Impact of Silicon Addition on the Development of Gelled Pork Lard Emulsions with Controlled Lipid Digestibility for Application as Fat Replacers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Macro- and Microstructure
2.2. Technological Properties during Chilling Storage
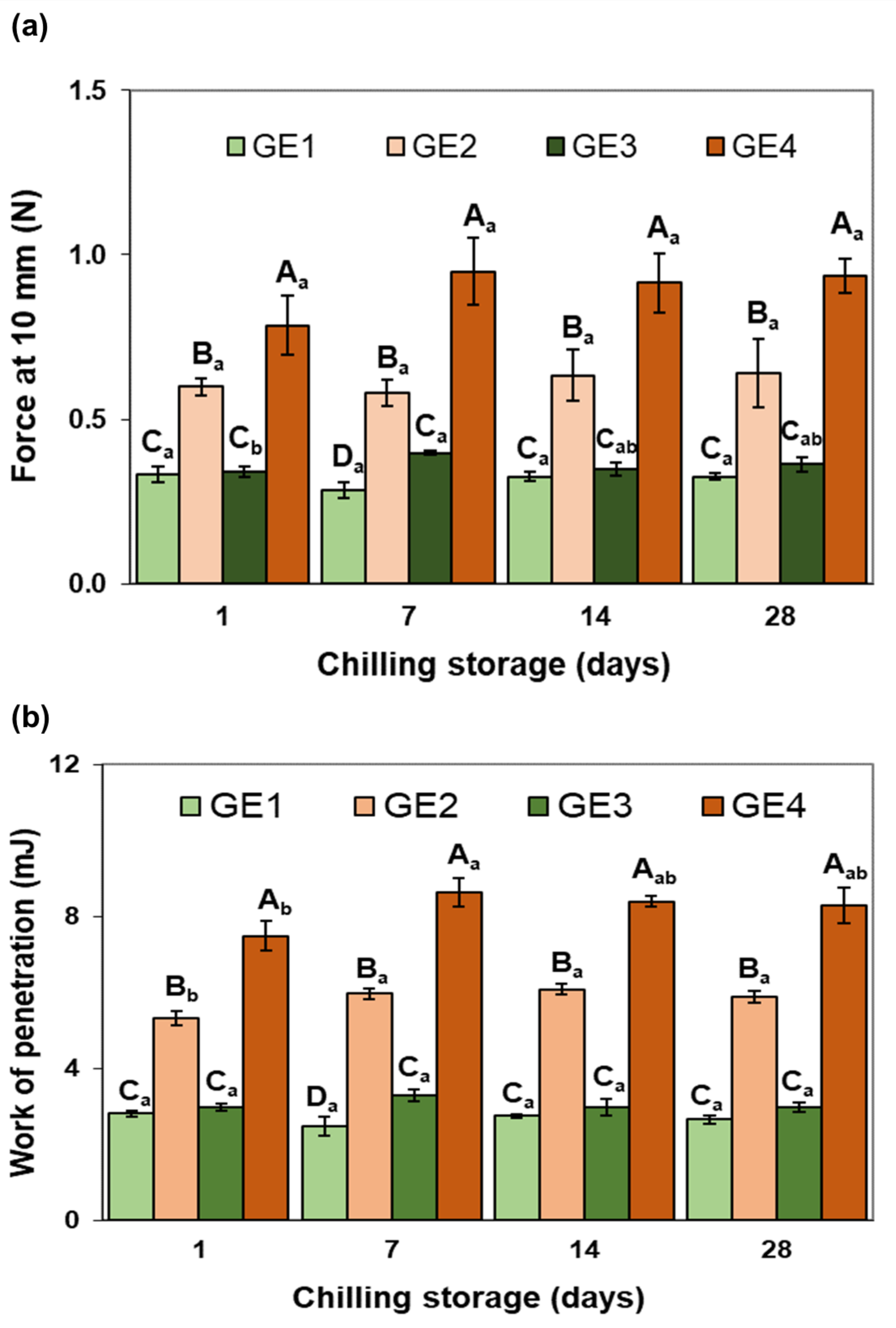
2.2.1. Instrumental Texture and Color
2.2.2. Gravitational and Thermal Stability
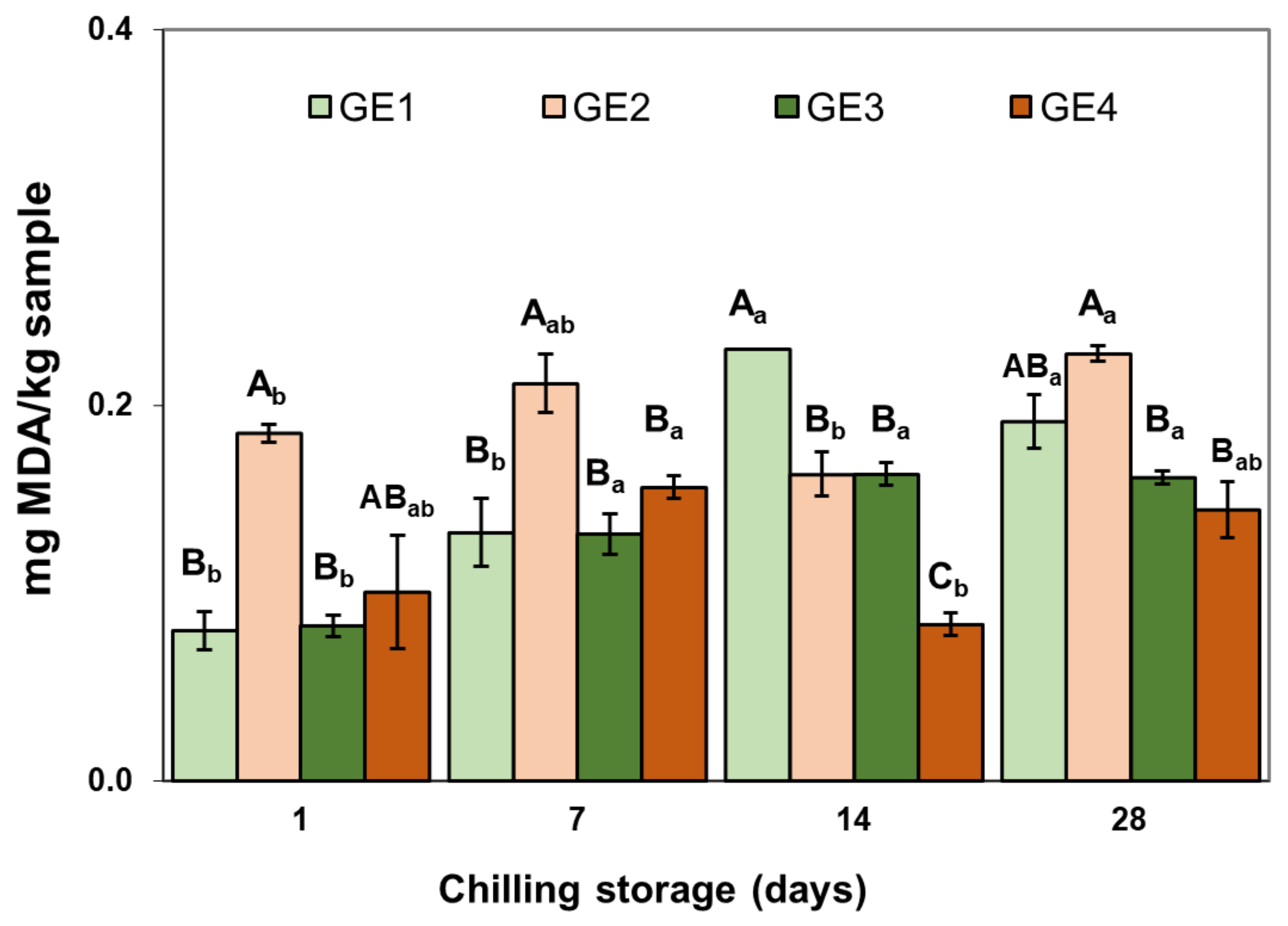
2.2.3. Lipid Oxidation
2.3. Extent of Lipolysis during In Vitro Digestion
2.4. Confocal Laser Scanning Microscopy of the Digested GE
3. Conclusions
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation of Gelled Emulsions (GE)
4.3. Microstructure
4.4. Instrumental Texture and Color
4.5. Gravitational and Thermal Stability
4.6. Determination of Thiobarbituric Acid Reactive Substances)
4.7. In Vitro Gastrointestinal Digestion (GID)
4.8. Fat Extraction, High-Performance Size-Exclusion Liquid Chromatography, and Extent of Lipolysis during In Vitro GID
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freire, M.; Bou, R.; Cofrades, S.; Jiménez-Colmenero, F. Technological characteristics of cold-set gelled double emulsion enriched with n-3 fatty acids: Effect of hydroxytyrosol addition and chilling storage. Food Res. Int. 2017, 100, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wright, A.J. Pectin and gastric pH interactively affect DHA-rich emulsion in vitro digestion microstructure, digestibility and bioaccessibility. Food Hydrocoll. 2018, 76, 49–59. [Google Scholar] [CrossRef]
- Michalski, M.C.; Genot, C.; Gayet, C.; Lopez, C.; Fine, F.; Joffre, F.; Vendeuvre, J.L.; Bouvier, J.; Chardigny, J.M.; Raynal-Ljutovac, K. Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog. Lipid Res. 2013, 52, 354–373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; McClements, D.J. New Mathematical Model for Interpreting pH-Stat Digestion Profiles: Impact of Lipid Droplet Characteristics on in vitro Digestibility. J. Agric. Food Chem. 2010, 58, 8085–8092. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Horne, D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog. Lipid Res. 2009, 48, 92–100. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Gaspar, A.L.C.; de Goes-Favoni, S.P. Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chem. 2015, 171, 315–322. [Google Scholar] [CrossRef]
- Gayoso, L.; Ansorena, D.; Astiasarán, I. DHA rich algae oil delivered by O/W or gelled emulsions: Strategies to increase its bioaccessibility. J. Sci. Food Agric. 2019, 9, 2251–2258. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Santana, R.C.; Perrechil, F.A.; Sato, A.C.K.; Cunha, R.L. Emulsifying properties of collagen fibers: Effect of pH, protein concentration and homogenization pressure. Food Hydrocoll. 2011, 25, 604–612. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, L.; Zhang, Y.; Li, H.; Zhao, D.; Cao, J.; Liu, X. Application of Emulsion Gels as Fat Substitutes in Meat Products. Foods 2022, 11, 1950. [Google Scholar] [CrossRef] [PubMed]
- Garcimartín, A.; Santos-López, J.A.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Silicon-Enriched Restructured Pork Affects the Lipoprotein Profile, VLDL Oxidation, and LDL Receptor Gene Expression in Aged Rats Fed an Atherogenic Diet. J. Nutr. 2015, 145, 2039–2045. [Google Scholar] [CrossRef]
- Garcimartín, A.; López-Oliva, M.E.; Macho-González, A.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Hypoglycaemic and hypotriglyceridaemic postprandial properties of organic silicon. J. Funct. Foods 2017, 29, 290–294. [Google Scholar] [CrossRef]
- Hernández-Martín, M.; Bocanegra, A.; Redondo-Castillejo, R.; Macho-González, A.; Sánchez-Muniz, F.J.; Benedí, J.; Bastida, S.; García-Fernández, R.A.; Garcimartín, A.; López-Oliva, M.E. Could Duodenal Molecular Mechanisms be Involved in the Hypocholesterolemic Effect of Silicon Used as Functional Ingredient in Late-Stage Type 2 Diabetes Mellitus? Mol. Nutr. Food Res. 2022, 66, 2200104. [Google Scholar] [CrossRef]
- Tedesco, E.; Benetti, F.; Pezzani, R. In vitro evaluation of different organic matrices used to modulate silicon bioavailability. FASEB J. 2020, 34, 12229–12238. [Google Scholar] [CrossRef]
- Cofrades, S.; Garcimartín, A.; Pérez-Mateos, M.; Saiz, A.; Redondo-Castillejo, R.; Bocanegra, A.; Benedí, J.; Álvarez, M.D. Stabilized soy protein emulsion enriched with silicon and containing or not methylcellulose as novel technological alternatives to reduce animal fat digestion. Food Res. Int. 2023, 170, 112833. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Dietz, K.J. Silicon as versatile playerin plantand human biology: Overlooked and poorly understood. Front. Plant Sci. 2015, 6, 994. [Google Scholar] [CrossRef] [PubMed]
- Montesano, F.F.; D’Imperio, M.; Parente, A.; Cardinali, A.; Renna, M.; Serio, F. Green bean biofortification for Si through soilless cultivation: Plant response and Si bioaccessibility in pods. Sci. Rep. 2016, 6, 31662. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Surh, J.; Decker, E.A.; McClements, D.J. Properties and stability of oil-in-water emulsions stabilized by fish gelatin. Food Hydrocoll. 2006, 20, 596–606. [Google Scholar] [CrossRef]
- Motoki, M.; Kumazawa, Y. Recent Research Trends in Transglutaminase Technology for Food Processing. Food Sci. Technol. Res. 2000, 6, 151–160. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kumazawa, Y.; Motoki, M. Strength of protein gels prepared with microbial transglutaminase as related to reaction conditions. J. Food Sci. 1994, 59, 866–871. [Google Scholar] [CrossRef]
- Motoki, M.; Seguro, K. Transglutaminase and its use for food processing. Trends in Food Sci. Technol. 1998, 9, 204–210. [Google Scholar] [CrossRef]
- Camino, N.A.; Pilosof, A.M.R. Hydroxypropylmethylcellulose at the oil–water interface. Part II. Submicron-emulsions as affected by pH. Food Hydrocoll. 2011, 25, 1051–1062. [Google Scholar] [CrossRef]
- Shi, A.; Feng, X.; Wang, Q.; Adhikari, B. Pickering and high internal phase Pickering emulsions stabilized by protein-based particles: A review of synthesis, application and prospective. Food Hydrocoll. 2020, 109, 106117. [Google Scholar] [CrossRef]
- Pintado, T.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Carmona, P.; Herrero, A.M. Oil-in-water emulsion gels stabilized with chia (Salvia hispanica L.) and cold gelling agents: Technological and infrared spectroscopic characterization. Food Chem. 2015, 185, 470–478. [Google Scholar] [CrossRef]
- Luo, N.; Ye, A.; Wolber, F.M.; Singh, H. Effect of Gel Structure on the In vitro Gastrointestinal Digestion Behaviour of Whey Protein Emulsion Gels and the Bioaccessibility of Capsaicinoids. Molecules 2021, 26, 1379. [Google Scholar] [CrossRef]
- Fontes-Candia, C.; Martínez, J.C.; López-Rubio, A.; Salvia-Trujillo, L.; Martín-Belloso, O.; Martínez-Sanz, M. Emulsion gels and oil-filled aerogels as curcumin carriers: Nanostructural characterization of gastrointestinal digestion products. Food Chem. 2022, 387, 132877. [Google Scholar] [CrossRef]
- Bellesi, F.A.; Martínez, M.J.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M.R. Comparative behavior of protein or polysaccharide stabilized emulsion under in vitro gastrointestinal conditions. Food Hydrocoll. 2016, 52, 47–56. [Google Scholar] [CrossRef]
- Infantes-García, M.R.; Verkempinck, S.H.E.; González-Fuentes, P.G.; Hendrickx, M.E.; Grauwet, T. Lipolysis products formation during in vitro gastric digestion is affected by the emulsion interfacial composition. Food Hydrocoll. 2021, 110, 106163. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]



| Gelled Emulsion | Number of Days | L* | a* | b* |
|---|---|---|---|---|
| GE1 | 1 | 88.08 ± 0.53 Aa | −0.21 ± 0.04 Bb | 5.94 ± 0.07 Ba |
| GE2 | 1 | 88.25 ± 0.11 Aa | −0.55 ± 0.08 Ab | 5.61 ± 0.13 Ca |
| GE3 | 1 | 86.50 ± 0.66 Bab | 0.05 ± 0.04 Ca | 6.28 ± 0.05 Aa |
| GE4 | 1 | 88.37 ± 0.57 Aa | −0.27 ± 0.02 Bab | 5.48 ± 0.11 Ca |
| GE1 | 7 | 88.24 ± 0.80 ABa | −0.10 ± 0.13 Bb | 5.48 ± 0.17 ABb |
| GE2 | 7 | 88.28 ± 0.42 ABa | −0.39 ± 0.08 Ab | 5.25 ± 0.06 Bb |
| GE3 | 7 | 87.41 ± 0.28 Ba | 0.14 ± 0.05 Ca | 5.88 ± 0.01 Abc |
| GE4 | 7 | 88.79 ± 0.64 Aa | −0.07 ± 0.11 BCb | 5.15 ± 0.19 Ba |
| GE1 | 14 | 87.98 ± 0.46 Aa | −0.05 ± 0.11 Cb | 5.71 ± 0.29 Aab |
| GE2 | 14 | 88.38 ± 0.30 Aa | −0.41 ± 0.03 Ab | 5.35 ± 0.12 ABa |
| GE3 | 14 | 86.56 ± 0.18 Bb | 0.33 ± 0.07 Ba | 5.62 ± 0.17 ABc |
| GE4 | 14 | 87.79 ± 0.48 Aa | 0.14 ± 0.18 BCb | 5.21 ± 0.13 Ba |
| GE1 | 28 | 87.66 ± 0.20 ABa | −0.61 ± 0.10 Ba | 5.85 ± 0.07 ABab |
| GE2 | 28 | 88.09 ± 0.50 Aa | −0.90 ± 0.07 Aa | 5.67 ± 0.18 Ba |
| GE3 | 28 | 86.90 ± 0.60 Bab | −0.30 ± 0.04 Cb | 6.10 ± 0.10 Aab |
| GE4 | 28 | 87.99 ± 0.53 Aa | −0.47 ± 0.10 BCa | 5.34 ± 0.15 Ca |
| Gelled Emulsion | TAG | DAG | MAG | FFA | MAG + FFA | Digestibility |
|---|---|---|---|---|---|---|
| Initial pork lard | 99.9 ± 0.0 | |||||
| GE1 | 10.41 ± 1.91 Bb | 11.47 ± 0.49 Ab | 15.96 ± 1.95 Aa | 62.16 ± 2.69 Aa | 78.12 ± 1.62 Aa | 89.58 ± 1.91 Aa |
| GE2 | 18.12 ± 1.64 Ab | 11.42 ± 0.85 Ab | 15.27 ± 0.22 Aa | 55.20 ± 0.93 Ba | 70.47 ± 1.07 Ba | 81.45 ± 1.77 Ba |
| GE3 | 14.84 ± 1.67 Ba | 12.32 ± 0.70 Aa | 14.53 ± 0.54 Ab | 58.31 ± 0.81 Ab | 72.84 ± 1.10 Ab | 85.14 ± 1.68 Ab |
| GE4 | 21.99 ± 3.89 Aa | 13.15 ± 0.54 Aa | 12.51 ± 1.19 Ab | 52.35 ± 2.98 Bb | 64.86 ± 4.17 Bb | 77.99 ± 3.90 Bb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofrades, S.; Hernández-Martín, M.; Garcimartín, A.; Saiz, A.; López-Oliva, M.E.; Benedí, J.; Álvarez, M.D. Impact of Silicon Addition on the Development of Gelled Pork Lard Emulsions with Controlled Lipid Digestibility for Application as Fat Replacers. Gels 2023, 9, 728. https://doi.org/10.3390/gels9090728
Cofrades S, Hernández-Martín M, Garcimartín A, Saiz A, López-Oliva ME, Benedí J, Álvarez MD. Impact of Silicon Addition on the Development of Gelled Pork Lard Emulsions with Controlled Lipid Digestibility for Application as Fat Replacers. Gels. 2023; 9(9):728. https://doi.org/10.3390/gels9090728
Chicago/Turabian StyleCofrades, Susana, Marina Hernández-Martín, Alba Garcimartín, Arancha Saiz, M. Elvira López-Oliva, Juana Benedí, and María Dolores Álvarez. 2023. "Impact of Silicon Addition on the Development of Gelled Pork Lard Emulsions with Controlled Lipid Digestibility for Application as Fat Replacers" Gels 9, no. 9: 728. https://doi.org/10.3390/gels9090728
APA StyleCofrades, S., Hernández-Martín, M., Garcimartín, A., Saiz, A., López-Oliva, M. E., Benedí, J., & Álvarez, M. D. (2023). Impact of Silicon Addition on the Development of Gelled Pork Lard Emulsions with Controlled Lipid Digestibility for Application as Fat Replacers. Gels, 9(9), 728. https://doi.org/10.3390/gels9090728








