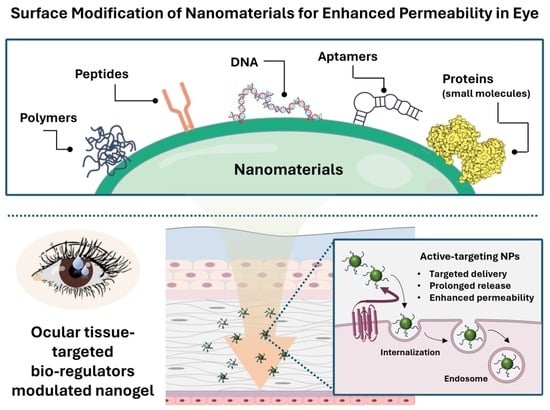
Advancements in Nanogels for Enhanced Ocular Drug Delivery: Cutting-Edge Strategies to Overcome Eye Barriers
Abstract
:1. Introduction
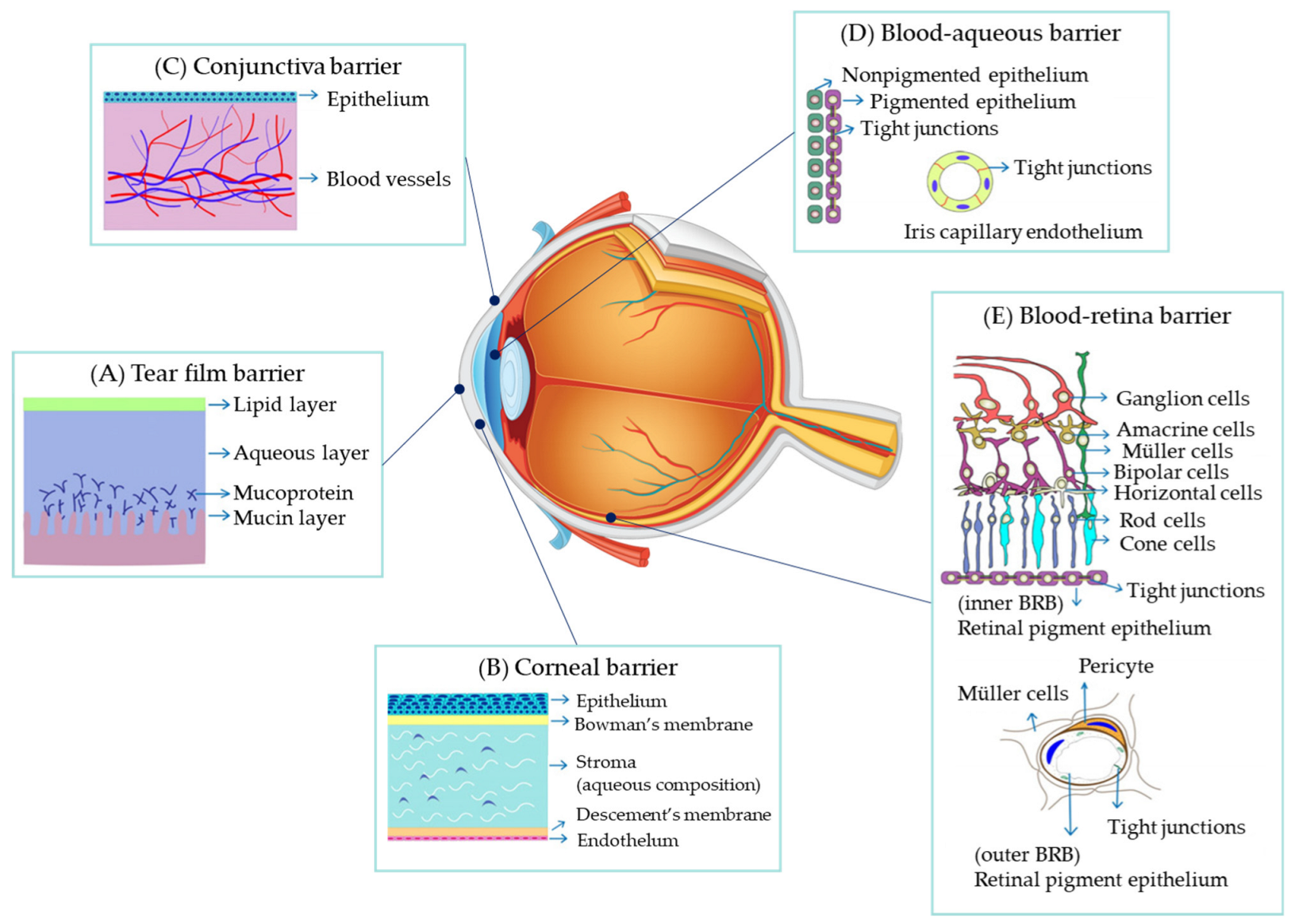
2. Barriers to Drug Administration for Ocular Diseases
2.1. Barriers to Drug Absorption into the Eye
2.2. Barriers to Movement to the Target Site
2.3. Barriers to Effect Manifestation at the Target Site
2.3.1. Lipid Bilayer of Cell Membranes
2.3.2. Receptor for Cell Membrane Penetration
3. Recent Progress and Challenges in Overcoming Biological Barriers with Nanomaterials
| Delivery Systems | Pros | Cons | Ref |
|---|---|---|---|
| Liposomes | Sustained drug release, improved bioavailability, biodegradable, biocompatible, and non-immunogenic | Poor stability, leakage, and fusion of drugs | [64,65] |
| Solid lipid nanoparticles (SLNs) | Drug loading capability for lipophilic and hydrophilic drugs, suitable for autoclaving sterilization, increased ocular bioavailability, and prolonged ocular retention time | Drug expulsion following polymeric transition during long storage | [66,67] |
| Polymeric nanoparticles | Increased ocular penetration, prolonged residence time, and simplicity of change | Burst effect and aggregation of particles and toxicity | [68,69,70,71,72] |
| Dendrimer | Improved drug penetration and effectiveness | Blurred vision and loss of eyesight | [73,74,75,76] |
| Stimuli-responsive gel | Sustained drug release, improved biocompatibility, and biodegradability, Versatility in design | Limited to smaller molecular weight drugs, poor stability, and temperature sensitivity (difficulty in retaining water) | [77,78,79] |
| Inorganic nanoparticle | Improved ocular penetration by small size, controlled release by physical and chemical properties (super-magnetism, photothermal, etc.) | Poor stability, bioavailability | [80,81,82,83] |
3.1. Continual Strategies in Indirect Modulation
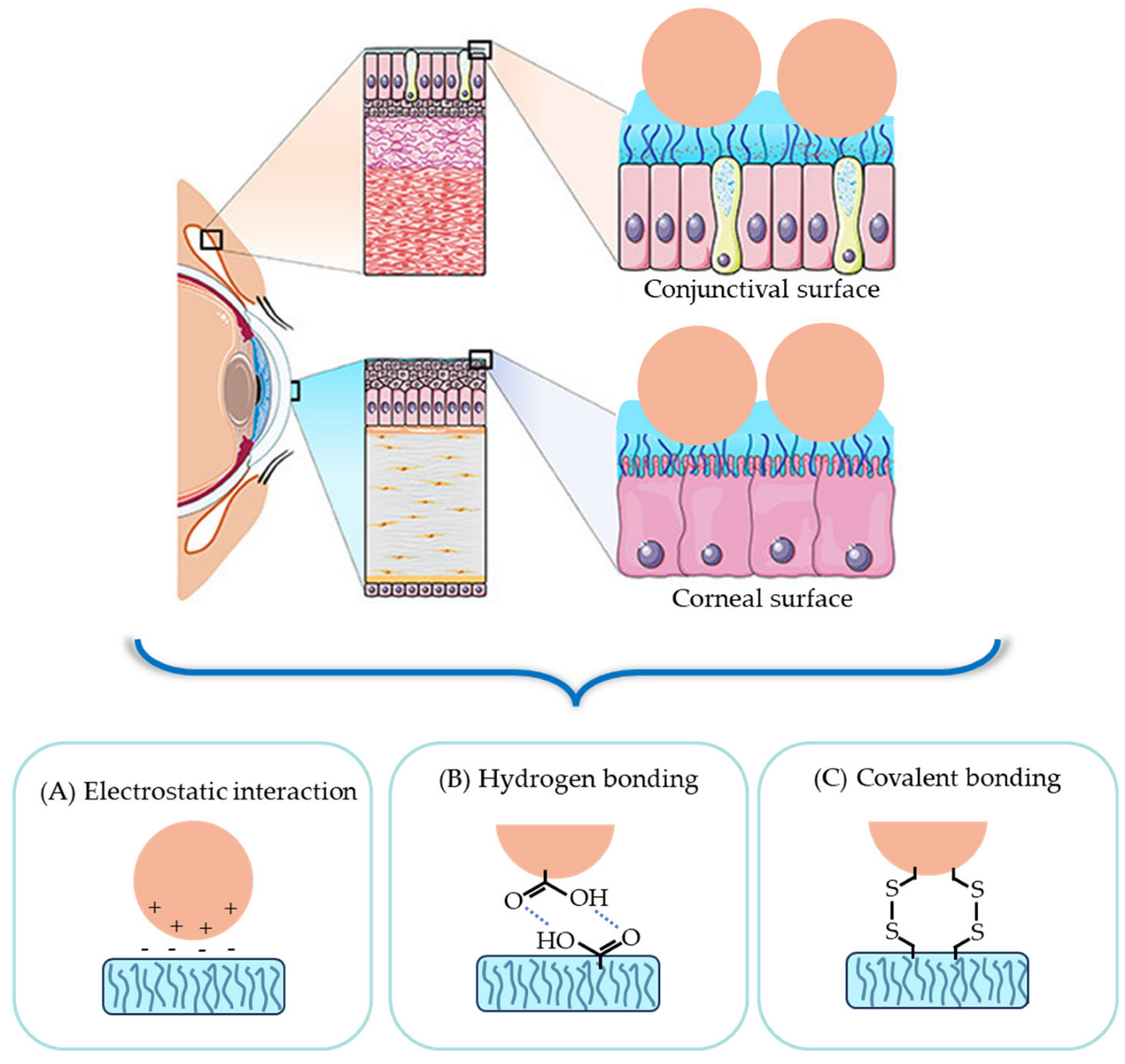
3.1.1. Leveraging Mucoadhesion for Effective Drug Adsorption
- Surface modification of electrostatic interaction with mucin
- Harnessing hydrogen bonds with carboxyl groups in mucin for nanocarrier surface modification
- Utilizing covalent bonds with cysteine-rich subdomains in mucin for nanocarrier surface modification
3.1.2. Optimizing Cellular Penetration and Uptake through Surface Interaction Adjustments
- Transforming junction protein–nanocarrier interactions for improved paracellular permeability
- Transforming cell–nanocarrier interactions for improved transcellular permeability
3.2. Improving Nanocarrier Drug Delivery through Specific Ligands as Modulators
3.2.1. Proteins and Short Peptides
3.2.2. Cell-Penetrating Peptides
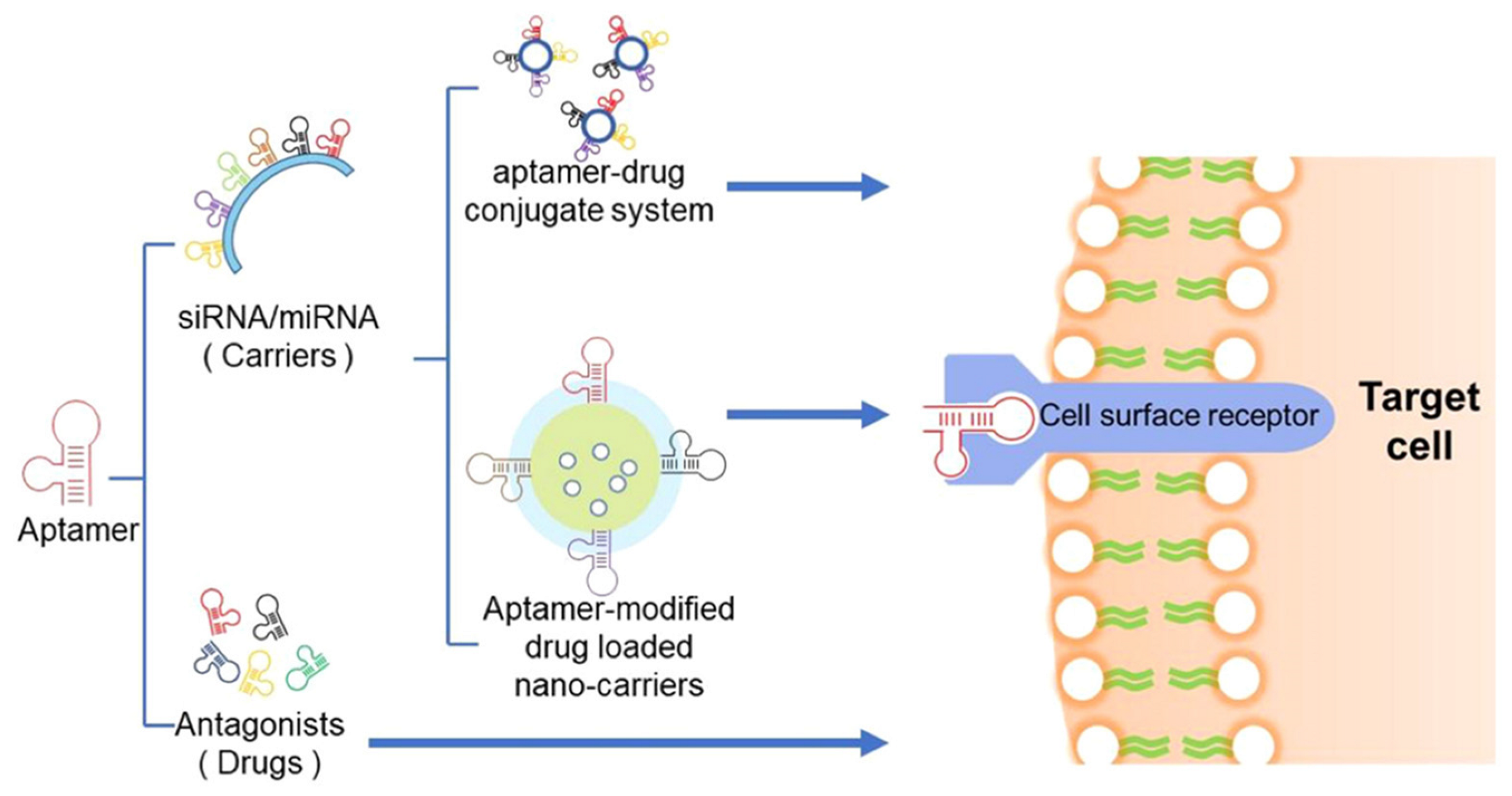
3.2.3. Aptamers
- Dual role of aptamers: serving as modulators and therapeutic molecules
- Aptamers as surface modifiers in drug carriers
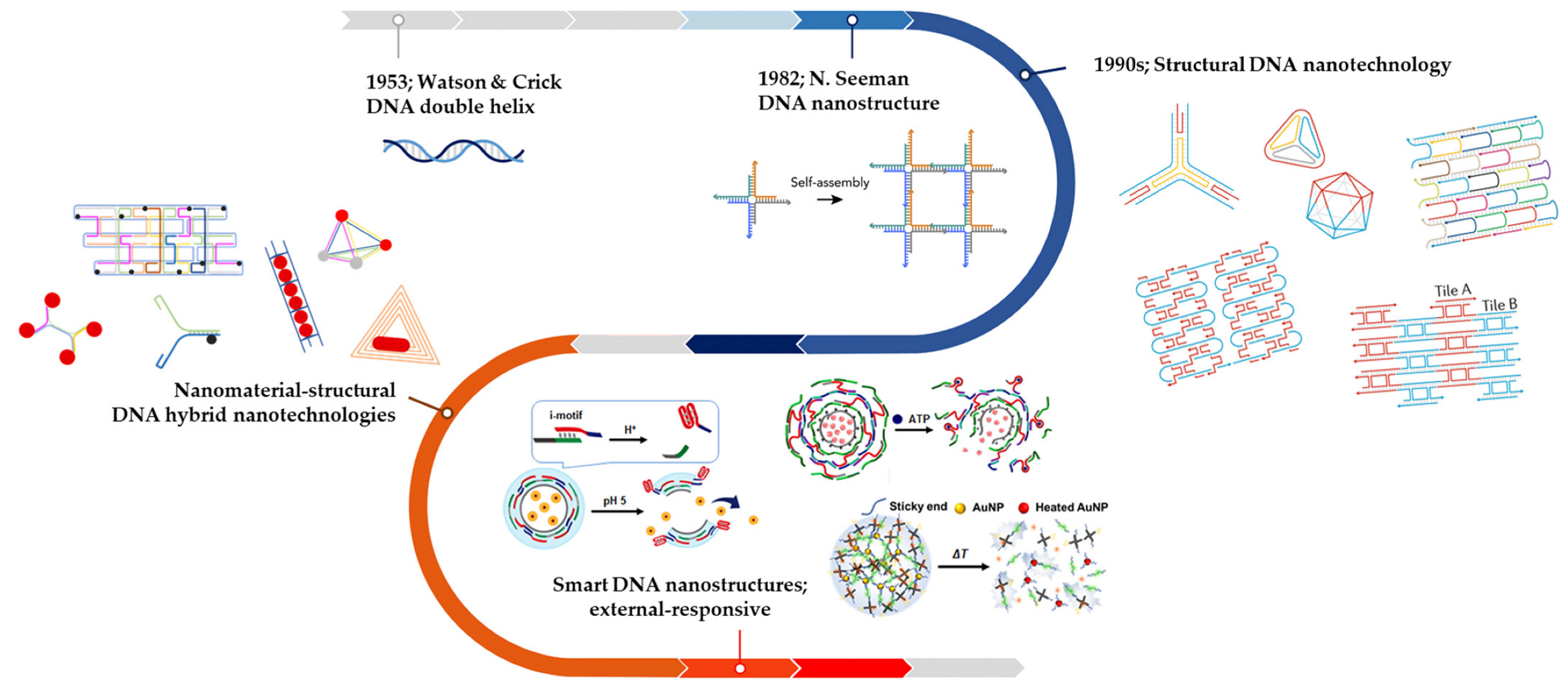
4. Exploring DNA Nanostructures as Innovative Vehicles for Ophthalmic Drug Delivery
4.1. Early DNA Nanostructures
4.1.1. Basic DNA Nanostructures: Polyhedron Assembly System
4.1.2. Hybrid Nanostructures for Stability
4.2. Smart DNA Nanostructures in Therapeutic Drug Delivery
4.2.1. Temperature-Responsive DNA Carrier
4.2.2. pH-Responsive DNA Carrier
4.2.3. Biomarker Molecule-Responsive DNA Carrier
4.3. Perspectives of DNA Nanocarriers for Ocular Drug Delivery
4.4. Challenges in Utilizing DNA Nanocarriers for Ocular Drug Delivery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Lee, J.; Rhee, Y.-S. Ophthalmic Dosage Forms for Drug Delivery to Posterior Segment. J. Pharm. Investig. 2022, 52, 161–173. [Google Scholar] [CrossRef]
- Gause, S.; Hsu, K.-H.; Shafor, C.; Dixon, P.; Powell, K.C.; Chauhan, A. Mechanistic Modeling of Ophthalmic Drug Delivery to the Anterior Chamber by Eye Drops and Contact Lenses. Adv. Colloid Interface Sci. 2016, 233, 139–154. [Google Scholar] [CrossRef]
- Trinh, H.M.; Cholkar, K.; Joseph, M.; Yang, X.; Mitra, A.K. Clear, Aqueous Topical Drop of Triamcinolone Acetonide. AAPS PharmSciTech 2017, 18, 2466–2478. [Google Scholar] [CrossRef]
- Ramos, M.S.; Xu, L.T.; Singuri, S.; Tafur, J.C.C.; Arepalli, S.; Ehlers, J.P.; Kaiser, P.K.; Singh, R.P.; Rachitskaya, A.V.; Srivastava, S.K.; et al. Patient-Reported Complications after Intravitreal Injection and Their Predictive Factors. Ophthalmol. Retin. 2021, 5, 625–632. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Lakdawala, D.H.; Shaikh, A.A.; Desai, A.R.; Choksi, H.H.; Vaidya, R.J.; Ranch, K.M.; Koli, A.R.; Vyas, B.A.; Shah, D.O. In Vitro and in Vivo Evaluation of Novel Implantation Technology in Hydrogel Contact Lenses for Controlled Drug Delivery. J. Control. Release 2016, 226, 47–56. [Google Scholar] [CrossRef]
- Arora, G.; Rastogi, V.; Singh, T.G.; Dhiman, S. Recent Advances in Nano-Formulations for Ophthalmic Drug Delivery. J. Pharm. Sci. Res. 2020, 12, 213–223. [Google Scholar]
- Srividya, B.; Cardoza, R.M.; Amin, P. Sustained Ophthalmic Delivery of Ofloxacin from a PH Triggered in Situ Gelling System. J. Control. Release 2001, 73, 205–211. [Google Scholar] [CrossRef]
- Sakurai, E.; Ozeki, H.; Kunou, N.; Ogura, Y. Effect of Particle Size of Polymeric Nanospheres on Intravitreal Kinetics. Ophthalmic Res. 2000, 33, 31–36. [Google Scholar] [CrossRef]
- Widjaja, L.K.; Bora, M.; Chan, P.N.P.H.; Lipik, V.; Wong, T.T.L.; Venkatraman, S.S. Hyaluronic Acid-Based Nanocomposite Hydrogels for Ocular Drug Delivery Applications. J. Biomed. Mater. Res. Part A 2014, 102, 3056–3065. [Google Scholar] [CrossRef]
- Qin, X.; Tian, L.; Zhang, H.; Chen, X.; Li, L. Evaluation of Corneal Elastic Modulus Based on Corneal Visualization Scheimpflug Technology. Biomed. Eng. Online 2019, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- De Hoon, I.; Barras, A.; Swebocki, T.; Vanmeerhaeghe, B.; Bogaert, B.; Muntean, C.; Abderrahmani, A.; Boukherroub, R.; De Smedt, S.; Sauvage, F.; et al. Influence of the Size and Charge of Carbon Quantum Dots on Their Corneal Penetration and Permeation Enhancing Properties. ACS Appl. Mater. Interfaces 2023, 15, 3760–3771. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Amin, M.M.; El-Korany, S.M.; Sayed, S. Corneal Targeted Fenticonazole Nitrate-Loaded Novasomes for the Management of Ocular Candidiasis: Preparation, in Vitro Characterization, Ex Vivo and in Vivo Assessments. Drug Deliv. 2022, 29, 2428–2441. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, X.; Li, H.; Lu, H.; Oswald, J.; Liu, Y.; Zeng, J.; Jin, C.; Peng, X.; Liu, J.; et al. IRGD Decorated Liposomes: A Novel Actively Penetrating Topical Ocular Drug Delivery Strategy. Nano Res. 2020, 13, 3105–3109. [Google Scholar] [CrossRef]
- Weng, Y.-H.; Ma, X.-W.; Che, J.; Li, C.; Liu, J.; Chen, S.-Z.; Wang, Y.-Q.; Gan, Y.-L.; Chen, H.; Hu, Z.-B.; et al. Nanomicelle-Assisted Targeted Ocular Delivery with Enhanced Antiinflammatory Efficacy In Vivo. Adv. Sci. 2018, 5, 1700455. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Managit, C.; Phanaksri, T.; Treesuppharat, W.; Fuongfuchat, A. Formulation Development and in Vitro Evaluation of Transferrin-Conjugated Liposomes as a Carrier of Ganciclovir Targeting the Retina. Int. J. Pharm. 2020, 577, 119084. [Google Scholar] [CrossRef]
- Drolet, D.W.; Green, L.S.; Gold, L.; Janjic, N. Fit for the Eye: Aptamers in Ocular Disorders. Nucleic Acid Ther. 2016, 26, 127–146. [Google Scholar] [CrossRef]
- Koirala, A.; Makkia, R.S.; Conley, S.M.; Cooper, M.J.; Naash, M.I. S/MAR-Containing DNA Nanoparticles Promote Persistent RPE Gene Expression and Improvement in RPE65-Associated LCA. Hum. Mol. Genet. 2013, 22, 1632–1642. [Google Scholar] [CrossRef]
- Farjo, R.; Skaggs, J.; Quiambao, A.B.; Cooper, M.J.; Naash, M.I. Efficient Non-Viral Ocular Gene Transfer with Compacted DNA Nanoparticles. PLoS ONE 2006, 1, e38. [Google Scholar] [CrossRef]
- Khar, R.; Warsi, M.; Akhter, S.; Ahmad, F.; Jain, G.; Mallick, N.; Pathan, S. Nano-vectors for the ocular delivery of nucleic acid-based therapeutics. Indian J. Pharm. Sci. 2010, 72, 675–688. [Google Scholar] [CrossRef]
- Kour, J.; Kumari, N.; Sapra, B. Ocular Prodrugs: Attributes and Challenges. Asian J. Pharm. Sci. 2021, 16, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C. Novel Eye Drop Delivery Systems: Advance on Formulation Design Strategies Targeting Anterior and Posterior Segments of the Eye. Pharmaceutics 2022, 14, 1150. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Joly-Chevrier, M.; Akbar, D.; Tran, S.D. Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1094. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Yamada, M.; Hato, S.; Nishida, T. Fluorophotometric Measurement of the Precorneal Residence Time of Topically Applied Hyaluronic Acid. Br. J. Ophthalmol. 2008, 92, 108–111. [Google Scholar] [CrossRef]
- Sebbag, L.; Allbaugh, R.A.; Wehrman, R.F.; Uhl, L.K.; Ben-Shlomo, G.; Chen, T.; Mochel, J.P. Fluorophotometric Assessment of Tear Volume and Turnover Rate in Healthy Dogs and Cats. J. Ocul. Pharmacol. Ther. 2019, 35, 497–502. [Google Scholar] [CrossRef]
- Snibson, G.R.; Greaves, J.L.; Soper, N.D.W.; Prydal, J.I.; Wilson, C.G.; Bron, A.J. Precorneal Residence Times of Sodium Hyaluronate Solutions Studied by Quantitative Gamma Scintigraphy. Eye 1990, 4, 594–602. [Google Scholar] [CrossRef]
- Meseguer, G.; Gurny, R.; Buri, P. In Vivo Evaluation of Dosage Forms: Application of Gamma Scintigraphy to Non-Enteral Routes of Administration. J. Drug Target. 1994, 2, 269–288. [Google Scholar] [CrossRef]
- Müller, L.; Jensen, B.P.; Bachmann, L.M.; Wong, D.; Wells, A.P. New Technique to Reduce Systemic Side Effects of Timolol Eye Drops: The Tissue Press Method—Cross-over Clinical Trial. Clin. Exp. Ophthalmol. 2020, 48, 24–30. [Google Scholar] [CrossRef]
- Nettey, H.; Darko, Y.; Bamiro, O.A.; Addo, R.T. Ocular Barriers. In Ocular Drug Delivery: Advances, Challenges and Applications; Addo, R.T., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–36. ISBN 978-3-319-47691-9. [Google Scholar]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Rizzolo, L.J. Development and Role of Tight Junctions in the Retinal Pigment Epithelium. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 2007; Volume 258, pp. 195–234. ISBN 0074-7696. [Google Scholar]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight Junction Proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef]
- Yi, X.; Wang, Y.; Yu, F.-S.X. Corneal Epithelial Tight Junctions and Their Response to Lipopolysaccharide Challenge. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4093–4100. [Google Scholar]
- Naylor, A.; Hopkins, A.; Hudson, N.; Campbell, M. Tight Junctions of the Outer Blood Retina Barrier. Int. J. Mol. Sci. 2020, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Li, S.; Chung, S.H.; Zhu, L.; Stayt, J.; Su, T.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Gillies, M.C. Tyrosine Phosphorylation of VE-Cadherin and Claudin-5 Is Associated with TGF-Β1-Induced Permeability of Centrally Derived Vascular Endothelium. Eur. J. Cell Biol. 2011, 90, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.G. Gap Junctions and Connexin-Interacting Proteins. Cardiovasc. Res. 2004, 62, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Trementozzi, A.N.; Zhao, C.; Smyth, H.; Cui, Z.; Stachowiak, J.C. Gap Junction-Mediated Delivery of Polymeric Macromolecules. ACS Biomater. Sci. Eng. 2022, 8, 1566–1572. [Google Scholar] [CrossRef]
- Trementozzi, A.N.; Hufnagel, S.; Xu, H.; Hanafy, M.S.; Rosero Castro, F.; Smyth, H.D.C.; Cui, Z.; Stachowiak, J.C. Gap Junction Liposomes for Efficient Delivery of Chemotherapeutics to Solid Tumors. ACS Biomater. Sci. Eng. 2020, 6, 4851–4857. [Google Scholar] [CrossRef]
- Supe, S.; Takudage, P.J. Methods for Evaluating Penetration of Drug into the Skin: A Review. Ski. Res. Technol. 2020, 27, 259–308. [Google Scholar] [CrossRef]
- Milanetti, E.; Raimondo, D.; Tramontano, A. Prediction of the Permeability of Neutral Drugs Inferred from Their Solvation Properties. Bioinformatics 2016, 32, 1163–1169. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.; Yang, H.; Xu, Q. Tailoring Combinatorial Lipid Nanoparticles for Intracellular Delivery of Nucleic Acids, Proteins, and Drugs. Acta Pharm. Sin. B 2022, 12, 2624–2639. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, G.; Yu, X.; Wei, T.; Farbiak, L.; Johnson, L.T.; Taylor, A.M.; Xu, J.; Hong, Y.; Zhu, H.; et al. Enhancing CRISPR/Cas Gene Editing through Modulating Cellular Mechanical Properties for Cancer Therapy. Nat. Nanotechnol. 2022, 17, 777–787. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, M.; Hur, S.; Cho, Y.; Park, J.; Jung, H.; Seo, Y.; Woo, H.A.; Nam, K.T.; Lee, K.; et al. Engineered Ionizable Lipid Nanoparticles for Targeted Delivery of RNA Therapeutics into Different Types of Cells in the Liver. Sci. Adv. 2023, 7, eabf4398. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Bellettato, C.M.; Scarpa, M. Possible Strategies to Cross the Blood–Brain Barrier. Ital. J. Pediatr. 2018, 44, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Bergström, C.A.S.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful Oral Delivery of Poorly Water-Soluble Drugs Both Depends on the Intraluminal Behavior of Drugs and of Appropriate Advanced Drug Delivery Systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Lee, M.-K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef]
- Vahedi, A.; Bigdelou, P.; Farnoud, A.M. Quantitative Analysis of Red Blood Cell Membrane Phospholipids and Modulation of Cell-Macrophage Interactions Using Cyclodextrins. Sci. Rep. 2020, 10, 15111. [Google Scholar] [CrossRef]
- BERMAN, E.R.; SCHWELL, H.; FEENEY, L. The Retinal Pigment Epithelium: Chemical Composition and Structure. Investig. Ophthalmol. Vis. Sci. 1974, 13, 675–687. [Google Scholar]
- Mannermaa, E.; Vellonen, K.-S.; Urtti, A. Drug Transport in Corneal Epithelium and Blood–Retina Barrier: Emerging Role of Transporters in Ocular Pharmacokinetics. Adv. Drug Deliv. Rev. 2006, 58, 1136–1163. [Google Scholar] [CrossRef]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D. Nanocarrier Mediated Retinal Drug Delivery: Overcoming Ocular Barriers to Treat Posterior Eye Diseases. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1473. [Google Scholar] [CrossRef]
- Schaeffer, L. Chapter 21—The Role of Functional Groups in Drug–Receptor Interactions. In The Practice of Medicinal Chemistry, 3rd ed.; Wermuth, C.G., Ed.; Academic Press: New York, NY, USA, 2008; pp. 464–480. ISBN 978-0-12-374194-3. [Google Scholar]
- Beuming, T.; Sherman, W. Current Assessment of Docking into GPCR Crystal Structures and Homology Models: Successes, Challenges, and Guidelines. J. Chem. Inf. Model. 2012, 52, 3263–3277. [Google Scholar] [CrossRef]
- Pogozheva, I.D.; Lomize, A.L.; Mosberg, H.I. Opioid Receptor Three-Dimensional Structures from Distance Geometry Calculations with Hydrogen Bonding Constraints. Biophys. J. 1998, 75, 612–634. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-H.; Park, K.-S.; Sohn, J.H.; Yeh, B.-I.; Ko, C.M.; Kong, I.D. Functional Expression of P2Y Receptors in WERI-Rb1 Retinoblastoma Cells. Korean J. Physiol. Pharmacol. 2011, 15, 61–66. [Google Scholar] [CrossRef]
- Maminishkis, A.; Jalickee, S.; Blaug, S.A.; Rymer, J.; Yerxa, B.R.; Peterson, W.M.; Miller, S.S. The P2Y2 Receptor Agonist INS37217 Stimulates RPE Fluid Transport In Vitro and Retinal Reattachment in Rat. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3555–3566. [Google Scholar]
- Rodrigues, F.S.C.; Campos, A.; Martins, J.; Ambrósio, A.F.; Campos, E.J. Emerging Trends in Nanomedicine for Improving Ocular Drug Delivery: Light-Responsive Nanoparticles, Mesoporous Silica Nanoparticles, and Contact Lenses. ACS Biomater. Sci. Eng. 2020, 6, 6587–6597. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-Based Ocular Drug Delivery Systems: Recent Advances and Future Prospects. J. Nanobiotechnol. 2023, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2015, 20, 1–11. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of Hyaluronic Acid as Carriers in Drug Delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Mayol, L.; Biondi, M.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Borzacchiello, A. Amphiphilic Hyaluronic Acid Derivatives toward the Design of Micelles for the Sustained Delivery of Hydrophobic Drugs. Carbohydr. Polym. 2014, 102, 110–116. [Google Scholar] [CrossRef]
- Rout, G.K.; Shin, H.-S.; Gouda, S.; Sahoo, S.; Das, G.; Fraceto, L.F.; Patra, J.K. Current Advances in Nanocarriers for Biomedical Research and Their Applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1053–1062. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, X.; Zhang, N.; Jiao, X.; Ma, Y.; Liu, R.; Liu, B.; Li, Z. Targeted Drug Delivery Vehicles Mediated by Nanocarriers and Aptamers for Posterior Eye Disease Therapeutics: Barriers, Recent Advances and Potential Opportunities. Nanotechnology 2022, 33, 162001. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, N.; Deckers, R.; Ozbakir, B.; Akthar, S.; Kok, R.J.; Lammers, T.; Storm, G. Influence of Cholesterol Inclusion on the Doxorubicin Release Characteristics of Lysolipid-Based Thermosensitive Liposomes. Int. J. Pharm. 2018, 548, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Swingle, K.L.; Hamilton, A.G.; Mitchell, M.J. Lipid Nanoparticle-Mediated Delivery of MRNA Therapeutics and Vaccines. Trends Mol. Med. 2021, 27, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Seyfoddin, A.; Shaw, J.; Al-Kassas, R. Solid Lipid Nanoparticles for Ocular Drug Delivery. Drug Deliv. 2010, 17, 467–489. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Matranga, M.A.; Cortinas, A.B.; Delcassian, D.; Daniel, K.B.; Langer, R.; Anderson, D.G. Glucose-Responsive Nanoparticles for Rapid and Extended Self-Regulated Insulin Delivery. ACS Nano 2020, 14, 488–497. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric Micelle Stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric Micelles and Alternative Nanonized Delivery Vehicles for Poorly Soluble Drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric Micelles in Drug Delivery: An Insight of the Techniques for Their Characterization and Assessment in Biorelevant Conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Surekha, B.; Kommana, N.S.; Dubey, S.K.; Kumar, A.V.P.; Shukla, R.; Kesharwani, P. PAMAM Dendrimer as a Talented Multifunctional Biomimetic Nanocarrier for Cancer Diagnosis and Therapy. Colloids Surf. B Biointerfaces 2021, 204, 111837. [Google Scholar] [CrossRef]
- Thiagarajan, G.; Greish, K.; Ghandehari, H. Charge Affects the Oral Toxicity of Poly(Amidoamine) Dendrimers. Eur. J. Pharm. Biopharm. 2013, 84, 330–334. [Google Scholar] [CrossRef]
- Han, M.; Huang-Fu, M.-Y.; Guo, W.-W.; Guo, N.-N.; Chen, J.; Liu, H.-N.; Xie, Z.-Q.; Lin, M.-T.; Wei, Q.-C.; Gao, J.-Q. MMP-2-Sensitive HA End-Conjugated Poly(Amidoamine) Dendrimers via Click Reaction To Enhance Drug Penetration into Solid Tumor. ACS Appl. Mater. Interfaces 2017, 9, 42459–42470. [Google Scholar] [CrossRef]
- Ficker, M.; Theeuwen, M.J.M.; Janaszewska, A.; Gorzkiewicz, M.; Svenningsen, S.W.; Klajnert-Maculewicz, B.; Christensen, J.B. Complexes of Indomethacin with 4-Carbomethoxy-Pyrrolidone PAMAM Dendrimers Show Improved Anti-Inflammatory Properties and Temperature-Dependent Binding and Release Profile. Mol. Pharm. 2018, 15, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Saha, D.; Majumdar, S.; Giri, L. Imaging Methods for the Assessment of a Complex Hydrogel as an Ocular Drug Delivery System for Glaucoma Treatment: Opportunities and Challenges in Preclinical Evaluation. Mol. Pharm. 2022, 19, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Yang, X.; Wang, Q.; Zhang, A.; Tang, B. Hydrogels-Based Ophthalmic Drug Delivery Systems for Treatment of Ocular Diseases. Mater. Sci. Eng. C 2021, 127, 112212. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold Nanoparticle Based Photothermal Therapy: Development and Application for Effective Cancer Treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Soenen, S.J.; Parak, W.J.; Rejman, J.; Manshian, B. (Intra)Cellular Stability of Inorganic Nanoparticles: Effects on Cytotoxicity, Particle Functionality, and Biomedical Applications. Chem. Rev. 2015, 115, 2109–2135. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, N. Surface Modification of Nanocarriers for Cancer Therapy. Curr. Nanosci. 2009, 5, 123–134. [Google Scholar] [CrossRef]
- Ahmed, A.; Sarwar, S.; Hu, Y.; Munir, M.U.; Nisar, M.F.; Ikram, F.; Asif, A.; Rahman, S.U.; Chaudhry, A.A.; Rehman, I.U. Surface-Modified Polymeric Nanoparticles for Drug Delivery to Cancer Cells. Expert Opin. Drug Deliv. 2021, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Naik, J.B.; Pardeshi, S.R.; Patil, R.P.; Patil, P.B.; Mujumdar, A. Mucoadhesive Micro-/Nano Carriers in Ophthalmic Drug Delivery: An Overview. Bionanoscience 2020, 10, 564–582. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Kaldybekov, D.B.; Filippov, S.K.; Radulescu, A.; Khutoryanskiy, V. V Maleimide-Decorated PEGylated Mucoadhesive Liposomes for Ocular Drug Delivery. Langmuir 2022, 38, 13870–13879. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Garg, A.; Ahmad, M.Z.; Alqahtani, A.A.; Walbi, I.A.; Ahmad, J. Recent Progress in Chitosan-Based Nanomedicine for Its Ocular Application in Glaucoma. Pharmaceutics 2023, 15, 681. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Yao, C.H.; Lue, S.J.; Yang, C.J.; Su, Y.H.; Huang, C.C.; Lai, J.Y. Amination-Mediated Nano Eye-Drops with Enhanced Corneal Permeability and Effective Burst Release for Acute Glaucoma Treatment. Chem. Eng. J. 2023, 451, 138620. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, J.; Zhang, X.; Pan, W. Nanostructured Lipid Carrier (NLC) Coated with Chitosan Oligosaccharides and Its Potential Use in Ocular Drug Delivery System. Int. J. Pharm. 2011, 403, 185–191. [Google Scholar] [CrossRef]
- Landucci, E.; Mazzantini, C.; Calvani, M.; Pellegrini-Giampietro, D.E.; Bergonzi, M.C. Evaluation of Conventional and Hyaluronic Acid-Coated Thymoquinone Liposomes in an In Vitro Model of Dry Eye. Pharmaceutics 2023, 15, 578. [Google Scholar] [CrossRef]
- Griesser, J.; Hetényi, G.; Bernkop-Schnürch, A. Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities? Polymers 2018, 10, 243. [Google Scholar] [CrossRef]
- Oh, S.; Wilcox, M.; Pearson, J.P.; Borrós, S. Optimal Design for Studying Mucoadhesive Polymers Interaction with Gastric Mucin Using a Quartz Crystal Microbalance with Dissipation (QCM-D): Comparison of Two Different Mucin Origins. Eur. J. Pharm. Biopharm. 2015, 96, 477–483. [Google Scholar] [CrossRef]
- Ludwig, A. The Use of Mucoadhesive Polymers in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef] [PubMed]
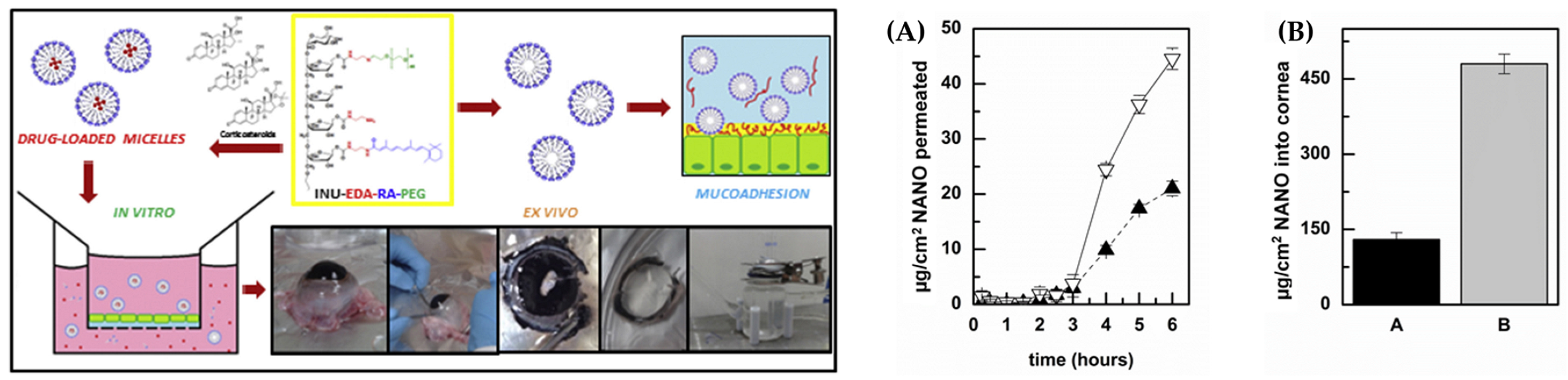
- Di Prima, G.; Bongiovì, F.; Palumbo, F.S.; Pitarresi, G.; Licciardi, M.; Giammona, G. Mucoadhesive PEGylated Inulin-Based Self-Assembling Nanoparticles: In Vitro and Ex Vivo Transcorneal Permeation Enhancement of Corticosteroids. J. Drug Deliv. Sci. Technol. 2019, 49, 195–208. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Ping, Q.; Xiao, Y.; Huang, X. Mucoadhesive Effect of Thiolated PEG Stearate and Its Modified NLC for Ocular Drug Delivery. J. Control. Release 2009, 137, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Irmukhametova, G.S.; Mun, G.A.; Khutoryanskiy, V. V Thiolated Mucoadhesive and PEGylated Nonmucoadhesive Organosilica Nanoparticles from 3-Mercaptopropyltrimethoxysilane. Langmuir 2011, 27, 9551–9556. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Development, Optimization, and In Vitro/In Vivo Characterization of Enhanced Lipid Nanoparticles for Ocular Delivery of Ofloxacin: The Influence of Pegylation and Chitosan Coating. AAPS PharmSciTech 2019, 20, 183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, A.; Zhu, L.; Yang, X.; Fang, G.; Tang, B. Cyclodextrin-Based Ocular Drug Delivery Systems: A Comprehensive Review. Coord. Chem. Rev. 2023, 476, 214919. [Google Scholar] [CrossRef]
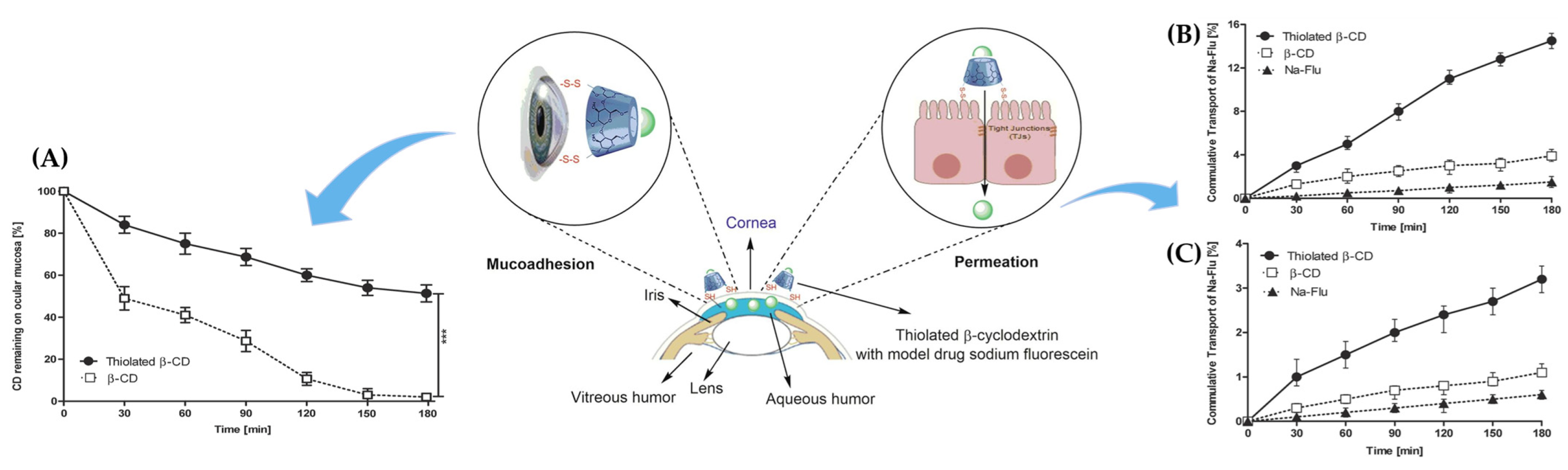
- Asim, M.H.; Ijaz, M.; Mahmood, A.; Knoll, P.; Jalil, A.; Arshad, S.; Bernkop-Schnürch, A. Thiolated Cyclodextrins: Mucoadhesive and Permeation Enhancing Excipients for Ocular Drug Delivery. Int. J. Pharm. 2021, 599, 120451. [Google Scholar] [CrossRef]
- Grassiri, B.; Knoll, P.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Bernkop-Schnürch, A. Thiolated Hydroxypropyl-β-Cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2612. [Google Scholar] [CrossRef]
- Ijaz, M.; Ahmad, M.; Akhtar, N.; Laffleur, F.; Bernkop-Schnürch, A. Thiolated α-Cyclodextrin: The Invisible Choice to Prolong Ocular Drug Residence Time. J. Pharm. Sci. 2016, 105, 2848–2854. [Google Scholar] [CrossRef]
- Jahan, F.; Zaman, S.U.; Akhtar, S.; Arshad, R.; Ibrahim, I.M.; Shahnaz, G.; Rahdar, A.; Pandey, S. Development of Mucoadhesive Thiomeric Chitosan Nanoparticles for the Targeted Ocular Delivery of Vancomycin against Staphylococcus Aureus Resistant Strains. Nanofabrication 2021, 6, 16–24. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M.; Zoidl, T. Thiolated Polymers—Thiomers: Synthesis and in Vitro Evaluation of Chitosan–2-Iminothiolane Conjugates. Int. J. Pharm. 2003, 260, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Hintzen, F.; Hauptstein, S.; Perera, G.; Bernkop-Schnürch, A. Synthesis and in Vitro Characterization of Entirely S-Protected Thiolated Pectin for Drug Delivery. Eur. J. Pharm. Biopharm. 2013, 85, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C.; Hauser, M.; Frey, A.; Jelkmann, M.; Laffleur, F.; Götzfried, S.K.; Gust, R.; Bernkop-Schnürch, A. Covalently Binding Mucoadhesive Polymers: N-Hydroxysuccinimide Grafted Polyacrylates. Eur. J. Pharm. Biopharm. 2019, 139, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, K.D.; Gilbert, J.C.; Jilma, B. Pharmacokinetics, Pharmacodynamics and Safety of Aptamers. Adv. Drug Deliv. Rev. 2018, 134, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.; Ragupathy, S.; Borchard, G. Target Specific Tight Junction Modulators. Adv. Drug Deliv. Rev. 2021, 171, 266–288. [Google Scholar] [CrossRef] [PubMed]
- Schuerer, N.; Stein, E.; Inic-Kanada, A.; Ghasemian, E.; Stojanovic, M.; Montanaro, J.; Bintner, N.; Hohenadl, C.; Sachsenhofer, R.; Barisani-Asenbauer, T. Effects of Chitosan and Chitosan N-Acetylcysteine Solutions on Conjunctival Epithelial Cells. J. EuCornea 2018, 1, 12–18. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting Across the Cell Membrane: An Overview for Small Molecules, Peptides, and Proteins. In Site-Specific Protein Labeling: Methods and Protocols; Gautier, A., Hinner, M.J., Eds.; Springer: New York, NY, USA, 2015; pp. 29–53. [Google Scholar]
- Mun, E.A.; Morrison, P.W.J.; Williams, A.C.; Khutoryanskiy, V.V. On the Barrier Properties of the Cornea: A Microscopy Study of the Penetration of Fluorescently Labeled Nanoparticles, Polymers, and Sodium Fluorescein. Mol. Pharm. 2014, 11, 3556–3564. [Google Scholar] [CrossRef]
- Nikolova, D.; Ruseva, K.; Tzachev, C.; Christov, L.; Vassileva, E. Novel Poly(Sulfobetaine Methacrylate) Based Carriers as Potential Ocular Drug Delivery Systems for Timolol Maleate. Polym. Int. 2022, 71, 662–667. [Google Scholar] [CrossRef]
- Ma, J.; Kang, K.; Yi, Q.; Zhang, Z.; Gu, Z. Multiple PH Responsive Zwitterionic Micelles for Stealth Delivery of Anticancer Drugs. RSC Adv. 2016, 6, 64778–64790. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of Polyethylene Glycol on the Surface of Nanoparticles for Targeted Drug Delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Park, J.; Zhang, Y.; Vykhodtseva, N.; Akula, J.D.; McDannold, N.J. Targeted and Reversible Blood-Retinal Barrier Disruption via Focused Ultrasound and Microbubbles. PLoS ONE 2012, 7, e42754. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gray, W.D.; Davis, M.E.; Luo, Y. Peptide- and Saccharide-Conjugated Dendrimers for Targeted Drug Delivery: A Concise Review. Interface Focus 2012, 2, 307–324. [Google Scholar] [CrossRef]
- Lajunen, T.; Hisazumi, K.; Kanazawa, T.; Okada, H.; Seta, Y.; Yliperttula, M.; Urtti, A.; Takashima, Y. Topical Drug Delivery to Retinal Pigment Epithelium with Microfluidizer Produced Small Liposomes. Eur. J. Pharm. Sci. 2014, 62, 23–32. [Google Scholar] [CrossRef]
- Youale, J.; Bigot, K.; Kodati, B.; Jaworski, T.; Fan, Y.; Nsiah, N.Y.; Pappenhagen, N.; Inman, D.M.; Behar-Cohen, F.; Bordet, T.; et al. Neuroprotective Effects of Transferrin in Experimental Glaucoma Models. Int. J. Mol. Sci. 2022, 23, 12753. [Google Scholar] [CrossRef]
- Chowers, I.; Wong, R.; Dentchev, T.; Farkas, R.H.; Iacovelli, J.; Gunatilaka, T.L.; Medeiros, N.E.; Presley, J.B.; Campochiaro, P.A.; Curcio, C.A.; et al. The Iron Carrier Transferrin Is Upregulated in Retinas from Patients with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Yefimova, M.G.; Jeanny, J.-C.; Guillonneau, X.; Keller, N.; Nguyen–Legros, J.; Sergeant, C.; Guillou, F.; Courtois, Y. Iron, Ferritin, Transferrin, and Transferrin Receptor in the Adult Rat Retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2343–2351. [Google Scholar]
- Baudouin, C.; Brignole, F.; Gastaud, P. Transferrin Receptor Expression by Retinal Pigment Epithelial Cells in Proliferative Vitreoretinopathy. Arch. Ophthalmol. 1991, 109, 1195–1196. [Google Scholar] [CrossRef]
- Singh, S.R.; Grossniklaus, H.E.; Kang, S.J.; Edelhauser, H.F.; Ambati, B.K.; Kompella, U.B. Intravenous Transferrin, RGD Peptide and Dual-Targeted Nanoparticles Enhance Anti-VEGF Intraceptor Gene Delivery to Laser-Induced CNV. Gene Ther. 2009, 16, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, M.; Han, Y.; Zhang, H.; Xu, L.; Xiang, Y. Targeting Therapy of Choroidal Neovascularization by Use of Polypeptide- and PEDF-Loaded Immunoliposomes under Ultrasound Exposure. J. Huazhong Univ. Sci. Technol. Med. Sci. 2010, 30, 798–803. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, J.; Zhao, L.; Mao, K.; Gu, Q.; Li, D.; Zhao, J.; Wu, X. RGD-Modified Multifunctional Nanoparticles Encapsulating Salvianolic Acid A for Targeted Treatment of Choroidal Neovascularization. J. Nanobiotechnol. 2021, 19, 196. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Attar, M. Antibody-Drug Conjugate (ADC) Research in Ophthalmology—A Review. Pharm. Res. 2015, 32, 3572–3576. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, S.; Jo, D.H.; Cho, C.S.; Kim, S.R.; Kang, D.; Chae, J.; Yoo, D.K.; Ha, S.; Chung, J.; et al. Specific Ablation of PDGFRβ-Overexpressing Pericytes with Antibody-Drug Conjugate Potently Inhibits Pathologic Ocular Neovascularization in Mouse Models. Commun. Med. 2021, 1, 58. [Google Scholar] [CrossRef]
- Eaton, J.S.; Miller, P.E.; Mannis, M.J.; Murphy, C.J. Ocular Adverse Events Associated with Antibody–Drug Conjugates in Human Clinical Trials. J. Ocul. Pharmacol. Ther. 2015, 31, 589–604. [Google Scholar] [CrossRef]
- Awasthy, B.; Maseeh, A.; Mukherji, P. Ocular Toxicities Associated with Anticancer Therapies: Are We Missing Something in the Blind Spot? J. Clin. Oncol. 2022, 40, e15001. [Google Scholar] [CrossRef]
- Eby, A.M.; Khan, R.; Ray, M.; Heilbrun, L.; Milanovic, C.; Jessica, J.; McDermott, M.; Heath, E. Corneal Toxicity of Antibody Drug Conjugate Chemotherapeutics. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2645. [Google Scholar]
- Domínguez-Llamas, S.; Caro-Magdaleno, M.; Mataix-Albert, B.; Avilés-Prieto, J.; Romero-Barranca, I.; Rodríguez-de-la-Rúa, E. Adverse Events of Antibody–Drug Conjugates on the Ocular Surface in Cancer Therapy. Clin. Transl. Oncol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Nhàn, N.T.T.; Maidana, D.E.; Yamada, K.H. Ocular Delivery of Therapeutic Agents by Cell-Penetrating Peptides. Cells 2023, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.N.; Cashman, S.M.; Kumar-Singh, R. Cell-Penetrating Peptide for Enhanced Delivery of Nucleic Acids and Drugs to Ocular Tissues Including Retina and Cornea. Mol. Ther. 2008, 16, 107–114. [Google Scholar] [CrossRef]
- Magzoub, M. Combating Proteins with Proteins: Engineering Cell-Penetrating Peptide Antagonists of Amyloid-β Aggregation and Associated Neurotoxicity. DNA Cell Biol. 2020, 39, 920–925. [Google Scholar] [CrossRef]
- Carreras-Badosa, G.; Maslovskaja, J.; Periyasamy, K.; Urgard, E.; Padari, K.; Vaher, H.; Tserel, L.; Gestin, M.; Kisand, K.; Arukuusk, P.; et al. NickFect Type of Cell-Penetrating Peptides Present Enhanced Efficiency for MicroRNA-146a Delivery into Dendritic Cells and during Skin Inflammation. Biomaterials 2020, 262, 120316. [Google Scholar] [CrossRef]
- Puvvula, P.K.; Moon, A.M. Novel Cell-Penetrating Peptides Derived From Scaffold-Attachment- Factor A Inhibits Cancer Cell Proliferation and Survival. Front. Oncol. 2021, 11, 621825. [Google Scholar] [CrossRef] [PubMed]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Z.; Li, M. Overcoming the Cellular Barriers and beyond: Recent Progress on Cell Penetrating Peptide Modified Nanomedicine in Combating Physiological and Pathological Barriers. Asian J. Pharm. Sci. 2022, 17, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.N.; Cashman, S.M.; Read, S.P.; Kumar-Singh, R. Cell Penetrating Peptide POD Mediates Delivery of Recombinant Proteins to Retina, Cornea and Skin. Vis. Res. 2010, 50, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Amit, C.; Muralikumar, S.; Janaki, S.; Lakshmipathy, M.; Therese, K.L.; Umashankar, V.; Padmanabhan, P.; Narayanan, J. Designing and Enhancing the Antifungal Activity of Corneal Specific Cell Penetrating Peptide Using Gelatin Hydrogel Delivery System. Int. J. Nanomed. 2019, 14, 605–622. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular Penetration of Fluorometholone-Loaded PEG-PLGA Nanoparticles Functionalized with Cell-Penetrating Peptides. Nanomedicine 2019, 14, 3089–3104. [Google Scholar] [CrossRef]
- Kudłak, B.; Wieczerzak, M. Aptamer Based Tools for Environmental and Therapeutic Monitoring: A Review of Developments, Applications, Future Perspectives. Crit. Rev. Environ. Sci. Technol. 2020, 50, 816–867. [Google Scholar] [CrossRef]
- Wan, L.-Y.; Yuan, W.-F.; Ai, W.-B.; Ai, Y.-W.; Wang, J.-J.; Chu, L.-Y.; Zhang, Y.-Q.; Wu, J.-F. An Exploration of Aptamer Internalization Mechanisms and Their Applications in Drug Delivery. Expert Opin. Drug Deliv. 2019, 16, 207–218. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T.; Feinsod, M.; Guyer, D.R. Pegaptanib for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef]
- Eyetech Study Group. Preclinical and phase 1A clinical evaluation of an anti-VEGF pegylated aptamer (EYE001) for the treatment of exudative age-related macular degeneration. Retina 2002, 22, 143–152. [Google Scholar] [CrossRef]
- Jo, N.; Mailhos, C.; Ju, M.; Cheung, E.; Bradley, J.; Nishijima, K.; Robinson, G.S.; Adamis, A.P.; Shima, D.T. Inhibition of Platelet-Derived Growth Factor B Signaling Enhances the Efficacy of Anti-Vascular Endothelial Growth Factor Therapy in Multiple Models of Ocular Neovascularization. Am. J. Pathol. 2006, 168, 2036–2053. [Google Scholar] [CrossRef]
- Li, X.; Figg, C.A.; Wang, R.; Jiang, Y.; Lyu, Y.; Sun, H.; Liu, Y.; Wang, Y.; Teng, I.-T.; Hou, W.; et al. Cross-Linked Aptamer–Lipid Micelles for Excellent Stability and Specificity in Target-Cell Recognition. Angew. Chem. Int. Ed. 2018, 57, 11589–11593. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Li, L.; Xian, G.; Wang, W.; Ma, D.; Xie, L. Investigation on Novel Chitosan Nanoparticle–Aptamer Complexes Targeting TGF-β Receptor II. Int. J. Pharm. 2013, 456, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Lohiya, G.; Katti, D.S. Carboxylated Chitosan-Mediated Improved Efficacy of Mesoporous Silica Nanoparticle-Based Targeted Drug Delivery System for Breast Cancer Therapy. Carbohydr. Polym. 2022, 277, 118822. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-Y.; Liu, Y.; Zhou, L.; Wong, M.-S.; Liu, J. Mucin-Targeting-Aptamer Functionalized Liposomes for Delivery of Cyclosporin A for Dry Eye Diseases. J. Mater. Chem. B 2023, 11, 4684–4694. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.; Lopes-Nunes, J.; Santos, F.M.; Campello, M.P.C.; Oliveira, M.C.; Paulo, A.; Tomaz, C.; Cruz, C. Assessment of Aptamer as a Potential Drug Targeted Delivery for Retinal Angiogenesis Inhibition. Pharmaceuticals 2023, 16, 751. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; He, Z.; Xiong, J.; Chao, J. Smart Drug Delivery Systems Based on DNA Nanotechnology. Chempluschem 2022, 87, e202100548. [Google Scholar] [CrossRef]
- Shin, S.W.; Yuk, J.S.; Chun, S.H.; Lim, Y.T.; Um, S.H. Hybrid Material of Structural DNA with Inorganic Compound: Synthesis, Applications, and Perspective. Nano Converg. 2020, 7, 2. [Google Scholar] [CrossRef]
- Chandrasekaran, A.R. Nuclease Resistance of DNA Nanostructures. Nat. Rev. Chem. 2021, 5, 225–239. [Google Scholar] [CrossRef]
- Kim, T.; Nam, K.; Kim, Y.M.; Yang, K.; Roh, Y.H. DNA-Assisted Smart Nanocarriers: Progress, Challenges, and Opportunities. ACS Nano 2021, 15, 1942–1951. [Google Scholar] [CrossRef]
- Huang, F.; Liao, W.-C.; Sohn, Y.S.; Nechushtai, R.; Lu, C.-H.; Willner, I. Light-Responsive and PH-Responsive DNA Microcapsules for Controlled Release of Loads. J. Am. Chem. Soc. 2016, 138, 8936–8945. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-C.; Lu, C.-H.; Hartmann, R.; Wang, F.; Sohn, Y.S.; Parak, W.J.; Willner, I. Adenosine Triphosphate-Triggered Release of Macromolecular and Nanoparticle Loads from Aptamer/DNA-Cross-Linked Microcapsules. ACS Nano 2015, 9, 9078–9086. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, T.; Kim, Y.M.; Yang, K.; Choi, I.; Roh, Y.H. Multifunctional DNA Nanogels for Aptamer-Based Targeted Delivery and Stimuli-Triggered Release of Cancer Therapeutics. Macromol. Rapid Commun. 2021, 42, e2000457. [Google Scholar] [CrossRef]
- Seeman, N.C. Nucleic Acid Junctions and Lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, B.; Zheng, T.; Zhou, T.; Guo, M.; Wang, Y.; Dong, Y. DNA Materials Assembled from One DNA Strand. Int. J. Mol. Sci. 2023, 24, 8177. [Google Scholar] [CrossRef]
- Jiang, S.; Ge, Z.; Mou, S.; Yan, H.; Fan, C. Designer DNA Nanostructures for Therapeutics. Chem 2021, 7, 1156–1179. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to Create Nanoscale Shapes and Patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef]
- Castro, C.E.; Kilchherr, F.; Kim, D.-N.; Shiao, E.L.; Wauer, T.; Wortmann, P.; Bathe, M.; Dietz, H. A Primer to Scaffolded DNA Origami. Nat. Methods 2011, 8, 221–229. [Google Scholar] [CrossRef]
- Wei, B.; Dai, M.; Yin, P. Complex Shapes Self-Assembled from Single-Stranded DNA Tiles. Nature 2012, 485, 623–626. [Google Scholar] [CrossRef]
- Yang, H.; Altvater, F.; de Bruijn, A.D.; McLaughlin, C.K.; Lo, P.K.; Sleiman, H.F. Chiral Metal–DNA Four-Arm Junctions and Metalated Nanotubular Structures. Angew. Chem. Int. Ed. 2011, 50, 4620–4623. [Google Scholar] [CrossRef]
- Tang, J.; Jia, X.; Li, Q.; Cui, Z.; Liang, A.; Ke, B.; Yang, D.; Yao, C. A DNA-Based Hydrogel for Exosome Separation and Biomedical Applications. Proc. Natl. Acad. Sci. USA 2023, 120, e2303822120. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, J.; Wang, D.; Zhu, H.; Maity, S.K.; Qu, X.; Bogaert, B.; Pei, H.; Zhang, H. Programmable and Multifunctional DNA-Based Materials for Biomedical Applications. Adv. Mater. 2018, 30, e1703658. [Google Scholar] [CrossRef]
- Chen, J.; Seeman, N.C. Synthesis from DNA of a Molecule with the Connectivity of a Cube. Nature 1991, 350, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, X.; Wu, X.; Li, Y.; Tang, W.; Yang, C.; Liu, J.; Ding, B. Chemically Modified DNA Nanostructures for Drug Delivery. Innovation 2022, 3, 100217. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Sun, W.; Shen, Z.; Seeman, N.C. A Nanomechanical Device Based on the B–Z Transition of DNA. Nature 1999, 397, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, N.R.; Ma, R.-I.; Seeman, N.C. An Immobile Nucleic Acid Junction Constructed from Oligonucleotides. Nature 1983, 305, 829–831. [Google Scholar] [CrossRef]
- Seeman, N.C. DNA in a Material World. Nature 2003, 421, 427–431. [Google Scholar] [CrossRef]
- Yan, H.; Park, S.H.; Finkelstein, G.; Reif, J.H.; LaBean, T.H. DNA-Templated Self-Assembly of Protein Arrays and Highly Conductive Nanowires. Science 2003, 301, 1882–1884. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, S.; Tian, C.; Yu, G.; Jiang, W.; Wang, G.; Mao, C. Retrosynthetic Analysis-Guided Breaking Tile Symmetry for the Assembly of Complex DNA Nanostructures. J. Am. Chem. Soc. 2016, 138, 13579–13585. [Google Scholar] [CrossRef]
- He, Y.; Ye, T.; Su, M.; Zhang, C.; Ribbe, A.E.; Jiang, W.; Mao, C. Hierarchical Self-Assembly of DNA into Symmetric Supramolecular Polyhedra. Nature 2008, 452, 198–201. [Google Scholar] [CrossRef]
- Yin, P.; Hariadi, R.F.; Sahu, S.; Choi, H.M.T.; Park, S.H.; LaBean, T.H.; Reif, J.H. Programming DNA Tube Circumferences. Science 2008, 321, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ong, L.L.; Shih, W.M.; Yin, P. Three-Dimensional Structures Self-Assembled from DNA Bricks. Science 2012, 338, 1177–1183. [Google Scholar] [CrossRef]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-Based Method for Rationally Assembling Nanoparticles into Macroscopic Materials. Nature 1996, 382, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, E.; Li, T.I.N.G.; Senesi, A.J.; Schmucker, A.L.; Pals, B.C.; de la Cruz, M.O.; Mirkin, C.A. DNA-Mediated Nanoparticle Crystallization into Wulff Polyhedra. Nature 2014, 505, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Rosi, N.L.; Wang, Y.; Huo, F.; Mirkin, C.A. Asymmetric Functionalization of Gold Nanoparticles with Oligonucleotides. J. Am. Chem. Soc. 2006, 128, 9286–9287. [Google Scholar] [CrossRef]
- Yang, H.; McLaughlin, C.K.; Aldaye, F.A.; Hamblin, G.D.; Rys, A.Z.; Rouiller, I.; Sleiman, H.F. Metal–Nucleic Acid Cages. Nat. Chem. 2009, 1, 390–396. [Google Scholar] [CrossRef]
- Mitra, D.; Di Cesare, N.; Sleiman, H.F. Self-Assembly of Cyclic Metal–DNA Nanostructures Using Ruthenium Tris(Bipyridine)-Branched Oligonucleotides. Angew. Chem. Int. Ed. 2004, 43, 5804–5808. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, Z.; Yao, C.; Yang, D. Construction of Rolling Circle Amplification-Based DNA Nanostructures for Biomedical Applications. Biomater. Sci. 2022, 10, 3054–3061. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, J.; Liu, H.; Zhao, B.; Yan, J.; Ma, Y.; Xiao, S.; Song, S.; Huang, Q.; Chao, J.; et al. Rolling Circle Amplification-Based DNA Origami Nanostructrures for Intracellular Delivery of Immunostimulatory Drugs. Small 2013, 9, 3082–3087. [Google Scholar] [CrossRef]
- Yan, J.; Hu, C.; Wang, P.; Zhao, B.; Ouyang, X.; Zhou, J.; Liu, R.; He, D.; Fan, C.; Song, S. Growth and Origami Folding of DNA on Nanoparticles for High-Efficiency Molecular Transport in Cellular Imaging and Drug Delivery. Angew. Chem. Int. Ed. 2015, 54, 2431–2435. [Google Scholar] [CrossRef]
- Zhao, T.-T.; Chen, Q.-Y.; Yang, H. Spectroscopic Study on the Formation of DNA-Ag Clusters and Its Application in Temperature Sensitive Vehicles of DOX. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 66–69. [Google Scholar] [CrossRef]
- Yu, Z.; Li, N.; Zheng, P.; Pan, W.; Tang, B. Temperature-Responsive DNA-Gated Nanocarriers for Intracellular Controlled Release. Chem. Commun. 2014, 50, 3494–3497. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, F.; Zhang, H.; Cheng, L.; Chen, K.; Shen, J.; Qi, J.; Deng, L.; He, C.; Santos, H.A.; et al. DNA-Grafted Hyaluronic Acid System with Enhanced Injectability and Biostability for Photo-Controlled Osteoarthritis Gene Therapy. Adv. Sci. 2021, 8, 2004793. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Wulf, V.; Vázquez-González, M.; Fadeev, M.; Willner, I. DNA-Based Hydrogels Loaded with Au Nanoparticles or Au Nanorods: Thermoresponsive Plasmonic Matrices for Shape-Memory, Self-Healing, Controlled Release, and Mechanical Applications. ACS Nano 2019, 13, 3424–3433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yuan, X.; Yu, J.; Huang, Y.; Shao, C.; Xiao, F.; Lin, L.; Li, Y.; Tian, L. Magnesium-Stabilized Multifunctional DNA Nanoparticles for Tumor-Targeted and PH-Responsive Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 15418–15427. [Google Scholar] [CrossRef]
- Song, L.; Ho, V.H.B.; Chen, C.; Yang, Z.; Liu, D.; Chen, R.; Zhou, D. Efficient, PH-Triggered Drug Delivery Using a PH-Responsive DNA-Conjugated Gold Nanoparticle. Adv. Healthc. Mater. 2013, 2, 275–280. [Google Scholar] [CrossRef]
- Li, Y.; Yue, S.; Cao, J.; Zhu, C.; Wang, Y.; Hai, X.; Song, W.; Bi, S. PH-Responsive DNA Nanomicelles for Chemo-Gene Synergetic Therapy of Anaplastic Large Cell Lymphoma. Theranostics 2020, 10, 8250–8263. [Google Scholar] [CrossRef]
- Yao, D.; Bhadra, S.; Xiong, E.; Liang, H.; Ellington, A.D.; Jung, C. Dynamic Programming of a DNA Walker Controlled by Protons. ACS Nano 2020, 14, 4007–4013. [Google Scholar] [CrossRef]
- Wang, H.; Peng, P.; Wang, Q.; Du, Y.; Tian, Z.; Li, T. Environment-Recognizing DNA-Computation Circuits for the Intracellular Transport of Molecular Payloads for MRNA Imaging. Angew. Chem. Int. Ed. 2020, 59, 6099–6107. [Google Scholar] [CrossRef]
- Li, B.L.; Setyawati, M.I.; Chen, L.; Xie, J.; Ariga, K.; Lim, C.-T.; Garaj, S.; Leong, D.T. Directing Assembly and Disassembly of 2D MoS2 Nanosheets with DNA for Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 15286–15296. [Google Scholar] [CrossRef]
- Ye, D.; Li, M.; Zhai, T.; Song, P.; Song, L.; Wang, H.; Mao, X.; Wang, F.; Zhang, X.; Ge, Z.; et al. Encapsulation and Release of Living Tumor Cells Using Hydrogels with the Hybridization Chain Reaction. Nat. Protoc. 2020, 15, 2163–2185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Hsu, M.-W.; Su, Y.-C.; Chang, H.-M.; Chang, C.-H.; Jan, J.-S. Naturally Derived DNA Nanogels as PH- and Glutathione-Triggered Anticancer Drug Carriers. Mater. Sci. Eng. C 2020, 114, 111025. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, C.; Cansiz, S.; Wu, C.; Xu, J.; Cui, C.; Liu, Y.; Hou, W.; Wang, Y.; Zhang, L.; et al. Self-Assembly of DNA Nanohydrogels with Controllable Size and Stimuli-Responsive Property for Targeted Gene Regulation Therapy. J. Am. Chem. Soc. 2015, 137, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, L.; Liu, Q.; Tian, R.; Shang, Y.; Liu, F.; Liu, S.; Zhao, S.; Han, Z.; Sun, J.; et al. A Tubular DNA Nanodevice as a SiRNA/Chemo-Drug Co-Delivery Vehicle for Combined Cancer Therapy. Angew. Chem. Int. Ed. 2021, 60, 2594–2598. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Meng, T.; Jiang, D.; Sun, J.; Yao, H.; Zhu, J.-J.; Min, Q. Nanomediator–Effector Cascade Systems for Amplified Protein Kinase Activity Imaging and Phosphorylation-Induced Drug Release In Vivo. Angew. Chem. Int. Ed. 2021, 60, 21565–21574. [Google Scholar] [CrossRef]
- Yang, Y.; Han, Y.; Sun, Q.; Cheng, J.; Yue, C.; Liu, Y.; Song, J.; Jin, W.; Ding, X.; de la Fuente, J.M.; et al. Au-SiRNA@ Aptamer Nanocages as a High-Efficiency Drug and Gene Delivery System for Targeted Lung Cancer Therapy. J. Nanobiotechnol. 2021, 19, 54. [Google Scholar] [CrossRef]
- Fakih, H.H.; Fakhoury, J.J.; Bousmail, D.; Sleiman, H.F. Minimalist Design of a Stimuli-Responsive Spherical Nucleic Acid for Conditional Delivery of Oligonucleotide Therapeutics. ACS Appl. Mater. Interfaces 2019, 11, 13912–13920. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, T.-T.; Li, S.-S.; Song, P.; Zhang, K.; Guan, Q.-Y.; Kang, B.; Xu, J.-J.; Chen, H.-Y. Endogenous MicroRNA-Triggered and Real-Time Monitored Drug Release via Cascaded Energy Transfer Payloads. Anal. Chem. 2017, 89, 10239–10247. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, Q.; Kang, X.; Guan, X. Probing Mercury(II)–DNA Interactions by Nanopore Stochastic Sensing. J. Phys. Chem. B 2013, 117, 4763–4769. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The Cellular and Molecular Basis of Hyperthermia. Crit. Rev. Oncol./Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Brahim, I.; Lamard, M.; Benyoussef, A.-A.; Quellec, G. Automation of Dry Eye Disease Quantitative Assessment: A Review. Clin. Exp. Ophthalmol. 2022, 50, 653–666. [Google Scholar] [CrossRef]
- Xing, Y.; Cheng, E.; Yang, Y.; Chen, P.; Zhang, T.; Sun, Y.; Yang, Z.; Liu, D. Self-Assembled DNA Hydrogels with Designable Thermal and Enzymatic Responsiveness. Adv. Mater. 2011, 23, 1117–1121. [Google Scholar] [CrossRef]
- Song, J.; Hwang, S.; Im, K.; Hur, J.; Nam, J.; Hwang, S.; Ahn, G.-O.; Kim, S.; Park, N. Light-Responsible DNA Hydrogel–Gold Nanoparticle Assembly for Synergistic Cancer Therapy. J. Mater. Chem. B 2015, 3, 1537–1543. [Google Scholar] [CrossRef]
- Thygesen, J.E.M.; Jensen, O.L. PH Changes of the Tear Fluid in the Conjunctival Sac during Postoperative Inflammation of the Human Eye. Acta Ophthalmol. 1987, 65, 134–136. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The Chemistry, Physiology and Pathology of PH in Cancer. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ouyang, Y.; Sohn, Y.S.; Nechushtai, R.; Pikarsky, E.; Fan, C.; Willner, I. PH- and MiRNA-Responsive DNA-Tetrahedra/Metal–Organic Framework Conjugates: Functional Sense-and-Treat Carriers. ACS Nano 2021, 15, 6645–6657. [Google Scholar] [CrossRef] [PubMed]
- Zenk, J.; Tuntivate, C.; Schulman, R. Kinetics and Thermodynamics of Watson–Crick Base Pairing Driven DNA Origami Dimerization. J. Am. Chem. Soc. 2016, 138, 3346–3354. [Google Scholar] [CrossRef] [PubMed]
- Nagda, R.; Park, S.; Jung, I.L.; Nam, K.; Yadavalli, H.C.; Kim, Y.M.; Yang, K.; Kang, J.; Thulstrup, P.W.; Bjerrum, M.J.; et al. Silver Nanoclusters Serve as Fluorescent Rivets Linking Hoogsteen Triplex DNA and Hairpin-Loop DNA Structures. ACS Nano 2022, 16, 13211–13222. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, S.; Lu, H.; Ying, J.Y. Acid-Resistant and Physiological PH-Responsive DNA Hydrogel Composed of A-Motif and i-Motif toward Oral Insulin Delivery. J. Am. Chem. Soc. 2022, 144, 5461–5470. [Google Scholar] [CrossRef]
- Fu, W.; Tang, L.; Wei, G.; Fang, L.; Zeng, J.; Zhan, R.; Liu, X.; Zuo, H.; Huang, C.Z.; Mao, C. Rational Design of PH-Responsive DNA Motifs with General Sequence Compatibility. Angew. Chem. Int. Ed. 2019, 58, 16405–16410. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.; Sotgia, F.; Lisanti, M.P. Tumor Microenvironment and Metabolic Synergy in Breast Cancers: Critical Importance of Mitochondrial Fuels and Function. Semin. Oncol. 2014, 41, 195–216. [Google Scholar] [CrossRef]
- Chen, W.-H.; Yu, X.; Liao, W.-C.; Sohn, Y.S.; Cecconello, A.; Kozell, A.; Nechushtai, R.; Willner, I. ATP-Responsive Aptamer-Based Metal–Organic Framework Nanoparticles (NMOFs) for the Controlled Release of Loads and Drugs. Adv. Funct. Mater. 2017, 27, 1702102. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, C.; Yu, J.; Li, Y.; Jiang, W.; Mao, C. Self-Assembly of Responsive Multilayered DNA Nanocages. J. Am. Chem. Soc. 2015, 137, 1730–1733. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Jiang, T.; Sun, W.; Gu, Z. ATP-Responsive DNA-Graphene Hybrid Nanoaggregates for Anticancer Drug Delivery. Biomaterials 2015, 50, 67–74. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Y.; Yuan, S.; Xu, X.; Wu, Z.; Wu, Z.; Qi, X. ATP Responsive DNA Nanogels Grown on Biocompatible Branches for Anticancer Drug Delivery. Soft Matter 2019, 15, 3655–3658. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Lin, L.; Xu, C.; Tian, H.; Chen, X. A GSH-Gated DNA Nanodevice for Tumor-Specific Signal Amplification of MicroRNA and MR Imaging–Guided Theranostics. Small 2019, 15, e1903016. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, D.; Zhou, Z.; Piao, Y.; Tang, J.; Shen, Y. Glutathione-Specific and Intracellularly Labile Polymeric Nanocarrier for Efficient and Safe Cancer Gene Delivery. ACS Appl. Mater. Interfaces 2020, 12, 14825–14838. [Google Scholar] [CrossRef]
- Zhang, Z.; Balogh, D.; Wang, F.; Sung, S.Y.; Nechushtai, R.; Willner, I. Biocatalytic Release of an Anticancer Drug from Nucleic-Acids-Capped Mesoporous SiO2 Using DNA or Molecular Biomarkers as Triggering Stimuli. ACS Nano 2013, 7, 8455–8468. [Google Scholar] [CrossRef]
- Woolcock, K. Structure of a DNA Enzyme. Nat. Struct. Mol. Biol. 2016, 23, 97. [Google Scholar] [CrossRef]
- Wang, C.; Sun, W.; Wright, G.; Wang, A.Z.; Gu, Z. Inflammation-Triggered Cancer Immunotherapy by Programmed Delivery of CpG and Anti-PD1 Antibody. Adv. Mater. 2016, 28, 8912–8920. [Google Scholar] [CrossRef]
- Yurke, B.; Turberfield, A.J.; Mills, A.P.; Simmel, F.C.; Neumann, J.L. A DNA-Fuelled Molecular Machine Made of DNA. Nature 2000, 406, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; O’Connell, M.; Wisdom, J.A.; Dai, H. Carbon Nanotubes as Multifunctional Biological Transporters and Near-Infrared Agents for Selective Cancer Cell Destruction. Proc. Natl. Acad. Sci. USA 2005, 102, 11600–11605. [Google Scholar] [CrossRef]
- Li, J.; Fan, C.; Pei, H.; Shi, J.; Huang, Q. Smart Drug Delivery Nanocarriers with Self-Assembled DNA Nanostructures. Adv. Mater. 2013, 25, 4386–4396. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in Ocular Applications and Their Potential Toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef]
- Prow, T.W.; Bhutto, I.; Kim, S.Y.; Grebe, R.; Merges, C.; McLeod, D.S.; Uno, K.; Mennon, M.; Rodriguez, L.; Leong, K.; et al. Ocular Nanoparticle Toxicity and Transfection of the Retina and Retinal Pigment Epithelium. Nanomedicine 2008, 4, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Conley, S.M.; Makkia, R.; Guo, J.; Cooper, M.J.; Naash, M.I. Comparative Analysis of DNA Nanoparticles and AAVs for Ocular Gene Delivery. PLoS ONE 2012, 7, e52189. [Google Scholar] [CrossRef]
- Willem de Vries, J.; Schnichels, S.; Hurst, J.; Strudel, L.; Gruszka, A.; Kwak, M.; Bartz-Schmidt, K.-U.; Spitzer, M.S.; Herrmann, A. DNA Nanoparticles for Ophthalmic Drug Delivery. Biomaterials 2018, 157, 98–106. [Google Scholar] [CrossRef]
- Schnichels, S.; Simmang, D.; Löscher, M.; Herrmann, A.; de Vries, J.W.; Spitzer, M.S.; Hurst, J. Lipid-DNA Nanoparticles as Drug-Delivery Vehicles for the Treatment of Retinal Diseases. Pharmaceutics 2023, 15, 532. [Google Scholar] [CrossRef]
- Schnichels, S.; Hurst, J.; De Vries, J.W.; Ullah, S.; Frol, K.; Gruszka, A.; Loscher, M.; Bartz-Schmidt, K.-U.; Spitzer, M.S.; Herrmann, A. Improved Treatment Options for Glaucoma with Brimonidine-Loaded Lipid DNA Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 9445–9456. [Google Scholar] [CrossRef]
- Schnichels, S.; Frößl, K.; de Vries, J.W.; Löscher, M.; Bartz-Schmidt, K.; Herrmann, A.; Spitzer, M.S.; Hurst, J. Brimonidine Loaded Lipid DNA-Nanoparticles as an Improved and Novel Treatment Option for Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1703. [Google Scholar]
- Schnichels, S.; Hurst, J.; de Vries, J.W.; Ullah, S.; Gruszka, A.; Kwak, M.; Löscher, M.; Dammeier, S.; Bartz-Schmidt, K.-U.; Spitzer, M.S.; et al. Self-Assembled DNA Nanoparticles Loaded with Travoprost for Glaucoma-Treatment. Nanomedicine 2020, 29, 102260. [Google Scholar] [CrossRef] [PubMed]
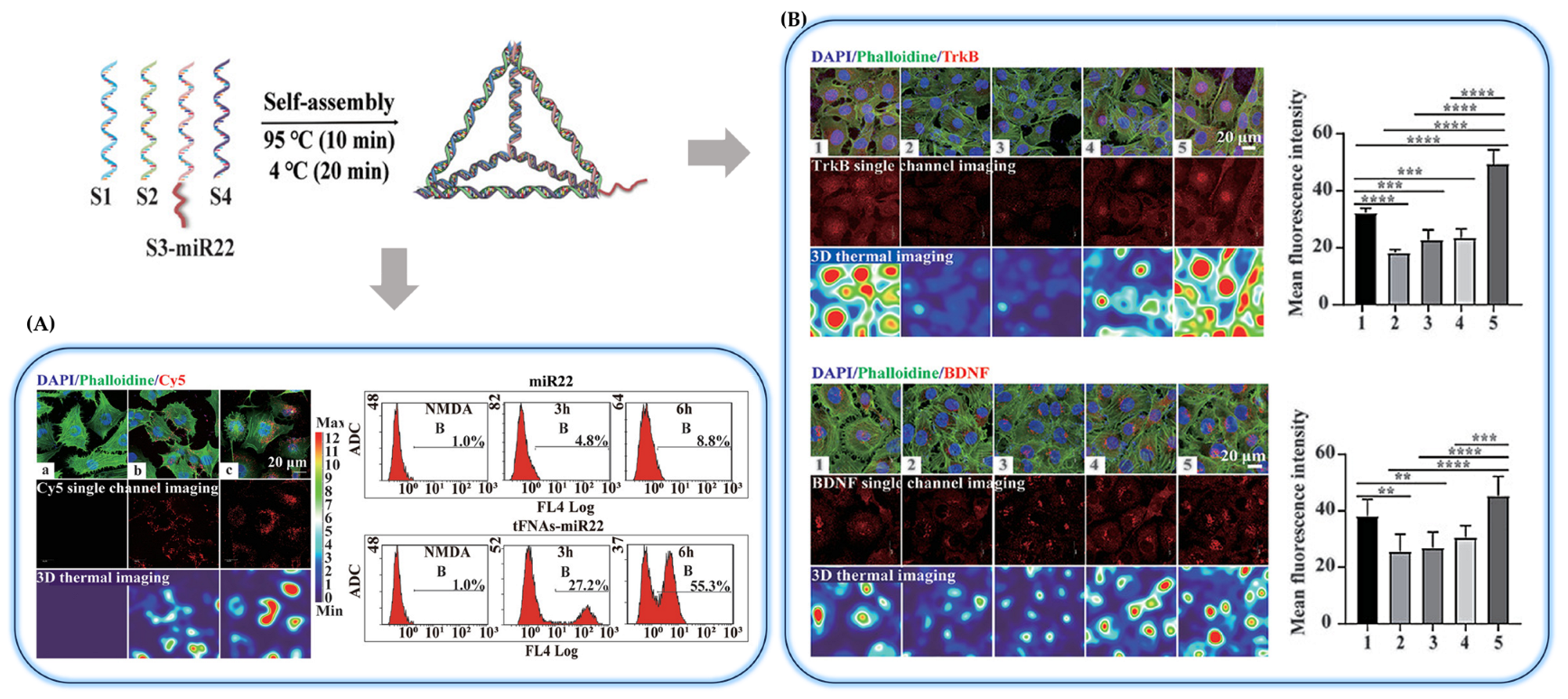
- Zhou, X.; Lai, Y.; Xu, X.; Wang, Q.; Sun, L.; Chen, L.; Li, J.; Li, R.; Luo, D.; Lin, Y.; et al. Tetrahedral Framework Nucleic Acids Inhibit Pathological Neovascularization and Vaso-Obliteration in Ischaemic Retinopathy via PI3K/AKT/MTOR Signalling Pathway. Cell Prolif. 2023, 56, e13407. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiao, L.; Yan, N.; Li, Y.; Wang, Y.; Qin, X.; Zhao, D.; Liu, M.; Li, N.; Lin, Y. The Neuroprotective Effect of MicroRNA-22-3p Modified Tetrahedral Framework Nucleic Acids on Damaged Retinal Neurons Via TrkB/BDNF Signaling Pathway. Adv. Funct. Mater. 2021, 31, 2104141. [Google Scholar] [CrossRef]
- Kansara, V.S.; Cooper, M.; Sesenoglu-Laird, O.; Muya, L.; Moen, R.; Ciulla, T.A. Suprachoroidally Delivered DNA Nanoparticles Transfect Retina and Retinal Pigment Epithelium/Choroid in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-Q.; Quiambao, A.B.; Fitzgerald, J.B.; Cooper, M.J.; Conley, S.M.; Naash, M.I. Ocular Delivery of Compacted DNA-Nanoparticles Does Not Elicit Toxicity in the Mouse Retina. PLoS ONE 2009, 4, e7410. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J. Novel Approaches for Retinal Drug and Gene Delivery. Transl. Vis. Sci. Technol. 2014, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Huang, S.; Britton, W.R.; Chen, J. MicroRNAs in Vascular Eye Diseases. Int. J. Mol. Sci. 2020, 21, 649. [Google Scholar] [CrossRef]
- Guan, C.; Zhu, X.; Feng, C. DNA Nanodevice-Based Drug Delivery Systems. Biomolecules 2021, 11, 1855. [Google Scholar] [CrossRef]






| Stimulus | Structures | Mechanisms | Target Diseases (Drug) | Ref. | |
|---|---|---|---|---|---|
| Temperature | DNA based silver nanoclusters | The anti-parallel four-strand structure forming DNA-AgNC is structured as an i-motif including C-quadruplex as the temperature changes. | Cancer (Dox) | [185] | |
| DNA-gated mesoporous silica nanocarriers | Change of the amino group on the surface of the MSNs acting as the valve | Cancer (Dox) | [186] | ||
| DNA-grafted HA with gold nanorod | NIR-triggered on-demand release of spherical nucleic acids by photo-thermal induced DNA dehybridization | Osteoarthritis (gene therapy) | [187] | ||
| DNA based hydrogels loaded with gold or silver nanoparticles | Thermoplastic properties of AuNPs and AuNRs trigger the dehybridization of the DNA duplexes | Cancer (Dox) | [188] | ||
| pH | Mg2+ aggregated functional DNAs from RCA (i-motif) | i-motif structure switch in response to pH changes | Cancer (Dox) | [189] | |
| MN/MC2 duplex with GNP (i-motif) | Cancer (Dox) | [190] | |||
| DNA polymer micelles (Hoogsteen-type triplexes) | Hoogsteen interaction switch in response to pH changes | Cancer (Dox) | [191] | ||
| Tetrameric DNA walker (triple-stranded structure) | - (Fluorescence) | [192] | |||
| Biomolecule | ATP | Framework nucleic acid (FNA) nanocarriers | ATP aptamer (ABA27) responding to ATP triggers the toehold-mediated strand displacement reaction | - (mRNA) | [193] |
| 2D MoS2 Nanosheets with DNA | Autonomously disassembled of structures in response to cancer cells’ heightened ATP metabolism | Cancer (Dox) | [194] | ||
| DNA hydrogels by aptamer-trigger-clamped hybridization chain reaction | Destruction of the hydrogel through the stimulus-response of ATP | Cancer (cloaking and decloaking of tumor cells) | [195] | ||
| GSH | DNA-DOX nanogels formed by Cross-linking kiwifruit-derived DNA | High GSH concentration cleaved the disulfide bonds of DTSSP-cross-linked DNA-DOX NGs | Cancer (Dox) | [196] | |
| DNA nanohydrogels were created through a self-assembly process using three kinds of building units | High GSH concentration cleaved the disulfide bonds of building units (Y-shaped monomers and a DNA linker) | Cancer (-) | [197] | ||
| DNA nanodevice functionalized with small interfering RNA (siRNA) | Mechanical opening and release of siRNA in response to intracellular GSH; cleaved the disulfide bonds | Cancer (Dox) | [198] | ||
| Enzymes | Artificial kinase-mediated cascade nanosystem composed of nanomediator (NM) and nanoeffector (NE) | Protein kinase-catalyzed phosphorylation to secondary mediator DNA | Cancer (Dox) | [199] | |
| Nanocarriers with double-stranded DNA and MMP-2 cleavable peptides | (MMP)-2 enzymes overexpressed in tumor tissue cleaved the peptide chain | Lung cancer (Dox) | [200] | ||
| Oligonucleotides | Spherical nucleic acid from monodisperse DNA–polymer conjugates | In the present of a particular cytoplasmic genetic marker, two triggers hybridize and release nucleic acid therapeutics. | - (Nucleic acid therapeutics) | [201] | |
| Drug delivery platform of carbon dots which were connected to a stem-loop molecular beacon | Overexpressed endogenous microRNA-21 released drugs by competitive hybridization with the molecular beacon | Cancer (Dox) | [202] | ||
| Metal ion | Loop size of the DNA hairpin | Formation of Thymine–Hg(II)–Thymine complexes by DNA–Hg(II) interactions | - (detection of mercury(II)) | [203] | |
| Structures | Effectiveness | Target Diseases | Ref. |
|---|---|---|---|
| DNA nanoparticles |
| Virus or infections | [230,231] |
| Lipid-DNA Nanoparticles |
| Retinal diseases or glaucoma | [232,233,234,235] |
| Tetrahedral framework nucleic acids |
| Optic neurodegenerative diseases (gene delivery) | [236,237] |
| Plasmid DNA nanoparticles compacted with PEG-substituted lysine 30-mer peptides |
| Retinal diseases | [238,239] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Noh, H. Advancements in Nanogels for Enhanced Ocular Drug Delivery: Cutting-Edge Strategies to Overcome Eye Barriers. Gels 2023, 9, 718. https://doi.org/10.3390/gels9090718
Lee H, Noh H. Advancements in Nanogels for Enhanced Ocular Drug Delivery: Cutting-Edge Strategies to Overcome Eye Barriers. Gels. 2023; 9(9):718. https://doi.org/10.3390/gels9090718
Chicago/Turabian StyleLee, Hyeonah, and Hyeran Noh. 2023. "Advancements in Nanogels for Enhanced Ocular Drug Delivery: Cutting-Edge Strategies to Overcome Eye Barriers" Gels 9, no. 9: 718. https://doi.org/10.3390/gels9090718
APA StyleLee, H., & Noh, H. (2023). Advancements in Nanogels for Enhanced Ocular Drug Delivery: Cutting-Edge Strategies to Overcome Eye Barriers. Gels, 9(9), 718. https://doi.org/10.3390/gels9090718