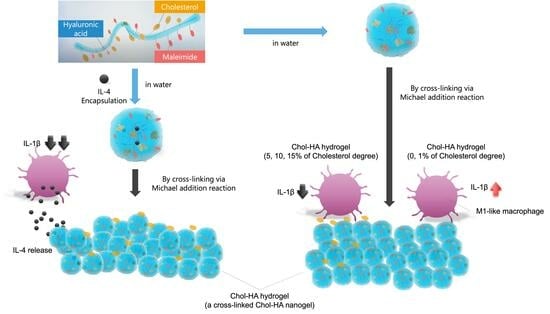
Preparation of Cholesterol-Modified Hyaluronic Acid Nanogel-Based Hydrogel and the Inflammatory Evaluation Using Macrophage-like Cells
Abstract
:1. Introduction
2. Results
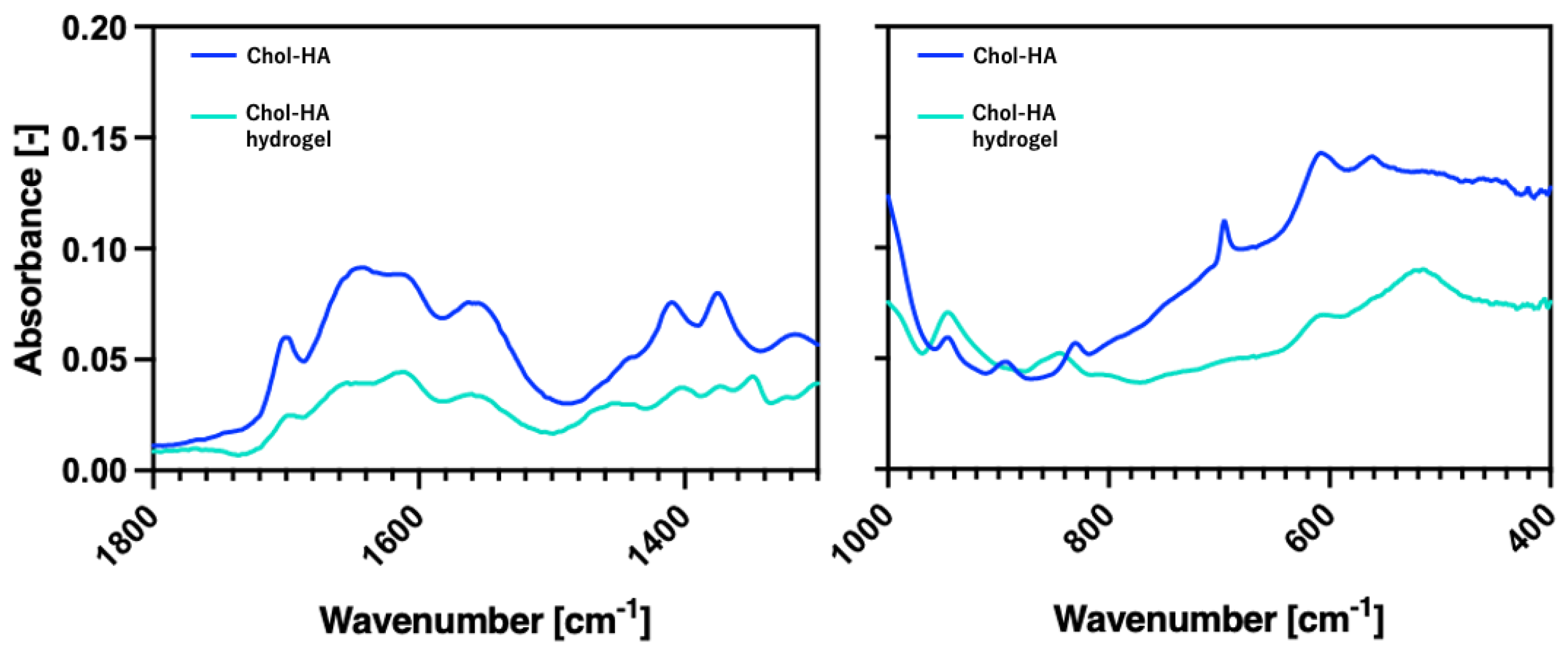
2.1. Synthesis of Chol-HA
2.2. Characterization of the Chol-HA Nanogel Using Dynamic Light Scattering (DLS)
2.3. Preparation of the Chol-HA Hydrogel
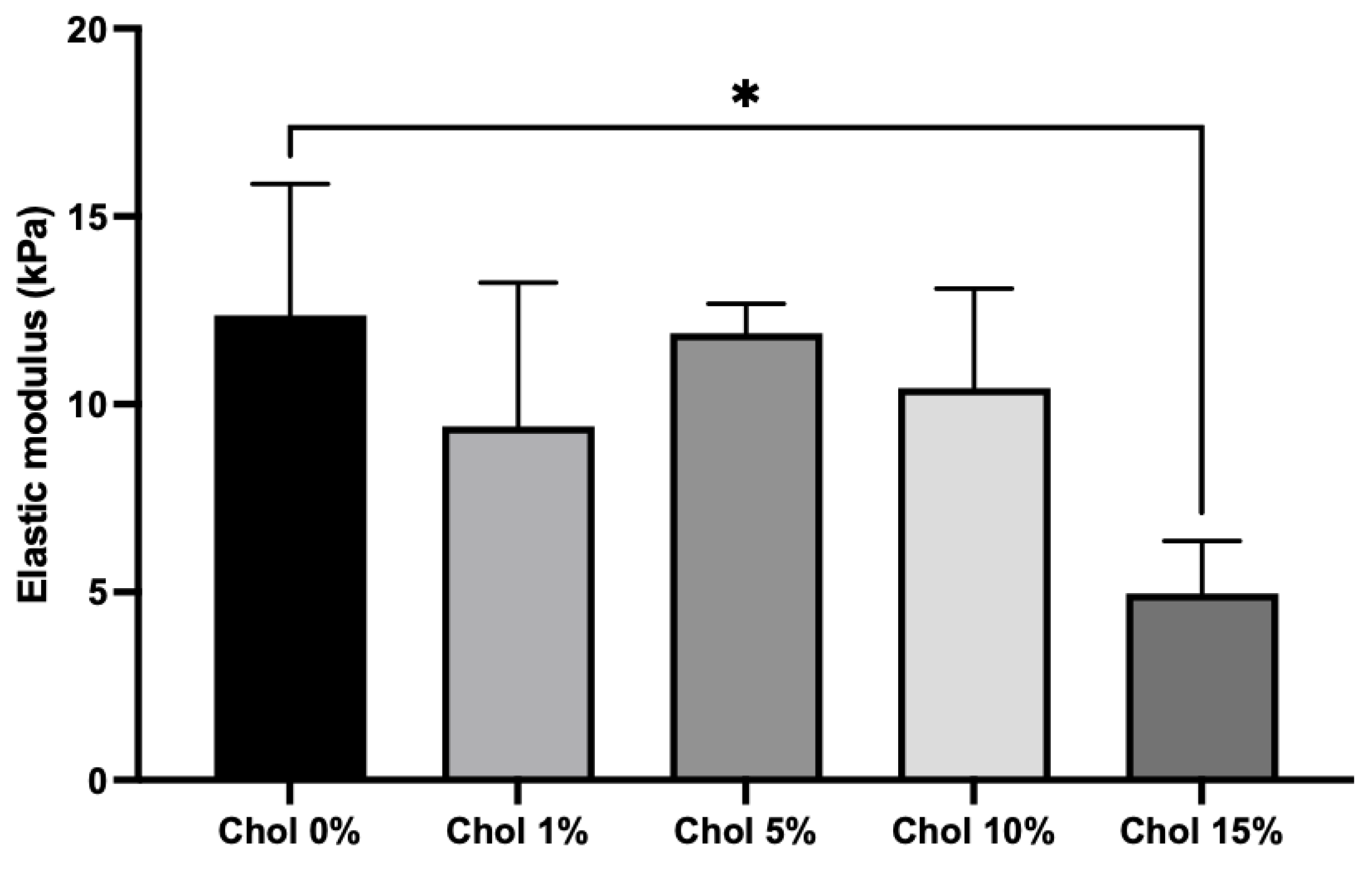
2.4. Mechanical Properties of the Chol-HA Hydrogel
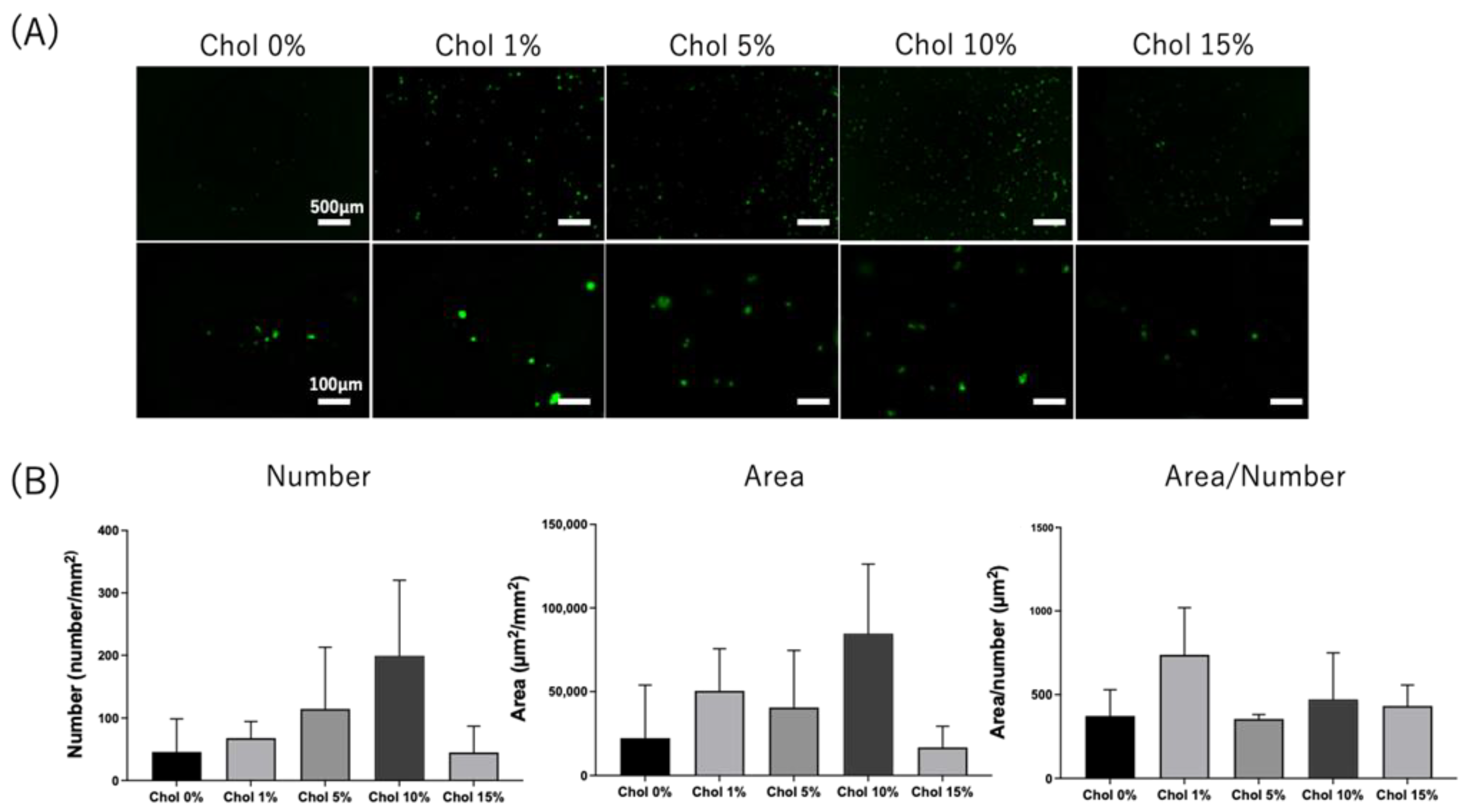
2.5. Cell Adhesion on the Chol-HA Hydrogel
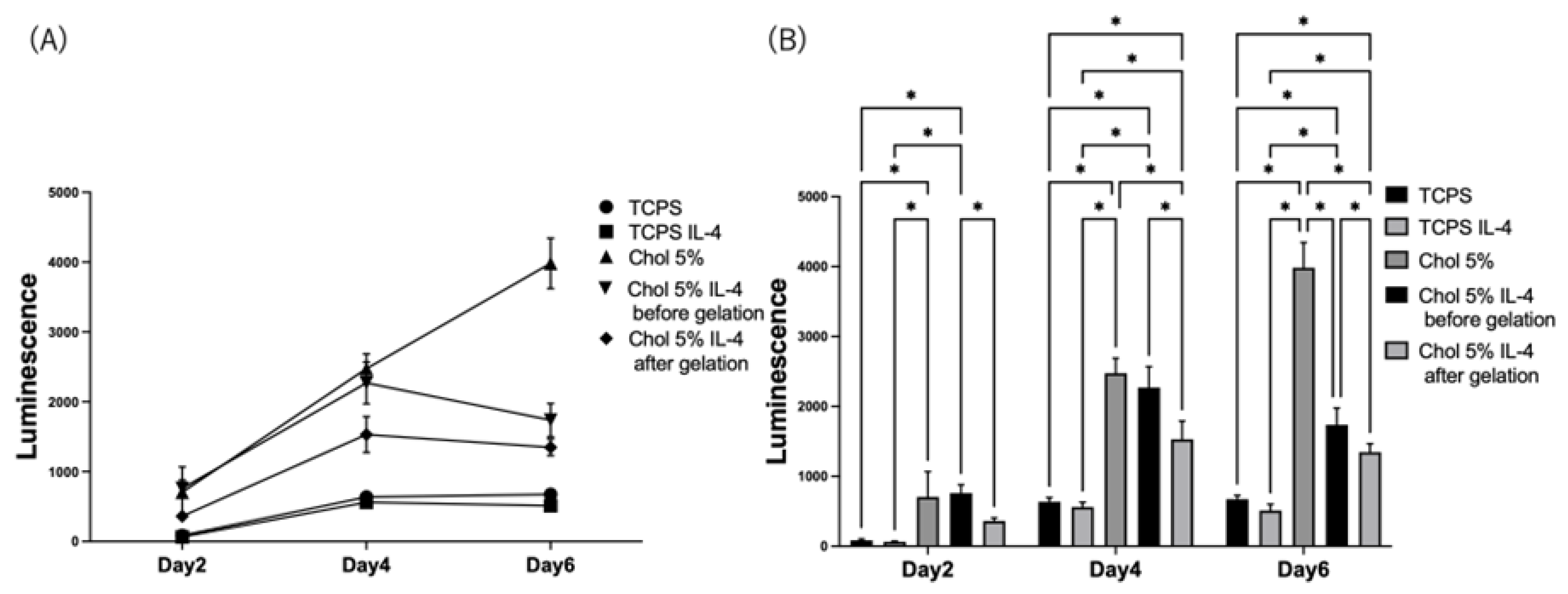
2.6. Pro-Inflammation Response of HiBiT-THP-1 to the Chol-HA Hydrogel
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Preparation of Hyaluronic Acid Modified with Cholesterol and Maleimide (Chol-HA)
5.3. Dynamic Light Scattering Measurement of Chol-HA Nanogel
5.4. Preparation of Chol-HA Nanogel-Based Hydrogel (Chol-HA Hydrogel)
5.5. Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopic Analysis
5.6. Encapsulation of IL-4 in the Chol-HA Hydrogel
5.7. Scanning Electron Microscope (SEM) Observation
5.8. Compression Test
5.9. Cell Culture
5.10. Cell Culture on the Chol-HA Hydrogel
5.11. Fluorescent Microscopic Observation of Cells on the Chol-HA Hydrogel
5.12. HiBiT Assay
5.13. Statical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Chol | Cholesterol |
| HA | Hyaluronic acid |
| HA-TBA | Hyaluronic acid tetra-n-butylammonium salt |
| DMT-MM | Hydrochloride 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride |
| FBS | Fetal bovine serum |
| TCPS | Tissue culture polystyrene |
| PMA | Phorbol 12-Myristate 13-Acetate |
| LPS | Lipopolysaccharide |
| IL-4 | Interleukin 4 |
References
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Extraction of Elastic Modulus and Hardness of the Soft Metallic Films on Hard Substrates by Nanoindentation. J. Control. Release 2000, 69, 169. [Google Scholar]
- Zhang, H.; Huang, S.; Yang, X.; Zhai, G. Current research on hyaluronic acid-drug bioconjugates. Eur. J. Med. Chem. 2014, 86, 310–317. [Google Scholar] [CrossRef]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef]
- Widjaja, L.K.; Bora, M.; Chan, P.N.P.H.; Lipik, V.; Wong, T.T.L.; Venkatraman, S.S. Hyaluronic acid-based nanocomposite hydrogels for ocular drug delivery applications. J. Biomed. Mater. Res. Part A 2014, 102, 3056–3065. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Sureerat, K.; Younghyen, J.; Hansoo, P. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 1–16. [Google Scholar]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, 41–56. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, X.; Ji, Y.; Yin, L.; Dong, Z.; Chen, F.; Zhong, M.; Shen, J.; Liu, Z.; Chang, L. Adjustable and ultrafast light-cured hyaluronic acid hydrogel: Promoting biocompatibility and cell growth. J. Mater. Chem. B 2020, 8, 5441–5450. [Google Scholar] [CrossRef]
- Zhu, J.; Li, F.; Wang, X.; Yu, J.; Wu, D. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kumar, S. CD44-Mediated Adhesion to Hyaluronic Acid Contributes to Mechanosensing and Invasive Motility. Mol. Cancer Res. 2014, 12, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef] [PubMed]
- Gloria, H.Á.; Kristina, N. Hyaluronan and its derivatives for ophthalmology: Recent advances and future perspectives. Carbohydr. Polym. 2021, 259, 117697. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Deguchi, S.; Moriguchi, N.; Yamaguchi, S.; Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar] [CrossRef]
- Hirakura, T.; Yasugi, K.; Nemoto, T.; Sato, M.; Shimoboji, T.; Aso, Y.; Morimoto, N.; Akiyoshi, K. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: New system for sustained delivery of protein with a chaperone-like function. J. Control. Release 2010, 142, 483–489. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Mukai, S.; Sasaki, Y.; Akiyoshi, K. Nanogel Tectonics for Tissue Engineering: Protein Delivery Systems with Nanogel Chaperones. Adv. Health Mater. 2018, 7, e1800729. [Google Scholar] [CrossRef]
- Lima, C.S.A.d.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef]
- Antonia-Nancy, H.; Iwatsuki, Y.; Yabuuchi, K.; Aso, S.; Katsumata, T.; Fukumoto, K.; Tanaka, Y.; Nakai, T.; Shimoboji, T.; Matsumoto, M.; et al. Self-assembled nanogels based on hyaluronic acid for antibody protection from heat denaturation. Biochem. Eng. J. 2023, 196, 108955. [Google Scholar] [CrossRef]
- Nakai, T.; Hirakura, T.; Sakurai, Y.; Shimoboji, T.; Ishigai, M.; Akiyoshi, K. Injectable Hydrogel for Sustained Protein Release by Salt-Induced Association of Hyaluronic Acid Nanogel. Macromol. Biosci. 2012, 12, 475–483. [Google Scholar] [CrossRef]
- Spiller, K.L.; Koh, T.J. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliv. Rev. 2017, 122, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.E.; García, A.J. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021, 133, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Matsushima, T.; Kurimoto, R.; Chiba, T.; Inutani, Y.; Asahara, H. Identification of chemical compounds regulating PD-L1 by introducing HiBiT-tagged cells. FEBS Lett. 2021, 595, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Bonam, S.R.; Rasala, S.; Wankar, J.; Bohara, R.A.; Bayry, J.; Rochev, Y.; Pandit, A. Recent advances and prospects of hyaluronan as a multifunctional therapeutic system. J. Control. Release 2021, 336, 598–620. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Liu, Y.; He, Y.; Wang, W.; Du, Y.; Yang, C.; Gao, F. CD44 clustering is involved in monocyte differentiation. Acta Biochim. Biophys. Sin. 2014, 46, 540–547. [Google Scholar] [CrossRef]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.B.; Dockrell, D.H. The Identification of Markers of Macrophage Differentiation in PMA-Stimulated THP-1 Cells and Monocyte-Derived Macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef]
- Hsieh, J.Y.; Keating, M.T.; Smith, T.D.; Meli, V.S.; Botvinick, E.L.; Liu, W.F. Matrix crosslinking enhances macrophage adhesion, migration, and inflammatory activation. APL Bioeng. 2019, 3, 016103. [Google Scholar] [CrossRef]
- Blakney, A.K.; Swartzlander, M.D.; Bryant, S.J. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A 2012, 100, 1375–1386. [Google Scholar] [CrossRef]
- Weiss, G.; Bogdan, C.; Hentze, M.W. Pathways for the regulation of macrophage iron metabolism by the anti-inflammatory cytokines IL-4 and IL-13. J. Immunol. 1997, 158, 420–425. [Google Scholar] [CrossRef]
- Schuerwegh, A.; Dombrecht, E.; Stevens, W.; Van Offel, J.; Bridts, C.; De Clerck, L. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthr. Cartil. 2003, 11, 681–687. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, Y.; Liang, Q.; Ge, C.; Yang, J.; Shan, B.; Liu, Y.; Zhou, X.; Yin, L. Inflammation-Instructed Hierarchical Delivery of IL-4/miR-21 Orchestrates Osteoimmune Microenvironment toward the Treatment of Rheumatoid Arthritis. Adv. Funct. Mater. 2021, 31, 2101033. [Google Scholar] [CrossRef]




| Cholesterol Incorporation (%) | Size (nm) | PDI (-) |
|---|---|---|
| 0 | 178 ± 4.2 | 0.525 ± 0.030 |
| 1 | 146 ± 2.4 | 0.467 ± 0.003 |
| 5 | 106 ± 1.4 | 0.398 ± 0.001 |
| 10 | 73 ± 1.8 | 0.335 ± 0.003 |
| 15 | 37 ± 0.3 | 0.291 ± 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yabuuchi, K.; Suzuki, M.; Liang, C.; Hashimoto, Y.; Kimura, T.; Akiyoshi, K.; Kishida, A. Preparation of Cholesterol-Modified Hyaluronic Acid Nanogel-Based Hydrogel and the Inflammatory Evaluation Using Macrophage-like Cells. Gels 2023, 9, 866. https://doi.org/10.3390/gels9110866
Yabuuchi K, Suzuki M, Liang C, Hashimoto Y, Kimura T, Akiyoshi K, Kishida A. Preparation of Cholesterol-Modified Hyaluronic Acid Nanogel-Based Hydrogel and the Inflammatory Evaluation Using Macrophage-like Cells. Gels. 2023; 9(11):866. https://doi.org/10.3390/gels9110866
Chicago/Turabian StyleYabuuchi, Kohei, Mika Suzuki, Chen Liang, Yoshihide Hashimoto, Tsuyoshi Kimura, Kazunari Akiyoshi, and Akio Kishida. 2023. "Preparation of Cholesterol-Modified Hyaluronic Acid Nanogel-Based Hydrogel and the Inflammatory Evaluation Using Macrophage-like Cells" Gels 9, no. 11: 866. https://doi.org/10.3390/gels9110866
APA StyleYabuuchi, K., Suzuki, M., Liang, C., Hashimoto, Y., Kimura, T., Akiyoshi, K., & Kishida, A. (2023). Preparation of Cholesterol-Modified Hyaluronic Acid Nanogel-Based Hydrogel and the Inflammatory Evaluation Using Macrophage-like Cells. Gels, 9(11), 866. https://doi.org/10.3390/gels9110866