Flow Structure and Force Generation on Flapping Wings at Low Reynolds Numbers Relevant to the Flight of Tiny Insects
Abstract
:1. Introduction
2. Methods
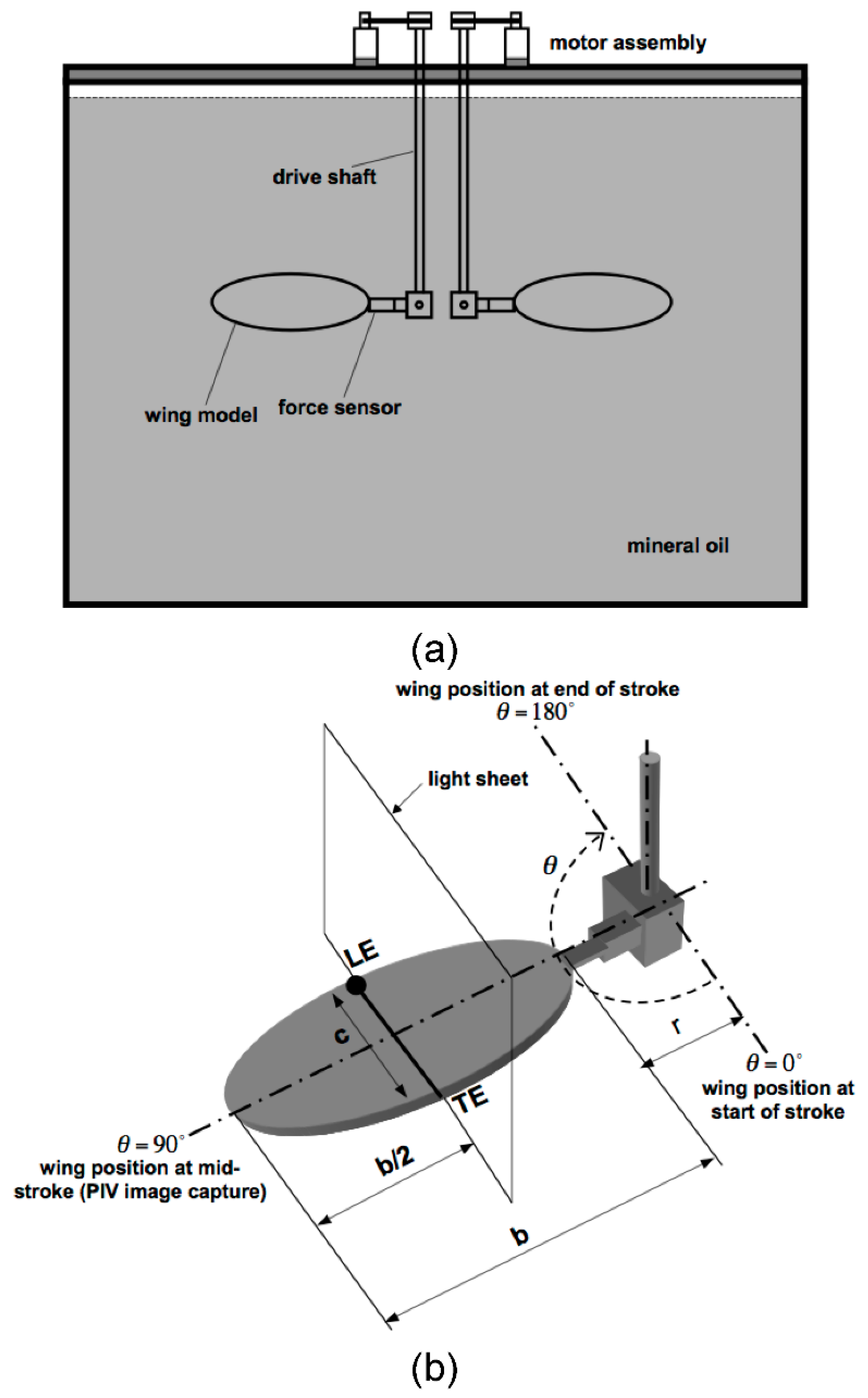
2.1. Dynamically Scaled Model Wing
2.2. Kinematics
2.3. Flow Visualization
2.4. Numerical Method
2.5. Computational Wing
2.6. Grid Convergence Study
3. Results
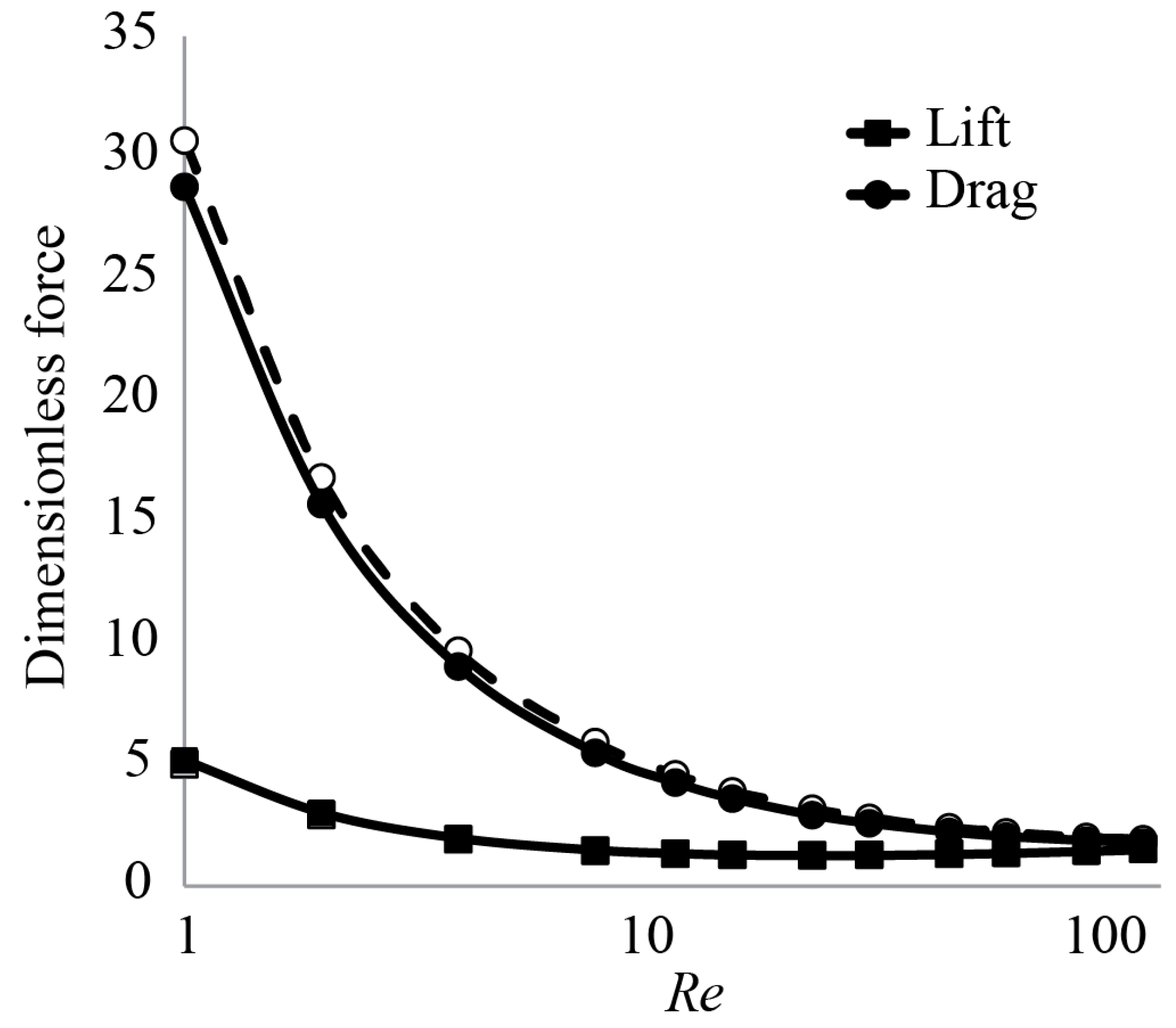
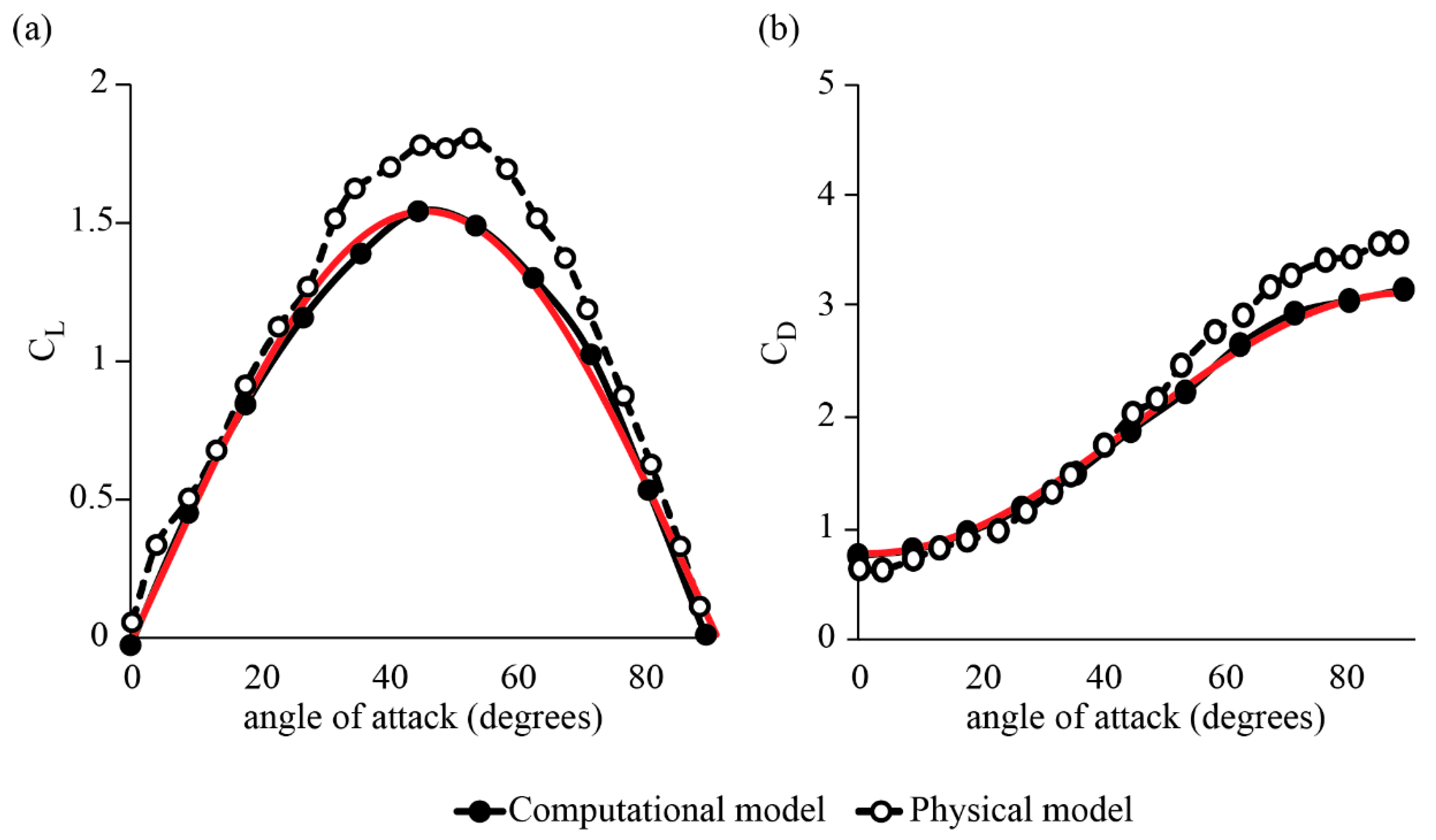
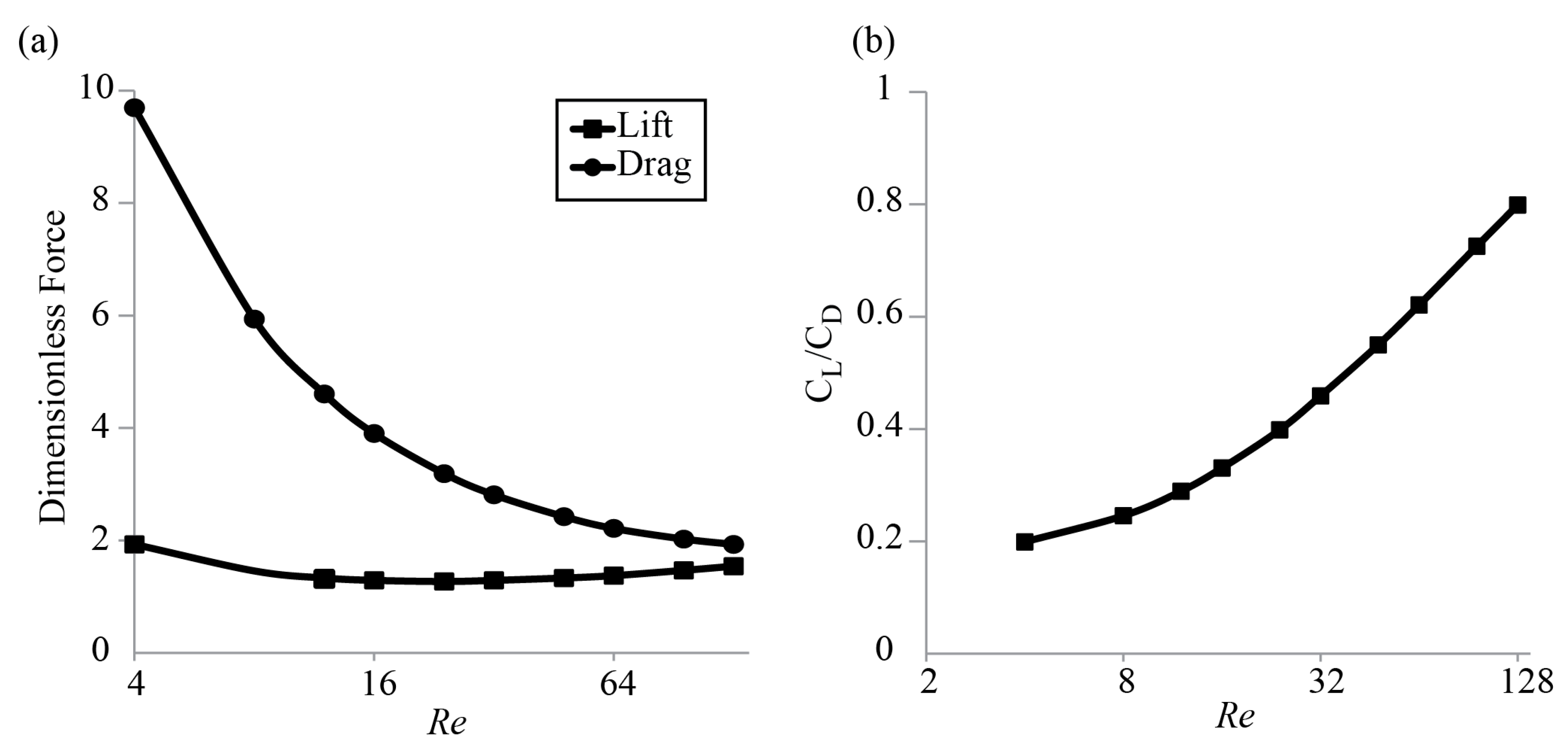
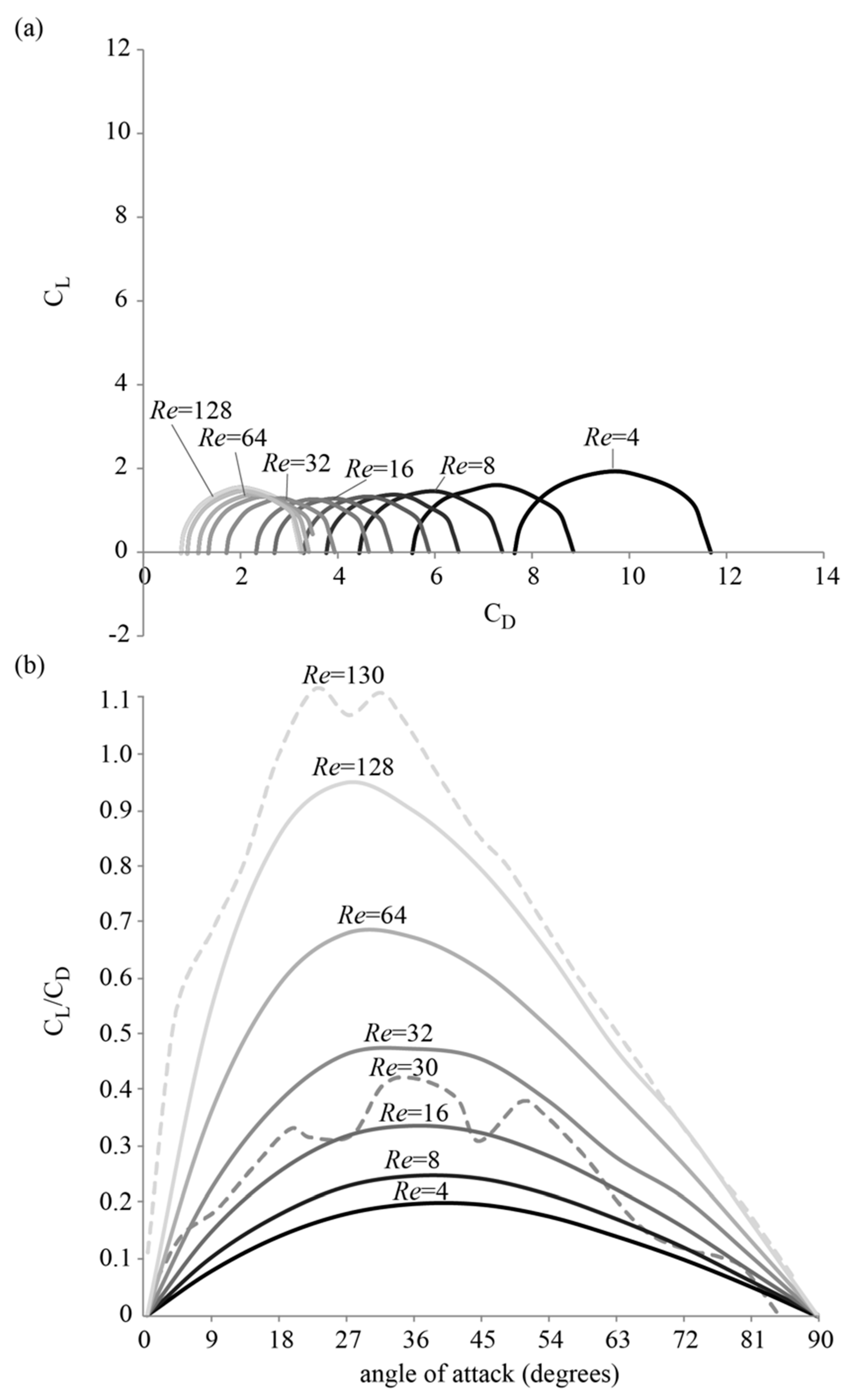
3.1. Forces around a Revolving Three-Dimensional Wing at Low Reynolds Numbers
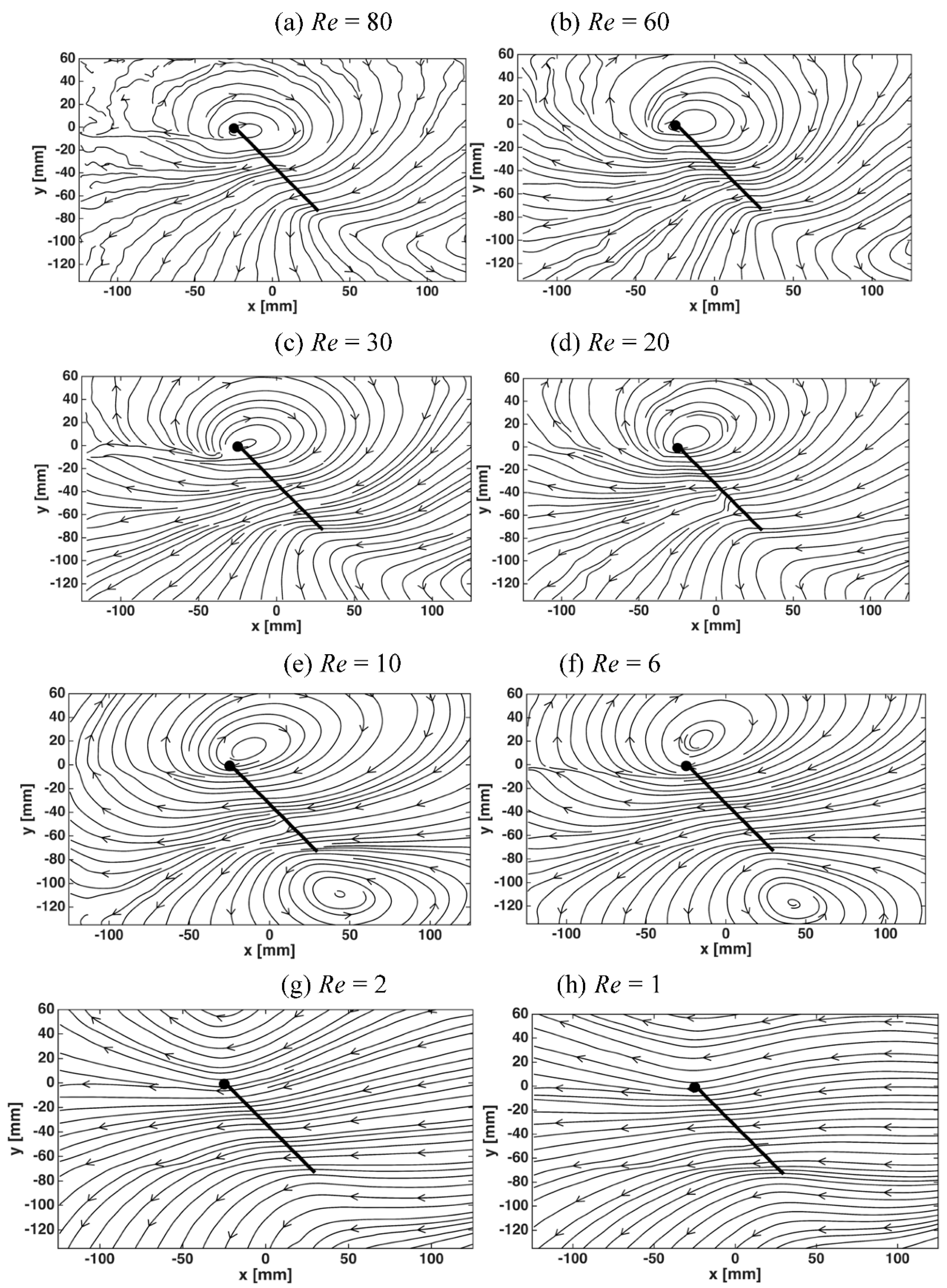
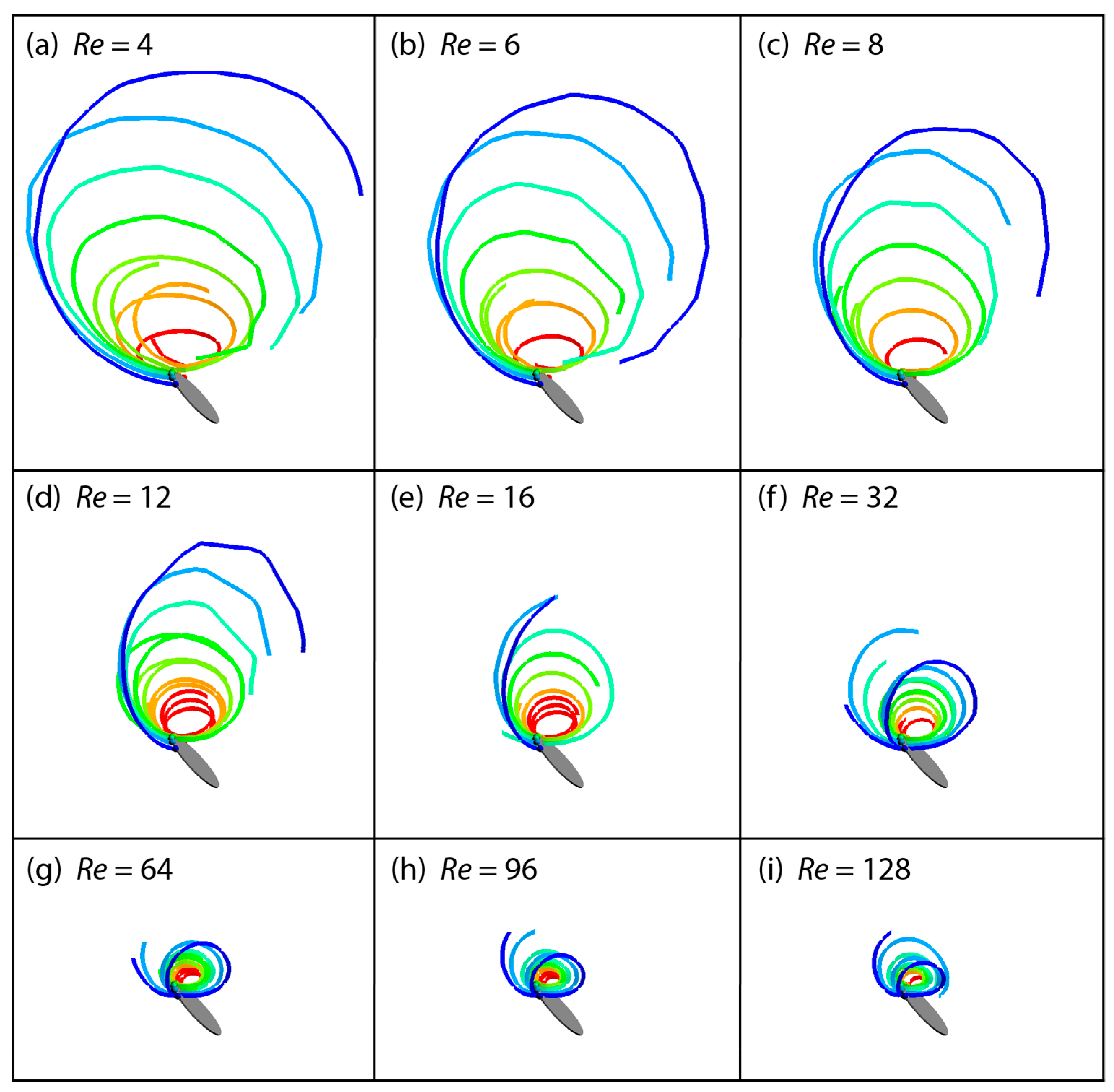
3.2. Flow Structures at Low Reynolds Numbers
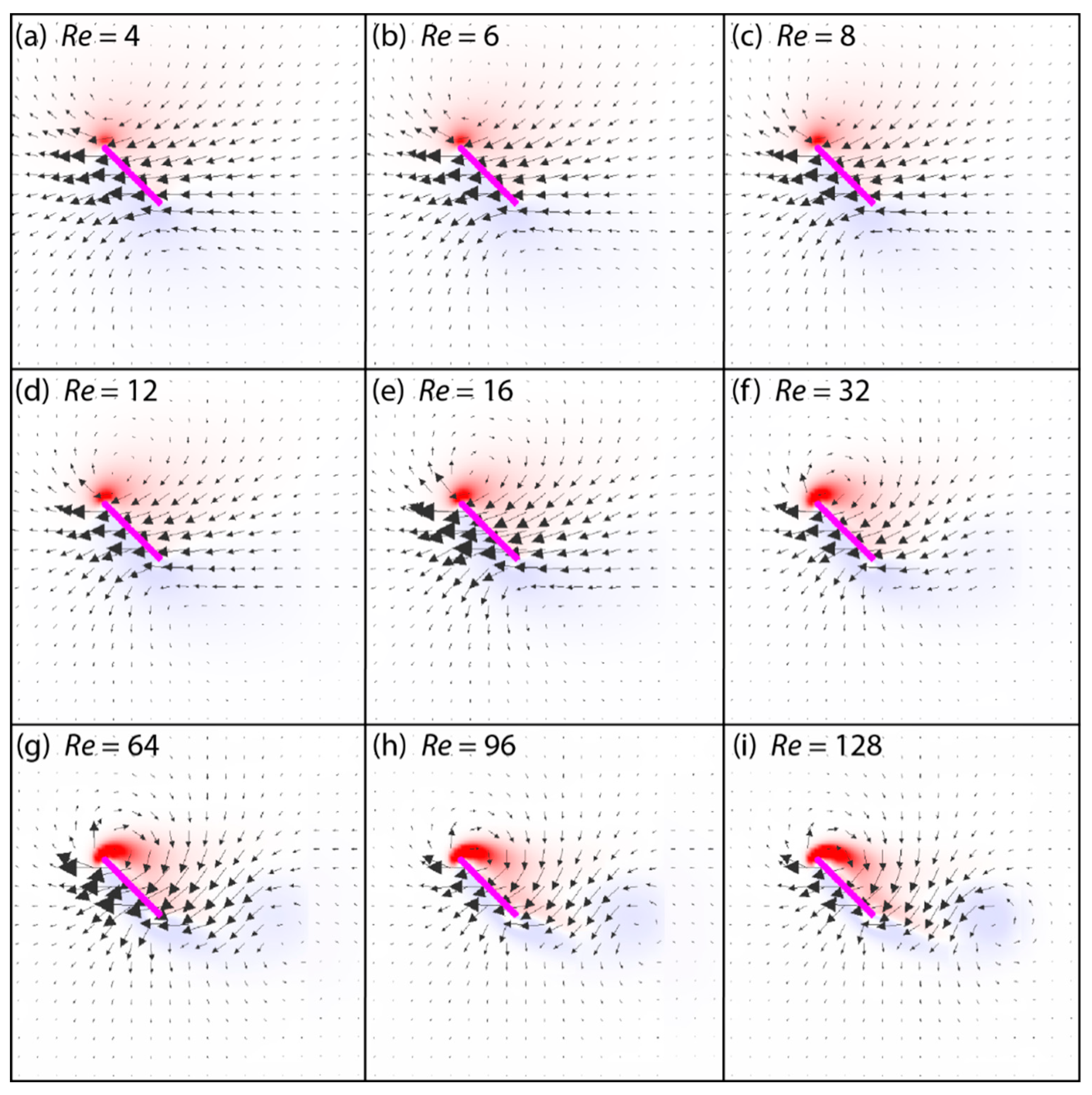
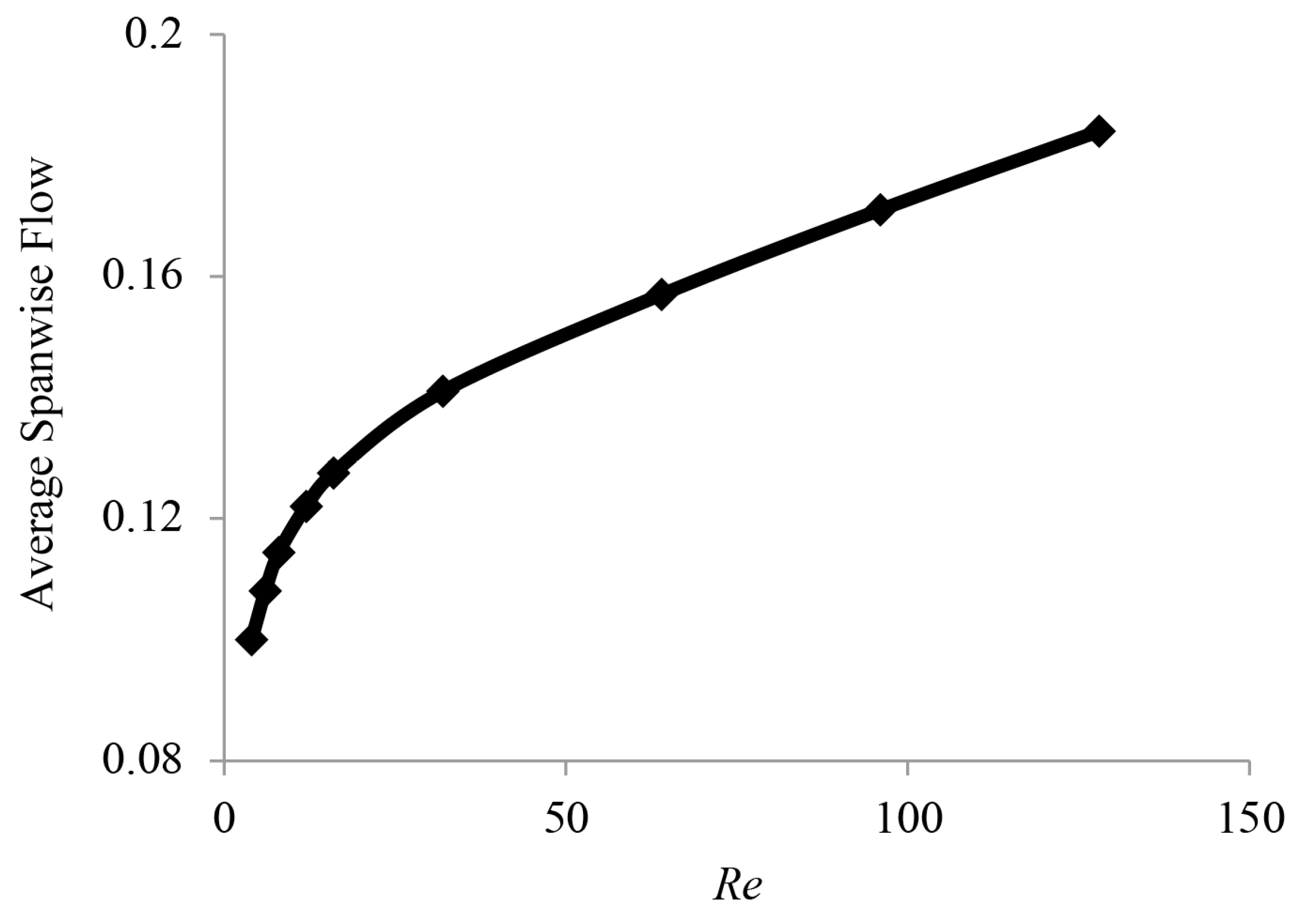
3.3. Spanwise Flow
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

Appendix C

References
- Huber, J.T.; Noyes, J.S. A new genus and species of fairyfly, Tinkerbella nana (Hymenoptera, Mymaridae), with comments on its sister genus Kikiki, and discussion on small size limits in arthropods. J. Hymenopt. Res. 2013, 32, 17–44. [Google Scholar] [CrossRef]
- Palmer, J.M.; Reddy, D.V.R.; Wightman, J.A.; Rao, G.V.R. New information on the thrips vectors of tomato spotted wilt virus in groundnut crops in India. Int. Arachis Newsl. 1990, 7, 24–25. [Google Scholar]
- Crespi, B.J.; Carmean, D.A.; Chapman, T.W. Ecology and evolution of galling thrips and their allies. Annu. Rev. Entomol. 1997, 42, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Dowton, M. Hymenoptera: Evolution, Biodiversity and Biological Control; Csiro Publishing: Clayton, Australia, 2000. [Google Scholar]
- Strickland, C.; Kristensen, N.P.; Miller, L.A. Inferring stratified parasitoid dispersal mechanisms and parameters from coarse data using mathematical and Bayesian methods. R. Soc. Interface 2017, 215, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- Shogren, C.; Paine, T.D. Economic Benefit for Cuban Laurel Thrips Biological Control. J. Econ. Entomol. 2016, 109, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Terry, I. Thrips and weevils as dual, specialist pollinators of the Australian cycad Macrozamia communis (Zamiaceae). Int. J. Plant Sci. 2001, 162, 1293–1305. [Google Scholar] [CrossRef]
- Terry, I. Thrips: The primeval pollinators. In Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera, Calabria, Italy, 2–7 July 2001; Australian National Insect Collection: Canberra, Australia, 2002. [Google Scholar]
- Jones, D.R. Plant viruses transmitted by thrips. Eur. J. Plant Pathol. 2005, 113, 119–157. [Google Scholar] [CrossRef]
- Ullman, D.E.; Meideros, R.; Campbell, L.R.; Whitfield, A.E.; Sherwood, J.L.; German, T.L. Thrips as vectors of tospoviruses. Adv. Bot. Res. 2002, 36, 113–140. [Google Scholar]
- Santhanakrishnan, A.; Robinson, A.K.; Jones, S.; Low, A.A.; Gadi, S.; Hedrick, T.L.; Miller, L.A. Clap and fling mechanism with interacting porous wings in tiny insect flight. J. Exp. Biol. 2014, 217, 3898–3909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis-Fogh, T. Quick estimates of flight fitness in hovering animals, including novel mechanisms for lift production. J. Exp. Biol. 1973, 59, 169–230. [Google Scholar]
- Bomphrey, R.J.; Nakata, T.; Phillips, N.; Walker, S.W. Smart wing rotation and trailing-edge vortices enable high frequency mosquito flight. Nature 2017, 544, 92–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidi, G.; Weihs, D. Flow around a Comb Wing in Low-Reynolds-Number Flow. AIAA J. 2012, 50, 249–253. [Google Scholar] [CrossRef]
- Ellington, C.P. Wing mechanics and take-off preparation of Thrips (Thysanoptera). J. Exp. Biol. 1980, 85, 129–136. [Google Scholar]
- Jones, S.K.; Yun, Y.J.; Hedrick, T.L.; Griffith, B.E.; Miller, L.A. Bristles reduce the force required to ‘fling’ wings apart in the smallest insects. J. Exp. Biol. 2016, 219, 3759–3772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolomenskiy, D.; Moffatt, H.; Farge, M.; Schneider, K. Two-and three-dimensional numerical simulations of the clap–fling–sweep of hovering insects. J. Fluids Struct. 2011, 27, 784–791. [Google Scholar] [CrossRef]
- Kolomenskiy, D.; Moffatt, H.K.; Farge, M.; Schneider, K. Vorticity generation during the clap–fling–sweep of some hovering insects. Theor. Comput. Fluid Dyn. 2010, 24, 209–215. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, D. Aerodynamics of a translating comb-like plate inspired by a fairyfly wing. Phys. Fluids 2017, 29, 081902. [Google Scholar] [CrossRef]
- Miller, L.A.; Peskin, C.S. When vortices stick: An aerodynamic transition in tiny insect flight. J. Exp. Biol. 2004, 207, 3073–3088. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.A.; Peskin, C.S. A computational fluid dynamics study of ’clap and fling’ in the smallest insects. J. Exp. Biol. 2005, 208, 17. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.A.; Peskin, C.S. Flexible clap and fling in tiny insect flight. J. Exp. Biol. 2009, 212, 3076–3090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Yu, X. Aerodynamic force generation in hovering flight in a tiny insect. AIAA J. 2006, 44, 1532–1540. [Google Scholar] [CrossRef]
- Sunada, S.; Takashima, H.; Hattori, T.; Yasuda, K.; Kawachi, K. Fluid-dynamic characteristics of a bristled wing. J. Exp. Biol. 2002, 205, 2737–2744. [Google Scholar] [PubMed]
- Wang, Z.J. Two dimensional mechanism for insect hovering. Phys. Rev. Lett. 2004, 85, 2216–2219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J. The role of drag in insect hovering. J. Exp. Biol. 2004, 207, 4147–4155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żbikowski, R. On aerodynamic modelling of an insect–like flapping wing in hover for micro air vehicles. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2002, 360, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Sun, M. Unsteady aerodynamic forces of a flapping wing. J. Exp. Biol. 2004, 207, 1137–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, N.; Gupta, A.; Sanghi, S.; Aono, H.; Shyy, W. Lift-drag and flow structures associated with the “clap and fling” motion. Phys. Fluids 2014, 26, 071906. [Google Scholar] [CrossRef]
- Jones, S.; Laurenza, R.; Hedrick, T.L.; Griffith, B.E.; Miller, L.A. Lift vs. drag based mechanisms for vertical force production in the smallest flying insects. J. Theor. Biol. 2015, 384, 105–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.J.; Birch, J.M.; Dickinson, M.H. Unsteady forces and flows in low Reynolds number hovering flight: Two-dimensional computations vs robotic wing experiments. J. Exp. Biol. 2004, 207, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Lentink, D.; Dickinson, M.H. Rotational accelerations stabilize leading edge vortices on revolving fly wings. J. Exp. Biol. 2009, 212, 2705–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Aono, H. Size effects on insect hovering aerodynamics: An integrated computational study. Bioinspir. Biomim. 2009, 4, 015002. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.D.; Lentink, D. Flapping wing aerodynamics: From insects to vertebrates. J. Exp. Biol. 2016, 219, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Ellington, C.P. The aerodynamics of hovering insect flight. III. Kinematics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 305, 41–78. [Google Scholar] [CrossRef]
- Ellington, C.P. The novel aerodynamics of insect flight: Applications to micro-air vehicles. J. Exp. Biol. 1999, 202, 3439–3448. [Google Scholar] [PubMed]
- Hedrick, T.L.; Combes, S.; Miller, L.A. Recent developments in the study of insect flight. Can. J. Zool. 2015, 92, 3898–3909. [Google Scholar] [CrossRef]
- Sane, S.P. The aerodynamics of insect flight. J. Exp. Biol. 2003, 206, 4191–4208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birch, J.M.; Dickson, W.B.; Dickinson, M.H. Force production and flow structure of the leading edge vortex on flapping wings at high and low Reynolds numbers. J. Exp. Biol. 2004, 207, 1063–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, M.H.; Lehmann, F.-O.; Sane, S.P. Wing rotation and the aerodynamic basis of insect flight. Science 1999, 284, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Sane, S.P.; Dickinson, M.H. The control of flight force by a flapping wing: Lift and drag production. J. Exp. Biol. 2001, 204, 2607–2626. [Google Scholar] [PubMed]
- Fauci, L.J.; Fogelson, A.L. Truncated Newton methods and the modeling of complex immersed elastic structures. Commun. Pure Appl. Math. 1993, 46, 787–818. [Google Scholar] [CrossRef]
- Fauci, L.J.; Peskin, C.S. A computational model of aquatic animal locomotion. J. Comput. Phys. 1988, 77, 85–108. [Google Scholar] [CrossRef]
- Herschlag, G.; Miller, L. Reynolds number limits for jet propulsion: A numerical study of simplified jellyfish. J. Theor. Biol. 2011, 285, 84–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, B.E.; Luo, X. Hybrid finite difference/finite element version of the immersed boundary method. Int. J. Numer. Methods Biomed. Eng. 2017, 33, e2888. [Google Scholar] [CrossRef] [PubMed]
- Geuzaine, C.; Remacle, J.F. Gmsh: A 3-D finite element mesh generator with built-in pre-and post-processing facilities. Int. J. Numer. Methods Eng. 2009, 79, 1309–1331. [Google Scholar] [CrossRef]
- Griffith, B.E.; Hornung, R.D.; McQueen, D.M.; Peskin, C.S. An adaptive, formally second order accurate version of the immersed boundary method. J. Comput. Phys. 2007, 223, 10–49. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; Karp-Boss, L.; Jumars, P.A.; Fauci, L. Hydrodynamic effects of spines: A different spin. Limnol. Oceanogr. Fluids Environ. 2011, 1, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Vo, G.D.; Heys, J. Biofilm Deformation in Response to Fluid Flow in Capillaries. Biotechnol. Bioeng. 2011, 108, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Tytell, E.D.; Hsu, C.Y.; Williams, T.L.; Cohen, A.H.; Fauci, L.J. Interactions between internal forces, body stiffness, and fluid environment in a neuromechanical model of lamprey swimming. Proc. Natl. Acad. Sci. USA 2010, 107, 19832–19837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, B.E. Immersed boundary model of aortic heart valve dynamics with physiological driving and loading conditions. Int. J. Numer. Methods Biomed. Eng. 2012, 28, 317–345. [Google Scholar] [CrossRef]
- Griffith, B.E.; Luo, X.Y.; McQueen, D.M.; Peskin, C.S. Simulating the fluid dynamics of natural and prosthetic heart valves using the immersed boundary method. Int. J. Appl. Mech. 2009, 1, 137–177. [Google Scholar] [CrossRef]
- Dickinson, M.H.; Gotz, K.G. Unsteady aerodynamic performance of model wings at low Reynolds numbers. J. Exp. Biol. 1993, 174, 45–64. [Google Scholar]
- Batchelor, G. An Introduction to Fluid Dynamics. Cambridge Mathematical Library, 2nd ed.; Cambridge University Press: Cambridge, UK, 2000; ISBN 978-0-521-66396-0. [Google Scholar]
- Usherwood, J.R.; Ellington, C.P. The aerodynamics of revolving wings: I. Model hawkmoth wings. J. Exp. Biol. 2002, 205, 1547–1564. [Google Scholar] [PubMed]
- Usherwood, J.R.; Ellington, C.P. The aerodynamics of revolving wings: II. Propeller force coefficients from mayfly to quail. J. Exp. Biol. 2002, 205, 1565–1576. [Google Scholar] [PubMed]
- Ellington, C.P.; van den Berg, C.; Willmott, A.P.; Thomas, A.L. Leading-edge vortices in insect flight. Nature 1996, 384, 626–630. [Google Scholar] [CrossRef]
- Birch, J.M.; Dickinson, M.H. Spanwise flow and the attachment of the leading-edge vortex on insect wings. Nature 2001, 412, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Harbig, R.; Sheridan, J.; Thompson, M. Reynolds number and aspect ratio effects on the leading-edge vortex for rotating insect wing planforms. J. Fluid Mech. 2013, 717, 166–192. [Google Scholar] [CrossRef]
- Aono, H.; Liang, F.; Liu, H. Near-and far-field aerodynamics in insect hovering flight: An integrated computational study. J. Exp. Biol. 2008, 211, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Ellington, C.P. The aerodynamics of hovering insect flight. IV. Aerodynamic mechanisms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 305, 79–113. [Google Scholar] [CrossRef]
- Lehmann, F.O.; Pick, S. The aerodynamic benefit of wing-wing interaction depends on stroke trajectory in flapping insect wings. J. Exp. Biol. 2007, 210, 1362–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, F.O.; Sane, S.P.; Dickinson, M. The aerodynamic effects of wing-wing interaction in flapping insect wings. J. Exp. Biol. 2005, 208, 3075–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lighthill, M.J. On the Weis-Fogh mechanism of lift generation. J. Fluid Mech. 1973, 60, 1–17. [Google Scholar] [CrossRef]
- Maxworthy, T. Experiments on the Weis-Fogh mechanism of lift generation by insects in hovering flight. Part 1. Dynamics of the fling. J. Fluid Mech. 1979, 93, 47–63. [Google Scholar] [CrossRef]
- Spedding, G.R.; Maxworthy, T. The generation of circulation and lift in a rigid two-dimensional fling. J. Fluid Mech. 1986, 165, 247–272. [Google Scholar] [CrossRef]
- Sun, M.; Xin, Y. Flows around two airfoils performing fling and subsequent translation and translation and subsequent clap. Acta Mech. Sin. 2003, 19, 13–117. [Google Scholar]
- Ellington, C. The aerodynamics of hovering insect flight. I. The quasi-steady analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 305, 1–15. [Google Scholar] [CrossRef]
- Imai, I. A New Method of Solving Oseen’s Equations and Its Application to the Flow Past anInclined Elliptic Cylinder. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1954, 224, 141–160. [Google Scholar] [CrossRef]
- In, K.M.; Choi, D.H.; Kim, M.-U. Two-dimensional viscous flow past a flat plate. Fluid Dyn. Res. 1995, 15, 13–24. [Google Scholar] [CrossRef]



| Dimensionless Parameter | Value |
|---|---|
| Chord Length | 0.0825 |
| Span Length | 0.165 |
| Radius of Wing Tip | 0.2011 |
| Sweep Distance (Radians) | |
| Final Simulation Time | 1 |
| Acceleration Time | 0.1 |
| Deceleration Time | 0.1 |
| Domain Length (L) | 1 |
| Number of Refinement Levels | 4 |
| Refinement Ratio | 1:4 |
| Time Step (dt) | 1 × 10−5 |
| Spatial Step Size (dx) | 1/256 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santhanakrishnan, A.; Jones, S.K.; Dickson, W.B.; Peek, M.; Kasoju, V.T.; Dickinson, M.H.; Miller, L.A. Flow Structure and Force Generation on Flapping Wings at Low Reynolds Numbers Relevant to the Flight of Tiny Insects. Fluids 2018, 3, 45. https://doi.org/10.3390/fluids3030045
Santhanakrishnan A, Jones SK, Dickson WB, Peek M, Kasoju VT, Dickinson MH, Miller LA. Flow Structure and Force Generation on Flapping Wings at Low Reynolds Numbers Relevant to the Flight of Tiny Insects. Fluids. 2018; 3(3):45. https://doi.org/10.3390/fluids3030045
Chicago/Turabian StyleSanthanakrishnan, Arvind, Shannon K. Jones, William B. Dickson, Martin Peek, Vishwa T. Kasoju, Michael H. Dickinson, and Laura A. Miller. 2018. "Flow Structure and Force Generation on Flapping Wings at Low Reynolds Numbers Relevant to the Flight of Tiny Insects" Fluids 3, no. 3: 45. https://doi.org/10.3390/fluids3030045






