [PubMed]
- Staruch, R.M.; Ganguly, M.; Tannock, I.F.; Hynynen, K.; Chopra, R. Enhanced drug delivery in rabbit vx2 tumours using thermosensitive liposomes and mri-controlled focused ultrasound hyperthermia. Int. J. Hyperther. 2012, 28, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Lili, C.; Zhaomei, M.; Paul, H.; Ma, C.M.; Annie, W.; Alan, P. MR-guided focused ultrasound: Enhancement of intratumoral uptake of [3 h]-docetaxel in vivo. Phys. Med. Biol. 2010, 55, 7399–7410. [Google Scholar]
- Paparel, P.; Chapelon, J.Y.; Bissery, A.; Chesnais, S.; Curiel, L.; Gelet, A. Influence of the docetaxel administration period (neoadjuvant or concomitant) in relation to hifu treatment on the growth of dunning tumors: Results of a preliminary study. Prostate Cancer Prostatic Dis. 2007, 11, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-S.; Liu, L.-X.; Gu, Y.-H.; Lin, Q.-F.; Guo, R.-H.; Shu, Y.-Q. The effect of endostatin and gemcitabine combined with hifu on the animal xenograft model of human pancreatic cancer. Biomed. Pharmacother. 2010, 64, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Paparel, P.; Curiel, L.; Chesnais, S.; Ecochard, R.; Chapelon, J.-Y.; Gelet, A. Synergistic inhibitory effect of high-intensity focused ultrasound combined with chemotherapy on dunning adenocarcinoma. BJU Int. 2005, 95, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhang, Y.; He, H.; Zhou, S.; Liu, Y.; Huang, P. Anticancer potency of cytotoxic drugs after exposure to high-intensity focused ultrasound in the presence of microbubbles and hematoporphyrin. Mol. Pharm. 2011, 8, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, L.; Zhang, S.; Xu, Y.; Fan, Y.; Zhang, L. Effects of high-intensity focused ultrasound on cisplatin-resistant human lung adenocarcinoma in vitro and in vivo. Acta Biochim. Biophys. Sin. 2017, 49, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-Y.; Cho, Y.J.; Choi, J.-J.; Choi, C.H.; Kim, T.-J.; Kim, B.-G.; Bae, D.-S.; Kim, Y.-S.; Lee, J.-W. The effect of high-intensity focused ultrasound in combination with cisplatin using a xenograft model of cervical cancer. Anticancer Res. 2012, 32, 5285–5289. [Google Scholar] [PubMed]
- Zhao, H.; Yang, G.; Wang, D.; Yu, X.; Zhang, Y.; Zhu, J.; Ji, Y.; Zhong, B.; Zhao, W.; Yang, Z.; et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs 2010, 21, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Yan, T.; Wang, G.; Zhao, W.; Zhang, T.; Zhou, D. High-intensity focused ultrasound therapy in combination with gemcitabine for unresectable pancreatic carcinoma. Ther. Clin. Risk Manag. 2016, 12, 687–691. [Google Scholar] [PubMed]
- Dudar, T.E.; Jain, R.K. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res. 1984, 44, 605–612. [Google Scholar] [PubMed]
- Bischof, J.C.; Padanilam, J.; Holmes, W.H.; Ezzell, R.M.; Lee, R.C.; Tompkins, R.G.; Yarmush, M.L.; Toner, M. Dynamics of cell membrane permeability changes at supraphysiological temperatures. Biophys. J. 1995, 68, 2608–2614. [Google Scholar] [CrossRef]
- Marmor, J.B. Interactions of hyperthermia and chemotherapy in animals. Cancer Res. 1979, 39, 2269–2276. [Google Scholar] [PubMed]
- Los, G.; van Vugt, M.J.H.; Pinedo, H.M. Response of peritoneal solid tumours after intraperitoneal chemohyperthermia treatment with cisplatin or carboplatin. Br. J. Cancer 1994, 69, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Bonnay, M.; Puizillou, J.M.; Antoun, S.; Demirdjian, S.; El Otmany, A.; Pignon, J.P.; Drouard-Troalen, L.; Ouellet, J.F.; Ducreux, M. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: Pharmacokinetics and tissue distribution. Ann. Oncol. 2002, 13, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Capitano, M.L.; Spernyak, J.A.; Schueckler, J.T.; Thomas, S.; Singh, A.K.; Evans, S.S.; Hylander, B.L.; Repasky, E.A. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 2011, 71, 3872–3880. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Horsman, M.R. Tumour perfusion and associated physiology: Characterization and significance for hyperthermia. Int. J. Hyperther. 2010, 26, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Werthmöller, N.; Frey, B.; Rückert, M.; Lotter, M.; Fietkau, R.; Gaipl, U.S. Combination of ionising radiation with hyperthermia increases the immunogenic potential of b16-f10 melanoma cells in vitro and in vivo. Int. J. Hyperther. 2016, 32, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.M.C. A review of immune therapy in cancer and a question: Can thermal therapy increase tumor response? Int. J. Hyperther. 2018, 34, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Grüll, H.; Langereis, S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using mri-guided high intensity focused ultrasound. J. Control. Release 2012, 161, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Browning, R.J.; Reardon, P.J.T.; Parhizkar, M.; Pedley, R.B.; Edirisinghe, M.; Knowles, J.C.; Stride, E. Drug delivery strategies for platinum-based chemotherapy. ACS Nano 2017, 11, 8560–8578. [Google Scholar] [CrossRef] [PubMed]
- Moy, A.J.; Tunnell, J.W. Combinatorial immunotherapy and nanoparticle mediated hyperthermia. Adv. Drug Deliv. Rev. 2017, 114, 175–183. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, Z.; Ran, H.; Zheng, Y.; Wang, D.; Xu, J.; Wang, Z.; Chen, Y.; Li, P. Nanoparticle-enhanced synergistic hifu ablation and transarterial chemoembolization for efficient cancer therapy. Nanoscale 2016, 8, 4324–4339. [Google Scholar] [CrossRef] [PubMed]
- Kopechek, J.A.; Park, E.; Mei, C.-S.; McDannold, N.J.; Porter, T.M. Accumulation of phase-shift nanoemulsions to enhance mr-guided ultrasound-mediated tumor ablation in vivo. J. Healthc. Eng. 2013, 4, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Qian, X.; Qian, K.; Zhang, W.; He, W.; Chen, Y.; Han, J.; Zhang, Y.; Yang, X.; Fan, L. Au nanoparticle-coated, plga-based hybrid capsules for combined ultrasound imaging and hifu therapy. J. Mater. Chem. B 2015, 3, 4213–4220. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Ran, H.; Zhou, Y.; Shen, H.; Chen, Y.; Chen, H.; Krupka, T.M.; Li, A.; Li, P.; et al. Superparamagnetic plga-iron oxide microcapsules for dual-modality us/mr imaging and high intensity focused us breast cancer ablation. Biomaterials 2012, 33, 5854–5864. [Google Scholar] [CrossRef] [PubMed]
- Thiriet, M.; Solovchuk, M.; Sheu, T.W.-H. Hifu treatment of liver cancer—Reciprocal effect of blood flow and us studied from a patient-specific configuration. In Computational Modeling of Objects Presented in Images. Fundamentals, Methods, and Applications: 4th International Conference, CompIMAGE 2014; Zhang, Y.J., Tavares, J.M.R.S., Eds.; Springer International Publishing: Pittsburgh, PA, USA, 2014; pp. 1–11. [Google Scholar]
- Harriet, L.-B.; Boon, T.; Eleanor, S.; Constantin, C.C. The effect of particle density on ultrasound-mediated transport of nanoparticles. Phys. Med. Biol. 2016, 61, 7906–7918. [Google Scholar]
- Raghavan, R. Theory for acoustic streaming in soft porous matter and its applications to ultrasound-enhanced convective delivery. J. Ther. Ultrasound 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Seidl, M.; Steinbach, P.; Wörle, K.; Hofstädter, F. Induction of stress fibres and intercellular gaps in human vascular endothelium by shock-waves. Ultrasonics 1994, 32, 397–400. [Google Scholar] [CrossRef]
- Hancock, H.A.; Smith, L.H.; Cuesta, J.; Durrani, A.K.; Angstadt, M.; Palmeri, M.L.; Kimmel, E.; Frenkel, V. Investigations into pulsed high-intensity focused ultrasound–enhanced delivery: Preliminary evidence for a novel mechanism. Ultrasound Med. Biol. 2009, 35, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Reinz, E.; Jenne, J.W.; Fatar, M.; Schmidt-Glenewinkel, H.; Hennerici, M.G.; Meairs, S. Reorganization of gap junctions after focused ultrasound blood-brain barrier opening in the rat brain. J. Cereb. Blood Flow Metab. 2010, 30, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.E.; Vo, H.; Angstadt, M.; Li, K.P.C.; Quinn, T.; Frenkel, V. Pulsed high intensity focused ultrasound mediated nanoparticle delivery: Mechanisms and efficacy in murine muscle. Ultrasound Med. Biol. 2009, 35, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Mesiwala, A.H.; Farrell, L.; Wenzel, H.J.; Silbergeld, D.L.; Crum, L.A.; Winn, H.R.; Mourad, P.D. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med. Biol. 2002, 28, 389–400. [Google Scholar] [CrossRef]
- Lizzi, F.L.; Muratore, R.; Deng, C.X.; Ketterling, J.A.; Alam, S.K.; Mikaelian, S.; Kalisz, A. Radiation-force technique to monitor lesions during ultrasonic therapy. Ultrasound Med. Biol. 2003, 29, 1593–1605. [Google Scholar] [CrossRef]
- Frenkel, V.; Deng, C.; O’Neill, B.E.; Quijano, J.; Stone, M.J.; Dromi, S.; Hunter, F.; Xie, J.; Quinn, T.P.; Wood, B.J.; et al. Pulsed-high intensity focused ultrasound (hifu) exposures for enhanced delivery of therapeutics: Mechanisms and applications. AIP Conf. Proc. 2006, 829, 528–532. [Google Scholar]
- Palmeri, M.L.; Sharma, A.C.; Bouchard, R.R.; Nightingale, R.W.; Nightingale, K.R. A finite-element method model of soft tissue response to impulsive acoustic radiation force. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Percutaneous penetration of anticancer agents: Past, present and future. Biomed. Pharmacother. 2016, 84, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Kim, Y.-S.; Yang, J.; Sun, W.C.; Park, H.; Chae, S.Y.; Namgung, M.-S.; Choi, K.-S. Pulsed high-intensity focused ultrasound therapy enhances targeted delivery of cetuximab to colon cancer xenograft model in mice. Ultrasound Med. Biol. 2013, 39, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, V.; Etherington, A.; Greene, M.; Quijano, J.; Xie, J.; Hunter, F.; Dromi, S.; Li, K.C.P. Delivery of liposomal doxorubicin (doxil) in a breast cancer tumor model: Investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Acad. Radiol. 2006, 13, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.O.; Casey, G.D.; Tangney, M.; Cashman, J.; Collins, C.G.; Soden, D.M.; O’Sullivan, G.C. Effective tumor treatment using optimized ultrasound-mediated delivery of bleomycin. Ultrasound Med. Biol. 2008, 34, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Ahn, Y.D.; Lee, J.Y. In vivo study of enhanced chemotherapy combined with ultrasound image-guided focused ultrasound (usgfus) treatment for pancreatic cancer in a xenograft mouse model. Eur. Radiol. 2018, 28, 3710–3718. [Google Scholar] [CrossRef] [PubMed]
- Sponheim, N.; Hoff, L.; Waaler, A.; Muan, B.; Morris, H.; Holm, S.; Myrum, M.; de Jong, N.; Skotland, T. Albunex-a new ultrasound contrast agent. In Proceedings of the International Conference onAcoustic Sensing and Imaging, London, UK, 29–30 March 1993; pp. 103–108. [Google Scholar]
- De Jong, N. Improvements in ultrasound contrast agents. IEEE Eng. Med. Biol. Mag. 1994, 15, 72–82. [Google Scholar] [CrossRef]
- Christiansen, C.; Vebner, A.J.; Muan, B.; Vik, H.; Haider, T.; Nicolaysen, H.; Skotland, T. Lack of an immune response to albunex, a new ultrasound contrast agent based on air-filled albumin microspheres. Int. Arch. Allergy Immunol. 1994, 104, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Arditi, M.; Barrau, M.-B.; Brochot, J.; Broillet, A.; Ventrone, R.; Yan, F. BR1: A new ultrasonographic contrast agent based on sulfur hexafluoride-filled microbubbles. Investig. Radiol. 1995, 30, 451–457. [Google Scholar] [CrossRef]
- Schneider, M. Sonovue, a new ultrasound contrast agent. Eur. Radiol. 1999, 9, S347–S348. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M. Characteristics of sonovue™. Echocardiography 1999, 16, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.X.; Sieling, F.; Pan, H.; Cui, J. Ultrasound-induced cell membrane porosity. Ultrasound Med. Biol. 2004, 30, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Browning, R.J.; Mulvana, H.; Tang, M.; Hajnal, J.V.; Wells, D.J.; Eckersley, R.J. Influence of needle gauge on in vivo ultrasound and microbubble-mediated gene transfection. Ultrasound Med. Biol. 2011, 37, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Browning, R.J.; Mulvana, H.; Tang, M.-X.; Hajnal, J.V.; Wells, D.J.; Eckersley, R.J. Effect of albumin and dextrose concentration on ultrasound and microbubble mediated gene transfection in vivo. Ultrasound Med. Biol. 2012, 38, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Alter, J.; Sennoga, C.A.; Lopes, D.M.; Eckersley, R.J.; Wells, D.J. Microbubble stability is a major determinant of the efficiency of ultrasound and microbubble mediated in vivo gene transfer. Ultrasound Med. Biol. 2009, 35, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Fokong, S.; Theek, B.; Wu, Z.; Koczera, P.; Appold, L.; Jorge, S.; Resch-Genger, U.; van Zandvoort, M.; Storm, G.; Kiessling, F.; et al. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J. Control. Release 2012, 163, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Sirsi, S.R.; Borden, M.A. State-of-the-art materials for ultrasound-triggered drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.E.; Allen, J.S.; Dayton, P.A.; Chomas, J.E.; Klibanov, A.L.; Ferrara, K.W. Experimental and theoretical evaluation of microbubble behavior: Effect of transmitted phase and bubble size. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Jeffries, K.; Browning, R.J.; Meng-Xing, T.; Dunsby, C.; Eckersley, R.J. In vivo acoustic super-resolution and super-resolved velocity mapping using microbubbles. IEEE Trans. Med. Imaging 2015, 34, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Couture, O.; Hingot, V.; Heiles, B.; Muleki-Seya, P.; Tanter, M. Ultrasound localization microscopy and super-resolution: A state of the art. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.S.; Lee, J.J.; Taha, A.A.; Avgerinos, E.; Chaer, R.A. Vascular applications of contrast-enhanced ultrasound imaging. J. Vasc. Surg. 2017, 66, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.Y.; Kennedy, A.M.; Shea, J.E.; Scaife, C.L.; Nam, K.-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J. Control. Release 2009, 138, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.; Nam, K.-H.; Gupta, R.; Gao, Z.; Mohan, P.; Payne, A.; Todd, N.; Liu, X.; Kim, T.; Shea, J.; et al. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J. Control. Release 2011, 153, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jin, Y.; Zhou, W.; Zhao, J. Ultrasound-triggered drug release and enhanced anticancer effect of doxorubicin-loaded poly(d,l-lactide-co-glycolide)-methoxy-poly(ethylene glycol) nanodroplets. Ultrasound Med. Biol. 2011, 37, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Kwan, J.J.; Shah, A.R.; Coussios, C.-C.; Carlisle, R.C. Exploitation of sub-micron cavitation nuclei to enhance ultrasound-mediated transdermal transport and penetration of vaccines. J. Control. Release 2016, 238, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.J.; Myers, R.; Coviello, C.M.; Graham, S.M.; Shah, A.R.; Stride, E.; Carlisle, R.C.; Coussios, C.C. Ultrasound-propelled nanocups for drug delivery. Small 2015, 11, 5305–5314. [Google Scholar] [CrossRef] [PubMed]
- Loughran, J.; Eckersley, R.J.; Tang, M.-X. Modeling non-spherical oscillations and stability of acoustically driven shelled microbubbles. J. Acoust. Soc. Am. 2012, 131, 4349–4357. [Google Scholar] [CrossRef] [PubMed]
- Hoff, L.; Sontum, P.C.; Hovem, J.M. Oscillations of polymeric microbubbles: Effect of the encapsulating shell. J. Acoust. Soc. Am. 2000, 107, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Sboros, V. Response of contrast agents to ultrasound. Adv. Drug Deliv. Rev. 2008, 60, 1117–1136. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Tachibana, S. The use of ultrasound for drug delivery. Echocardiography 2001, 18, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Van Wamel, A.; Kooiman, K.; Harteveld, M.; Emmer, M.; ten Cate, F.J.; Versluis, M.; de Jong, N. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. J. Control. Release 2006, 112, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bekeredjian, R.; Grayburn, P.A.; Shohet, R.V. Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine. J. Am. Coll. Cardiol. 2005, 45, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Hernot, S.; Klibanov, A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Wang, H.E.; Lin, G.L.; Lin, H.H.; Wong, T.T. Evaluation of the increase in permeability of the blood-brain barrier during tumor progression after pulsed focused ultrasound. Int. J. Nanomed. 2012, 7, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Dimcevski, G.; Kotopoulis, S.; Bjånes, T.; Hoem, D.; Schjøtt, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; McCormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 2016, 243, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Lentacker, I.; Geers, B.; Demeester, J.; De Smedt, S.C.; Sanders, N.N. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: Cytotoxicity and mechanisms involved. Mol. Ther. 2009, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lentacker, I.; De Smedt, S.C.; Sanders, N.N. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter 2009, 5, 2161–2170. [Google Scholar] [CrossRef]
- Rapoport, N.; Gao, Z.; Kennedy, A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J. Natl. Cancer Inst. 2007, 99, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, Y.; Ran, H.; Tan, J.; Lin, Y.; Zhang, Q.; Ren, J.; Wang, Z. Ultrasound triggered drug release from 10-hydroxycamptothecin-loaded phospholipid microbubbles for targeted tumor therapy in mice. J. Control. Release 2012, 162, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Yamaguchi, K.; Feril Jr, L.B.; Endo, H.; Ogawa, K.; Tachibana, K.; Nakayama, J. Synergistic inhibition of malignant melanoma proliferation by melphalan combined with ultrasound and microbubbles. Ultrason. Sonochem. 2011, 18, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Sanches, P.G.; Rossin, R.; Böhmer, M.; Tiemann, K.; Grüll, H. Real-time imaging and kinetics measurements of focused ultrasound-induced extravasation in skeletal muscle using spect/ct. J. Control. Release 2013, 168, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Yao-Sheng, T.; Fotios, V.; James, J.C.; Thomas, D.; Kirsten, S.; Elisa, E.K. In vivo transcranial cavitation threshold detection during ultrasound-induced blood–brain barrier opening in mice. Phys. Med. Biol. 2010, 55, 6141–6155. [Google Scholar]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Targeted disruption of the blood–brain barrier with focused ultrasound: Association with cavitation activity. Phys. Med. Biol. 2006, 51, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Wible, J.H.; Galen, K.P.; Wojdyla, J.K.; Hughes, M.S.; Klibanov, A.L.; Brandenburger, G.H. Microbubbles induce renal hemorrhage when exposed to diagnostic ultrasound in anesthetized rats. Ultrasound Med. Biol. 2002, 28, 1535–1546. [Google Scholar] [CrossRef]
- Timbie, K.; Zhang, C.; Nance, E.; Song, J.; Miller, W.; Hanes, J.; Price, R. Ultrasound-mediated delivery of brain-penetrating nanoparticles across the blood-tumor barrier. J. Ther. Ultrasound 2015, 3, P34. [Google Scholar] [CrossRef]
- Burgess, A.; Dubey, S.; Yeung, S.; Hough, O.; Eterman, N.; Aubert, I.; Hynynen, K. Alzheimer disease in a mouse model: Mr imaging–guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology 2014, 273, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused ultrasound-mediated drug delivery through the blood–brain barrier. Expert Rev. Neurother. 2015, 15, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Suppiah, S.; Surendrakumar, S.; Bigioni, L.; Lipsman, N. Low-intensity mr-guided focused ultrasound mediated disruption of the blood-brain barrier for intracranial metastatic diseases. Front. Oncol. 2018, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Schlicher, R.K.; Radhakrishna, H.; Tolentino, T.P.; Apkarian, R.P.; Zarnitsyn, V.; Prausnitz, M.R. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med. Biol. 2006, 32, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Lentacker, I.; Wang, N.; Vandenbroucke, R.E.; Demeester, J.; De Smedt, S.C.; Sander, N.N. Ultrasound exposure of lipoplex loaded microbubbles facilitates direct cytoplasmic entry of the lipoplexes. Mol. Pharm. 2009, 6, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.B.; Shohet, R.V. Tiny bubbles and endocytosis? Circ. Res. 2009, 104, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Meijering, B.D.M.; Juffermans, L.J.M.; van Wamel, A.; Henning, R.H.; Zuhorn, I.S.; Emmer, M.; Versteilen, A.M.G.; Paulus, W.J.; van Gilst, W.H.; Kooiman, K.; et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ. Res. 2009, 104, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Chumakova, O.V.; Liopo, A.V.; Andreev, V.G.; Cicenaite, I.; Evers, B.M.; Chakrabarty, S.; Pappas, T.C.; Esenaliev, R.O. Composition of plga and pei/DNA nanoparticles improves ultrasound-mediated gene delivery in solid tumors in vivo. Cancer Lett. 2008, 261, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, L.; Li, T.; Shen, X.; Yan, J.; Zuo, L.; Wu, C.; Liu, Y. Multifunctional plga nanobubbles as theranostic agents: Combining doxorubicin and p-gp sirna co-delivery into human breast cancer cells and ultrasound cellular imaging. J. Biomed. Nanotechnol. 2015, 11, 2124–2136. [Google Scholar] [CrossRef] [PubMed]
- Eggen, S.; Fagerland, S.-M.; Mørch, Ý.; Hansen, R.; Søvik, K.; Berg, S.; Furu, H.; Bøhn, A.D.; Lilledahl, M.B.; Angelsen, A.; et al. Ultrasound-enhanced drug delivery in prostate cancer xenografts by nanoparticles stabilizing microbubbles. J. Control. Release 2014, 187, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, H.; Sheng, Y.; Kamila, S.; Logan, K.; Thomas, K.; Callan, B.; Taylor, M.A.; Love, M.; O’Rourke, D.; Kelly, P.; et al. Gemcitabine loaded microbubbles for targeted chemo-sonodynamic therapy of pancreatic cancer. J. Control. Release 2018, 279, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mullin, L.B.; Phillips, L.C.; Dayton, P.A. Nanoparticle delivery enhancement with acoustically activated microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-G.; Xu, H.-X.; Chen, H.-R. Nano/microparticles and ultrasound contrast agents. World J. Radiol. 2013, 5, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Esenaliev, R. Plga nanoparticles for ultrasound-mediated gene delivery to solid tumors. J. Drug Deliv. 2012, 2012, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.W.; Hsiang, Y.-H.J.; Alexander, E.; Kilbanov, A.L.; Price, R.J. Covalently linking poly(lactic-co-glycolic acid) nanoparticles to microbubbles before intravenous injection improves their ultrasound-targeted delivery to skeletal muscle. Small 2011, 7, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.W.; Alexander, E.; Timbie, K.; Kilbanov, A.L.; Price, R.J. Ultrasound-activated agents comprised of 5fu-bearing nanoparticles bonded to microbubbles inhibit solid tumor growth and improve survival. Mol. Ther. 2014, 22, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jia, Y.; Qu, F.; Sun, Y.; Wang, P.; Zhang, K.; Xu, C.; Liu, Q.; Wang, X. Ultrasound-responsive polymeric micelles for sonoporation-assisted site-specific therapeutic action. ACS Appl. Mater. Interfaces 2017, 9, 25706–25716. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Mulvana, H.; Browning, R.J.; Luan, Y.; Jong, N.D.; Tang, M.X.; Eckersley, R.J.; Stride, E. Characterization of contrast agent microbubbles for ultrasound imaging and therapy research. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 232–251. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browning, R.J.; Stride, E. Microbubble-Mediated Delivery for Cancer Therapy. Fluids 2018, 3, 74. https://doi.org/10.3390/fluids3040074
Browning RJ, Stride E. Microbubble-Mediated Delivery for Cancer Therapy. Fluids. 2018; 3(4):74. https://doi.org/10.3390/fluids3040074
Chicago/Turabian StyleBrowning, Richard J., and Eleanor Stride. 2018. "Microbubble-Mediated Delivery for Cancer Therapy" Fluids 3, no. 4: 74. https://doi.org/10.3390/fluids3040074
APA StyleBrowning, R. J., & Stride, E. (2018). Microbubble-Mediated Delivery for Cancer Therapy. Fluids, 3(4), 74. https://doi.org/10.3390/fluids3040074




