Comparison of Hydroxypropyl Methylcellulose and Alginate Gel Films with Meloxicam as Fast Orodispersible Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
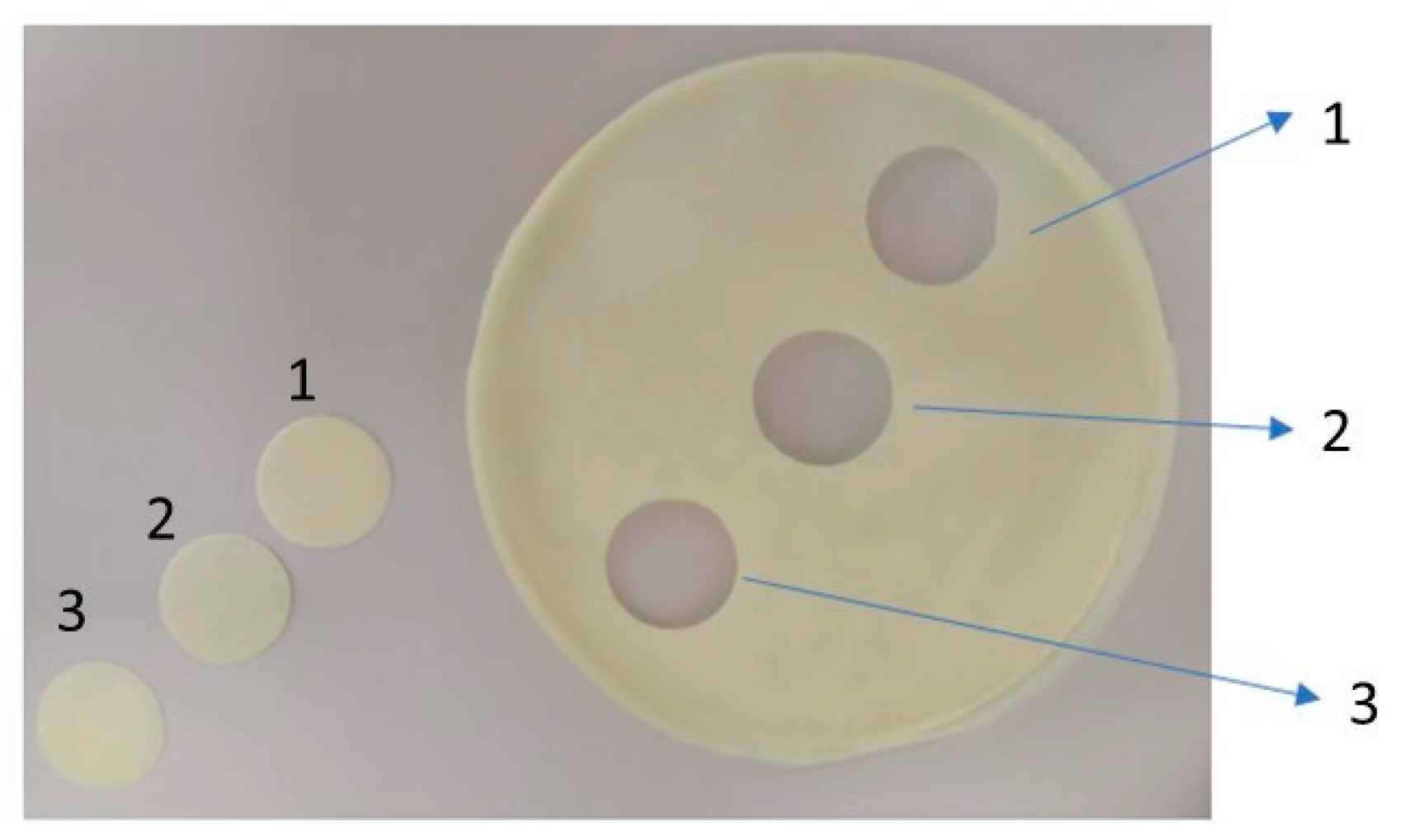
2.1. Appearance of Gel Films
2.2. Mass Uniformity of the Gel Films
2.3. Determination of Loading of Meloxicam in Films
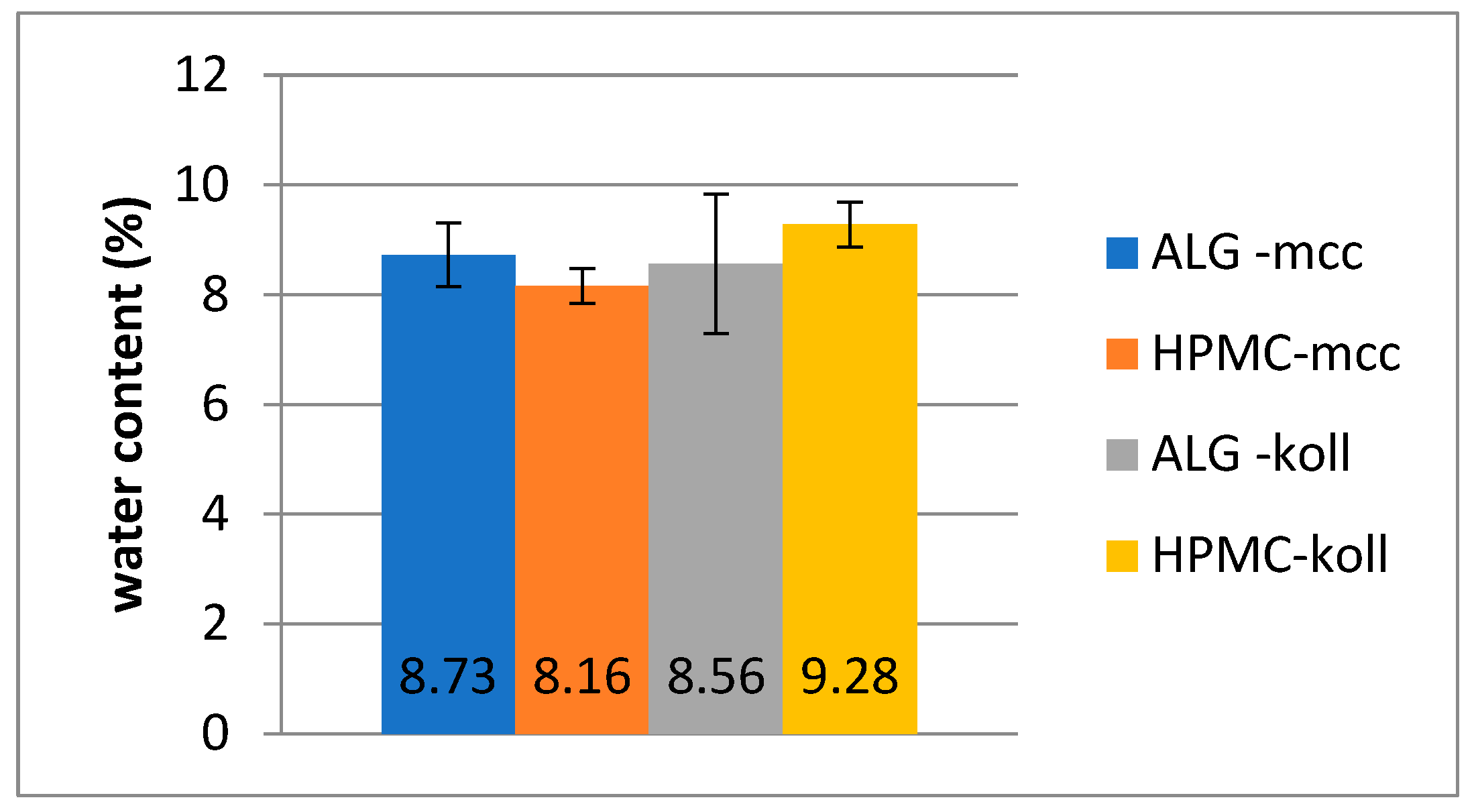
2.4. Water Content
2.5. Disintegration Time
2.6. Water Uptake
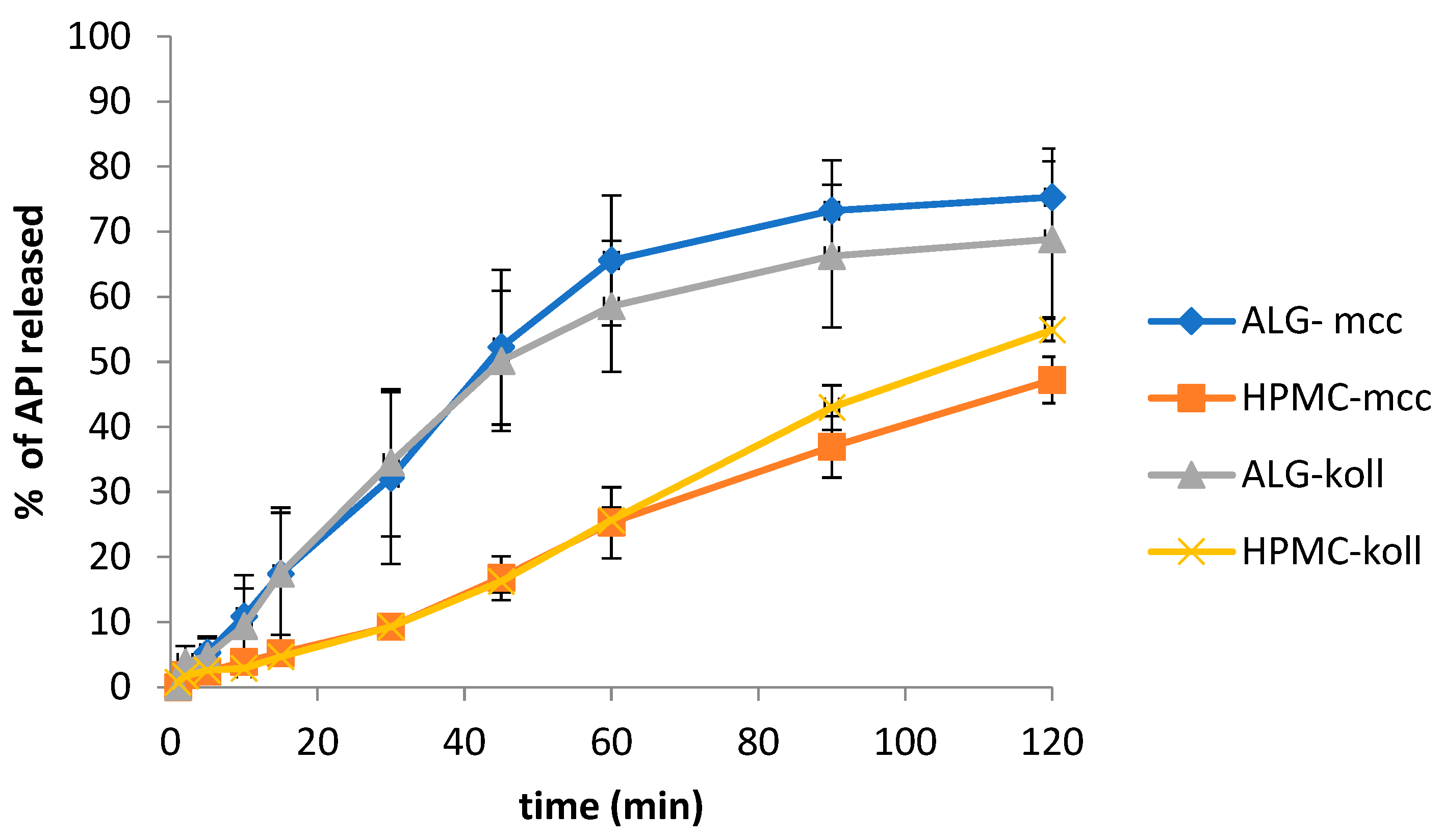
2.7. Dissolution of MLX from Gel Films
2.8. Comparison of Release Profiles with DDSolver Software
3. Conclusions and Perspectives
4. Materials and Methods
4.1. Materials
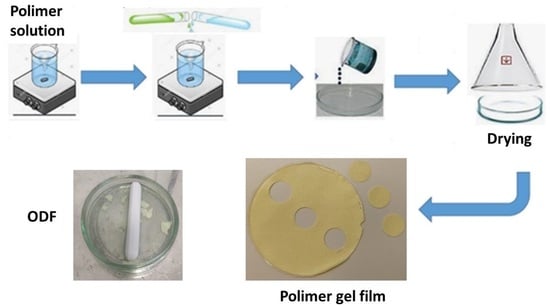
4.2. Preparation of Polymer Gel Films
4.3. Characterization of Prepared Gel Films
4.3.1. Appearance, Size, and Mass Uniformity of the Gel Films
4.3.2. Water Content
4.3.3. Content of MLX in Gel Films
4.3.4. Disintegration Time of Gel Films
4.3.5. Wettability of the Films
4.3.6. Dissolution Study
4.3.7. Comparison of Release Profiles for the Similarities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olechno, K.; Basa, A.; Winnicka, K. Success Depends on Your Backbone’—About the Use of Polymers as Essential Materials Forming Orodispersible Films. Materials 2021, 14, 2021. [Google Scholar] [CrossRef]
- Schiele, J.T.; Quinzler, R.; Klimm, H.D.; Pruszydlo, M.G.; Haefeli, W.E. Difficulties swallowing solid oral dosage forms in a general practice population: Prevalence, causes, and relationship to dosage forms. Eur. J. Clin. Pharm. 2013, 69, 937–948. [Google Scholar] [CrossRef]
- Visser, J.C.; Wibier, L.; Mekhaeil, M.; Woerdenbag, H.J.; Taxis, K. Orodispersible films as a personalized dosage form for nursing home residents, an exploratory study. Int. J. Clin. Pharm. 2020, 42, 436–444. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simões, S. Oral films: Current status and future perspectives: I––Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef]
- Garsuch, J.B.V. Comparative investigations on different polymers for the preparation of fast-dissolving oral films. J. Pharm. Pharmacol. 2010, 62, 539–545. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.J.; Shin, D.; Jo, K.; Lee, L. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Umemura, K.; Tahara, K.; Takeuchi, H. Formulation design of hydroxypropyl cellulose films for use as orally disintegrating dosage forms. J. Drug Deliv. Sci. Technol. 2018, 46, 93–100. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Size, Shape and Other Physical Attributes of Generic Tablets and Capsules; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2015; pp. 1–7. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 20 May 2023).
- Salawi, A. An Insight into Preparatory Methods and Characterization of Orodispersible Film—A Review. Pharmaceuticals 2022, 15, 844. [Google Scholar] [CrossRef]
- Amin, M.; Gangurde, A.; Alai, V. Oral Film Technology: Challenges and Future Scope for Pharmaceutical Industry. Ijppr. Human 2015, 3, 183–203. [Google Scholar]
- Cilurzo, F.; Musazzi, U.M.; Franzé, S.; Selmin, F.; Minghetti, P. Orodispersible dosage forms: Biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 2018, 23, 251–259. [Google Scholar] [CrossRef]
- Gupta, M.S.; Gowda, D.V.; Kumar, T.P.; Rosenholm, J.M. A Comprehensive Review of Patented Technologies to Fabricate Orodispersible Films: Proof of Patent Analysis (2000–2020). Pharmaceutics 2022, 14, 820. [Google Scholar] [CrossRef]
- Özakar, R.S.; Özakar, E. Current Overview of Oral Thin Films. Turk. J. Pharm. Sci. 2021, 18, 111–121. [Google Scholar] [CrossRef]
- Scarpa, M.; Stegemann, S.; Hsiao, W.K.; Pichler, H.; Gaisford, S.; Bresciani, M.; Paudel, A.; Orlu, M. Orodispersible films: Towards drug delivery in special populations. Int. J. Pharm. 2017, 523, 327–335. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Khalid, G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2020, 576, 118963. [Google Scholar] [CrossRef]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlíidalová, E.; Turánek, J.; Mašek, J. Hypromellose—A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef]
- Dharmalingam, K.; Anandalakshmi, R. Fabrication, characterization and drug loading efficiency of citric acid crosslinked NaCMC-HPMC hydrogel films for wound healing drug delivery applications. Int. J. Biol. Macromol. 2019, 134, 815–829. [Google Scholar] [CrossRef]
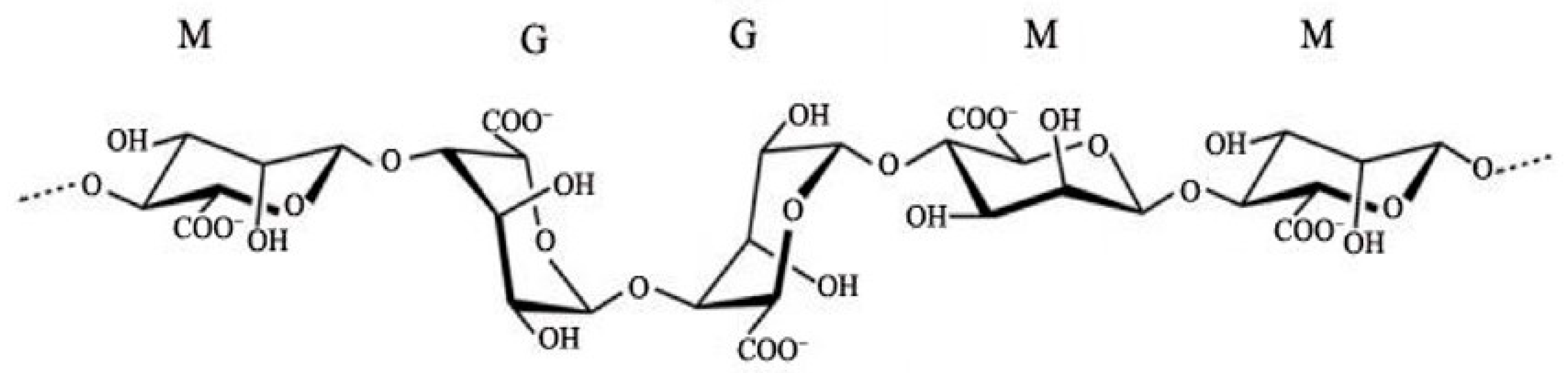
- Sanchez-Ballester, N.M.; Bataille, B.; Soulairol, I. Sodium alginate and alginic acid as pharmaceutical excipients for tablet formulation: Structure-function relationship. Carbohydr. Polym. 2021, 270, 118399. [Google Scholar] [CrossRef]
- Abourehab, M.S.A.; Rajendran, R.R.; Singh, A.; Prmanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Armal, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef]
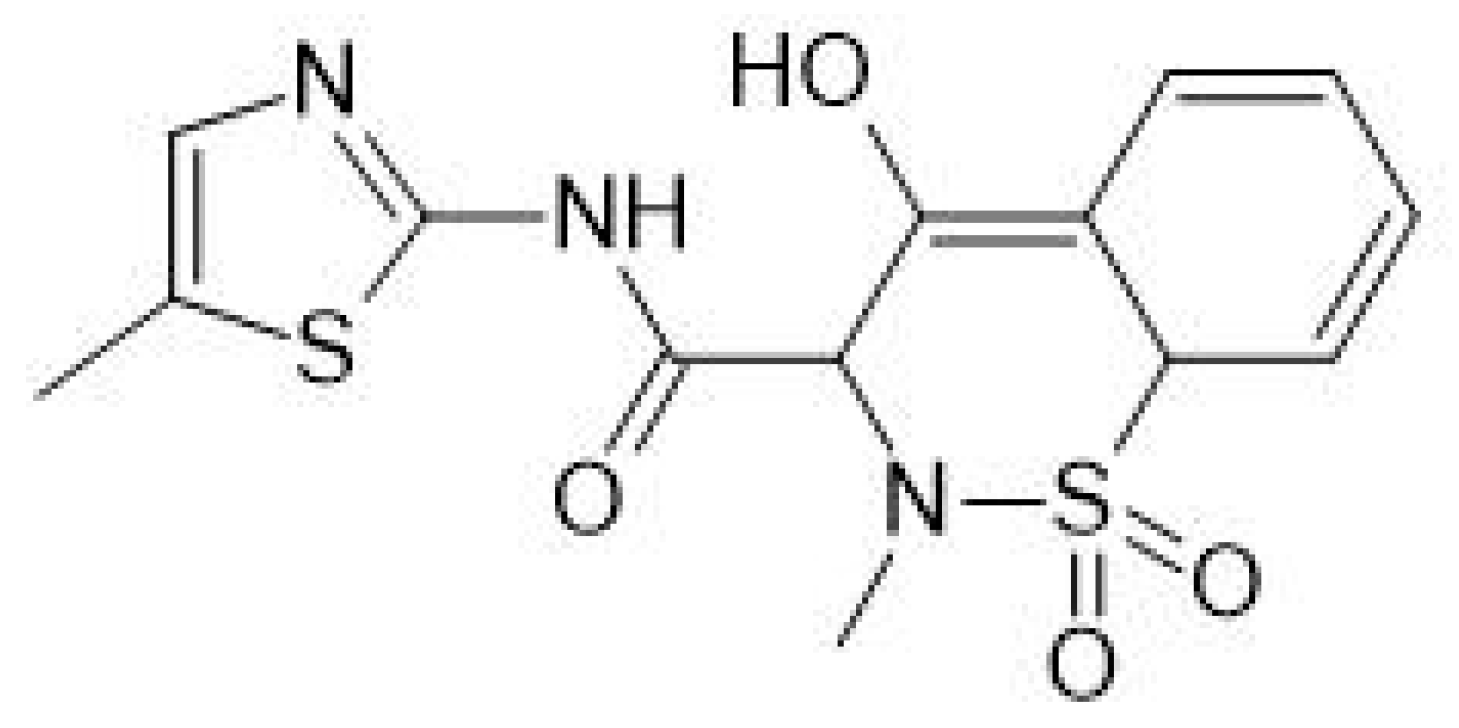
- Khalil, N.J.; Aldosari, K.F. Meloxicam. Profiles Drug Subst. Excip. Relat. Methodol. 2020, 45, 159–197. [Google Scholar] [CrossRef]
- Naidu, N.B.; Chowdary, K.P.R.; Murthy, K.V.R.; Satyanarayana, V.; Hayman, A.R.; Becket, G. Physicochemical characterization and dissolution properties of meloxicam–cyclodextrin binary systems. J. Pharm. Biomed. Anal. 2004, 35, 75–86. [Google Scholar] [CrossRef]
- Seedher, N.; Bhatia, S. Solubility Enhancement of Cox-2 Inhibitors Using Various Solvent Systems. AAPS PharmSciTech 2003, 4, 33. [Google Scholar] [CrossRef]
- Luger, P.; Daneck, K.; Engel, W.; Trummlitz, G.; Wagner, K. Structure and physicochemical properties of meloxicam, a new NSAID. Eur. J. Pharm. Sci. 1996, 4, 175–187. [Google Scholar] [CrossRef]
- Scarpa, M.; Paudel, A.; Kloprogge, F.; Hsiao, W.K.; Bresciani, M.; Gaisford, S.; Orlu, M. Key acceptability attributes of orodispersible films. Eur. J. Pharm. Biopharm. 2018, 125, 131–140. [Google Scholar] [CrossRef]
- Gorle, A.P.; Gattani, S.G. Development and evaluation of ocular drug delivery system. Pharm. Dev. Technol. 2010, 15, 46–52. [Google Scholar] [CrossRef]
- Song, Q.; Shen, C.H.; Shena, B.; Liana, W.; Liu, X.; Dai, B.; Yuan, H. Development of a fast dissolving sublingual film containing meloxicam nanocrystals for enhanced dissolution and earlier absorption. J. Drug Del. Sci. Technol. 2018, 43, 243–252. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Aamir, M.N.; Shah, M.S.; Ali, L.; Anwer, K.; Javaid, Z. Formulation design, characterization and in vitro drug release study of orodispersible film comprising BCS class II drugs. Pak. J. Pharm. Sci. 2020, 33, 343–353. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263. [Google Scholar] [CrossRef]
- Muselík, J.; Komersová, A.; Kubová, K.; Matzick, K.; Skalická, B. Critical Overview of FDA and EMA Statistical Methods to Compare In Vitro Drug Dissolution Profiles of Pharmaceutical Products. Pharmaceutics 2021, 13, 1703. [Google Scholar] [CrossRef]
- Kumar, R.S.; Annu, K. Superdisintegrant: Crucial elements for mouth dissolving tablets. J. Drug Deliv. Therap. 2019, 9, 461–468. [Google Scholar] [CrossRef]
- Bishal, A.; Ali, K.A.; Bandyopadhyay, B.; Bandyopadhyay, R.; Debnath, B. Study of different super-disintegrants and their use as a magic ingredient for different immediate-release tablets. J. Pharm. Negat. Res. 2022, 13, 1222. [Google Scholar]
- Shobana, K.; Subramanian, L.; Rajesh, M.; Sivaranjani, K. A Review on Superdisintegrants. Int. J. Pharm. Sci. Rev. Res. 2020, 65, 149–154. [Google Scholar] [CrossRef]
- Shruthi, B.K.; Chandrakala, V.; Srinivasan, S. Role of Superdisintegrants in Rapid Dissolving Oral Films. Int. J. Pharm. Sci. Rev. Res. 2022, 75, 110–116. [Google Scholar]




| Properties | Meloxicam | Publ. | ||
|---|---|---|---|---|
| state | yellow solid state | [20] | ||
| molecular mass (g/mol) | 351.401 | [21] | ||
| BCS class | II | [22] | ||
| melting temperature (°C) | 255 | |||
| solubility (mg/mL) | water | 0.012 | [22,23] | |
| glycerol | 0.138 | |||
| NaOH | pH = 7.4 | 0.062 | ||
| pH = 9.6 | 2.615 | |||
| pH = 10.7 | 17.900 | |||
| Ethanol | 0.354 | |||
| PEG-400 | 3.763 | |||
| pKa | pH 0–3 | 1.09 | [23] | |
| pH 2.5–6.5 | 4.18 | |||
| logP | pH = 2 | 2.43 | ||
| pH = 4 | 2.34 | |||
| pH = 6 | 1.01 | |||
| pH = 7 | 0.07 | |||
| Ingredient * | ALG-mcc | ALG-coll | HPMC-mcc | HPMC-koll |
|---|---|---|---|---|
| Weight (g) | ||||
| sodium alginate | 3.0 | 3.0 | - | - |
| HPMC | - | 3.0 | 3.0 | |
| kollidon | - | 2.0 | - | 2.0 |
| microcrystalline cellulose | 2.0 | - | 2.0 | - |
| meloxicam | 0.5 | 0.5 | 0.5 | 0.5 |
| glicerol | 5.0 | 5.0 | 5.0 | 5.0 |
| aspartame | 0.275 | 0.275 | 0.275 | 0.275 |
| Series | ALG-mcc | ALG-koll | HPMC-mcc | HPMC-koll |
|---|---|---|---|---|
| Shape | Round | |||
| Diameter | 19 mm | |||
| Surface of upper side | Rough | Rough | smooth | Rough |
| Surface of lower side | smooth | |||
| Color | Yellow | |||
| Elasticity | High elasticity Does not break when bent No trace when bend is visible | Low elasticity Does not break when bent There is a trace of the fold | ||
| Property | Series | ||||
|---|---|---|---|---|---|
| ALG-mcc | HPMC-mcc | ALG-koll | HPMC-koll | ||
| Mass (g) | Xśr ± SD | 0.096 ± 0.030 | 0.117 ± 0.011 | 0.108 ± 0.033 | 0.095 ± 0.020 |
| Content of MLX (mg) | Determined Xśr ± SD | 4.85 ± 0.40 | 5.53 ± 0.51 | 4.11 ± 0.34 | 5.16 ± 1.29 |
| theoretical | 4.62 | 5.98 | 5.21 | 5.51 | |
| Water content (%) | Xśr ± SD | 8.73 ± 0.58 | 8.16 ± 0.32 | 8.56 ± 1.27 | 9.28 ± 0.41 |
| Disintegration time (s) | Xśr ± SD | 107 ± 40 | 160 ± 62 | 69 ± 49 | 74 ± 14 |
| Comparing Series | Similarity Coefficient f2 | ||
|---|---|---|---|
| HPMC-mcc | ALG-koll | HPMC-koll | |
| ALG-mcc | 31.05 | 69.53 * | 32.55 |
| HPMC-mcc | 33.97 | 74.21 * | |
| ALG-koll | 35.43 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadach, B.; Misek, M.; Ferlak, J. Comparison of Hydroxypropyl Methylcellulose and Alginate Gel Films with Meloxicam as Fast Orodispersible Drug Delivery. Gels 2023, 9, 687. https://doi.org/10.3390/gels9090687
Jadach B, Misek M, Ferlak J. Comparison of Hydroxypropyl Methylcellulose and Alginate Gel Films with Meloxicam as Fast Orodispersible Drug Delivery. Gels. 2023; 9(9):687. https://doi.org/10.3390/gels9090687
Chicago/Turabian StyleJadach, Barbara, Martyna Misek, and Jan Ferlak. 2023. "Comparison of Hydroxypropyl Methylcellulose and Alginate Gel Films with Meloxicam as Fast Orodispersible Drug Delivery" Gels 9, no. 9: 687. https://doi.org/10.3390/gels9090687
APA StyleJadach, B., Misek, M., & Ferlak, J. (2023). Comparison of Hydroxypropyl Methylcellulose and Alginate Gel Films with Meloxicam as Fast Orodispersible Drug Delivery. Gels, 9(9), 687. https://doi.org/10.3390/gels9090687