Abstract
An evolving field, nanotechnology has made its mark in the fields of nanoscience, nanoparticles, nanomaterials, and nanomedicine. Specifically, metal nanoparticles have garnered attention for their diverse use and applicability to dressings for wound healing due to their antimicrobial properties. Given their convenient integration into wound dressings, there has been increasing focus dedicated to investigating the physical, mechanical, and biological characteristics of these nanoparticles as well as their incorporation into biocomposite materials, such as hydrogel scaffolds for use in lieu of antibiotics as well as to accelerate and ameliorate healing. Though rigorously tested and applied in both medical and non-medical applications, further investigations have not been carried out to bring metal nanoparticle–hydrogel composites into clinical practice. In this review, we provide an up-to-date, comprehensive review of advancements in the field, with emphasis on implications on wound healing in in vivo experiments.
1. Introduction
Wound healing is an intricate physiological process consisting of a series of molecular and cellular events that facilitate the regeneration of the skin, a protective barrier against the external environment. Since its inception, hydrogels have advanced the field of wound healing, insofar as to promote damaged tissue healing within a hydrated milieu [1]. As well, the integration of therapeutic nanoparticles (NP) and biomolecules into hydrogels for local wound application has been shown to enhance and accelerate healing [2,3]. In this era of increasing antibiotic resistance, nanoparticles with antimicrobial properties are mainstay alternatives to the incorporation of antibiotics into hydrogels. The literature also contains evidence for the application of other inclusions such as metals, growth factor-releasing nanoparticles, and enzyme-releasing nanoparticles. Continuing advancements in hydrogel synthesis and nanoparticle integration offer a vast range of possibilities for creating more effective therapeutic gels.
Chronic wounds are increasingly a cause of public health concern. Indeed, under the growing population of patients with obesity and advanced age, the burden of pressure sores, venous insufficiency, and diabetes is expected to increase along with secondary instances of chronic wounds [4]. As such, a corresponding rise in treatment costs for chronic wounds is expected to strain current systems of healthcare delivery [5]. If undertreated, the persistence of chronic wounds can lead to delayed healing, progression of infectious wound infiltration, and reduced patient quality of life [5]. This outcome is especially likely in underserved populations, thereby grounding chronic wounds as a growing global health issue. Hydrogels are uniquely positioned to offer cost-effective and optimized local treatment for chronic wounds; moreover, they are non-toxic and non-irritant, biocompatible, easily applicable, and cost-effective [6]. Therefore, additional research and literature synthesis are required to support the calculated expansion of this rapidly evolving field.
Of importance, the incorporation of metal nanoparticles into hydrogels has been shown to enhance antimicrobial properties and accelerate healing [7]. Silver nanoparticles are a notable example that have demonstrated efficacy in reducing bacterial growth to promote wound healing [6,7]. One study reporting the application of guar gum hydrogels with embedded silver nanoparticles as wound dressings in a rat model demonstrates > 40% wound healing in the setting of 60% antibacterial efficacy, altogether with only 20% local cytotoxicity, after 12 days post-incision [8]. Another study reports on the spectrum of antimicrobial activity of silver nanoparticle inclusions in Swiss Albino mice, noting rapid healing with insignificant scarring after 48 h, exhibiting a stronger bactericidal selectivity against S. aureus than E. coli (92% overall bacterial reduction) [9]. Another report demonstrates additional properties of silver nanoparticles such as enhanced wound re-epithelialization, cell proliferation, and reduced tissue inflammation [2]. Zinc is another example of a metal nanoparticle that has exhibited antimicrobial properties when incorporated into hydrogel scaffolds. Similarly efficacious results with respect to antimicrobial activity and wound healing have been demonstrated with gold nanoparticle applications [10]. Although beyond the scope of this review, metal nanoparticles embedded in hydrogels have been studied for applications other than wound healing, such as for drug delivery, tissue engineering, cancer treatments, and imaging, each of which deserves further study [11]. Nonetheless, the application of metal nanoparticles for wound healing shows tremendous promise for clinical application to accelerate and ameliorate wound healing. However, further research is required to elucidate the therapeutic capabilities of these nanoparticles through the optimization of their design and delivery to wounded tissue. With recent advances in the application of metal nanoparticles into hydrogel scaffolds, there is a need to summarize new results to inform promising future areas of research. The rationale for this study was therefore to summarize the effects of metal nanoparticle-embedded hydrogel scaffolds with antimicrobial properties for wound healing in the literature on in vitro and in vivo results.
The primary objective of this review was to identify the corpus of nanoparticle-embedded hydrogels that, to date, have been investigated for their antimicrobial properties in wound healing. The secondary objective was to synthesize current evidence on key therapeutic properties as they pertain to each nanoparticle agent.
2. Methods
This systematic review abides by Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The protocol for this review was prospectively registered with PROSPERO (identifier: 375393). As recommended by article 2.4 of the Tri-Council Policy Statement, IRB/Ethics Committee approval was not required for this study since data were extracted from published primary research. This research adheres to the tenets of the Declaration of Helsinki, and where appropriate, adheres to PRISMA reporting guidelines.
2.1. Eligibility Criteria for Considering Studies for This Review
We included any in vivo study that investigated the effect of metallic nanoparticles embedded in hydrogel scaffolds to enhance antimicrobial properties during wound healing. No preference was discerned for exposures, such as infection-induced pathogens. There were no restrictions on the type of hydrogel scaffolding. Studies were excluded if they investigated non-metal nanoparticle agents, such as polymeric or solid lipid nanoparticles. Additionally, studies focusing on other parameters of hydrogel efficacy, such as the promotion of angiogenesis, were not recommended for study inclusion. Non-English-written studies were also excluded.
2.2. Search Methods for Identifying Studies
On 30 October 2022, Ovid MEDLINE and EMBASE were searched by SS using the study eligibility criteria. The search strategy was developed in consultation with an institutional librarian. Notable search restrictions included the English language. Study record management and eligibility assessment were completed in Covidence (Melbourne, Australia). Figure 1. depicts the PRISMA flow diagram for study inclusion for this review.
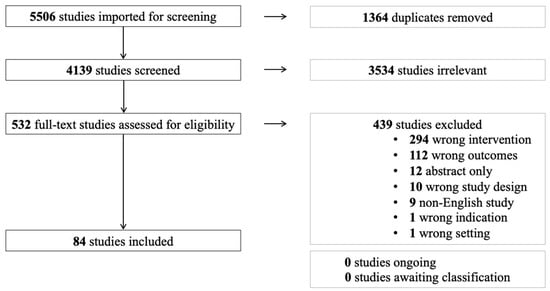
Figure 1.
PRISMA flow diagram for study inclusion.
Excluded studies fell under the following categories: wrong intervention, where studies did not include metal nanoparticles embedded in hydrogel scaffolds; wrong outcomes, where studies did not include review wound healing outcomes; wrong indication, where studies did not have the primary objective to investigate the effect of the intervention on wound healing; wrong study design, where in vivo investigations were not conducted; abstract only; non-English studies; and wrong setting, where studies discussed hydrogel preparation.
2.3. Data Collection and Risk of Bias Assessment
Title and abstract screening were completed by two independent reviewers (FB and JD) using Covidence (Melbourne, Australia). Studies were excluded if they were in vitro studies or if they did not describe wound healing outcomes with hydrogel scaffolding. Full-text articles were subsequently evaluated by two independent review authors (FB and JD), with reasons documented for study exclusion. Data extraction proceeded independently and in duplicate (FB, HS, JD, and LR). Variables recommended for extraction included hydrogel types; notable structural and in vitro properties; antimicrobial agent delivered; particle size; dosage delivered; rate of release; antimicrobial capacity; inhibition zone; cell type; cytotoxic effects; validated animal model; application method for wound healing; and year of publication. Conflicts at any stage in these review steps were adjudicated by a lead study author (SS and BT). For title and abstract screening, full-text evaluation, and data extraction, all review authors (SS, BT, FB, and JD) evaluated the first 100 records to calibrate inter-rater inconsistencies. Risk of bias evaluation was not conducted as the included studies were overwhelmingly conducted in a controlled non-clinical setting.
2.4. Data Synthesis and Analysis
The primary objective of this review was to produce a summary catalogue of nanoparticle-embedded hydrogels that, to date, have been investigated for their antimicrobial properties in wound healing in vivo. The secondary objective was to synthesize the evidence of study findings for each nanoparticle agent. A thematic analysis approach was used to summarize nanoparticles features as they relate to hydrogel types; notable structural and in vitro properties; antimicrobial agent delivered; particle size; dosage delivered; rate of release; antimicrobial capacity; inhibition zone; cell type; cytotoxic effects; validated animal model; application method for wound healing; and year of publication. The thematic analysis was reviewed by all study authors. All results that were applicable to each secondary outcome were sought from each study.
3. Wound Healing
3.1. Normal Wound Healing
In order to understand aberrant healing, normal wound repair and healing processes must be understood. Defined as an injury to the body involving laceration or breaking of a membrane usually with damage to underlying tissues, a wound can be acute or chronic [12]. In the acute phase of normal wound healing, there are four principal phases: hemostasis, inflammation, proliferation, and remodeling. Various cell types are involved in the healing process, such as fibroblasts and keratinocytes as well as proteins, hormones, cytokines, and enzymes [13].
Immediately following injury, there is bleeding. In the hemostatic phase of wound healing, vasoconstriction of arterial vessels occurs. Subsequent hypoxia and acidosis within the wound result in relaxation of the blood vessels with bleeding ensuing once more. Exposure of blood to the subendothelial tissues allows for the activation of the proteolytic cleavage and clotting cascades [14]. Platelets aggregate to form a hemostatic plug, releasing growth factors such as platelet-derived growth factor (PDGF), transforming growth factor-ß (TGF-ß), epidermal growth factor (EGF), and insulin-like growth factors (IGF), which trigger angiogenesis and activate and fibroblasts, endothelial cells, macrophages, and neutrophils [15]. Serotonin, histamine, and other vasoactive amines are released by mast cells and platelets, resulting in vasodilation and increased vascular permeability [15].
The inflammatory phase begins shortly after, with the activation of the complement cascade resulting in the attraction of leukocytes to the site of injury. Neutrophils and macrophages play a role in the removal of bacteria, damaged tissue, and foreign bodies from the site, thereby preventing infection [16]. Activation of macrophages results in further release of growth factors, which initiate subsequent cellular reactions [17]. Lymphocytes are attracted at 72 h post-injury [15].
The proliferation phase begins 72 h to approximately 2 weeks post-injury. Fibroblasts migrate and deposit extracellular tissue matrix (ECM), including fibrous proteins, collagen, polysaccharides, proteoglycans, glycosaminoglycans, and fibronectin, which appear as granulation tissue [18]. During this time, collagen synthesis and deposition simultaneous occur, with the collagen type dependent on the nature of the injury and healing factors present [15]. Wound contraction occurs by myofibroblasts via interactions with the ECM through the shrinking of connective tissue, effectively bringing wound edges closer together [19].
Lastly, the remodeling phase occurs, which may extend beyond 1–2 years [15]. Collagen bundles increase in diameter and become more organized within the matrix and matrix metalloproteinase enzymes are reduced [15,19].
3.2. Delayed Wound Healing
In healthy individuals, standard healing of acute wounds takes approximately 2–3 weeks followed by the remodeling phase, which takes 1–2 years [16]. Chronic wounds are generally defined as those taking longer than 6 weeks to heal or that have frequent recurrence [4]. As wound healing is an intricate process, a deviation in the physiological healing process may result in arrest in one of the four phases, resulting in a halt of healing progression. Many factors can contribute to delayed healing, including but not limited to chronic disease, wound infection, persistence of foreign bodies, vascular insufficiency, diabetes, neurological deficits, nutritional deficiencies, chronic irritation and trauma [4]. With chronic wounds, pathological damage extends beyond the area of compromised skin integrity, resulting in pathological damage to surrounding tissues as well [20].
3.3. Infected Wound Healing
As the majority of wound beds are moist, warm, and nutritious, they are highly susceptible to infection and provide optimized environments for the growth and colonization of bacteria [21]. The most predominant species found in infected wounds include Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Enterobacter cloacae, Klebsiella species, Streptococcus species, and Proteus species [22]. Bacteria are present on virtually all open wounds [4]. When the growth of bacteria does not exceed host defenses, wound beds are considered colonized and may actually hasten wound healing via the promotion of wound bed perfusion [23]. Once host defenses are no longer able to maintain the balance between bacterial growth and death, the wound becomes infected and enters a non-healing state [23]. Infected wounds are often polymicrobial, with Staphylococcus aureus and anaerobes being the most common [22]. Infected wounds pose a burden to both the patient as well as the health care system due to increased costs of care [24].
Most commercial products are not catered towards wounds with active infection. Such wounds progress to requiring systemic antibiotic therapy; however, this also has complications such as systemic toxicity, associated with end organ damage [25]. Additionally, systemic therapy does not penetrate well into ischemic or necrotic tissues. The use of local antibiotics, usually topically, has grown more popular, with Acticoat, high-density polyethylene mesh with nanocrystalline silver coating, as a prime example [21]. However, with the increasing use of antibiotics, antibiotic-resistant microorganisms have considerably increased [26]. This is an issue encountered both systemically and topically. As such, new strategies have been sought out to curb wound infections without the risks of antibiotics as listed above.
4. Current Wound Dressing Materials and Limitations
There is a longstanding history of wound dressings that dates back to ancient times, all of which demonstrate designs to manipulate the wound environment for the facilitation of wound healing [4]. Today, a myriad of wound care products are available, all majorly created with the understanding that a moist environment is crucial for cell migration and the facilitation of wound healing and contraction [16]. Further, these products aim to remove excess exudate, minimize trauma on removal, and protect against contaminants [4]. Currently, the following dressing materials described below are available [4]:
Peri-wound protection: Protects the integrity of the tissue surrounding the wound; includes ointments and barrier creams.
Gauze: Provides absorption but promotes desiccation of the wound base, which hinders healing. Moreover, binding of the gauze to the wound bed often results in trauma upon removal [27]. Moreover, as gauze dressings can become fully saturated with wound exudate, they are not effective barriers against infection. Modern gauze dressings have substances embedded to optimize the wound environment.
Films: Adhesive, non-absorbent, semi-occlusive dressings that can be used as primary or secondary dressings that facilitate the exchange between oxygen and water vapor between the wound bed and the external environment while still providing barrier functions against infection.
Foams: Non-adhesive, absorbent, semi-occlusive dressings that protect against shear with non-traumatic application and removal.
Hydrogels: Water-based, facilitating moist wound-healing environments. Application and removal cause minimal trauma to the wound bed. Further, hydrogels promote autolytic debridement.
Hydrocolloids: Adhesive, absorbent, occlusive dressings that are gel-forming, able to absorb large amounts of exudate while still maintaining moist wound-healing environments.
While wound dressings shield wounds from external contamination, functionalization and delivery of antibiotic properties directly to the wound bed have been investigated in efforts to accelerate and ameliorate the healing of infected wounds [28]. The incorporation of bioactive agents with such dressings has grown in popularity with increasing potential to be used for wound treatment. Due to their localized effect, this method is postulated to be more effective in the medicinal treatment of non-healing chronic wounds [29]. Despite this, the direct application of bioactive agents is still found to be more effective due to faster absorption [30].
5. Biopolymers in Wound Healing
As previously described, many different varieties of antibacterial wound dressings have been developed for wound treatment, including gauze, films, foams, hydrocolloids, alginates, collagens, and hydrogels. One issue with common wound dressings is their propensity to adhere to the wound site and edges, disrupting healing processes and causing damage to the wound bed [31]. Hydrogels, three-dimensional scaffold networks, are promising dressings as they provide suitable environments for cellular growth and adhesion [6]. They have high water content and also swell in hydrated environments, thus being able to absorb wound exudate while still maintaining a moist wound environment [4]. As hydrogels can retain large amounts of water, they are flexible and elastic [31]. Another issue with common scaffolds is that they have to be implanted at the wound bed, whereas hydrogels are able to conform to wound topography, filling all defects of the wound from the bottom up [6].
Hydrogel dressings can be made from many materials. Most commonly, polymers of synthetic molecules are used, such as polyacrylamide or polyvinylpyrrolidine [6]. These polymers are non-toxic and can maintain their shape; however, they have limited tissue adhesion properties and, as such, must be combined with other materials for the adjustment of their mechanical properties [31].
Another commonly used biopolymer is cellulose, a component of plant cell walls that is cost-efficient and ameliorates wound healing through the release of growth factors to stimulate granulation tissue formation, re-epithelialization, and angiogenesis as well as proliferation and movement of fibroblasts to the wound bed [32,33]. While more costly, chitin is another frequently used polysaccharide biopolymer for the formation of hydrogels [34]. Chitin is transformed into chitosan, which is soluble in aqueous solutions. In addition to its strong mechanical properties for wound healing, it also offers some antimicrobial properties as it is a cation, thus being able to treat infections through the disruption of cell membranes [35,36].
Alginate is another biopolymer that can be used in the preparation of hydrogels, but also in other dressings such as foams due to its absorbent properties [37,38,39,40]. It has good cytocompatibility and is non-toxic. It can be used in combination with other biopolymers, such as synthetic polymers, to enhance the biological and mechanical properties of the 3D hydrogel scaffold [41,42]. Gelatin, another naturally occurring polymer, is derived from collagen and is also used for its biocompatibility and biodegradable properties [43]. Likewise, fibrin, derived from fibrinogen, offers similar properties when used for wound healing, including reduction in inflammation, promotion of cell adhesion properties, and immunomodulation [43].
Starch, another biopolymer derived from plants, comes from various sources in many different shapes and sizes [44,45,46,47]. Apart from application in hydrogel scaffolds, given their cytocompatibility, low toxicity, and biodegradable nature, starches can be used in many other applications, including in drug delivery systems [44,45,46,47].
All the polymers mentioned above, both biological and synthetic, have been used in conjunction with metal nanoparticles for an enhanced healing effect. Other notable materials observed to be used in such combinations include dextran, elastin, silica, tannic acid, and lignin.
6. Nanotechnology for Wound Healing
Nanotechnology is defined as the manipulation of materials on an atomic or molecular scale [48]. Ever evolving, nanotechnology has revolutionized many industries, especially within the fields of nanoscience, nanoparticles, nanomaterials, and nanomedicine. Specifically, the field of nanomedicine has risen in popularity with myriad applications, including vaccine production, wearable devices, implants, drug delivery, and antibacterial applications [49]. In tissue engineering and regenerative medicine, nanomaterials have shown low toxicity and customizability, making them versatile agents to incorporate into medical practice [49]. For instance, metal nanoparticles such as silver (Ag) [50], gold (Au) [51], copper (Cu) [52], and zinc oxide (ZnO) [53] have demonstrated marked antimicrobial properties. While these intrinsic properties are advantageous for wound healing, these metal nanoparticles can also display anti-infective properties within drug-delivery vehicles.
7. Nanoparticles Used in Wound Healing
Metal nanoparticles have been considered in clinical applications for reasons including small size, high surface-to-volume ratio, shape, stability, low toxicity, and economic reasons, given their affordability [54,55]. Additionally, they can conveniently integrate into wound dressings [54]. One of the primary mechanisms in which antibacterial activity is offered by metal nanoparticles is through their bacteriostatic properties via attachment to DNA or RNA, via electrostatic interactions, halting further replication [56]. MicroRNAs, short, non-coding RNA molecules that have regulatory roles in gene expression, play a large role in wound healing processes, including inflammation, angiogenesis, cell proliferation, and ECM remodeling. In aberrant wound healing, such as infectious states, microRNAs can be targeted by metal nanoparticles through encapsulation, shielding charge groups and allowing for cellular uptake [57]. The modulation of microRNA allows for the enhancement of gene expression factors, promoting the production of factors essential for wound healing. Further, targeted delivery of these therapeutic agents minimizes off-target effects [57].
Another mechanism is through bactericidal properties via the creation of reactive oxygen species [56]. When embedded in hydrogel scaffolds, a substitute is created for damaged ECM which facilitates fibroblast proliferation and matrix formation for enhanced regeneration and repair [58,59]. As such, these nanoparticles can be used in lieu of antibiotics and are thought to accelerate and ameliorate healing while preventing infection [54,55]. A graphical summary of wound healing mechanisms per nanoparticle is depicted in Figure 2.
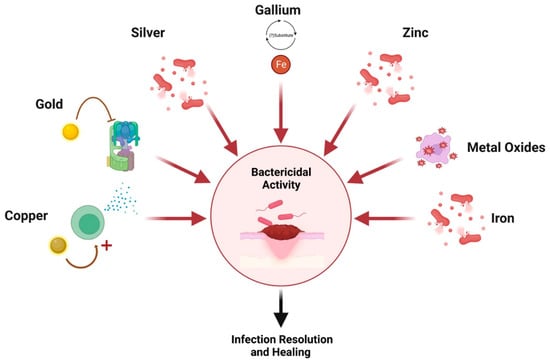
Figure 2.
Nanoparticle mechanisms of action. Created with Biorender.com.
7.1. Silver (Ag) Nanoparticles
The use of silver for the treatment of wounds and infection prevention dates back to at least 4000 B.C.E. with documented medical applications dating back to the 1700s; however, in large quantities, silver can also impair healing due to its toxic effects on keratinocytes and fibroblasts [60,61]. Today, silver continues to serve many applications in wound healing. For example, silver nitrate is used as a commonplace treatment for chronic wounds while silver sulfadiazine is used for burns. Nanotechnology has changed the use of silver for wound healing with the creation of silver nanoparticles (AgNPs), which are the most commonly used metal nanoparticles in wound management with many applications, including wound infections, ulcers, and burns. Known for their wide range of antimicrobial activity, effective against bacteria, viruses, fungi, and protozoa, as well as promotion of wound healing, AgNPs have been shown to disturb quorum sensing, effectively reducing biofilm formation [62,63,64].
The antibacterial effect is demonstrated via bactericidal and inhibitory mechanisms. In terms of bactericidal activity, apoptosis is induced in bacteria through AgNP interactions with sulfur and phosphorous-containing proteins, effectively disrupting cell membranes [65]. Moreover, as DNA consists of sulfur and phosphorous, AgNPs act on these bases to destroy DNA, further facilitating the apoptosis of bacterial cells [65]. Moreover, the continuous release of AgNPs, specifically at lower pH whereby acidic environments facilitate the oxidation of AgNPs to Ag+, negatively charged proteins are bound to, allowing for disruption of bacterial cell walls and membrane [65]. Through this mechanism, cell respiration is also disrupted through damage to bacterial mitochondria [66,67]. Despite these cytotoxic effects, which are AgNP-dose- and size-dependent, the proliferation of fibroblasts and keratinocytes is not affected [68]. In terms of inhibitory mechanisms, the presence of AgNPs in the wound environment allows for the formation of reactive oxygen species (ROS), which further disrupt bacterial cell viability through oxidative stress [66,67]. Wound healing is accelerated through these antibacterial properties as microbes can delay all stages of wound healing.
In addition to the antimicrobial properties of AgNPs, they also promote wound healing [69,70,71,72]. Firstly, they assist in the differentiation of fibroblasts into myofibroblasts, which allows for wound contractility [73]. Moreover, they stimulate the proliferation and relocation of keratinocytes to the wound bed [74]. As such, quicker wound epithelialization and scarless wound healing are promoted [73]. Accelerated and complete healing with increased epithelialization was observed in a study wherein an AgNP hydrogel was applied to a partial-thickness cutaneous wound in mice [75,76].
AgNPs also have anti-inflammatory effects through cytokine modulation, reducing levels that allow for decreased lymphocyte infiltration, further enhancing re-epithelialization [52,77]. One study demonstrated a significant reduction in inflammatory cytokines and oxidative stress, effectively promoting healing, while another study in a burn wound model in mice demonstrated reduced interleukin-6 (IL-6) and neutrophils and increased the levels of IL-10, vascular endothelial growth factor, and TGF-ß [50].
The summary of all in vivo studies related to AgNP-loaded hydrogels is shown in Table 1.

Table 1.
Summary of Characteristics and Findings of Included Trials for Silver Nanoparticles.
7.2. Gold (Au) Nanoparticles
AuNPs are commonly used in tissue regeneration, wound healing, and drug delivery of bioactive compounds due to their biocompatibility, high surface reactivity, and antioxidative effects [118,119]. While some antimicrobial effects are seen, unlike AgNPs, AuNPs do not offer much antimicrobial activity alone [120].
Antimicrobial action is demonstrated via two principal mechanisms, similar to AgNPs: bactericidal and inhibitory. Cell death is induced via the disruption of ATP synthase, leading to decreased ATP stores and an eventual collapse in energy metabolism [121]. This is due to the ability of AuNPs to alter membrane potential on entry into the cell [121]. Additionally, the creation of ROS is facilitated by AuNPs, further facilitating cell death. The smaller the size of the AuNPs, the greater the surface area and interface for interaction with microbes, demonstrating a stronger antimicrobial effect [122].
While some antibacterial effects are seen, AuNPs are principally used in tissue repair given their anti-inflammatory properties via cytokine modulation and antioxidant properties [123,124]. Substantial antioxidant properties are seen as AuNPs are able to bind free radicals such as nitric oxide (NO) or hydroxyl (OH-) [125,126,127]. This strong catalytic activity in free radical scavenging is further observed through the ability of AuNPs to increase nuclear factor erythroid 2-related factor (NRF2), which allows for antioxidant gene activation [128,129]. Furthermore, while being able to facilitate the creation of ROS, they are also able to receive electrons and remove or deactivate ROS, with greater effects seen the higher the surface area of the AuNPs is [119].
In addition to tissue repair, wound healing is found to be accelerated and ameliorated with the use of AuNPs through the promotion of collagen expression, growth factors, vascular endothelial growth factor (VEGF), fibroblast proliferation, decreased cellular apoptosis, and angiogenesis [67,130].
Despite these beneficial effects, AuNPs must usually be incorporated with other biomolecules for efficacy in wound healing applications. Examples include the incorporation of AuNPs in chitosan or gelatin for the enhancement of wound healing or in collagen for a similar effect [51,52]. One study of a rat full-thickness excisional wound model demonstrated accelerated healing and wound closure with improved hemostasis and re-epithelization compared to the Tegaderm dressing and pure chitosan hydrogel controls in a chitosan-AuNP hydrogel [131]. Recent studies have also incorporated phototherapy in conjunction with AuNPs to achieve antimicrobial activity [53,132].
The summary of all in vivo studies related to AuNP-loaded hydrogels is shown in Table 2.

Table 2.
Summary of Characteristics and Findings of Included Trials for Gold Nanoparticles.
7.3. Copper (Cu) and Copper Oxide (CuO)
Previous studies have demonstrated that CuNPs have antimicrobial activity as well as properties that facilitate tissue repair. CuNPs have shown antibacterial activity against bacterial strains such as Escherichia coli and Staphylococcus aureus but also fungicidal effects [138,139,140,141,142,143]. The principal mechanism of action is through adhesion of the CuNP to bacteria due to their opposing electrical charges, resulting in a reduction reaction that weakens and destroys the bacterial cell wall. CuNPs have also shown antibacterial activity through the enhancement of immunity with the promotion of interleukin-2 (IL2) production, as well as its ability to serve as a cofactor for various enzymes such as cytochrome oxidase [144]. Additionally, CuNPs have an influence on cytokine regulation, thus also having anti-inflammatory properties [145]. In terms of tissue repair, ECM synthesis is promoted through the stimulation of ECM components such as fibrinogen and fibroblasts as well as the production of integrins and collagen [146,147]. One study observing the use of a CuNP-embedded hydrogel in the treatment of full-thickness excisional wounds in rats demonstrated an accelerated wound healing rate [52]. Despite these positive effects, CuNPs are prone to rapid oxidation, promotion of the production of free radicals, and instability, thus limiting its use [148,149].
CuO NPs have been used in multiple biomedical settings, such as in drug delivery, as anti-cancer agents, and wound healing given their biocompatibility, low toxicity, and antimicrobial properties [150,151]. The specific mechanism for the antibacterial effects of CuO remains unknown; however, it is postulated that it is related to the generation of ROS within bacterial cells [152]. However, with CuO NPs, antibacterial activity was partially related to bacterial properties. For example, different effects were noted with increased bactericidal activity in Gram-negative organisms, such as E. coli, compared to Gram-positive organisms, such as S. aureus [153]. Despite these antibacterial properties, one concern is toxicity, the induction of oxidative stress, and subsequent DNA and mitochondrial damage [154,155].
The summary of all in vivo studies related to Cu and CuO NP-loaded hydrogels is shown in Table 3 and Table 4, respectively.

Table 3.
Summary of Characteristics and Findings of Included Trials for Copper Nanoparticles.

Table 4.
Summary of Characteristics and Findings of Included Trials for Copper Oxide Nanoparticles.
7.4. Zinc (Zn) and Zinc Oxide (ZnO)
Zn and ZnO NPs are some of the most commonly used NPs in wound healing applications due to their anti-inflammatory and antimicrobial properties [166]. As inorganic agents, they are more stable than their organic agent counterparts. They are also advantageous in their ability to remain within the wound bed for longer periods of time [166,167]. The antimicrobial effects of Zn and ZnO NPs are due to disruption of cell membranes and oxidant injury [166,167]. Zinc also serves as a cofactor for metalloproteinases and other enzymatic complexes, promoting migration of keratinocytes and regeneration of the ECM [166,167]. A previous study examining full-thickness wounds in a rat model showed accelerated and ameliorated healing compared to control with improved re-epithelialization as well as increased collagen deposition and tissue granulation [167]. Moreover, both Zn and ZnO NPs have demonstrated good biocompatibility and low cytotoxicity [166].
Like other NPs, the Zn and ZnO NP effect is dependent on the size, surface-area-to-volume ratio, and concentration of the NPs [168]. Smaller NPs have been shown to be more cytotoxic given their larger surface-area-to-volume ratio, whereas larger NPs demonstrate increased cytocompatibility [169]. In fact, a previous study demonstrated that ZnO NPs are highly compatible with fibroblast cells and promote their growth, migration, and adhesion [142].
Summaries of all in vivo studies related to Zn and ZnO NP loaded hydrogels are shown in Table 5 and Table 6, respectively.

Table 5.
Summary of Characteristics and Findings of Included Trials for Zinc Nanoparticles.

Table 6.
Summary of Characteristics and Findings of Included Trials for Zinc Oxide Nanoparticles.
7.5. Other Metal Oxides
Metal oxide NPs include zinc oxide (ZnO), copper oxide (CuO), cerium oxide (CeO2), manganese oxide (MnO2), and titanium oxide (TiO2). These NPs have antioxidant properties and have been shown to facilitate wound healing through the restriction of ROS, inhibiting apoptosis [166,167].
7.5.1. Titanium Oxide (TiO2)
A study of the antimicrobial effects of TiO2 NPs demonstrated little effect but showed accelerated wound healing in a full-thickness excisional wound model [183].
7.5.2. Cerium Oxide (CeO2)
CeO2 NPs have the highest antioxidant activity of all NPs and are most active in the scavenging of free radicals [184]. This is due to the oxygen vacancies of CeO2, leading to the reduction of Cerium from Ce+4 to Ce+3, for example [184].
7.5.3. Manganese Oxide (MnO2)
MnO2 is also a potential candidate to be used in nanoparticle–hydrogel composites. Known to relieve oxidative stress, MnO2 is able to catalytically decompose H2O2 into O2, thus effectively providing a targeted approach to hypoxic relief [185]. One study evaluating MnO2 nanoparticles in the healing of chronic diabetic wounds in vivo demonstrated the eradication of biofilms, attenuation of hyperglycemia, hemostasis, and the creation of an optimized wound environment which reduced inflammation, accelerated granulation tissue formation and re-epithelialization, and accelerated wound healing [185]. The summary of all in vivo studies related to MnO2 NP-loaded hydrogels is shown in Table 7.

Table 7.
Summary of Characteristics and Findings of Included Trials for Manganese Nanoparticles.
7.6. Iron (Fe) Nanoparticles (FeNP)
Less commonly used in antibacterial wound dressing applications, iron nanoparticles have been shown to induce bacterial death, membrane damage, DNA degradation, and lipid peroxidation [187,188,189]. One study demonstrated high antibacterial activities against S. aureus and E. coli both in vitro and in an in vivo infected full-thickness excisional wound model in mice where accelerated wound healing and anti-inflammatory properties were observed [187].
The summary of all in vivo studies related to FeNP-loaded hydrogels is shown in Table 8.

Table 8.
Summary of Characteristics and Findings of Included Trials for Iron Nanoparticles.
7.7. Gallium (Ga) Nanoparticles (GaNP)
Gallium is very infrequently used in wound healing applications. Given that gallium and iron have equal ionic radii, one study hypothesized that the substitution of iron with gallium would impair bacterial iron metabolism and exert an antimicrobial effect [194]. This has previously been observed in vitro, whereby gallium resulted in reduced bacterial survival [194,195,196]. Moreover, given the inability of gallium to be reduced in physiological environments, a property not shared with iron, gallium also disrupts enzyme activity [197]. A 2022 study by Qin et al. demonstrated the good antimicrobial effect of gallium embedded in an alginate-base hydrogel, with good biocompatibility against NIH3T3 cells in vitro as well as accelerated wound healing with good biocompatibility, angiogenesis, and collagen deposition compared to the control in an S. aureus infected full-thickness excisional wound model in mice [194].
The summary of all in vivo studies related to GaNP-loaded hydrogels is shown in Table 9.

Table 9.
Summary of Characteristics and Findings of Included Trials for Gallium Nanoparticles.
7.8. Combinations of Metal Nanoparticles
Occasionally, metal NPs can be used in conjunction with each other to provide synergistic antibacterial effects. For example, one study investigated the effects of AgNP and CuNP within a chitosan hydrogel, demonstrating good antibacterial activity against S. aureus and E. coli with good biocompatibility and accelerated healing compared to control in an S. aureus infected full-thickness excisional wound model in type 1 diabetic rats [198]. Another study looked at the synergy between ZnO and AgNPs, demonstrating an excellent bactericidal effect against E. coli and S. aureus [199]. Interestingly, AgNPs were observed to exhibit a small amount of cytotoxicity when tested against mouse calvarial (MC3T3-E1) cells alone, but when used in conjunction with ZnO NPs, lower cytotoxicity was seen [199]. In an S. aureus infected partial thickness wound model in rats, the release of Ag+ and Zn2+ was found to stimulate immune function to produce a large number of white blood cells and neutrophils (2–4 times more than the control), thereby producing the synergistic antibacterial effects and accelerated wound healing [200].
The summary of all in vivo studies related to combinations of metal-NP-loaded hydrogels is shown in Table 10.

Table 10.
Summary of Characteristics and Findings of Included Trials for Combinations of Nanoparticles.
8. Challenges and Future Directions
While there are myriad advantages in the addition of metal NPs to hydrogel scaffolds, there are limitations to their use as well. Principally, cytotoxicity is of concern as the interactions of NPs and cells have not yet fully been elicited [65]. While exploration into the cytotoxicity of NPs has been investigated, the field lacks uniformity such that tests of different cell types have demonstrated different cytotoxic responses. Further, there are multiple factors that influence NP activity, including the size, shape, concentration within the hydrogel, and surface charge [93]. Another concern regarding NPs is release and uptake. Poor release or uptake limits the efficacy and quality of treatment provided whereas excess release can result in deposition in surrounding tissues or organs, toxicity, or other undesired effects [31,65,202]. For example, overuse of silver-containing dressings impair healing processes due to cytotoxicity against keratinocytes and fibroblasts as well as systemic adverse effects such as argyria [202,203,204]. Thus, despite an extensive repertoire of current studies, future investigations and development would benefit from further toxicity and cytocompatibility assessments both in vivo and in vitro and iterate on current formulations for hydrogels with metal nanoparticles. We also recommend continued translational research on the application of these dressings in clinical practice.
9. Conclusions
The main aim of this review is to highlight the antimicrobial activity of metal nanoparticles embedded within hydrogel scaffolds for wound healing. Here, we elaborately discussed how these nanoparticles interact with pathogens but also with the wound environment to eradicate infection and enhance the healing process. When used in conjunction, hydrogels and metal nanoparticles demonstrate a synergistic effect. Excellent activity was demonstrated by select metal nanoparticles embedded within hydrogels. Accelerated and ameliorated wound healing was also observed with the promotion of re-epithelialization, angiogenesis, increased deposition of collagen and granulation tissue, and downregulation of inflammatory processes. Despite the large body of research in the field, there is a gap in preclinical and clinical studies, which, if addressed, could facilitate the use of these dressings in common clinical practice.
Author Contributions
Conceptualization, S.S.-O., N.A. and B.T.; Methodology, S.S.-O., N.A. and B.T.; Software, S.S.-O. and B.T.; Validation, S.S.-O., N.A. and B.T.; Formal Analysis, S.S.-O. and B.T.; Investigation, S.S.-O. and B.T.; Resources, S.S.-O., N.A. and B.T.; Data Curation, J.D., F.B., H.S. and L.R.; Writing—Original Draft Preparation, S.S.-O., N.A. and B.T.; Writing—Review and Editing, S.S.-O., N.A. and B.T.; Visualization, S.S.-O., N.A. and B.T.; Supervision, S.S.-O. and N.A.; Project Administration, S.S.-O. and N.A. All authors have read and agreed to the published version of the manuscript.
Conceptualization, S.S.-O., N.A. and B.T.; Methodology, S.S.-O., N.A. and B.T.; Software, S.S.-O. and B.T.; Validation, S.S.-O., N.A. and B.T.; Formal Analysis, S.S.-O. and B.T.; Investigation, S.S.-O. and B.T.; Resources, S.S.-O., N.A. and B.T.; Data Curation, J.D., F.B., H.S. and L.R.; Writing—Original Draft Preparation, S.S.-O., N.A. and B.T.; Writing—Review and Editing, S.S.-O., N.A. and B.T.; Visualization, S.S.-O., N.A. and B.T.; Supervision, S.S.-O. and N.A.; Project Administration, S.S.-O. and N.A. All authors have read and agreed to the published version of the manuscript.
Conceptualization, S.S.-O., N.A. and B.T.; Methodology, S.S.-O., N.A. and B.T.; Software, S.S.-O. and B.T.; Validation, S.S.-O., N.A. and B.T.; Formal Analysis, S.S.-O. and B.T.; Investigation, S.S.-O. and B.T.; Resources, S.S.-O., N.A. and B.T.; Data Curation, J.D., F.B., H.S. and L.R.; Writing—Original Draft Preparation, S.S.-O., N.A. and B.T.; Writing—Review and Editing, S.S.-O., N.A. and B.T.; Visualization, S.S.-O., N.A. and B.T.; Supervision, S.S.-O. and N.A.; Project Administration, S.S.-O. and N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study extracts data in its entirety from published primary research. Under Article 2.4 of the Tri-Council Policy Statement, this study is exempt from institutional review board approval. This study will not identify any individual or generate new forms of identifiable information.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hartmeier, P.R.; Pham, N.B.; Velankar, K.Y.; Issa, F.; Giannoukakis, N.; Meng, W.S. Hydrogel Dressings for Chronic Wound Healing in Diabetes: Beyond Hydration. J. Pharm. Drug. Deliv. Res. 2021, 10, 1000197. [Google Scholar] [PubMed]
- Haidari, H.; Bright, R.; Strudwick, X.L.; Garg, S.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Multifunctional ultrasmall AgNP hydrogel accelerates healing of S. aureus infected wounds. Acta Biomater. 2021, 128, 420–434. [Google Scholar] [CrossRef] [PubMed]
- El-Ezz, D.A.; Abdel-Rahman, L.H.; Al-Farhan, B.S.; Mostafa, D.A.; Ayad, E.G.; Basha, M.T.; Abdelaziz, M.; Abdalla, E.M. Enhanced In Vivo Wound Healing Efficacy of a Novel Hydrogel Loaded with Copper (II) Schiff Base Quinoline Complex (CuSQ) Solid Lipid Nanoparticles. Pharmaceuticals 2022, 15, 978. [Google Scholar] [CrossRef] [PubMed]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J.; Deptuła, M.; Karpowicz, P.; Wardowska, A.; Sass, P.; Sosnowski, P.; Mieczkowska, A.; Filipowicz, N.; Dzierżyńska, M.; et al. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Ma, L.; Tan, Y.; Chen, X.; Ran, Y.; Tong, Q.; Tang, L.; Su, W.; Wang, X.; Li, X. Injectable oxidized alginate/carboxylmethyl chitosan hydrogels functionalized with nanoparticles for wound repair. Carbohydr. Polym. 2022, 293, 119733. [Google Scholar] [CrossRef]
- Xiang, J.; Bai, Y.; Huang, Y.; Lang, S.; Li, J.; Ji, Y.; Peng, B.; Liu, G. A zwitterionic silver nanoparticle-incorporating injectable hydrogel with a durable and efficient antibacterial effect for accelerated wound healing. J. Mater. Chem. B 2022, 10, 7979–7994. [Google Scholar] [CrossRef]
- Bhubhanil, S.; Talodthaisong, C.; Khongkow, M.; Namdee, K.; Wongchitrat, P.; Yingmema, W.; Hutchison, J.A.; Lapmanee, S.; Kulchat, S. Enhanced wound healing properties of guar gum/curcumin-stabilized silver nanoparticle hydrogels. Sci. Rep. 2021, 11, 21836. [Google Scholar] [CrossRef]
- Anjum, S.; Gupta, A.; Sharma, D.; Gautam, D.; Bhan, S.; Sharma, A.; Kapil, A.; Gupta, B. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater. Sci. Eng. C 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hikmat, S.; Abu Ghith, D.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Int. J. Pharm. 2019, 565, 174–186. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Wound Definition & Meaning—Merriam-Webster. Available online: https://www.merriam-webster.com/dictionary/wound (accessed on 10 January 2023).
- Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Shiffman, M.A.; Low, M. (Eds.) Recent Clinical Techniques, Results, and Research in Wounds. In Chronic Wounds, Wound Dressings and Wound Healing; Springer International Publishing: Cham, Switzerland, 2021; Volume 6. [Google Scholar] [CrossRef]
- Harper, D.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2014, 32, 445–450. [Google Scholar] [CrossRef]
- Stunova, A.; Vistejnova, L. Dermal fibroblasts—A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.B.; Barbul, A. GENERAL PRINCIPLES OF WOUND HEALING. Surg. Clin. N. Am. 1997, 77, 509–528. [Google Scholar] [CrossRef]
- Farrow, W. Phlebolymphedema–A Common Underdiagnosed and Undertreated Problem in the Wound Care Clinic. J. Am. Coll. Certif. Wound Spéc. 2010, 2, 14–23. [Google Scholar] [CrossRef]
- Mittal, A.; Kumar, N. A new, bioactive, antibacterial-eluting, composite graft for infection-free wound healing. Wound Repair Regen. 2014, 22, 527–536. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound Microbiology and Associated Approaches to Wound Management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- The Wound Infection Continuum and Its Application to Clinical Practice. Wound Manag. Prev. 2003, 49, 1–7. Available online: https://www.hmpgloballearningnetwork.com/site/wmp/content/the-wound-infection-continuum-and-its-application-clinical-practice (accessed on 11 January 2023).
- Kumar, M.S.; Kirubanandan, S.; Sripriya, R.; Sehgal, P.K. Triphala Promotes Healing of Infected Full-Thickness Dermal Wound. J. Surg. Res. 2008, 144, 94–101. [Google Scholar] [CrossRef]
- Shanmugasundaram, N.; Uma, T.S.; Lakshmi, T.S.R.; Babu, M. Efficiency of controlled topical delivery of silver sulfadiazine in infected burn wounds. J. Biomed. Mater. Res. Part A 2009, 89A, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Horton, R. Antibiotics: Achieving the balance between access and excess. Lancet 2016, 387, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D. Formation of the Scab and the Rate of Epithelization of Superficial Wounds in the Skin of the Young Domestic Pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- Falabella, A.F.; Schachner, L.A.; Valencia, I.C.; Eaglstein, W.H. The use of tissue-engineered skin (Apligraf) to treat a newborn with epidermolysis bullosa. Arch. Dermatol. 1999, 135, 1219–1222. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Matsumoto, S.; Tanaka, R.; Okada, K.; Arita, K.; Hyakusoku, H.; Miyamoto, M.; Tabata, Y.; Mizuno, H. The Effect of Control-released Basic Fibroblast Growth Factor in Wound Healing: Histological Analyses and Clinical Application. Plast. Reconstr. Surg. Glob. Open 2013, 1, e44. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti. Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive chitosan–agarose hydrogel for skin regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Saarai, A.; Sedlacek, T.; Kasparkova, V.; Kitano, T.; Saha, P. On the characterization of sodium alginate/gelatine-based hydrogels for wound dressing. J. Appl. Polym. Sci. 2012, 126, E79–E88. [Google Scholar] [CrossRef]
- Straccia, M.C.; D’Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate Hydrogels Coated with Chitosan for Wound Dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and Characterization of Films Based on Alginate and Aloe Vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Dantas, M.; Cavalcante, D.; Araújo, F.; Barretto, S.; Aciole, G.; Pinheiro, A.; Ribeiro, M.; Lima-Verde, I.; Melo, C.; Cardoso, J.; et al. Improvement of dermal burn healing by combining sodium alginate/chitosan-based films and low level laser therapy. J. Photochem. Photobiol. B Biol. 2011, 105, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical Biopolymers, their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE21–ZE25. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Powell, H.M.; Boyce, S.T. Fiber density of electrospun gelatin scaffolds regulates morphogenesis of dermal–epidermal skin substitutes. J. Biomed. Mater. Res. Part A 2008, 84, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Alobi, N.O.; Sunday, E.A.; Magu, T.O.; Oloko, G.O.; Nyong, B.E. Analysis of Starch from Non-Edible Root and Tubers as Sources of Raw Materials for the Synthesis of Biodegradable Starch Plastics. Rochester, NY, USA, 27 November 2018. Available online: https://papers.ssrn.com/abstract=3291118 (accessed on 20 January 2023).
- Mendes, S.C.; Reis, R.; Bovell, Y.P.; Cunha, A.; van Blitterswijk, C.A.; de Bruijn, J.D. Biocompatibility testing of novel starch-based materials with potential application in orthopaedic surgery: A preliminary study. Biomaterials 2001, 22, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, H.S.; Gama, F.M.; Reis, R.L. In Vitro Assessment of the Enzymatic Degradation of Several Starch Based Biomaterials. Biomacromolecules 2003, 4, 1703–1712. [Google Scholar] [CrossRef]
- Gomes, M.E.; Sikavitsas, V.I.; Behravesh, E.; Reis, R.L.; Mikos, A.G. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. J. Biomed. Mater. Res. 2003, 67, 87–95. [Google Scholar] [CrossRef]
- Definition of Nanotechnology. Available online: https://www.merriam-webster.com/dictionary/nanotechnology (accessed on 11 January 2023).
- Nanotechnology in Medicine. Available online: https://onlinelibrary.wiley.com/doi/epub/10.1002/9781119769897 (accessed on 11 January 2023).
- Tian, J.; Wong, K.K.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Cliu, J.F.; Tam, P.K. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [CrossRef]
- Chen, S.-A.; Chen, H.-M.; Yao, Y.-D.; Hung, C.-F.; Tu, C.-S.; Liang, Y.-J. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur. J. Pharm. Sci. 2012, 47, 875–883. [Google Scholar] [CrossRef]
- Tiwari, M.; Narayanan, K.; Thakar, M.B.; Jagani, H.V.; Rao, J.V. Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 2014, 8, 230–237. [Google Scholar] [CrossRef]
- Barui, A.K.; Veeriah, V.; Mukherjee, S.; Manna, J.; Patel, A.K.; Patra, S.; Pal, K.; Murali, S.; Rana, R.K.; Chatterjee, S.; et al. Zinc oxide nanoflowers make new blood vessels. Nanoscale 2012, 4, 7861–7869. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef]
- Barroso, A.; Mestre, H.; Ascenso, A.; Simões, S.; Reis, C. Nanomaterials in wound healing: From material sciences to wound healing applications. Nano Sel. 2020, 1, 443–460. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA delivery through nanoparticles. J. Control. Release 2019, 313, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Yates, C.C.; Hebda, P.; Wells, A. Skin Wound Healing and Scarring: Fetal Wounds and Regenerative Restitution. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care—Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef]
- Politano, A.D.; Campbell, K.T.; Rosenberger, L.H.; Sawyer, R.G. Use of Silver in the Prevention and Treatment of Infections: Silver Review. Surg. Infect. 2013, 14, 8–20. [Google Scholar] [CrossRef]
- Cameron, P.; Gaiser, B.K.; Bhandari, B.; Bartley, P.M.; Katzer, F.; Bridle, H. Silver Nanoparticles Decrease the Viability of Cryptosporidium parvum Oocysts. Appl. Environ. Microbiol. 2016, 82, 431–437. [Google Scholar] [CrossRef]
- Navani, N.K.; Lambadi, P.R.; Sharma, T.K.; Kumar, P.; Vasnani, P.; Thalluri, S.M.; Bisht, N.; Pathania, R. Facile biofunctionalization of silver nanoparticles for enhanced antibacterial properties, endotoxin removal, and biofilm control. Int. J. Nanomed. 2015, 10, 2155–2171. [Google Scholar] [CrossRef]
- Singh, B.R.; Singh, B.N.; Singh, A.; Khan, W.; Naqvi, A.H.; Singh, H.B. Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci. Rep. 2015, 5, 13719. [Google Scholar] [CrossRef]
- Guide to the Quality and Safety of Tissues and Cells for Human Application—European Directorate for the Quality of Medi-cines & HealthCare—EDQM. European Directorate for the Quality of Medicines & HealthCare. Available online: https://www.edqm.eu/en/guide-to-the-quality-and-safety-of-tissues-and-cells-for-human-application1 (accessed on 20 January 2023).
- Butler, K.S.; Peeler, D.J.; Casey, B.J.; Dair, B.J.; Elespuru, R.K. Silver nanoparticles: Correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis 2015, 30, 577–591. [Google Scholar] [CrossRef]
- Franková, J.; Pivodová, V.; Vágnerová, H.; Juránová, J.; Ulrichova, J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J. Appl. Biomater. Funct. Mater. 2016, 14, e137–e142. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.Y.; Cheung, S.O.F.; Huang, L.; Niu, J.; Tao, C.; Ho, C.-M.; Che, C.-M.; Tam, P.K.H. Further Evidence of the Anti-inflammatory Effects of Silver Nanoparticles. Chemmedchem 2009, 4, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Núñez, N.V.; Turrent, L.D.C.I.; Padilla, C.R. Bactericidal Effect of Silver Nanoparticles against Multidrug-Resistant Bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Kreytsberg, G.N.; Gracheva, I.E.; Kibrik, B.S.; Golikov, I.V. Antituberculous effect of silver nanoparticles. J. Phys. Conf. Ser. 2011, 291, 012030. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef]
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chemother. 2007, 59, 587–590. [Google Scholar] [CrossRef]
- Liu, X.; Lee, P.-Y.; Ho, C.-M.; Lui, V.C.H.; Chen, Y.; Che, C.-M.; Tam, P.K.H.; Wong, K.K.Y. Silver Nanoparticles Mediate Differential Responses in Keratinocytes and Fibroblasts during Skin Wound Healing. Chemmedchem 2010, 5, 468–475. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Unnithan, A.R.; Sasikala, A.R.K.; Samarikhalaj, M.; Thomas, R.G.; Jeong, Y.Y.; Nasseri, S.; Murugesan, P.; Wu, D.; Park, C.H.; et al. Mussel-Inspired Electrospun Nanofibers Functionalized with Size-Controlled Silver Nanoparticles for Wound Dressing Application. ACS Appl. Mater. Interfaces 2015, 7, 12176–12183. [Google Scholar] [CrossRef]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled Release of Biologically Active Silver from Nanosilver Surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Central Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A. Therapeutic potential of green synthesized silver nanoparticles loaded PVA hydrogel patches for wound healing. J. Drug Deliv. Sci. Technol. 2019, 54, 101308. [Google Scholar] [CrossRef]
- Bharathi, S.; Ramesh, B.; Kumaran, S.; Radhakrishnan, M.; Saravanan, D.; Pugazhvendan, S.R.; Nalinasundari, M.S. Development of nanobiomaterial for wound healing based on silver nanoparticles loaded on chitosan hydrogel. 3 Biotech 2021, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, S.; Zhao, W.; Zhao, C. A rapid-triggered approach towards antibacterial hydrogel wound dressing with synergic photothermal and sterilization profiles. Biomater. Adv. 2022, 138, 212873. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fan, D.; Li, G.; He, L.; Qin, X.; Zhao, B.; Wang, Q.; Liang, W. Antibacterial, Adhesive, and Conductive Hydrogel for Diabetic Wound Healing. Macromol. Biosci. 2022, 23, 2200349. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, E.; Sadati, S.A.; Yousefiasl, S.; Sartorius, R.; Zafari, M.; Rezakhani, L.; Alizadeh, M.; Zare, E.N.; Omidghaemi, S.; Ghanavatinejad, F.; et al. Cell loaded hydrogel containing Ag-doped bioactive glass–ceramic nanoparticles as skin substitute: Antibacterial properties, immune response, and scarless cutaneous wound regeneration. Bioeng. Transl. Med. 2022, 7, e10386. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Koh, T.W.; Bhatnagar, S. Synthesis, Characterization, Antibacterial and Wound Healing Efficacy of Silver Nanoparticles From Azadirachta indica. Front. Microbiol. 2021, 12, 611560. [Google Scholar] [CrossRef]
- Choudhary, M.; Chhabra, P.; Tyagi, A.; Singh, H. Scar free healing of full thickness diabetic wounds: A unique combination of silver nanoparticles as antimicrobial agent, calcium alginate nanoparticles as hemostatic agent, fresh blood as nutrient/growth factor supplier and chitosan as base matrix. Int. J. Biol. Macromol. 2021, 178, 41–52. [Google Scholar] [CrossRef]
- De Lima, G.G.; De Lima, D.W.F.; De Oliveira, M.J.A.; Lugão, A.B.; Alcântara, M.T.S.; Devine, D.M.; De Sá, M.J.C. Synthesis and in Vivo Behavior of PVP/CMC/Agar Hydrogel Membranes Impregnated with Silver Nanoparticles for Wound Healing Applications. ACS Appl. Bio Mater. 2018, 1, 1842–1852. [Google Scholar] [CrossRef]
- Cao, C.; Yang, N.; Zhao, Y.; Yang, D.; Hu, Y.; Yang, D.; Song, X.; Wang, W.; Dong, X. Biodegradable hydrogel with thermo-response and hemostatic effect for photothermal enhanced anti-infective therapy. Nano Today 2021, 39, 101165. [Google Scholar] [CrossRef]
- Deng, P.; Chen, F.; Zhang, H.; Chen, Y.; Zhou, J. Conductive, Self-Healing, Adhesive, and Antibacterial Hydrogels Based on Lignin/Cellulose for Rapid MRSA-Infected Wound Repairing. ACS Appl. Mater. Interfaces 2021, 13, 52333–52345. [Google Scholar] [CrossRef] [PubMed]
- Diniz, F.R.; Maia, R.C.A.P.; Andrade, L.R.; Andrade, L.N.; Chaud, M.V.; Da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; Da Costa, L.P.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Chandra, A.; Mazumder, A.; Mazumder, R. Green synthesis of silver nanoparticles using Arnebia nobilis root extract and wound healing potential of its hydrogel. Asian J. Pharm. 2014, 8, 95. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Park, C.H.; Kim, C.S. In Situ Synthesis of Antimicrobial Silver Nanoparticles within Antifouling Zwitterionic Hydrogels by Catecholic Redox Chemistry for Wound Healing Application. Biomacromolecules 2016, 17, 1213–1223. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Wu, X.; Ren, Y.; Li, Z.; Ren, J. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Chen, S.; Ge, W.; Zhao, Y.; Xu, X.; Wang, S.; Zhang, J. Riclin-Capped Silver Nanoparticles as an Antibacterial and Anti-Inflammatory Wound Dressing. Int. J. Nanomed. 2022, 17, 2629–2641. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Hong, Y.-L.; Wu, T.-L. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111385. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Yang, H.; Shu, Y.; Li, K.; Chen, K.; Xiao, W.; Liao, X. Preparation and antibacterial properties of an AgBr@SiO2/GelMA composite hydrogel. Biomed. Mater. 2022, 17, 025005. [Google Scholar] [CrossRef]
- Liu, K.; Dai, L.; Li, C. A lignocellulose-based nanocomposite hydrogel with pH-sensitive and potent antibacterial activity for wound healing. Int. J. Biol. Macromol. 2021, 191, 1249–1254. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, J.; Lv, M.; She, K.; Sun, J.; Lu, Q.; Han, C.; Ding, S.; Zhao, S.; Wang, G.; et al. Photocrosslinking silver nanoparticles–aloe vera–silk fibroin composite hydrogel for treatment of full-thickness cutaneous wounds. Regen. Biomater. 2021, 8, rbab048. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, A.K.M.F.; Oliveira, A.C.D.J.; Quelemes, P.V.; Plácido, A.; Da Silva, F.V.; Oliveira, I.S.; De Almeida, M.P.; Amorim, A.D.G.N.; Delerue-Matos, C.; Oliveira, R.D.C.M.D.; et al. In Situ Synthesis of Silver Nanoparticles in a Hydrogel of Carboxymethyl Cellulose with Phthalated-Cashew Gum as a Promising Antibacterial and Healing Agent. Int. J. Mol. Sci. 2017, 18, 2399. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Sun, X.; Lin, X.; Yi, W.; Jiang, J. An injectable metal nanoparticle containing cellulose derivative-based hydrogels: Evaluation of antibacterial and in vitro-vivo wound healing activity in children with burn injuries. Int. Wound J. 2021, 19, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Zhou, J.; Kong, L.; Dai, Y.; Zhang, X.; Song, W.; Zhu, C. An injectable photo-cross-linking silk hydrogel system augments diabetic wound healing in orthopaedic surgery through spatiotemporal immunomodulation. J. Nanobiotechnol. 2022, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Higuera, A.; Rodríguez-Beas, C.; Villalobos-Noriega, J.M.A.; Arizmendi-Grijalva, A.; Ochoa-Sánchez, C.; Larios-Rodríguez, E.; Martínez-Soto, J.M.; Rodríguez-León, E.; Ibarra-Zazueta, C.; Mora-Monroy, R.; et al. Hydrogel with silver nanoparticles synthesized by Mimosa tenuiflora for second-degree burns treatment. Sci. Rep. 2021, 11, 11312. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Mugade, M.; Patole, M.; Pokharkar, V. Bioengineered mannan sulphate capped silver nanoparticles for accelerated and targeted wound healing: Physicochemical and biological investigations. Biomed. Pharmacother. 2017, 91, 95–110. [Google Scholar] [CrossRef]
- Shi, G.; Chen, W.; Zhang, Y.; Dai, X.; Zhang, X.; Wu, Z. An Antifouling Hydrogel Containing Silver Nanoparticles for Modulating the Therapeutic Immune Response in Chronic Wound Healing. Langmuir 2018, 35, 1837–1845. [Google Scholar] [CrossRef]
- Hu, J.; Tao, M.; Sun, F.; Chen, C.; Chen, G.; Wang, G. Multifunctional hydrogel based on dopamine-modified hyaluronic acid, gelatin and silver nanoparticles for promoting abdominal wall defect repair. Int. J. Biol. Macromol. 2022, 222, 55–64. [Google Scholar] [CrossRef]
- Ren, Y.; Ailierken, A.; Zhao, L.; Lin, Z.; Jiang, J.; Li, B.; Wang, J.; Hua, J.; Tu, Q. hUC-MSCs lyophilized powder loaded polysaccharide ulvan driven functional hydrogel for chronic diabetic wound healing. Carbohydr. Polym. 2022, 288, 119404. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Mussel-inspired adhesive bilayer hydrogels for bacteria-infected wound healing via NIR-enhanced nanozyme therapy. Colloids Surfaces B Biointerfaces 2021, 210, 112230. [Google Scholar] [CrossRef]
- Kong, F.; Fan, C.; Yang, Y.; Lee, B.H.; Wei, K. 5-hydroxymethylfurfural-embedded poly (vinyl alcohol)/sodium alginate hybrid hydrogels accelerate wound healing. Int. J. Biol. Macromol. 2019, 138, 933–949. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xia, D.-L.; Chen, Y.-P.; Li, X.-D.; Chen, C.; Wang, Y.-F.; Shen, L.; Hu, Y.-L.; Gu, H.-Y. Evaluation of a two-stage antibacterial hydrogel dressing for healing in an infected diabetic wound: ANTIBACTERIAL HYDROGEL DRESSING FOR DIABETIC WOUND HEALING. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Fan, Z. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, C.; Jiang, Y.; Huang, J.; Liu, Y.; Nthumba, P.M.; Gu, G.; Wu, X.; Zhao, Y.; Ren, J. Engineering an adhesive based on photosensitive polymer hydrogels and silver nanoparticles for wound healing. J. Mater. Chem. B 2020, 8, 5756–5764. [Google Scholar] [CrossRef]
- Deng, M.; Wu, Y.; Ren, Y.; Song, H.; Zheng, L.; Lin, G.; Wen, X.; Tao, Y.; Kong, Q.; Wang, Y. Clickable and smart drug delivery vehicles accelerate the healing of infected diabetic wounds. J. Control. Release 2022, 350, 613–629. [Google Scholar] [CrossRef]
- Xiao, L.; Hui, F.; Tian, T.; Yan, R.; Xin, J.; Zhao, X.; Jiang, Y.; Zhang, Z.; Kuang, Y.; Li, N.; et al. A Novel Conductive Antibacterial Nanocomposite Hydrogel Dressing for Healing of Severely Infected Wounds. Front. Chem. 2021, 9, 787886. [Google Scholar] [CrossRef]
- Du, T.; Xiao, Z.; Cao, J.; Wei, L.; Li, C.; Jiao, J.; Song, Z.; Liu, J.; Du, X.; Wang, S. NIR-activated multi-hit therapeutic Ag2S quantum dot-based hydrogel for healing of bacteria-infected wounds. Acta Biomater. 2022, 145, 88–105. [Google Scholar] [CrossRef]
- Yan, X.; Fang, W.-W.; Xue, J.; Sun, T.-C.; Dong, L.; Zha, Z.; Qian, H.; Song, Y.-H.; Zhang, M.; Gong, X.; et al. Thermoresponsive in Situ Forming Hydrogel with Sol–Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Song, T.; Zhang, B.; Li, D.; Xiao, Y.; Zhang, X. A chitosan-based multifunctional hydrogel containing in situ rapidly bioreduced silver nanoparticles for accelerating infected wound healing. J. Mater. Chem. B 2022, 10, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xu, Y.; Zhou, F.; Hu, Y.; Zhao, J.; Liu, Z.; Zhai, Q.; Qi, S.; Zhang, Z.; Chen, L. Bio-functional hydrogel with antibacterial and anti-inflammatory dual properties to combat with burn wound infection. Bioeng. Transl. Med. 2022, 8, e10373. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Song, Y.; Wang, G.; Murray, R.W. Does Core Size Matter in the Kinetics of Ligand Exchanges of Monolayer-Protected Au Clusters? J. Am. Chem. Soc. 2005, 127, 2752–2757. [Google Scholar] [CrossRef] [PubMed]
- BarathManiKanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.K.; Youn, H.S.; Eom, S.; Gurunathan, S. RAesneatrcih-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Zain, N.M.; Stapley, A.; Shama, G. Green synthesis of silver and copper nanoparticles using ascorbic acid and chitosan for antimicrobial applications. Carbohydr. Polym. 2014, 112, 195–202. [Google Scholar] [CrossRef]
- Muller, A.P.; Ferreira, G.K.; Pires, A.J.; Silveira, G.D.B.; de Souza, D.L.; Brandolfi, J.D.A.; de Souza, C.T.; Paula, M.M.; Silveira, P.C.L. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of Alzheimer’s type. Mater. Sci. Eng. C 2017, 77, 476–483. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Rong, H.; Li, W.; Luo, Y.; Tian, K.; Quan, D.; Wang, Y.; Jiang, L. Effect of composite SiO 2 @AuNPs on wound healing: In vitro and vivo studies. J. Colloid Interface Sci. 2015, 445, 312–319. [Google Scholar] [CrossRef]
- Victor, E.G.; Silveira, P.C.; Possato, J.C.; da Rosa, G.L.; Munari, U.B.; de Souza, C.T.; Pinho, R.A.; da Silva, L.; Streck, E.L.; Paula, M.M. Pulsed ultrasound associated with gold nanoparticle gel reduces oxidative stress parameters and expression of pro-inflammatory molecules in an animal model of muscle injury. J. Nanobiotechnol. 2012, 10, 11. [Google Scholar] [CrossRef]
- Medhe, S.; Bansal, P.; Srivastava, M.M. Enhanced antioxidant activity of gold nanoparticle embedded 3,6-dihydroxyflavone: A combinational study. Appl. Nanosci. 2014, 4, 153–161. [Google Scholar] [CrossRef]
- Leu, J.-G.; Chen, S.-A.; Chen, H.-M.; Wu, W.-M.; Hung, C.-F.; Yao, Y.-D.; Tu, C.-S.; Liang, Y.-J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 767–775. [Google Scholar] [CrossRef]
- Esumi, K.; Houdatsu, H.; Yoshimura, T. Antioxidant Action by Gold−PAMAM Dendrimer Nanocomposites. Langmuir 2004, 20, 2536–2538. [Google Scholar] [CrossRef]
- Rattanata, N.; Daduang, S.; Wongwattanakul, M.; Leelayuwat, C.; Limpaiboon, T.; Lekphrom, R.; Sandee, A.; Boonsiri, P.; Chio-Srichan, S.; Daduang, J. Gold Nanoparticles Enhance the Anticancer Activity of Gallic Acid against Cholangiocarcinoma Cell Lines. Asian Pac. J. Cancer Prev. 2015, 16, 7143–7147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lai, G.; Fu, L.; Zhang, H.; Yu, A. Enzymatically catalytic deposition of gold nanoparticles by glucose oxidase-functionalized gold nanoprobe for ultrasensitive electrochemical immunoassay. Biosens. Bioelectron. 2015, 71, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Haupenthal, D.P.D.S.; Mendes, C.; Silveira, G.D.B.; Zaccaron, R.P.; Corrêa, M.E.A.B.; Nesi, R.T.; Pinho, R.; Paula, M.M.D.S.; Silveira, P.C.L. Effects of treatment with gold nanoparticles in a model of acute pulmonary inflammation induced by lipopolysaccharide. J. Biomed. Mater. Res. Part A 2019, 108, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Tuhin, R.H.; Begum, M.M.; Rahman, S.; Karim, R.; Begum, T.; Ahmed, S.U.; Mostofa, R.; Hossain, A.; Abdel-Daim, M.; Begum, R. Wound healing effect of Euphorbia hirta linn. (Euphorbiaceae) in alloxan induced diabetic rats. BMC Complement. Altern. Med. 2017, 17, 423. [Google Scholar] [CrossRef]
- Batool, Z.; Muhammad, G.; Iqbal, M.M.; Aslam, M.S.; Raza, M.A.; Sajjad, N.; Abdullah, M.; Akhtar, N.; Syed, A.; Elgorban, A.M.; et al. Hydrogel assisted synthesis of gold nanoparticles with enhanced microbicidal and in vivo wound healing potential. Sci. Rep. 2022, 12, 6575. [Google Scholar] [CrossRef]
- Kaul, S.; Sagar, P.; Gupta, R.; Garg, P.; Priyadarshi, N.; Singhal, N.K. Mechanobactericidal, Gold Nanostar Hydrogel-Based Bandage for Bacteria-Infected Skin Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 44084–44097. [Google Scholar] [CrossRef]
- Shang, K.; Tao, L.; Jiang, S.; Yan, J.; Hu, S.; Yang, G.; Ma, C.; Cheng, S.; Wang, X.; Yin, J. Highly flexible hydrogel dressing with efficient antibacterial, antioxidative, and wound healing performances. Biomater. Sci. 2022, 10, 1373–1383. [Google Scholar] [CrossRef]
- Pérez-Rafael, S.; Ivanova, K.; Stefanov, I.; Puiggalí, J.; del Valle, L.J.; Todorova, K.; Dimitrov, P.; Hinojosa-Caballero, D.; Tzanov, T. Nanoparticle-driven self-assembling injectable hydrogels provide a multi-factorial approach for chronic wound treatment. Acta Biomater. 2021, 134, 131–143. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, Y.; Zhou, Y.; Tan, C.; Liu, M. Gold@Halloysite nanotubes-chitin composite hydrogel with antibacterial and hemostatic activity for wound healing. Bioact. Mater. 2023, 20, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Melamed, E.; Kiambi, P.; Okoth, D.; Honigber, I.; Tamir, E.; Borkow, G. Healing of Chronic Wounds by Copper Oxide-Impregnated Wound Dressings—Case Series. Medicina 2021, 57, 296. [Google Scholar] [CrossRef]
- Baek, J.H.; Yoo, M.A.; Koh, J.S.; Borkow, G. Reduction of facial wrinkles depth by sleeping on copper oxide-containing pillowcases: A double blind, placebo controlled, parallel, randomized clinical study: Wrinkles reduction with copper pillowcases. J. Cosmet. Dermatol. 2012, 11, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Dykes, P. Increase in skin surface elasticity in normal volunteer subjects following the use of copper oxide impregnated socks. Ski. Res. Technol. 2014, 21, 272–277. [Google Scholar] [CrossRef]
- Barani, M.; Rahdar, A.; Mukhtar, M.; Razzaq, S.; Qindeel, M.; Olam, S.A.H.; Paiva-Santos, A.C.; Ajalli, N.; Sargazi, S.; Balakrishnan, D.; et al. Recent application of cobalt ferrite nanoparticles as a theranostic agent. Mater. Today Chem. 2022, 26, 101131. [Google Scholar] [CrossRef]
- Manuja, A.; Raguvaran, R.; Kumar, B.; Kalia, A.; Tripathi, B. Accelerated healing of full thickness excised skin wound in rabbits using single application of alginate/acacia based nanocomposites of ZnO nanoparticles. Int. J. Biol. Macromol. 2020, 155, 823–833. [Google Scholar] [CrossRef]
- El-Abeid, S.E.; Ahmed, Y.; Daròs, J.-A.; Mohamed, M.A. Reduced Graphene Oxide Nanosheet-Decorated Copper Oxide Nanoparticles: A Potent Antifungal Nanocomposite against Fusarium Root Rot and Wilt Diseases of Tomato and Pepper Plants. Nanomaterials 2020, 10, 1001. [Google Scholar] [CrossRef]
- Hopkins, R.G.; Failla, M.L. Copper Deficiency Reduces Interleukin-2 (IL-2) Production and IL-2 mRNA in Human T-Lymphocytes. J. Nutr. 1997, 127, 257–262. [Google Scholar] [CrossRef]
- Kornblatt, A.P.; Nicoletti, V.G.; Travaglia, A. The neglected role of copper ions in wound healing. J. Inorg. Biochem. 2016, 161, 1–8. [Google Scholar] [CrossRef]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67, S952–S959. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J.; Dardik, R.; Eidelman, A.I.; Lavie, Y.; Grunfeld, Y.; Ikher, S.; Huszar, M.; Zatcoff, R.C.; Marikovsky, M. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen. 2010, 18, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, D.; Krans, T.; Santo, C.E.; Elowsky, C.G.; Domaille, D.W.; Chang, C.J.; Grass, G. Mechanisms of Contact-Mediated Killing of Yeast Cells on Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 416–426. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjugate Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef]
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2013, 60, 208–337. [Google Scholar] [CrossRef]
- Ungur, G.; Hruza, J. Influence of copper oxide on the formation of polyurethane nanofibers via electrospinning. Fibers Polym. 2015, 16, 621–628. [Google Scholar] [CrossRef]
- Xiao, Y.; Reis, L.A.; Feric, N.; Knee, E.J.; Gu, J.; Cao, S.; Laschinger, C.; Londono, C.; Antolovich, J.; McGuigan, A.P.; et al. Diabetic wound regeneration using peptide-modified hydrogels to target re-epithelialization. Proc. Natl. Acad. Sci. USA 2016, 113, E5792–E5801. [Google Scholar] [CrossRef]
- Marrazzo, P.; O’leary, C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Yang, Y.; Qiao, L.; Hu, J.; Zhang, J.; Guo, B. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater. 2022, 146, 119–130. [Google Scholar] [CrossRef]
- Guo, G.; Sun, J.; Wu, Y.; Wang, J.; Zou, L.Y.; Huang, J.J.; Ren, K.-F.; Liu, C.-M.; Wu, Z.L.; Zheng, Q.; et al. Tough complex hydrogels transformed from highly swollen polyelectrolyte hydrogels based on Cu2+ coordination with anti-bacterial properties. J. Mater. Chem. B 2022, 10, 6414–6424. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian Lemraski, E.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, M.S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In vitro and in vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Li, Z.; Zheng, Y.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Noninvasive rapid bacteria-killing and acceleration of wound healing through photothermal/photodynamic/copper ion synergistic action of a hybrid hydrogel. Biomater. Sci. 2018, 6, 2110–2121. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Chen, J.; Guo, J.; Yuan, M.; Wan, G.; Yan, C.; Li, W.; Machens, H.-G.; Rinkevich, Y.; et al. Calcium ion cross-linked sodium alginate hydrogels containing deferoxamine and copper nanoparticles for diabetic wound healing. Int. J. Biol. Macromol. 2022, 202, 657–670. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef]
- Xu, Q.; Chang, M.; Zhang, Y.; Wang, E.; Xing, M.; Gao, L.; Huan, Z.; Guo, F.; Chang, J. PDA/Cu Bioactive Hydrogel with “Hot Ions Effect” for Inhibition of Drug-Resistant Bacteria and Enhancement of Infectious Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 31255–31269. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xia, A.; Wu, S.; Shen, J. Facile Synthesis of the Cu, N-CDs@GO-CS Hydrogel with Enhanced Antibacterial Activity for Effective Treatment of Wound Infection. Langmuir 2021, 37, 7928–7935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, D.; Ji, N.; Lee, S.; Wang, G.; Zheng, Y.; Zhang, X.; Yang, L.; Qin, Z.; Yang, Y. Bioinspired Design of Sericin/Chitosan/Ag@MOF/GO Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing. Polymers 2021, 13, 2812. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, Z.; Zare, E.N.; Salimi, F.; Goudarzi, I.; Tay, F.R.; Makvandi, P. Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef]
- Jaiswal, M.; Koul, V.; Dinda, A.K. In vitro and in vivo investigational studies of a nanocomposite-hydrogel-based dressing with a silver-coated chitosan wafer for full-thickness skin wounds. J. Appl. Polym. Sci. 2016, 133, 21. [Google Scholar] [CrossRef]
- Yadid, M.; Feiner, R.; Dvir, T. Gold Nanoparticle-Integrated Scaffolds for Tissue Engineering and Regenerative Medicine. Nano Lett. 2019, 19, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Czyżowska, A.; Barbasz, A. A review: Zinc oxide nanoparticles–friends or enemies? Int. J. Environ. Health Res. 2020, 32, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Niranjan, R.; Thangam, R.; Madhan, B.; Pandiyarasan, V.; Ramachandran, C.; Oh, D.-H.; Venkatasubbu, G.D. Investigations on the antimicrobial activity and wound healing potential of ZnO nanoparticles. Appl. Surf. Sci. 2019, 479, 1169–1177. [Google Scholar] [CrossRef]
- Lin, S.; Pei, L.; Zhang, W.; Shu, G.; Lin, J.; Li, H.; Xu, F.; Tang, H.; Peng, G.; Zhao, L.; et al. Chitosan-poloxamer-based thermosensitive hydrogels containing zinc gluconate/recombinant human epidermal growth factor benefit for antibacterial and wound healing. Mater. Sci. Eng. C 2021, 130, 112450. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, Y.; Wang, X.; Peng, J.; Xu, Y.; Chang, J. Multifunctional Hydrogels Prepared by Dual Ion Cross-Linking for Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 16054–16062. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Qi, J.; Hu, L.; Ouyang, D.; Wang, H.; Sun, Q.; Lin, L.; You, L.; Tang, B. A cannabidiol-containing alginate based hydrogel as novel multifunctional wound dressing for promoting wound healing. Biomater. Adv. 2021, 134, 112560. [Google Scholar] [CrossRef]
- Wang, S.; Liu, R.; Bi, S.; Zhao, X.; Zeng, G.; Li, X.; Wang, H.; Gu, J. Mussel-inspired adhesive zwitterionic composite hydrogel with antioxidant and antibacterial properties for wound healing. Colloids Surf. B Biointerfaces 2022, 220, 112914. [Google Scholar] [CrossRef]
- Zheng, L.; Jin, Q. Development of Gelatin Methacryloyl Hydrogel loaded ZnS Nanoparticles Patches for In vivo wound healing care, In vitro drug release and free radical scavenging evaluations. J. Drug Deliv. Sci. Technol. 2022, 71, 103290. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, W.; Xu, P.; Fu, X.; Yu, X.; Chen, L.; Leng, F.; Yu, C.; Yang, Z. Glucose-responsive multifunctional metal–organic drug-loaded hydrogel for diabetic wound healing. Acta Biomater. 2021, 140, 206–218. [Google Scholar] [CrossRef]
- Yao, S.; Zhao, Y.; Xu, Y.; Jin, B.; Wang, M.; Yu, C.; Guo, Z.; Jiang, S.; Tang, R.; Fang, X.; et al. Injectable Dual-Dynamic-Bond Cross-Linked Hydrogel for Highly Efficient Infected Diabetic Wound Healing. Adv. Healthc. Mater. 2022, 11, 2200516. [Google Scholar] [CrossRef]
- Yang, E.; Hou, W.; Liu, K.; Yang, H.; Wei, W.; Kang, H.; Dai, H. A multifunctional chitosan hydrogel dressing for liver hemostasis and infected wound healing. Carbohydr. Polym. 2022, 291, 119631. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Milan, P.B.; Amoupour, M. Enhanced antimicrobial and full-thickness wound healing efficiency of hydrogels loaded with heparinized ZnO nanoparticles: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 166, 200–212. [Google Scholar] [CrossRef]
- Tavakoli, S.; Mokhtari, H.; Kharaziha, M.; Kermanpur, A.; Talebi, A.; Moshtaghian, J. A multifunctional nanocomposite spray dressing of Kappa-carrageenan-polydopamine modified ZnO/L-glutamic acid for diabetic wounds. Mater. Sci. Eng. C 2020, 111, 110837. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Wang, R.; Yuan, P.; Fan, Z.; Yang, S. Preparation and properties of ZnO/sodium alginate bi-layered hydrogel films as novel wound dressings. New J. Chem. 2019, 43, 8684–8693. [Google Scholar] [CrossRef]
- Yin, X.; Huang, S.; Xu, S.; Chang, L.; Zhao, X.; Chen, Z.; Mei, X.; Gao, X. Preparation of pro-angiogenic, antibacterial and EGCG-modified ZnO quantum dots for treating bacterial infected wound of diabetic rats. Biomater. Adv. 2022, 133, 112638. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar] [CrossRef]
- Zhang, F.; Chan, S.-W.; Spanier, J.E.; Apak, E.; Jin, Q.; Robinson, R.D.; Herman, I.P. Cerium oxide nanoparticles: Size-selective formation and structure analysis. Appl. Phys. Lett. 2002, 80, 127–129. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, W.; Li, M.; Li, N.; Li, X.; Qin, X.; Wang, X.; Yu, J.; Li, F.; Huang, L.; et al. Mussel-inspired multifunctional hydrogel dressing with hemostasis, hypoglycemic, photothermal antibacterial properties on diabetic wounds. Biomater. Sci. 2022, 10, 4796–4814. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, W.; Li, M.; Li, N.; Li, X.; Qin, X.; Wang, X.; Yu, J.; Li, F.; Huang, L.; et al. Multifunctional hydrogel platform for biofilm scavenging and O2 generating with photothermal effect on diabetic chronic wound healing. J. Colloid Interface Sci. 2022, 617, 542–556. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, Z.; Lu, J.; Wang, T.; Wang, W.; Fan, L.; Xi, J.; Han, B. Ferrous iron-induced formation of glycyrrhizic acid hydrogels for Staphylococcus aureus-infected wound healing. Colloids Surf. B Biointerfaces 2023, 221, 112977. [Google Scholar] [CrossRef]
- Xi, J.; An, L.; Huang, Y.; Jiang, J.; Wang, Y.; Wei, G.; Xu, Z.; Fan, L.; Gao, L. Ultrasmall FeS2 Nanoparticles-Decorated Carbon Spheres with Laser-Mediated Ferrous Ion Release for Antibacterial Therapy. Small 2021, 17, e2005473. [Google Scholar] [CrossRef]
- Shen, X.; Ma, R.; Huang, Y.; Chen, L.; Xu, Z.; Li, D.; Meng, X.; Fan, K.; Xi, J.; Yan, X.; et al. Nano-decocted ferrous polysulfide coordinates ferroptosis-like death in bacteria for anti-infection therapy. Nano Today 2020, 35, 100981. [Google Scholar] [CrossRef]
- Han, N.; Xu, Z.; Cui, C.; Li, Y.; Zhang, D.; Xiao, M.; Fan, C.; Wu, T.; Yang, J.; Liu, W. A Fe3+-crosslinked pyrogallol-tethered gelatin adhesive hydrogel with antibacterial activity for wound healing. Biomater. Sci. 2020, 8, 3164–3172. [Google Scholar] [CrossRef]
- Li, Y.; Yang, K.; Wang, Z.; Xiao, J.; Tang, Z.; Li, H.; Yi, W.; Li, Z.; Luo, Y.; Li, J.; et al. Rapid In Situ Deposition of Iron-Chelated Polydopamine Coating on the Polyacrylamide Hydrogel Dressings for Combined Photothermal and Chemodynamic Therapy of Skin Wound Infection. ACS Appl. Bio. Mater. 2022, 5, 4541–4553. [Google Scholar] [CrossRef]
- Ma, M.; Zhong, Y.; Jiang, X. An injectable photothermally active antibacterial composite hydroxypropyl chitin hydrogel for promoting the wound healing process through photobiomodulation. J. Mater. Chem. B 2021, 9, 4567–4576. [Google Scholar] [CrossRef]
- Guo, S.; Yao, M.; Zhang, D.; He, Y.; Chang, R.; Ren, Y.; Guan, F. One-Step Synthesis of Multifunctional Chitosan Hydrogel for Full-Thickness Wound Closure and Healing. Adv. Healthc. Mater. 2022, 11, 2101808. [Google Scholar] [CrossRef]
- Qin, J.; Li, M.; Yuan, M.; Shi, X.; Song, J.; He, Y.; Mao, H.; Kong, D.; Gu, Z. Gallium(III)-Mediated Dual-Cross-Linked Alginate Hydrogels with Antibacterial Properties for Promoting Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 22426–22442. [Google Scholar] [CrossRef]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Choi, S.-R.; Switzer, B.; Britigan, B.E.; Narayanasamy, P. Gallium Porphyrin and Gallium Nitrate Synergistically Inhibit Mycobacterial Species by Targeting Different Aspects of Iron/Heme Metabolism. ACS Infect. Dis. 2020, 6, 2582–2591. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, S.; Cai, B.; Wang, Y.; Deng, D.; Wang, X. An injectable and self-healing hydrogel with antibacterial and angiogenic properties for diabetic wound healing. Biomater. Sci. 2022, 10, 3480–3492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Li, Z.; Zhu, S.; Yuan, X.; Cui, Z.; Yang, X.; Chu, P.K.; Wu, S. Nano Ag/ZnO-Incorporated Hydroxyapatite Composite Coatings: Highly Effective Infection Prevention and Excellent Osteointegration. ACS Appl. Mater. Interfaces 2018, 10, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lv, Y.; Yu, C.; Zhang, W.; Song, S.; Li, Y.; Chong, Y.; Huang, J.; Zhang, Z. Au-Pt nanozyme-based multifunctional hydrogel dressing for diabetic wound healing. Biomater. Adv. 2022, 137, 212869. [Google Scholar] [CrossRef]
- Poon, V.K.M.; Burd, A. In vitro cytotoxity of silver: Implication for clinical wound care. Burns 2004, 30, 140–147. [Google Scholar] [CrossRef]
- Innes, M.E.; Umraw, N.; Fish, J.S.; Gomez, M.; Cartotto, R.C. The use of silver coated dressings on donor site wounds: A prospective, controlled matched pair study. Burns 2001, 27, 621–627. [Google Scholar] [CrossRef]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver--a review. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).