Alginate Gel-Based Carriers for Encapsulation of Carotenoids: On Challenges and Applications
Abstract
1. Introduction
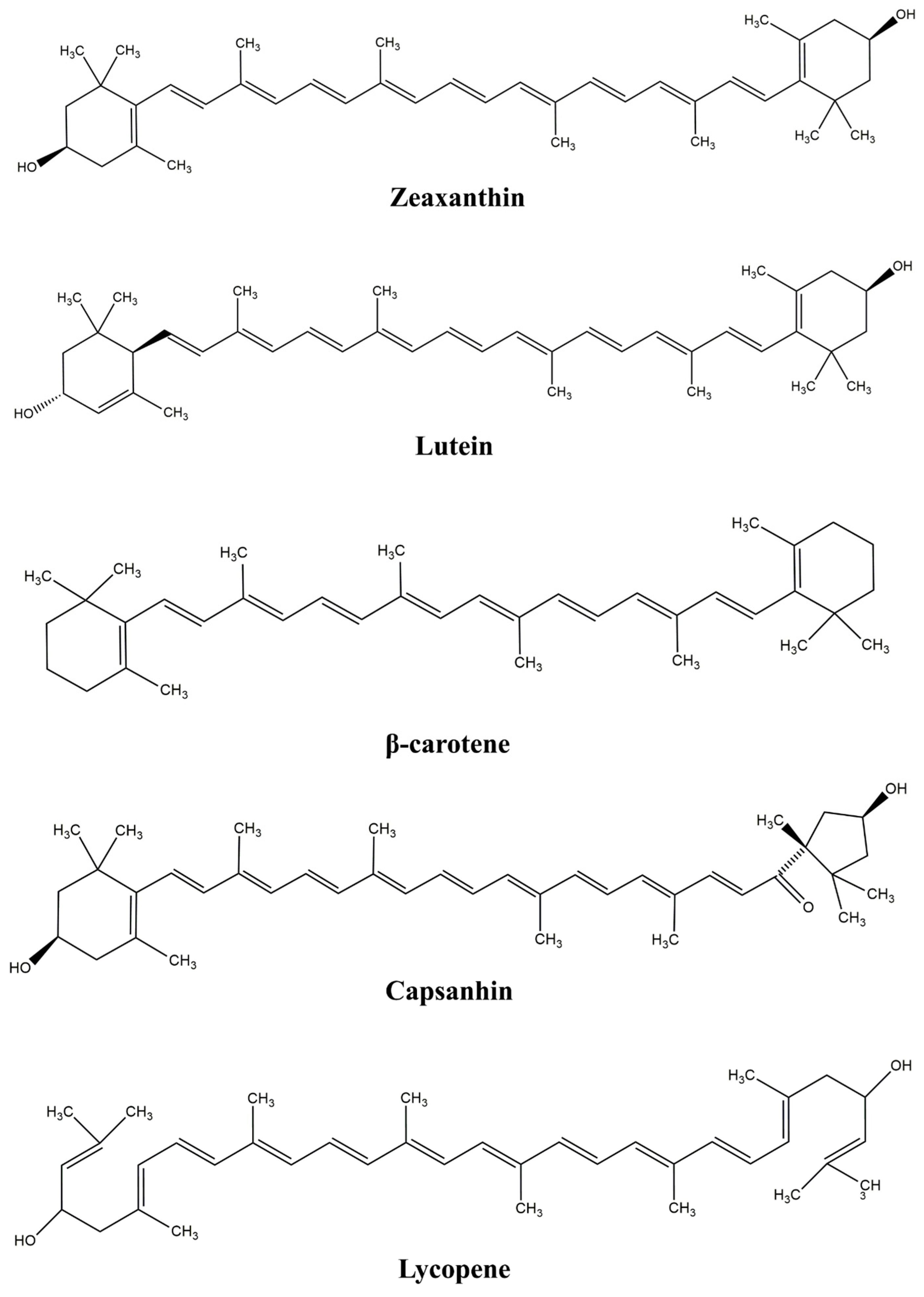
2. Carotenoids
3. Encapsulation
4. Main Physical and Chemical Properties of Alginate Important for Gel Formation
4.1. Sources
4.2. Chemical Structure
4.3. Alginate Gelation
4.3.1. Gel Formation
4.3.2. Physical Properties of Alginates
4.3.3. Chemical Modifications
5. Raman Spectroscopy and Other Methods in Alginate and Carotenoids Analysis
- Non or small preparative technique
- Small amount of sample
- Non-destructive
- Not interfered by water
- Spectra is acquired within a second
- Fast
- Relatively simple
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milivojevic, M.; Pajic-Lijakovic, I.; Levic, S.; Nedovic, V.; Bugarski, B. Alginic Acid: Sources, Modifications and Main Applications. In Alginic Acid—Chemical Structure, Uses and Health Benefits; Moore, A., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 45–88. [Google Scholar]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B. Biological Macromolecules in Cell Encapsulation. In Biological Macromolecules; Elsevier: Amsterdam, The Netherlands, 2022; pp. 491–528. [Google Scholar]
- Sampaio, G.L.A.; Pacheco, S.; Ribeiro, A.P.O.; Galdeano, M.C.; Gomes, F.S.; Tonon, R.V. Encapsulation of a Lycopene-Rich Watermelon Concentrate in Alginate and Pectin Beads: Characterization and Stability. LWT 2019, 116, 108589. [Google Scholar] [CrossRef]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B. Recent Advances in Alginates as Material for Biomedical Applications. In Alginates; Apple Academic Press: New York, NY, USA, 2019; pp. 25–88. [Google Scholar]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate Gel Particles–A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Girón-Hernández, J.; Gentile, P.; Benlloch-Tinoco, M. Impact of Heterogeneously Crosslinked Calcium Alginate Networks on the Encapsulation of β-Carotene-Loaded Beads. Carbohydr. Polym. 2021, 271, 118429. [Google Scholar] [CrossRef]
- Aguirre Calvo, T.R.; Santagapita, P.R. Pink Grapefruit Lycopene Encapsulated in Alginate-Based Beads: Stability towards Freezing and Drying. Int. J. Food Sci. Technol. 2019, 54, 368–375. [Google Scholar] [CrossRef]
- Barankevicz, G.B.; Novello, D.; Resende, J.T.; Schwarz, K.; Santos, E.F. Características Físicas e Químicas Da Polpa de Híbridos de Tomateiro, Durante o Armazenamento Congelado. Hortic. Bras. 2015, 33, 7–11. [Google Scholar] [CrossRef][Green Version]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B.; Nayak, A.K.; Hasnain, M.S. Gellan Gum in Drug Delivery Applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–186. [Google Scholar]
- García-Armenta, E.; Picos-Corrales, L.A.; Gutiérrez-López, G.F.; Gutiérrez-Dorado, R.; Perales-Sánchez, J.X.K.; García-Pinilla, S.; Reynoso-García, F.; Martínez-Audelo, J.M.; Armenta-Manjarrez, M.A. Preparation of Surfactant-Free Emulsions Using Amaranth Starch Modified by Reactive Extrusion. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125550. [Google Scholar] [CrossRef]
- Souza, A.L.R.; Hidalgo-Chávez, D.W.; Pontes, S.M.; Gomes, F.S.; Cabral, L.M.C.; Tonon, R.V. Microencapsulation by Spray Drying of a Lycopene-Rich Tomato Concentrate: Characterization and Stability. LWT 2018, 91, 286–292. [Google Scholar] [CrossRef]
- Soukoulis, C.; Cambier, S.; Hoffmann, L.; Bohn, T. Chemical Stability and Bioaccessibility of β-Carotene Encapsulated in Sodium Alginate o/w Emulsions: Impact of Ca2+ Mediated Gelation. Food Hydrocoll. 2016, 57, 301–310. [Google Scholar] [CrossRef]
- Robin, A.-L.; Sankhla, D. European Legislative Framework Controlling the Use of Food Additives. In Essential Guide to Food Additives; The Royal Society of Chemistry: London, UK, 2013; pp. 44–64. [Google Scholar]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-Loaded Nanocarriers: A Comprehensive Review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide Based Nanogels in the Drug Delivery System: Application as the Carrier of Pharmaceutical Agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Rostami, M.; Yousefi, M.; Khezerlou, A.; Aman Mohammadi, M.; Jafari, S.M. Application of Different Biopolymers for Nanoencapsulation of Antioxidants via Electrohydrodynamic Processes. Food Hydrocoll. 2019, 97, 105170. [Google Scholar] [CrossRef]
- Tavares, L.; Zapata Noreña, C.P.; Barros, H.L.; Smaoui, S.; Lima, P.S.; Marques de Oliveira, M. Rheological and Structural Trends on Encapsulation of Bioactive Compounds of Essential Oils: A Global Systematic Review of Recent Research. Food Hydrocoll. 2022, 129, 107628. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Bioactivity and Protective Effects of Natural Carotenoids. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; McClements, D.J.; Zou, L. Encapsulation of β-Carotene-Loaded Oil Droplets in Caseinate/Alginate Microparticles: Enhancement of Carotenoid Stability and Bioaccessibility. J. Funct. Foods 2018, 40, 527–535. [Google Scholar] [CrossRef]
- Soukoulis, C.; Bohn, T. A Comprehensive Overview on the Micro- and Nano-Technological Encapsulation Advances for Enhancing the Chemical Stability and Bioavailability of Carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Goodwin, T.W. Nature and Distribution of Carotenoids. Food Chem. 1980, 5, 3–13. [Google Scholar] [CrossRef]
- Alcaíno, J.; Baeza, M.; Cifuentes, V. Carotenoid Distribution in Nature. In Carotenoids in Nature: Biosynthesis, Regulation and Function; Springer: Cham, Switzerland, 2016; pp. 3–33. [Google Scholar]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A Global Perspective on Carotenoids: Metabolism, Biotechnology, and Benefits for Nutrition and Health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Toragall, V.; Jayapala, N.; Vallikannan, B. Chitosan-Oleic Acid-Sodium Alginate a Hybrid Nanocarrier as an Efficient Delivery System for Enhancement of Lutein Stability and Bioavailability. Int. J. Biol. Macromol. 2020, 150, 578–594. [Google Scholar] [CrossRef]
- Dutta, D.; Chaudhuri, U.; Chakraborty, R. Structure, Health Benefits, Antioxidant Property and Processing and Storage of Carotenoids. Afr. J. Food Agric. Nutr. Dev. 2011, 4, 13. [Google Scholar] [CrossRef]
- Hirschberg, J. Carotenoid Biosynthesis in Flowering Plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I. Carotenoids in Nature: Insights from Plants and Beyond. Funct. Plant Biol. 2011, 38, 833. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W. The Role of Xanthophyll Cycle Carotenoids in the Protection of Photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Polívka, T.; Frank, H.A. Molecular Factors Controlling Photosynthetic Light Harvesting by Carotenoids. Acc. Chem. Res. 2010, 43, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Green, B.R.; Parson, W.W. Advances in Photosynthesis and Respiration. In Light-Harvesting Antennas in Photosynthesis; Springer: Cham, Switzerland, 2009. [Google Scholar]
- Havaux, M. Carotenoid Oxidation Products as Stress Signals in Plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Structures and Analysis of Carotenoid Molecules. In Carotenoids in Nature: Biosynthesis, Regulation and Function; Springer: Cham, Switzerland, 2016; pp. 71–108. [Google Scholar]
- Britton, G. Structure and Properties of Carotenoids in Relation to Function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, Zeaxanthin, and Meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-Based Nutritional Interventions against Ocular Disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef]
- Khalid, M.; Saeed-ur-Rahman; Bilal, M.; Iqbal, H.M.N.; Huang, D. Biosynthesis and Biomedical Perspectives of Carotenoids with Special Reference to Human Health-Related Applications. Biocatal. Agric. Biotechnol. 2019, 17, 399–407. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, Inflammation, and Oxidative Stress—Implications of Cellular Signaling Pathways and Relation to Chronic Disease Prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S.; Daglia, M.; Rengasamy, K.R. Dietary Carotenoids in Cancer Chemoprevention and Chemotherapy: A Review of Emerging Evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef]
- Kolašinac, M.; Stevanović, P.D.; Kilibarda, N.; Kostić, Ž. Carotenoids: New Applications of “Old” Pigments. Phyton 2021, 90, 1041–1062. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, M.; Zhang, X.; Li, Z.; Guo, Y.; He, H.; Liang, B.; Li, X.; Ji, C. Design of Protein-Polysaccharide Multi-Scale Composite Interfaces to Modify Lipid Digestion. Trends Food Sci. Technol. 2022, 127, 38–48. [Google Scholar] [CrossRef]
- Jain, S.; Winuprasith, T.; Suphantharika, M. Encapsulation of Lycopene in Emulsions and Hydrogel Beads Using Dual Modified Rice Starch: Characterization, Stability Analysis and Release Behaviour during in-Vitro Digestion. Food Hydrocoll. 2020, 104, 105730. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, L.; Yang, S.; Wen, J.; Zhang, Y.; Jiang, L.; Sui, X. Producing Mixed-Soy Protein Adsorption Layers on Alginate Microgels to Controlled-Release β-Carotene. Food Res. Int. 2023, 164, 112319. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; McClements, D.J.; Jin, Z.; Miao, M. Biological Macromolecules for Nutrients Delivery. In Biological Macromolecules; Elsevier: Amsterdam, The Netherlands, 2022; pp. 455–477. [Google Scholar]
- Wei, Y.; Sun, C.; Dai, L.; Zhan, X.; Gao, Y. Structure, Physicochemical Stability and in Vitro Simulated Gastrointestinal Digestion Properties of β-Carotene Loaded Zein-Propylene Glycol Alginate Composite Nanoparticles Fabricated by Emulsification-Evaporation Method. Food Hydrocoll. 2018, 81, 149–158. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. The Impact of PH on Mechanical Properties, Storage Stability and Digestion of Alginate-Based and Soy Protein Isolate-Stabilized Emulsion Gel Beads with Encapsulated Lycopene. Food Chem. 2022, 372, 131262. [Google Scholar] [CrossRef]
- Celli, G.B.; Teixeira, A.G.; Duke, T.G.; Brooks, M.S.-L. Encapsulationof Lycopene from Watermelon in Calcium-Alginate Microparticles Using an Optimised Inverse-Gelation Method by Response Surface Methodology. Int. J. Food Sci. Technol. 2016, 51, 1523–1529. [Google Scholar] [CrossRef]
- Shu, J.; McClements, D.J.; Luo, S.; Ye, J.; Liu, C. Effect of Internal and External Gelation on the Physical Properties, Water Distribution, and Lycopene Encapsulation Properties of Alginate-Based Emulsion Gels. Food Hydrocoll. 2023, 139, 108499. [Google Scholar] [CrossRef]
- Xu, P.; Song, J.; Dai, Z.; Xu, Y.; Li, D.; Wu, C. Effect of Ca2+ Cross-Linking on the Properties and Structure of Lutein-Loaded Sodium Alginate Hydrogels. Int. J. Biol. Macromol. 2021, 193, 53–63. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E. Carotenoids Microencapsulation by Spray Drying Method and Supercritical Micronization. Food Res. Int. 2017, 99, 891–901. [Google Scholar] [CrossRef]
- Dajic Stevanovic, Z.; Sieniawska, E.; Glowniak, K.; Obradovic, N.; Pajic-Lijakovic, I. Natural Macromolecules as Carriers for Essential Oils: From Extraction to Biomedical Application. Front. Bioeng. Biotechnol. 2020, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; McClements, D.J. Encapsulation of β-Carotene in Alginate-Based Hydrogel Beads: Impact on Physicochemical Stability and Bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef]
- Liao, P.; Dai, S.; Lian, Z.; Tong, X.; Yang, S.; Chen, Y.; Qi, W.; Peng, X.; Wang, H.; Jiang, L. The Layered Encapsulation of Vitamin B2 and β-Carotene in Multilayer Alginate/Chitosan Gel Microspheres: Improving the Bioaccessibility of Vitamin B2 and β-Carotene. Foods 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.-B.; Maruf, A.; Das, P.R.; Nam, S.-H. A Review of Encapsulation of Carotenoids Using Spray Drying and Freeze Drying. Crit. Rev. Food Sci. Nutr. 2020, 60, 3547–3572. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Bai, F.; Wu, Y.; Ye, Q.; Liang, D.; Shi, C.; Zhang, X. Development and Evaluation of Lutein-loaded Alginate Microspheres with Improved Stability and Antioxidant. J. Sci. Food Agric. 2019, 99, 5195–5201. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jiang, J.; Gu, X.; Li, X.; Liu, T. Encapsulation of β-Carotene in Calcium Alginate Hydrogels Templated by Oil-in-Water-in-Oil (O/W/O) Double Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125548. [Google Scholar] [CrossRef]
- Jalali-Jivan, M.; Rostamabadi, H.; Assadpour, E.; Tomas, M.; Capanoglu, E.; Alizadeh-Sani, M.; Kharazmi, M.S.; Jafari, S.M. Recent Progresses in the Delivery of β-Carotene: From Nano/Microencapsulation to Bioaccessibility. Adv. Colloid Interface Sci. 2022, 307, 102750. [Google Scholar] [CrossRef]
- Roll Zimmer, T.B.; Barboza Mendonça, C.R.; Zambiazi, R.C. Methods of Protection and Application of Carotenoids in Foods—A Bibliographic Review. Food Biosci. 2022, 48, 101829. [Google Scholar] [CrossRef]
- Dos Santos, P.P.; de Aguiar Andrade, L.; Flôres, S.H.; de Oliveira Rios, A. Nanoencapsulation of Carotenoids: A Focus on Different Delivery Systems and Evaluation Parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Bagherzadeh, M.; Dinarvand, R.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Hamblin, M.R.; et al. The Colorful World of Carotenoids: A Profound Insight on Therapeutics and Recent Trends in Nano Delivery Systems. Crit. Rev. Food Sci. Nutr. 2022, 62, 3658–3697. [Google Scholar] [CrossRef] [PubMed]
- Boonlao, N.; Ruktanonchai, U.R.; Anal, A.K. Enhancing Bioaccessibility and Bioavailability of Carotenoids Using Emulsion-Based Delivery Systems. Colloids Surf. B Biointerfaces 2022, 209, 112211. [Google Scholar] [CrossRef] [PubMed]
- Pajic-Lijakovic, I.; Milivojevic, M.; Levic, S.; Trifkovic, K.; Balanc, B.; Nedovic, V.; Stevanovic-Dajic, Z.; Radosevic, R.; Bugarski, B. Matrix Resistance Stress Reduction—Prerequisite for Achieving Higher Concentration of Immobilized Cells. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 281–306. [Google Scholar]
- Hasnain, M.S.; Ahmed, S.A.; Alkahtani, S.; Milivojevic, M.; Kandar, C.C.; Dhara, A.K.; Nayak, A.K. Biopolymers for Drug Delivery. In Advanced Biopolymeric Systems for Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Advances in Material Research and Technology; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-46922-1. [Google Scholar]
- Levic, S.; Djordjevic, V.; Rajic, N.; Milivojevic, M.; Bugarski, B.; Nedovic, V. Entrapment of Ethyl Vanillin in Calcium Alginate and Calcium Alginate/Poly(Vinyl Alcohol) Beads. Chem. Pap. 2013, 67, 221–228. [Google Scholar] [CrossRef]
- Aguirre Calvo, T.R.; Busch, V.M.; Santagapita, P.R. Stability and Release of an Encapsulated Solvent-Free Lycopene Extract in Alginate-Based Beads. LWT 2017, 77, 406–412. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Bušić, A.; Barišić, L.; Vrsaljko, D.; Karlović, S.; Špoljarić, I.; Vojvodić, A.; Mršić, G.; Komes, D. Emulsion Templated Microencapsulation of Dandelion (Taraxacum officinale L.) Polyphenols and β-Carotene by Ionotropic Gelation of Alginate and Pectin. Food Hydrocoll. 2016, 57, 139–152. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Y.; Yuan, F.; Gao, Y.; Mao, L. Effect of Interfacial Compositions on the Physical Properties of Alginate-Based Emulsion Gels and Chemical Stability of Co-Encapsulated Bioactives. Food Hydrocoll. 2021, 111, 106389. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Z.; Guo, P.; Guo, Q.; Zhang, H.; Jiang, L.; Xia, N.; Xiao, B. Tuning Egg Yolk Granules/Sodium Alginate Emulsion Gel Structure to Enhance β-Carotene Stability and in Vitro Digestion Property. Int. J. Biol. Macromol. 2023, 232, 123444. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, S.; Gan, H.; Zhang, H.; Xia, N.; Jiang, L.; Ren, H.; Zhang, X. Investigation of the Formation Mechanism and β-Carotene Encapsulation Stability of Emulsion Gels Based on Egg Yolk Granules and Sodium Alginate. Food Chem. 2023, 400, 134032. [Google Scholar] [CrossRef]
- Smidsrod, O.; Skjakbrk, G. Alginate as Immobilization Matrix for Cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Wood, R.J. Bioavailability: Definition, General Aspects and Fortificants. In Encyclopedia of Human Nutrition, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2005. [Google Scholar]
- Leach, G.; Oliveira, G.; Morais, R. Production of a Carotenoid-Rich Product by Alginate Entrapment and Fluid-Bed Drying OfDunaliella Salina. J. Sci. Food Agric. 1998, 76, 298–302. [Google Scholar] [CrossRef]
- Roman, M.J.; Burri, B.J.; Singh, R.P. Release and Bioaccessibility of β-Carotene from Fortified Almond Butter during in Vitro Digestion. J. Agric. Food Chem. 2012, 60, 9659–9666. [Google Scholar] [CrossRef] [PubMed]
- Guedes Silva, K.C.; Feltre, G.; Dupas Hubinger, M.; Kawazoe Sato, A.C. Protection and Targeted Delivery of β-Carotene by Starch-Alginate-Gelatin Emulsion-Filled Hydrogels. J. Food Eng. 2021, 290, 110205. [Google Scholar] [CrossRef]
- Ye, H.; Chen, T.; Huang, M.; Ren, G.; Lei, Q.; Fang, W.; Xie, H. Exploration of the Microstructure and Rheological Properties of Sodium Alginate-Pectin-Whey Protein Isolate Stabilized Β-Carotene Emulsions: To Improve Stability and Achieve Gastrointestinal Sustained Release. Foods 2021, 10, 1991. [Google Scholar] [CrossRef]
- Xia, C.; Han, L.; Zhang, C.; Xu, M.; Liu, Z.; Chen, Y.; Zhu, Y.; Yu, M.; Wu, W.; Yin, S.; et al. Preparation and Optimization of Pickering Emulsion Stabilized by Alginate-Lysozyme Nanoparticles for β-Carotene Encapsulation. Colloid Polym. Sci. 2022, 300, 1291–1300. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.; Shang, W.; Yan, J.; Julian McClements, D.; Xiao, H.; Wu, H.; Zhu, B. Modulation of Physicochemical Stability and Bioaccessibility of β-Carotene Using Alginate Beads and Emulsion Stabilized by Scallop (Patinopecten Yessoensis) Gonad Protein Isolates. Food Res. Int. 2020, 129, 108875. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Magyar, A.; Kispert, L.D. Photochemical and Optical Properties of Water-Soluble Xanthophyll Antioxidants: Aggregation vs Complexation. J. Phys. Chem. B 2013, 117, 10173–10182. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, Physical and Biological Properties of Alginates and Their Biomedical Implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Indergaard, M.; Skjåk-Bræk, G. Characteristics of Alginate from Laminaria Digitata Cultivated in a High-Phosphate Environment. In Twelfth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 1987; pp. 541–549. [Google Scholar]
- Haug, A.; Smidsrød, O. Strontium–Calcium Selectivity of Alginates. Nature 1967, 215, 757. [Google Scholar] [CrossRef]
- Rehm, B.H.A. Alginates: Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2009; Volume 13, ISBN 978-3-540-92678-8. [Google Scholar]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Vold, I.M.N.; Kristiansen, K.A.; Christensen, B.E. A Study of the Chain Stiffness and Extension of Alginates, In Vitro Epimerized Alginates, and Periodate-Oxidized Alginates Using Size-Exclusion Chromatography Combined with Light Scattering and Viscosity Detectors. Biomacromolecules 2006, 7, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Larsen, B. A study on the Constitution of Alginic Acid by Partial Acid Hydrolysis. In Proceedings of the Fifth International Seaweed Symposium, Halifax, August 25–28, 1965; Elsevier: Cham, Switzerland, 1966; pp. 271–277. [Google Scholar]
- George, M.; Abraham, T.E. Polyionic Hydrocolloids for the Intestinal Delivery of Protein Drugs: Alginate and Chitosan—A Review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Ouwerx, C.; Velings, N.; Mestdagh, M.; Axelos, M.A. Physico-Chemical Properties and Rheology of Alginate Gel Beads Formed with Various Divalent Cations. Polym. Gels Netw. 1998, 6, 393–408. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Grasdalen, H.; Smidsrød, O. Inhomogeneous Polysaccharide Ionic Gels. Carbohydr. Polym. 1989, 10, 31–54. [Google Scholar] [CrossRef]
- Thu, B.; Bruheim, P.; Espevik, T.; Smidsrød, O.; Soon-Shiong, P.; Skjåk-Bræk, G. Alginate Polycation Microcapsules. Biomaterials 1996, 17, 1031–1040. [Google Scholar] [CrossRef]
- Pajic-Lijakovic, I.; Plavsic, M.; Nedovic, V.; Bugarski, B. Ca-Alginate Hydrogel Rheological Changes Caused by Yeast Cell Growth Dynamics. In Microbiology Book Series, Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010. [Google Scholar]
- Hertzberg, S.; Jensen, A. Studies of Alginate-Immobilized Marine Microalgae. Bot. Mar. 1989, 32, 267–274. [Google Scholar] [CrossRef]
- Hartmann, M.; Dentini, M.; Ingar Draget, K.; Skjåk-Bræk, G. Enzymatic Modification of Alginates with the Mannuronan C-5epimerase AlgE4 Enhances Their Solubility at Low PH. Carbohydr. Polym. 2006, 63, 257–262. [Google Scholar] [CrossRef]
- Al-Shamkhani, A.; Duncan, R. Radioiodination of Alginate via Covalently-Bound Tyrosinamide Allows Monitoring of Its Fate In Vivo. J. Bioact. Compat. Polym. 1995, 10, 4–13. [Google Scholar] [CrossRef]
- Zimmermann, U.; Klöck, G.; Federlin, K.; Hannig, K.; Kowalski, M.; Bretzel, R.G.; Horcher, A.; Entenmann, H.; Sieber, U.; Zekorn, T. Production of Mitogen-Contamination Free Alginates with Variable Ratios of Mannuronic Acid to Guluronic Acid by Free Flow Electrophoresis. Electrophoresis 1992, 13, 269–274. [Google Scholar] [CrossRef]
- Otterlei, M.; Østgaard, K.; Skjåk-Bræk, G.; Smidsrød, O.; Soon-Shiong, P.; Espevik, T. Induction of Cytokine Production from Human Monocytes Stimulated with Alginate. J. Immunother. 1991, 10, 286–291. [Google Scholar] [CrossRef]
- Leonard, M.; De Boisseson, M.R.; Hubert, P.; Dalençon, F.; Dellacherie, E. Hydrophobically Modified Alginate Hydrogels as Protein Carriers with Specific Controlled Release Properties. J. Control. Release 2004, 98, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Smidsrød, O.; Haug, A.; Larsen, B. The Influence of PH on the Rate of Hydrolysis of Acidic Polysaccharides. Acta Chem. Scand. 1966, 20, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Larsen, B. The Solubility of Alginate at Low PH. Acta Chem. Scand. 1963, 17, 1653–1662. [Google Scholar] [CrossRef]
- Leo, W.J.; McLoughlin, A.J.; Malone, D.M. Effects of Sterilization Treatments on Some Properties of Alginate Solutions and Gels. Biotechnol. Prog. 1990, 6, 51–53. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Effect of Ca 2+, Ba 2+, and Sr 2+ on Alginate Microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Baños, F.G.D.; Díez Peña, A.I.; Hernánez Cifre, J.G.; López Martínez, M.C.; Ortega, A.; García de la Torre, J. Influence of Ionic Strength on the Flexibility of Alginate Studied by Size Exclusion Chromatography. Carbohydr. Polym. 2014, 102, 223–230. [Google Scholar] [CrossRef]
- Cook, M.T.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of Probiotic Bacteria into Alginate Hydrogels. In Hydrogels in Cell-Based Therapies; The Royal Society of Chemistry: London, UK, 2014; pp. 95–111. [Google Scholar]
- Onsøyen, E. Alginate: Prodiction, Composition, Physicochemical Properties, Physiological Effects, Safety, and Food Applications. In Handbook of Dietary Fiber; Cho, S.S., Dreher, M.L., Eds.; Taylor and Francis, Marcel Deckker, Inc.: New York, NY, USA, 2001; pp. 639–654. [Google Scholar]
- Mohan, N.; Nair, P.D. Novel Porous, Polysaccharide Scaffolds for Tissue Engineering Applications. Trends. Biomater. Artif. Organs. 2005, 18, 219–224. [Google Scholar]
- Pajic-Lijakovic, I.; Milivojevic, M.; Levic, S.; Trifkovic, K.; Stevanovic-Dajic, Z.; Radosevic, R.; Nedovic, V.; Bugarski, B. Matrix Resistance Stress: A Key Parameter for Immobilized Cell Growth Regulation. Process Biochem. 2017, 52, 30–43. [Google Scholar] [CrossRef]
- Lee, K.Y.; Rowley, J.A.; Eiselt, P.; Moy, E.M.; Bouhadir, K.H.; Mooney, D.J. Controlling Mechanical and Swelling Properties of Alginate Hydrogels Independently by Cross-Linker Type and Cross-Linking Density. Macromolecules 2000, 33, 4291–4294. [Google Scholar] [CrossRef]
- Drury, J.L.; Dennis, R.G.; Mooney, D.J. The Tensile Properties of Alginate Hydrogels. Biomaterials 2004, 25, 3187–3199. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Molecular Engineering as an Approach to Design New Functional Properties of Alginate. Biomacromolecules 2007, 8, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Chater, P.I.; Wilcox, M.D.; Brownlee, I.A.; Pearson, J.P. Alginate as a Protease Inhibitor in Vitro and in a Model Gut System; Selective Inhibition of Pepsin but Not Trypsin. Carbohydr. Polym. 2015, 131, 142–151. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research Progress on Chemical Modification of Alginate: A Review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Galant, C.; Kjøniksen, A.-L.; Nguyen, G.T.M.; Knudsen, K.D.; Nyström, B. Altering Associations in Aqueous Solutions of a Hydrophobically Modified Alginate in the Presence of β-Cyclodextrin Monomers. J. Phys. Chem. B 2006, 110, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Salomonsen, T.; Jensen, H.M.; Stenbæk, D.; Engelsen, S.B. Chemometric Prediction of Alginate Monomer Composition: A Comparative Spectroscopic Study Using IR, Raman, NIR and NMR. Carbohydr. Polym. 2008, 72, 730–739. [Google Scholar] [CrossRef]
- Salomonsen, T.; Jensen, H.M.; Stenbæk, D.; Engelsen, S.B. Rapid Determination of Alginate Monomer Compostion Using Raman Spectroscopy and Chemometrics. In Gums and Stabilisers for the Food Industry 14; The Royal Society of Chemistry: London, UK, 2008; pp. 543–551. [Google Scholar]
- Lengert, E.; Yashchenok, A.M.; Atkin, V.; Lapanje, A.; Gorin, D.A.; Sukhorukov, G.B.; Parakhonskiy, B.V. Hollow Silver Alginate Microspheres for Drug Delivery and Surface Enhanced Raman Scattering Detection. RSC Adv. 2016, 6, 20447–20452. [Google Scholar] [CrossRef]
- Campos-Vallette, M.M.; Chandía, N.P.; Clavijo, E.; Leal, D.; Matsuhiro, B.; Osorio-Román, I.O.; Torres, S. Characterization of Sodium Alginate and Its Block Fractions by Surface-Enhanced Raman Spectroscopy. J. Raman Spectrosc. 2009, 41, 758–763. [Google Scholar] [CrossRef]
- Schmid, T.; Messmer, A.; Yeo, B.-S.; Zhang, W.; Zenobi, R. Towards Chemical Analysis of Nanostructures in Biofilms II: Tip-Enhanced Raman Spectroscopy of Alginates. Anal. Bioanal. Chem. 2008, 391, 1907–1916. [Google Scholar] [CrossRef]
- Kaczor, A.; Baranska, M. Structural Changes of Carotenoid Astaxanthin in a Single Algal Cell Monitored in Situ by Raman Spectroscopy. Anal. Chem. 2011, 83, 7763–7770. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M.; Baranski, R. Potential of NIR-FT-Raman Spectroscopy in Natural Carotenoid Analysis. Biopolymers 2005, 77, 212–221. [Google Scholar] [CrossRef]
- Kolašinac, S.; Pećinar, I.; Danojević, D.; Stevanović, Z.D. Raman Spectroscopy Coupled with Chemometric Modeling Approaches for Authentication of Different Paprika Varieties at Physiological Maturity. LWT 2022, 162, 113402. [Google Scholar] [CrossRef]
- Kolašinac, S.; Pećinar, I.; Danojević, D.; Aćić, S.; Stevanović, Z.D. Raman Spectroscopic-based Chemometric Modeling in Assessment of Red Pepper Ripening Phases and Carotenoids Accumulation. J. Raman Spectrosc. 2021, 52, 1598–1605. [Google Scholar] [CrossRef]
- Straksys, A.; Kavleiskaja, T.; Gruskiene, R.; Badokas, K.; Sereikaite, J. New β-Carotene-Xylan Complexes: Preparation and Characterization. Cellulose 2022, 29, 8705–8718. [Google Scholar] [CrossRef]
- Savic Gajic, I.M.; Savic, I.M.; Gajic, D.G.; Dosic, A. Ultrasound-Assisted Extraction of Carotenoids from Orange Peel Using Olive Oil and Its Encapsulation in Ca-Alginate Beads. Biomolecules 2021, 11, 225. [Google Scholar] [CrossRef]
- Jafari, S.M.; Esfanjani, A.F. Instrumental Analysis and Characterization of Nanocapsules. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier: Amsterdam, The Netherlands, 2017; pp. 524–544. [Google Scholar]
- Preetz, C.; Hauser, A.; Hause, G.; Kramer, A.; Mäder, K. Application of Atomic Force Microscopy and Ultrasonic Resonator Technology on Nanoscale: Distinction of Nanoemulsions from Nanocapsules. Eur. J. Pharm. Sci. 2010, 39, 141–151. [Google Scholar] [CrossRef]
| Component | Main Sources | Benefits | Limitations | Refs. |
|---|---|---|---|---|
| β-carotene | Carrots, sweet potatoes, tomatoes, apricots, squash, mangoes, yams, spinach, green peppers, green plants, and other fruits and vegetables. | Highest pro-vitamin A activity among the carotenoids, strong antioxidant that scavenges free radicals and reduces cancer, cataracts, infections, cardiovascular, and other chronic diseases, protects skin health, improves vision, strengthens immune system, and has immunomodulatory action. | Chemically unstable with high tendency for chemical degradation due to light, heat, and oxygen exposure due to large number of conjugated double bonds in its structure, high melting point, crystallinity at room temperature, extremely low water solubility, and very low (and variable) oral bioavailability (less than 10% from orally taken food). | [14,20,44,45] |
| Lycopene | Found in high concentrations in tomatoes, pink grapefruit, pink guava, papaya, and watermelon, also in apricots, barriers, plums, carrots, green peppers, red cabbage, passion fruit, and a large number of red-colored fruits, vegetables, and microorganisms. | Lycopene structure has a higher number of conjugated double bonds than other carotenoids, which makes it the most potent antioxidant among carotenoids (lycopene from watermelon had significantly higher antioxidant activity than from tomato). It possesses immunomodulatory and anti-inflammatory action, enhances immune system, prevents some types of cancer, cardiovascular, chronic, and degenerative diseases and hepatic fibrogenesis. | Chemically very unstable to oxygen, light, heat, and humidity exposure due to the presence of double bonds in its structure, easily oxidized and isomerized, destroyed at acidic pH, stable at basic pH, low water solubility induces low bioavailability, restricting its incorporation in different foods and beverages. | [3,14,44,46,47,48] |
| Lutein | Widely distributed in algae, fungi and bacteria, plants, especially in dark green leafy vegetables (broccoli, spinach, kale), orange-yellow fruits and vegetables (petals of the marigold flower, honeydew melon, mango, yellow corn, carrots, potatoes,) and egg yolks. | Lutein concentration is highest in the retina (~500 times higher than in other tissues), enhances eye health (proven retinal and macular protection against oxidative stress), maintains skin health, anti-diabetic, anti-obese, anti-cancer, anti-inflammatory antioxidant, antiatherogenic, anti-ageing, and immunomodulatory properties, prevents atherosclerosis, reduces prostate cancer risk. | Chemically unstable (sensitive to light, heat, pH, and oxidative stress, undergoes degradation due to interactions with other ingredients in the food matrix) crystalline at room temperature, very low water solubility, low bioavailability (in addition to the strongly hydrophobic nature of different nutrients, enzymes, and low pH in the GIT also decrease absorption), which all limit food and pharmacy applications. | [14,25,44,49] |
| Zeaxanthin | Egg, oranges, corn, honeydew melon, dark green leafy vegetables, bacteria. | Concentrated in macula prevents (as lutein) visual loss from age-related macular degeneration, prevents oxidation, cell and DNA damage, anti-ageing, prevents cardiovascular diseases, improves respiratory system and overall health. | Similar to lutein | [14,35,42] |
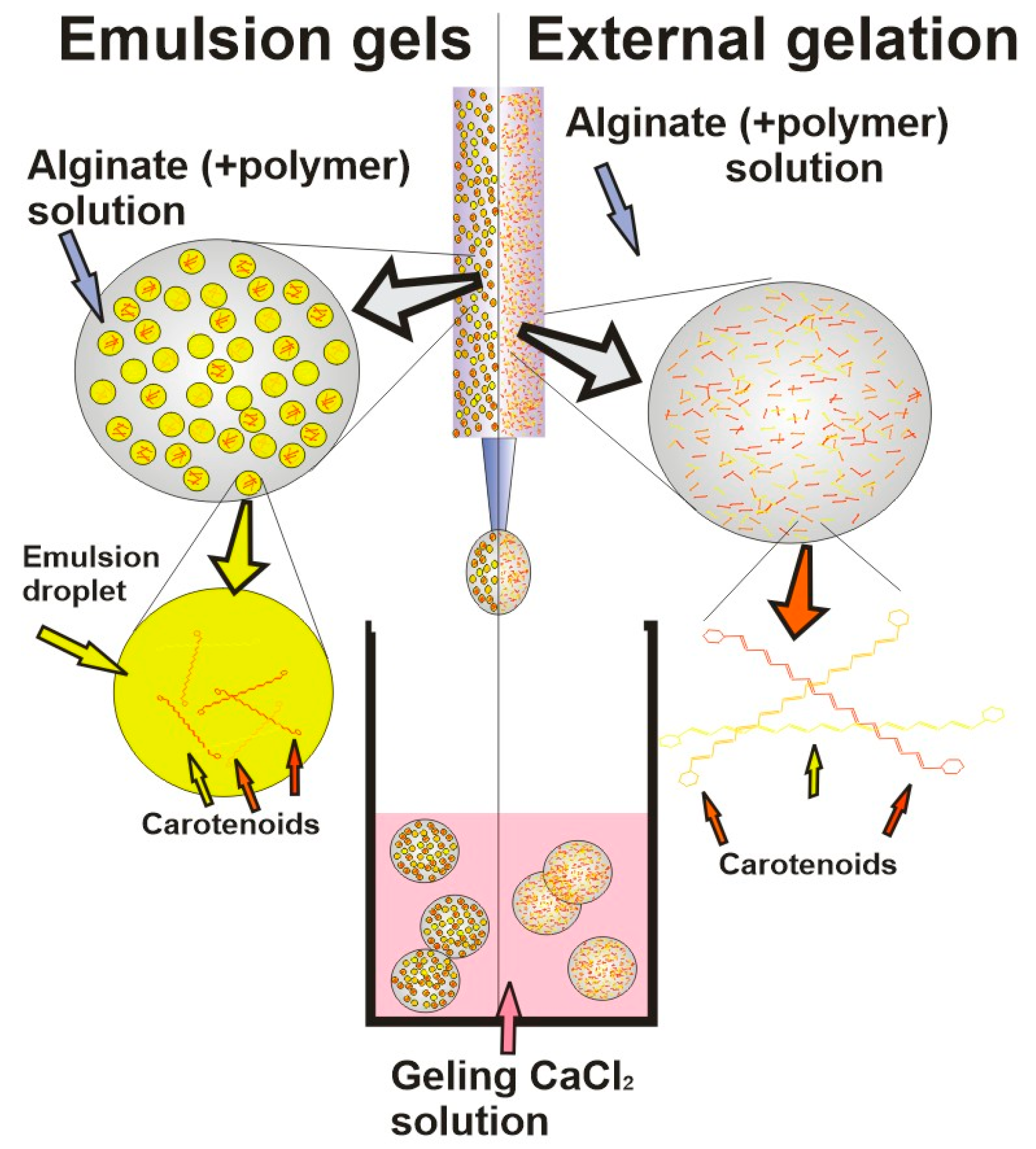
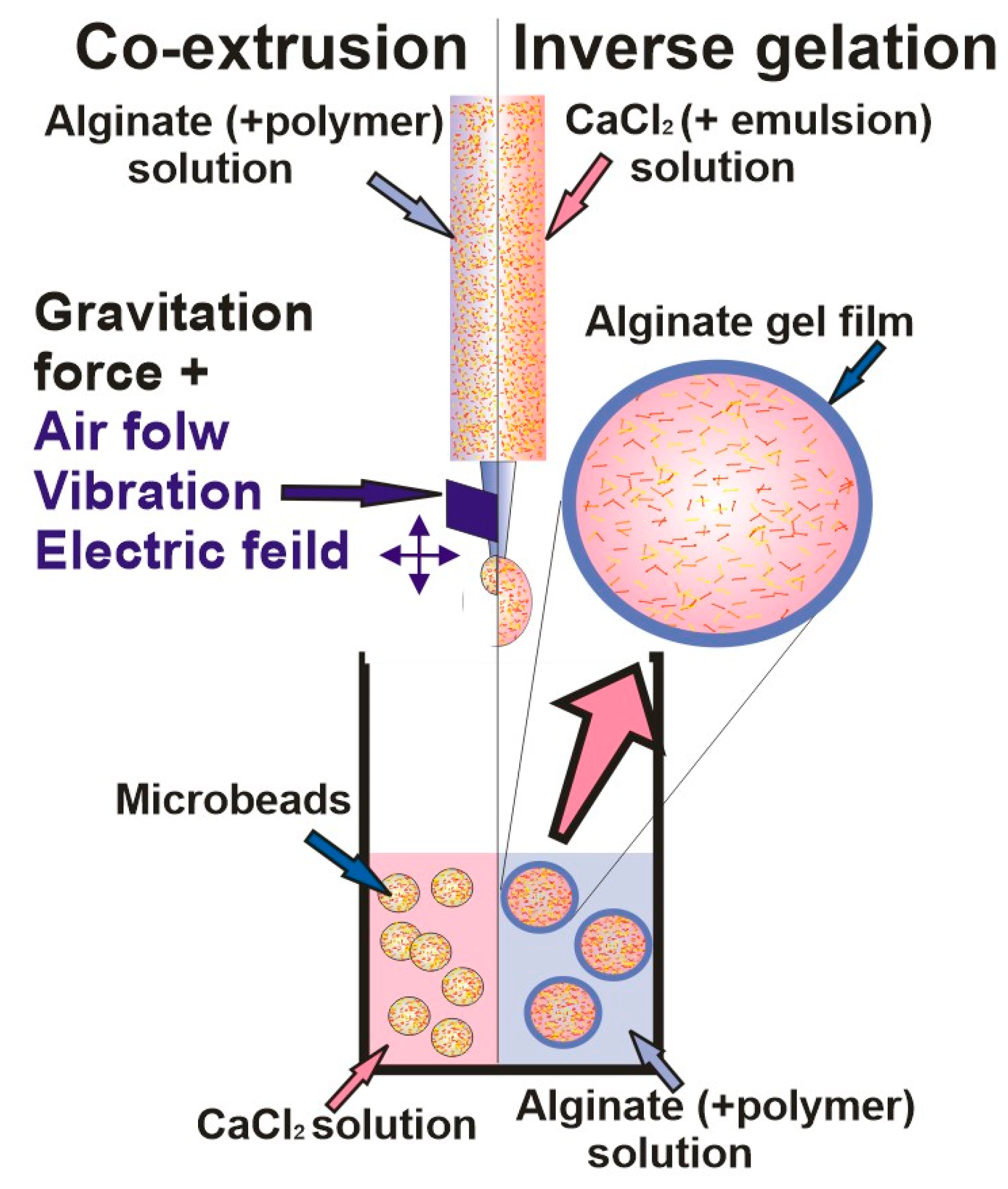
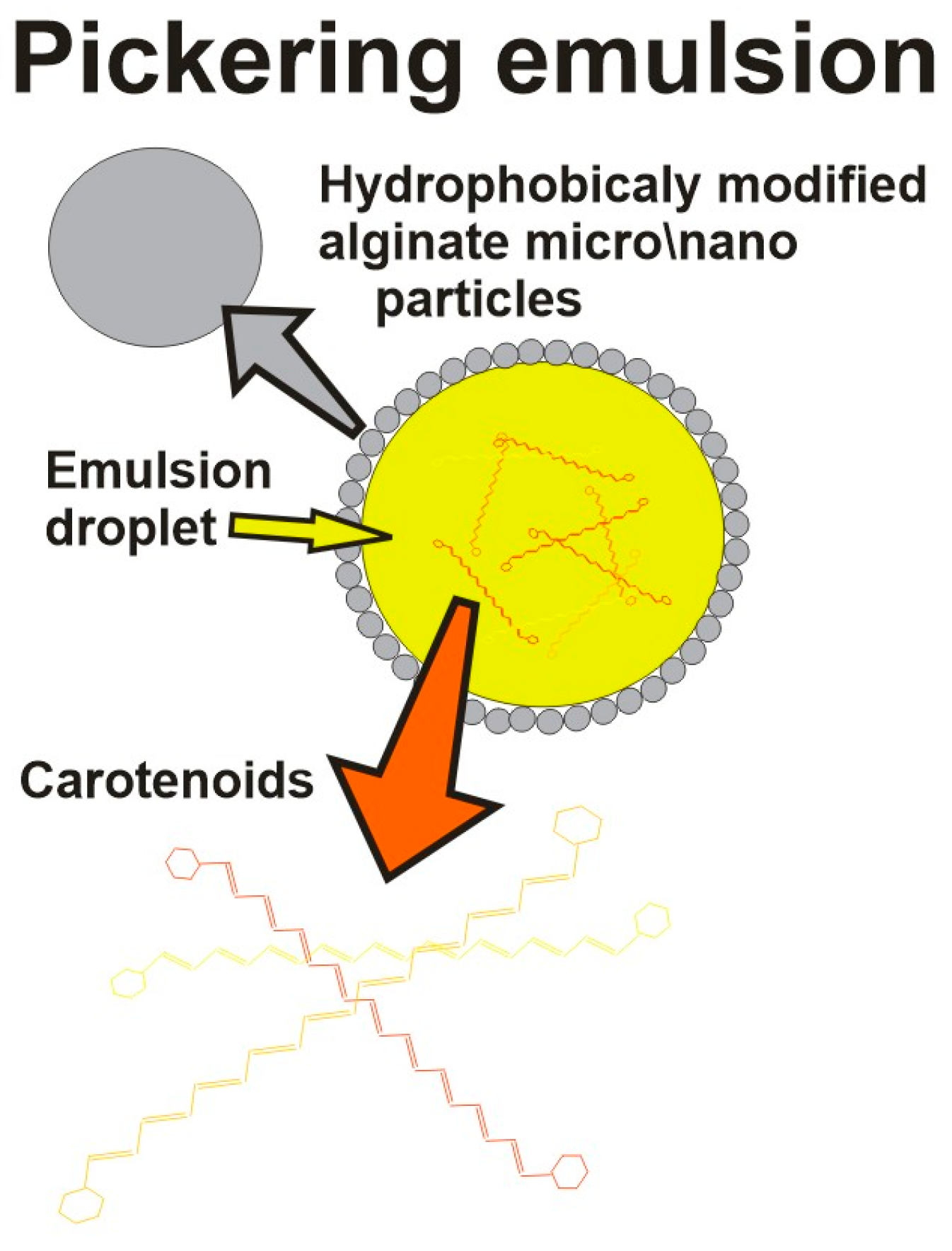
| Encapsulating Method | Properties/Drawbacks | Refs. |
|---|---|---|
External gelation
|
| [65,66] |
Inverse gelation
|
| [47,67] |
Emulsion gels
|
| [46,68,69] |
Co-extrusion microgels
|
| [70] |
Pickering emulsions
|
| [71] |
| Component | Encapsulation System | Benefits | Limitations | Refs. |
|---|---|---|---|---|
| β-carotene | Dried alginate beads |
| [72] | |
| Alginate beads |
|
| [6,51] | |
| Whey protein isolate (WPI)-alginate-chitosan capsules |
| [73] | ||
| Alginate o/w emulsions |
|
| [11] | |
| Whey proteins and HPMC-reinforced alginate hydrogels templated by emulsions |
| - | [60] | |
| Oil droplets in caseinate/alginate microparticles |
| - | [19] | |
| Emulsion gels based on egg yolk granules and sodium alginate |
|
| [63] | |
| Zeinpropylene glycol alginate composite nanoparticles |
| - | [44] | |
| Multilayer Alginate/Chitosan Gel Microspheres |
| - | [52] | |
| Starch-alginate-gelatin emulsion-filled hydrogels |
| - | [74] | |
| Alginate-Pectin-Whey Protein Isolate Stabilized Emulsions |
| - | [75] | |
| Pickering emulsion stabilized by alginate-lysozyme nanoparticles |
| [76] | ||
| Scallop gonad protein isolates stabilized emulsion in alginate beads |
|
| [77] | |
| Emulsion gel from egg yolk granules/sodium alginate bilayers emulsion |
|
| [62] | |
| Lycopene | Wet and dried alginate beads |
| [3] | |
| Internally and externally gelled alginate-based emulsion gels |
| [45] | ||
| Alginate-based emulsion gels containing protein-coated droplets |
| [46] | ||
| Alginate beads with added sugars and galactomannans |
| [59] | ||
| Alginate beads with modified rice starch |
|
| [40] | |
| inverse-gelated alginate microparticles |
| [47] | ||
| Alginate beads with added sugars and galactomannans |
| [7,59]] | ||
| Lutein | Chitosan-oleic acid- alginate nanocarrier |
| [24] | |
| Alginate hydrogels |
| [48] | ||
| Alginate microspheres |
| [54] |
| Type of Analysis | Laser Wavelength (nm) | Laser Power (mW) | The Aim of the Paper | References |
|---|---|---|---|---|
| FT-Raman | 1064 | 200 | Determination of Alginate Monomer Composition | [114] |
| Confocal Raman | 785 | 0.03 | Analysis of Hollow Silver Alginate Microspheres for Drug Delivery and Surface | [115] |
| SERS | 633 | 0.2 | Analysis of sodium alginates and their hetero- and homopolymeric fractions | [116] |
| SERS, TERS | 532 | 0.2 | Analysis of calcium alginate fibers and their network structures | [116,117] |
| Na Alginate + Ag | Ca Alginate + Ag | Na Alginate | Ca Alginate | Assignments | References |
|---|---|---|---|---|---|
| 645–647 cm−1 | Ring deformation | [116] | |||
| 751–766 cm−1 | Ring breathing | [116] | |||
| 799–811 cm−1 | 812 cm−1 | 807 cm−1 | 816 cm−1 | δ C–O–H, skeletal (ν C–C, ν C–O, δ C–C–H, δ C–C–O) | [117] |
| 876–879 cm−1 | 831 cm−1 | 888 cm−1 | 888 cm−1 | [117] | |
| 933 cm−1 | 870 cm−1 | 954 cm−1 | 959 cm−1 | [117] | |
| 1132 cm−1 | 1104 cm−1 | 1098 cm−1 | 1088 cm−1 | Glycosidic ring breathing mode | [117] |
| 1281 cm−1 | 1266 cm−1 | 1300 cm−1 | 1300 cm−1 | Carboxylate stretching vibration: Symmetric stretching or C–O single bond stretching vibration | [115,117] |
| 1329, 1337, 1374, 1384, 1388 cm−1 | 1385, 1397 cm−1 | 1413 cm−1 | 1433 cm−1 | Symmetric carboxylate stretching vibration | [117] |
| 1358 cm−1 | Aromatic stretching | [115] | |||
| 1492, 1502, 1524, 1526, 1554, 1561, 1571 cm−1 | 1610 cm−1 | 1625 cm−1 | 1625 cm−1 | Asymmetric carboxylate stretching vibration | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milivojević, M.; Popović, A.; Pajić-Lijaković, I.; Šoštarić, I.; Kolašinac, S.; Stevanović, Z.D. Alginate Gel-Based Carriers for Encapsulation of Carotenoids: On Challenges and Applications. Gels 2023, 9, 620. https://doi.org/10.3390/gels9080620
Milivojević M, Popović A, Pajić-Lijaković I, Šoštarić I, Kolašinac S, Stevanović ZD. Alginate Gel-Based Carriers for Encapsulation of Carotenoids: On Challenges and Applications. Gels. 2023; 9(8):620. https://doi.org/10.3390/gels9080620
Chicago/Turabian StyleMilivojević, Milan, Aleksandra Popović, Ivana Pajić-Lijaković, Ivan Šoštarić, Stefan Kolašinac, and Zora Dajić Stevanović. 2023. "Alginate Gel-Based Carriers for Encapsulation of Carotenoids: On Challenges and Applications" Gels 9, no. 8: 620. https://doi.org/10.3390/gels9080620
APA StyleMilivojević, M., Popović, A., Pajić-Lijaković, I., Šoštarić, I., Kolašinac, S., & Stevanović, Z. D. (2023). Alginate Gel-Based Carriers for Encapsulation of Carotenoids: On Challenges and Applications. Gels, 9(8), 620. https://doi.org/10.3390/gels9080620









