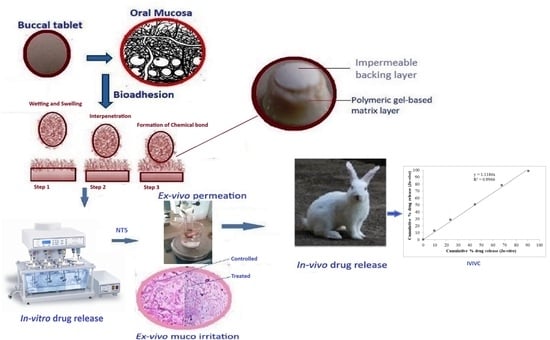
Development and Characterization of Gel-Based Buccoadhesive Bilayer Formulation of Nifedipine
Abstract
:1. Introduction
2. Results and Discussions
2.1. Drug Polymer Interaction Studies through DSC
2.2. Physicochemical Evaluation of Buccoadhesive Bilayered Formulations
Surface pH
2.3. Swelling Index
2.4. Ex Vivo Buccoadhesive Strength
2.5. In Vitro Drug Release Study
2.6. Ex Vivo Permeation Studies
2.7. Ex Vivo Muco Irritation by Histological Examination
2.8. In Vivo Drug Release Study
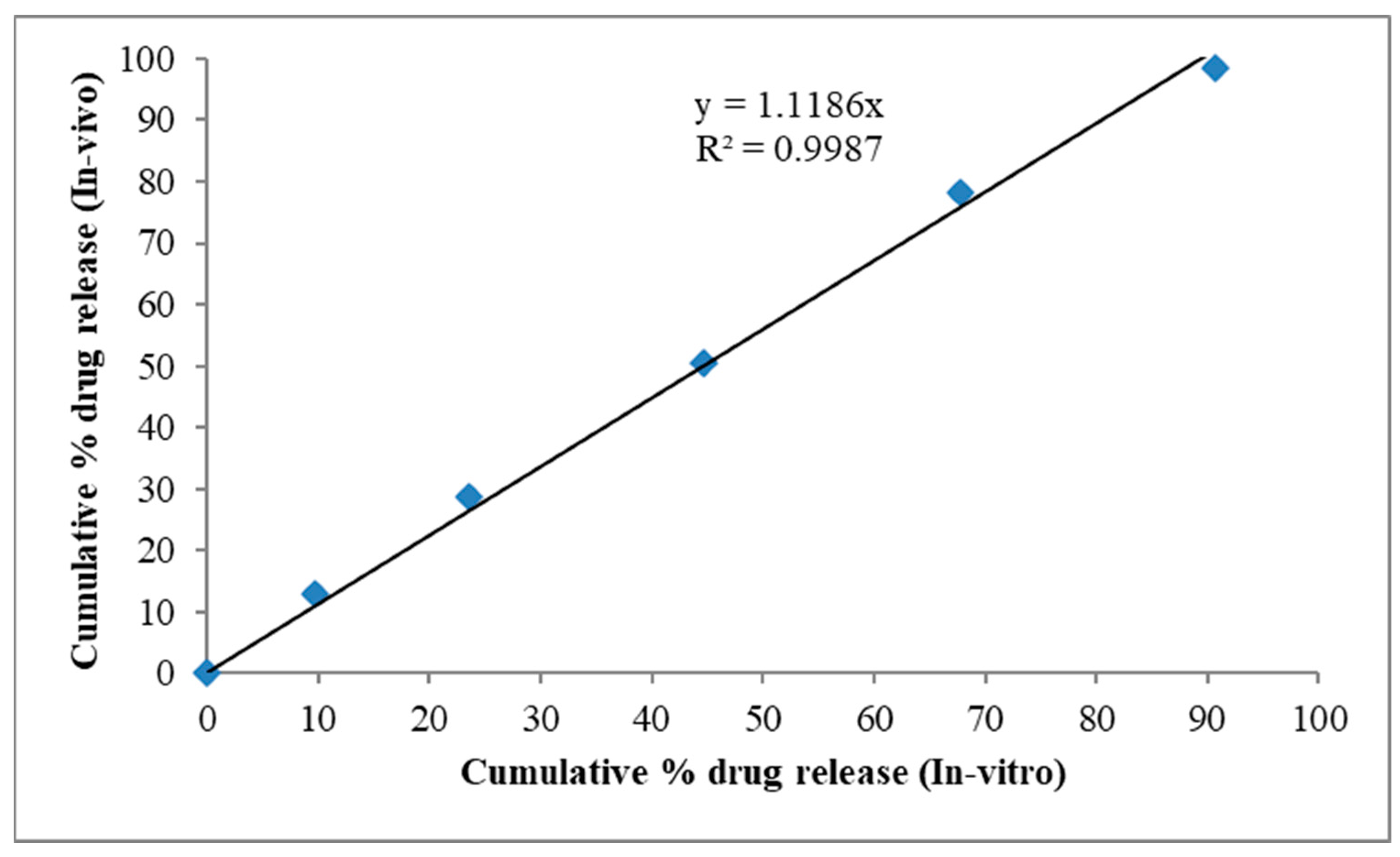
2.9. In Vitro—In Vivo Correlation
2.10. Stability Study
3. Conclusions
4. Materials and Methods
4.1. Drug Polymer Interaction Studies through Differential Scanning Calorimetry (DSC)
4.2. Preparation of Buccoadhesive Bilayer Formulations of Nifedipine
4.3. Physicochemical Evaluation of Buccoadhesive Bilayered Formulations
Surface pH
4.4. Swelling Index (SI)
4.5. Ex Vivo Buccoadhesive Strength
4.6. In Vitro Drug Release Study
4.7. Ex Vivo Permeation Studies
4.8. Ex Vivo Muco Irritation by Histological Examination
4.9. In Vivo Drug Release Study
4.10. In Vitro—In Vivo Correlation
4.11. Stability Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayan, M.F.; Salem, M.S.; Bayan, R.F. Development and in Vitro Evaluation of a Large-Intestinal Drug Delivery System. Res. J. Pharm. Technol. 2022, 15, 35–39. [Google Scholar] [CrossRef]
- Golshani, S.; Vatanara, A.; Amin, M. Recent Advances in Oral Mucoadhesive Drug Delivery. J. Pharm. Pharm. Sci. 2022, 25, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.R.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Bayan, M.F.; Marji, S.M.; Salem, M.S.; Begum, M.Y.; Chidambaram, K.; Chandrasekaran, B. Development of polymeric-based formulation as potential smart colonic drug delivery system. Polymers 2022, 14, 3697. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Smart, J.D. The Basics and Underlying Mechanisms of Mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Avinash, V.D.; Karishma, N.; Pharate, R.M.; Datir, M. Recent Advances in Mucoadhesive Buccal Drug Delivery System and Its Marketed Scope and Opportunities. Int. J. Adv. Pharm. Sci. 2018, 1, 86–104. [Google Scholar]
- Puri, V.; Sharma, A.; Maman, P.; Rathore, N.; Singh, I. Overview of Mucoadhesive Biopolymers for Buccal Drug Delivery Systems. Int. J. Appl. Pharm. 2019, 11, 18–29. [Google Scholar] [CrossRef]
- Birudaraj, R.; Berner, B.; Shen, S.; Li, X. Buccal Permeation of Buspirone: Mechanistic Studies on Transport Pathways. J. Pharm. Sci. 2005, 94, 70–78. [Google Scholar] [CrossRef]
- Price, T.M.; Blauer, K.L.; Hansen, M.; Stanczyk, F.; Lobo, R.; Bates, G.W. Single-Dose Pharmacokinetics of Sublingual versus Oral Administration of Micronized 17 Beta-Estradiol. Obstet. Gynecol. 1997, 89, 340–345. [Google Scholar] [CrossRef]
- Okur, N.Ü.; Bülbül, E.Ö.; Yağcılar, A.P.; Siafaka, P.I. Current Status of Mucoadhesive Gel Systems for Buccal Drug Delivery. Curr. Pharm. Des. 2021, 27, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Drug Delivery Applications of Chitin and Chitosan: A Review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Ritzoulis, C.; Bouropoulos, N.; Tzetzis, D.; Andreadis, D.A.; Boetker, J.; Rantanen, J.; Fatouros, D.G. Unidirectional Drug Release from 3D Printed Mucoadhesive Buccal Films Using FDM Technology: In Vitro and Ex Vivo Evaluation. Eur. J. Pharm. Biopharm. 2019, 144, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Roy, R.; Pathan, H.K.; Sharma, A.; Ghosh, S.; Kumar, S. Formulation and Evaluation of Polyherbal Aqueous Gel from Psidium Guajava, Piper Betel and Glycerrhiza Glabra Extract for Mouth Ulcer Treatment. Res. J. Pharmacogn. Phytochem. 2020, 12, 145–148. [Google Scholar] [CrossRef]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G.J. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef]
- Choe, S.-H.; Choi, E.-Y.; Hyeon, J.-Y.; Keum, B.R.; Choi, I.S.; Kim, S.-J. Effect of Nifedipine, a Calcium Channel Blocker, on the Generation of Nitric Oxide and Interleukin-1β by Murine Macrophages Activated by Lipopolysaccharide from Prevotella Intermedia. Naunyn. Schmiedebergs Arch. Pharmacol. 2021, 394, 59–71. [Google Scholar] [CrossRef]
- Jin, H.; Ngo, H.V.; Park, C.; Lee, B.-J. Mucoadhesive Buccal Tablet of Leuprolide and Its Fatty Acid Conjugate: Design, in Vitro Evaluation and Formulation Strategies. Int. J. Pharm. 2023, 639, 122963. [Google Scholar] [CrossRef]
- Javed, Q.U.; Syed, M.A.; Arshad, R.; Rahdar, A.; Irfan, M.; Raza, S.A.; Shahnaz, G.; Hanif, S.; Díez-Pascual, A.M. Evaluation and Optimization of Prolonged Release Mucoadhesive Tablets of Dexamethasone for Wound Healing: In Vitro–In Vivo Profiling in Healthy Volunteers. Pharmaceutics 2022, 14, 807. [Google Scholar]
- Ikram, M.; Gilhotra, N.; Gilhotra, R.M. Formulation and Optimization of Mucoadhesive Buccal Patches of Losartan Potassium by Using Response Surface Methodology. Adv. Biomed. Res. 2015, 4, 239. [Google Scholar]
- Hardy, I.J.; Cook, W.G.; Melia, C.D. Compression and Compaction Properties of Plasticised High Molecular Weight Hydroxypropylmethylcellulose (HPMC) as a Hydrophilic Matrix Carrier. Int. J. Pharm. 2006, 311, 26–32. [Google Scholar] [CrossRef]
- Giang, H.N.; Le, A.T.K.; Huynh, T.N.A.; Phung, T.K.; Sakai, W. Effect of Additives on Fabrication and Properties of Hydroxypropyl Methylcellulose-Based Hydrogels. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P. Drug-Polymers Composite Matrix Tablets: Effect of Hydroxypropyl Methylcellulose (HPMC) K-Series on Porosity, Compatibility, and Release Behavior of the Tablet Containing a BCS Class I Drug. Polymers 2022, 14, 3406. [Google Scholar] [CrossRef] [PubMed]
- Bayan, M.F.; Jaradat, A.; Alyami, M.H.; Naser, A.Y. Smart pellets for controlled delivery of 5-Fluorouracil. Molecules 2022, 28, 306. [Google Scholar] [CrossRef] [PubMed]
- Mane, P.P.; Bushetti, S.S.; Keshavshetti, G.G. Development and In Vitro Evaluation of Mucoadhesive Buccal Films of Nebivolol. Indian J. Pharm. Sci. 2014, 76, 166–169. [Google Scholar]
- Patel, H.; Srinatha, A.; Sridhar, B.K. External Cross-Linked Mucoadhesive Microbeads for Prolonged Drug Release: Development and In Vitro Characterization. Indian J. Pharm. Sci. 2014, 76, 437–444. [Google Scholar]
- Di Prima, G.; Conigliaro, A.; De Caro, V. Mucoadhesive Polymeric Films to Enhance Barbaloin Penetration Into Buccal Mucosa: A Novel Approach to Chemoprevention. AAPS PharmSciTech 2019, 20, 18. [Google Scholar] [CrossRef]
- Bayan, M.F. Drug release control and enhancement using carriers with different concentrations of Capmul® MCM C8. Int. J. Appl. Pharm. 2021, 13, 249–252. [Google Scholar] [CrossRef]
- El-Houssiny, A.S.; Ward, A.A.; Mostafa, D.M.; Abd-El-Messieh, S.L.; Abdel-Nour, K.N.; Darwish, M.M.; Khalil, W.A. Drug–Polymer Interaction between Glucosamine Sulfate and Alginate Nanoparticles: FTIR, DSC and Dielectric Spectroscopy Studies. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 25014. [Google Scholar] [CrossRef]
- Koirala, S.; Nepal, P.; Ghimire, G.; Basnet, R.; Rawat, I.; Dahal, A.; Pandey, J.; Parajuli-Baral, K. Formulation and Evaluation of Mucoadhesive Buccal Tablets of Aceclofenac. Heliyon 2021, 7, e06439. [Google Scholar] [CrossRef]
- Razzaq, S.; Hanif, S.; Syed, M.A.; Iqbal, J.; Hassan, S.S.; Raza, S.A.; Riaz, H.; Abid, F. Development and Evaluation of Mucoadhesive Buccal Tablet Containing Metronidazole for the Treatment of Periodontitis and Gingivitis. Pak. J. Pharm. Sci. 2018, 31, 1903–1910. [Google Scholar]
- Peddapalli, H.; Bakshi, V.; Boggula, N. Formulation, In Vitro and Ex Vivo Characterization of Mucoadhesive Buccal Tablets for Antihypertensive Drug. Asian J. Pharm. Clin. Res. 2018, 11, 402–411. [Google Scholar] [CrossRef]
- Chandarana, D.A.; Patel, K.S.; Patel, S.C.; Patel, D.R.; Prajapati, S.T. Formulation and Evaluation of Mucoadhesive Buccal Tablets of Carvedilol. Int. J. Appl. Pharm. 2020, 12, 170–181. [Google Scholar] [CrossRef]
- Bakr, F.; Soliman, M.; Elsabbagh, H. Formulation and In-Vitro, Ex-Vivo, and In-Vivo Evaluation of Mucoadhesive Buccal Tablets Containing Labetalol Hydrochloride for Enhancement of Systemic Bioavailability. J. Adv. Pharm. Res. 2022, 6, 15–27. [Google Scholar] [CrossRef]
- Alagusundaram, M.; Chetty, C.M.; Dhachinamoorthi, D. Development and Evaluation of Novel-Trans-Buccoadhesive Films of Famotidine. J. Adv. Pharm. Technol. Res. 2011, 2, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Attama, A.A.; Akpa, P.A.; Onugwu, L.E.; Igwilo, G. Novel Buccoadhesive Delivery System of Hydrochlorothiazide Formulated with Ethyl Cellulose-Hydroxypropyl Methylcellulose Interpolymer Complex. Sci. Res. Essay 2008, 3, 26–33. [Google Scholar]
- Ribeiro, L.; Ferreira, D.C.; Veiga, F.J.B. In Vitro Controlled Release of Vinpocetine-Cyclodextrin-Tartaric Acid Multicomponent Complexes from HPMC Swellable Tablets. J. Control. Release 2005, 103, 325–339. [Google Scholar] [CrossRef]
- Alagusundaram, M.; Chetty, C.M.; Dhachinamoorthi, D. Development and Evaluation of Novel Trans- Buccoadhesive Bilayer Tablets of Famotidine. Asian J. Pharm. 2014, 5, 150–156. [Google Scholar] [CrossRef]
- Giannola, L.I.; De Caro, V.; Giandalia, G.; Siragusa, M.G.; Tripodo, C.; Florena, A.M.; Campisi, G. Release of Naltrexone on Buccal Mucosa: Permeation Studies, Histological Aspects and Matrix System Design. Eur. J. Pharm. Biopharm. 2007, 67, 425–433. [Google Scholar] [CrossRef]
- Zewail, M.B.; Asaad, G.F.; Swellam, S.M.; Abd-Allah, S.M.; Hosny, S.K.; Sallah, S.K.; Eissa, J.E.; Mohamed, S.S.; El-Dakroury, W.A. Design, Characterization and in Vivo Performance of Solid Lipid Nanoparticles (SLNs)-Loaded Mucoadhesive Buccal Tablets for Efficient Delivery of Lornoxicam in Experimental Inflammation. Int. J. Pharm. 2022, 624, 122006. [Google Scholar] [CrossRef]
- Shin, S.C.; Bum, J.P.; Choi, J.S. Enhanced Bioavailability by Buccal Administration of Triamcinolone Acetonide from the Bioadhesive Gels in Rabbits. Int. J. Pharm. 2000, 209, 37–43. [Google Scholar] [CrossRef]
- Kassem, M.A.A.; Elmeshad, A.N.; Fares, A.R. Enhanced Bioavailability of Buspirone Hydrochloride via Cup and Core Buccal Tablets: Formulation and in Vitro/in Vivo Evaluation. Int. J. Pharm. 2014, 463, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Majid, H.; Bartel, A.; Burckhardt, B.B. Predictivity of Standardized and Controlled Permeation Studies: Ex Vivo—In Vitro—In Vivo Correlation for Sublingual Absorption of Propranolol. Eur. J. Pharm. Biopharm. 2021, 169, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rawas-Qalaji, M.; Bafail, R.; Cagliani, R.; Haider, M.; Hussain, Z. Assessment of Epinephrine Sublingual Stability and Permeability Pathways to Enhance Its Permeability for the Treatment of Anaphylaxis. Eur. J. Pharm. Sci. 2021, 167, 106025. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.M.; Prajapati, B.G.; Patel, M.M. Formulation, Evaluation, and Comparison of Bilayered and Multilayered Mucoadhesive Buccal Devices of Propranolol Hydrochloride. AAPS PharmSciTech 2007, 8, 22. [Google Scholar] [CrossRef]
- Shardor Ambarish, G.; Shirsand, S.B.; Shirsand, S.S.; Keshavshetti, G.; Fatima, A.; Aute, S.K. Bilayer Buccal Tablets of Furosemide: Design and Evaluation. Manipal J. Pharm. Sci. 2021, 24, 5. [Google Scholar]










| Formulation Code | Thickness (mm) ± SD | Weight Variation mg ± SD | Hardness (Kg/cm2) | Friability (%) | Drug Content In mg | Surface pH ± SD |
|---|---|---|---|---|---|---|
| NT1 | 2.38 ± 0.03 | 149 ± 0.52 | 4.1 ± 0.12 | 0.78 ± 0.03 | 19.91 ± 0.41 | 6.78 ± 0.06 |
| NT2 | 2.39 ± 0.02 | 149 ± 0.76 | 4.0 ± 0.21 | 0.69 ± 0.03 | 19.85 ± 0.19 | 6.76 ± 0.03 |
| NT3 | 2.39 ± 0.03 | 150 ± 0.69 | 4.1 ± 0.32 | 0.67 ± 0.04 | 20.32 ± 0.21 | 6.68 ± 0.02 |
| NT4 | 2.38 ± 0.05 | 149 ± 0.74 | 4.0 ± 0.28 | 0.65 ± 0.04 | 20.26 ± 0.41 | 6.76 ± 0.04 |
| NT5 | 2.42 ± 0.03 | 150 ± 0.22 | 4.2 ± 0.24 | 0.59 ± 0.01 | 20.02 ± 0.15 | 6.80 ± 0.02 |
| NT6 | 2.38 ± 0.04 | 149 ± 0.89 | 4.1 ± 0.24 | 0.84 ± 0.02 | 20.19 ± 0.01 | 6.75 ± 0.06 |
| NT7 | 2.39 ± 0.07 | 149 ± 0.98 | 4.1 ± 0.36 | 0.67 ± 0.04 | 19.78 ± 0.22 | 6.69 ± 0.06 |
| NT8 | 2.40 ± 0.02 | 149 ± 0.76 | 4.3 ± 0.29 | 0.63 ± 0.03 | 19.79 ± 0.65 | 6.72 ± 0.06 |
| NT9 | 2.38 ± 0.02 | 150 ± 0.87 | 4.2 ± 0.34 | 0.72 ± 0.01 | 20.21 ± 0.31 | 6.75 ± 0.04 |
| NT10 | 2.41 ± 0.02 | 150 ± 0.26 | 4.1 ± 0.51 | 0.74 ± 0.03 | 20.15 ± 0.15 | 6.79 ± 0.04 |
| Parameters | 1st Month | 2nd Month | 3rd Month | p Value |
|---|---|---|---|---|
| Physical appearance | No Change | No Change | No Change | - |
| Buccoadhesive strength | 34.88 ± 1.09 ns | 35.3 ± 1.09 ns | 36 ± 0.34 ns | 0.1539 |
| In vitro drug release | 98.06 ± 0.55 ns | 98.13 ± 0.32 ns | 98.26 ± 0.5 ns | 0.8709 |
| Formulation Code | NT1 | NT2 | NT3 | NT4 | NT5 | NT6 | NT7 | NT8 | NT9 | NT10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ingredients (mg) | Nifedipine | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| HPMC K 100 | 25 | - | 12.5 | 12.5 | 25 | - | 6.25 | 25 | 6.25 | 37.5 | |
| SCMC | 12.5 | 25 | - | 25 | - | 12.5 | 6.25 | 6.25 | 25 | - | |
| PVP K 30 | - | 12.5 | 25 | - | 12.5 | 25 | 25 | 6.25 | 6.25 | - | |
| CP 934 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Mg. stearate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | |
| Lactose | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | |
| Mannitol | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | |
| EC | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | |
| Total weight in mg | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alagusundaram, M.; Jain, N.K.; Begum, M.Y.; Parameswari, S.A.; Nelson, V.K.; Bayan, M.F.; Chandrasekaran, B. Development and Characterization of Gel-Based Buccoadhesive Bilayer Formulation of Nifedipine. Gels 2023, 9, 688. https://doi.org/10.3390/gels9090688
Alagusundaram M, Jain NK, Begum MY, Parameswari SA, Nelson VK, Bayan MF, Chandrasekaran B. Development and Characterization of Gel-Based Buccoadhesive Bilayer Formulation of Nifedipine. Gels. 2023; 9(9):688. https://doi.org/10.3390/gels9090688
Chicago/Turabian StyleAlagusundaram, M., Nem Kumar Jain, M. Yasmin Begum, S. Angala Parameswari, Vinod Kumar Nelson, Mohammad F. Bayan, and Balakumar Chandrasekaran. 2023. "Development and Characterization of Gel-Based Buccoadhesive Bilayer Formulation of Nifedipine" Gels 9, no. 9: 688. https://doi.org/10.3390/gels9090688
APA StyleAlagusundaram, M., Jain, N. K., Begum, M. Y., Parameswari, S. A., Nelson, V. K., Bayan, M. F., & Chandrasekaran, B. (2023). Development and Characterization of Gel-Based Buccoadhesive Bilayer Formulation of Nifedipine. Gels, 9(9), 688. https://doi.org/10.3390/gels9090688