Poly(vinyl alcohol)/Pullulan Composite Hydrogels as a Potential Platform for Wound Dressing Applications
Abstract
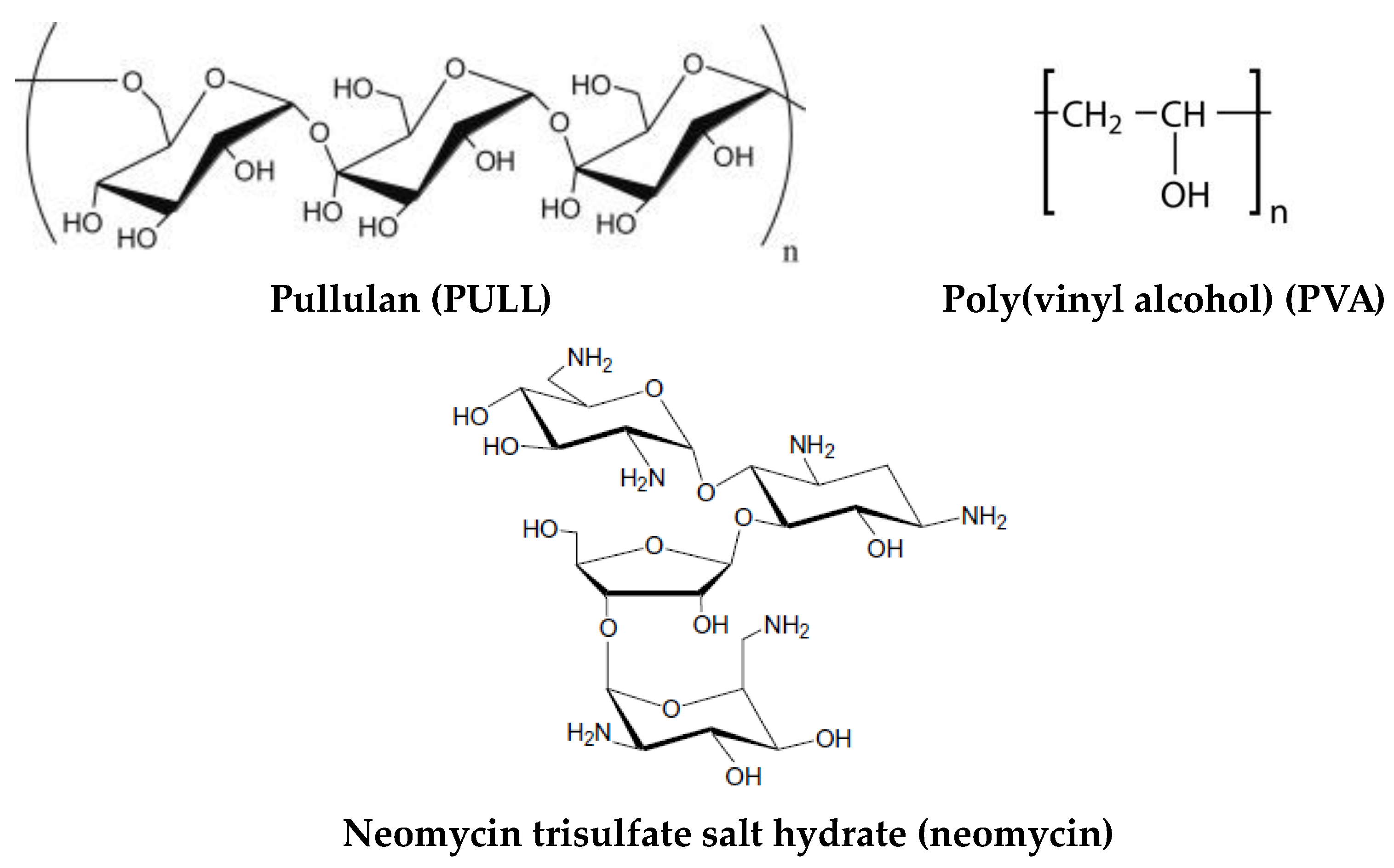
1. Introduction
2. Results and Discussion
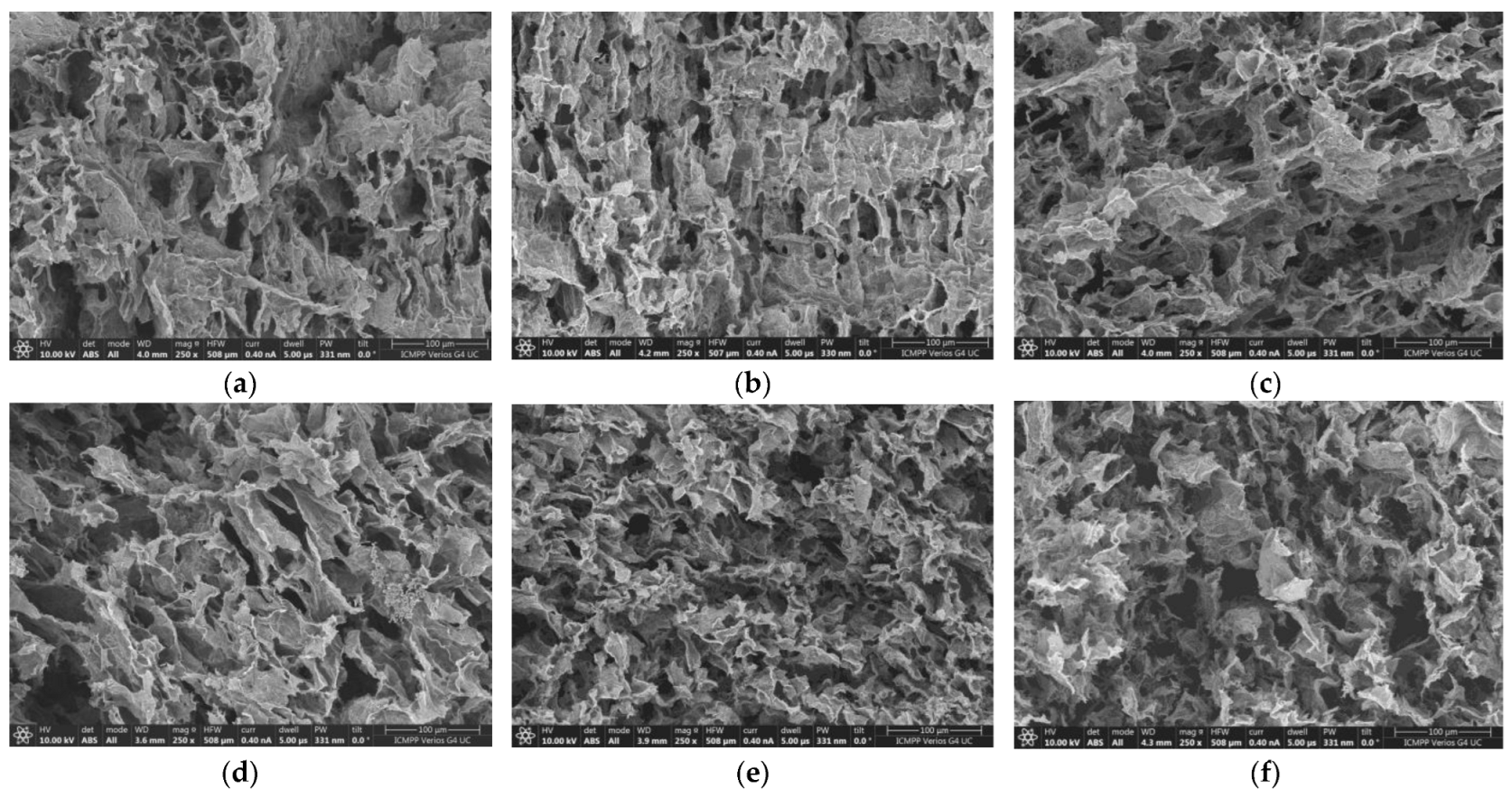
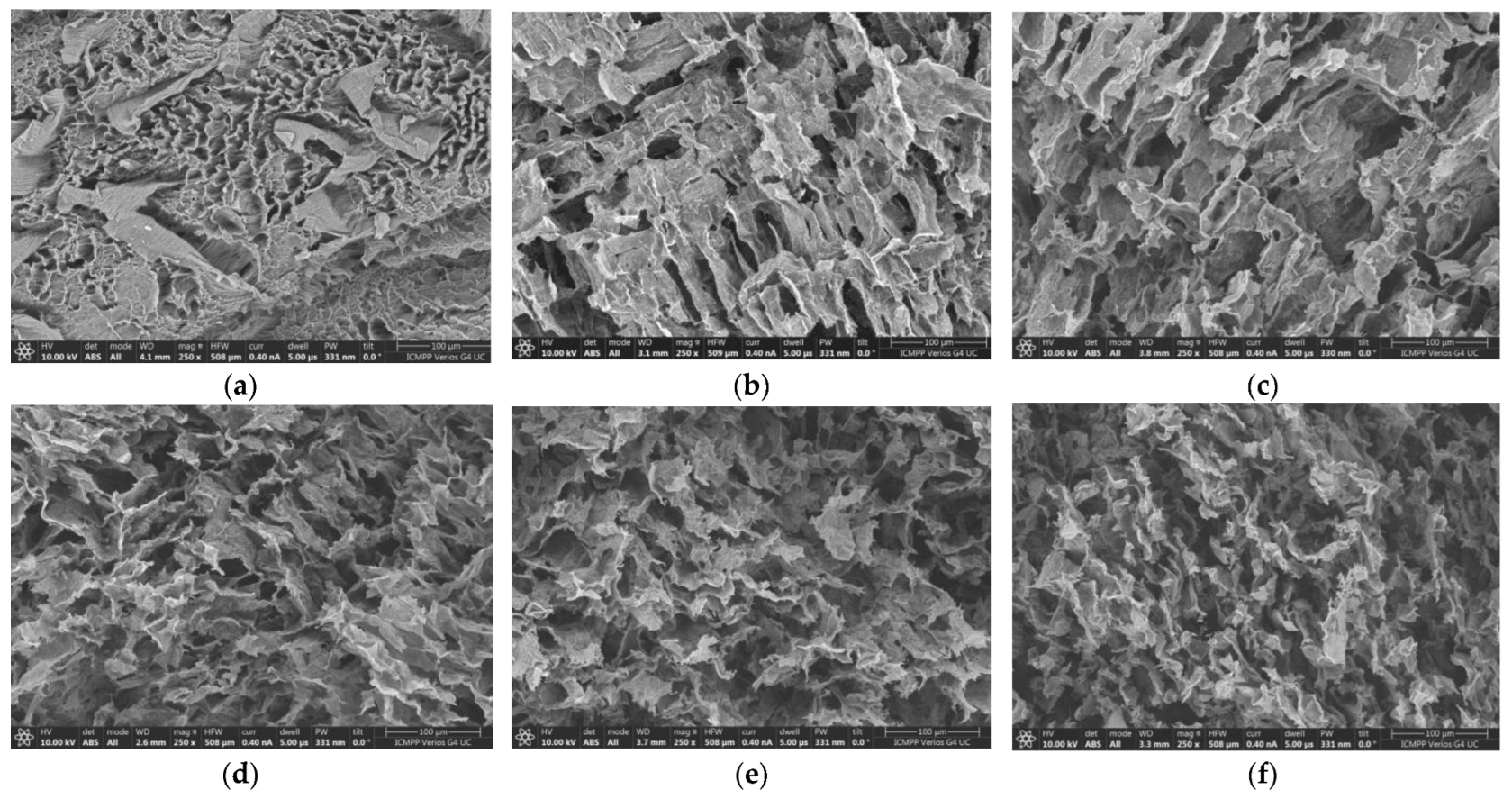
2.1. SEM Analysis
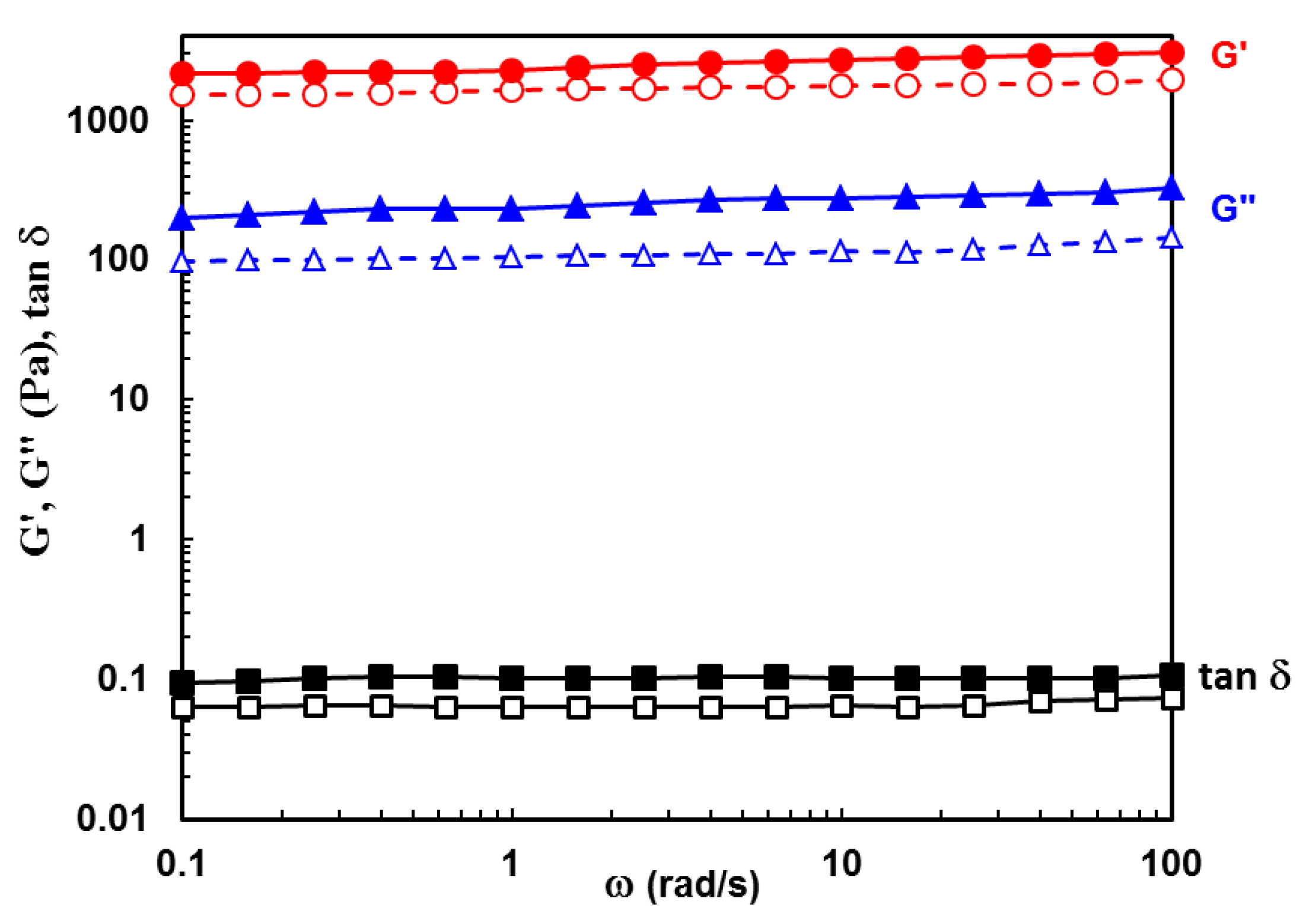
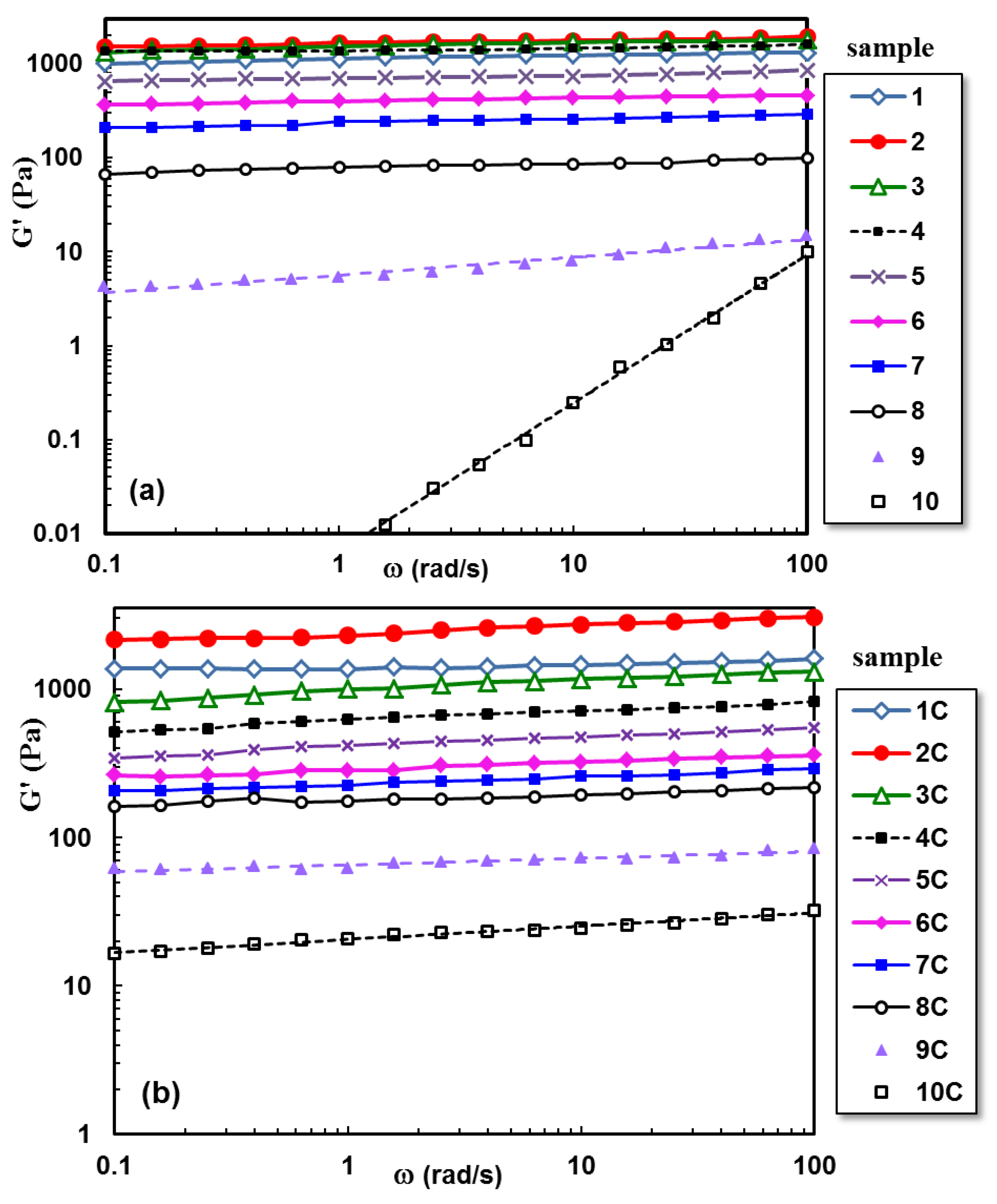
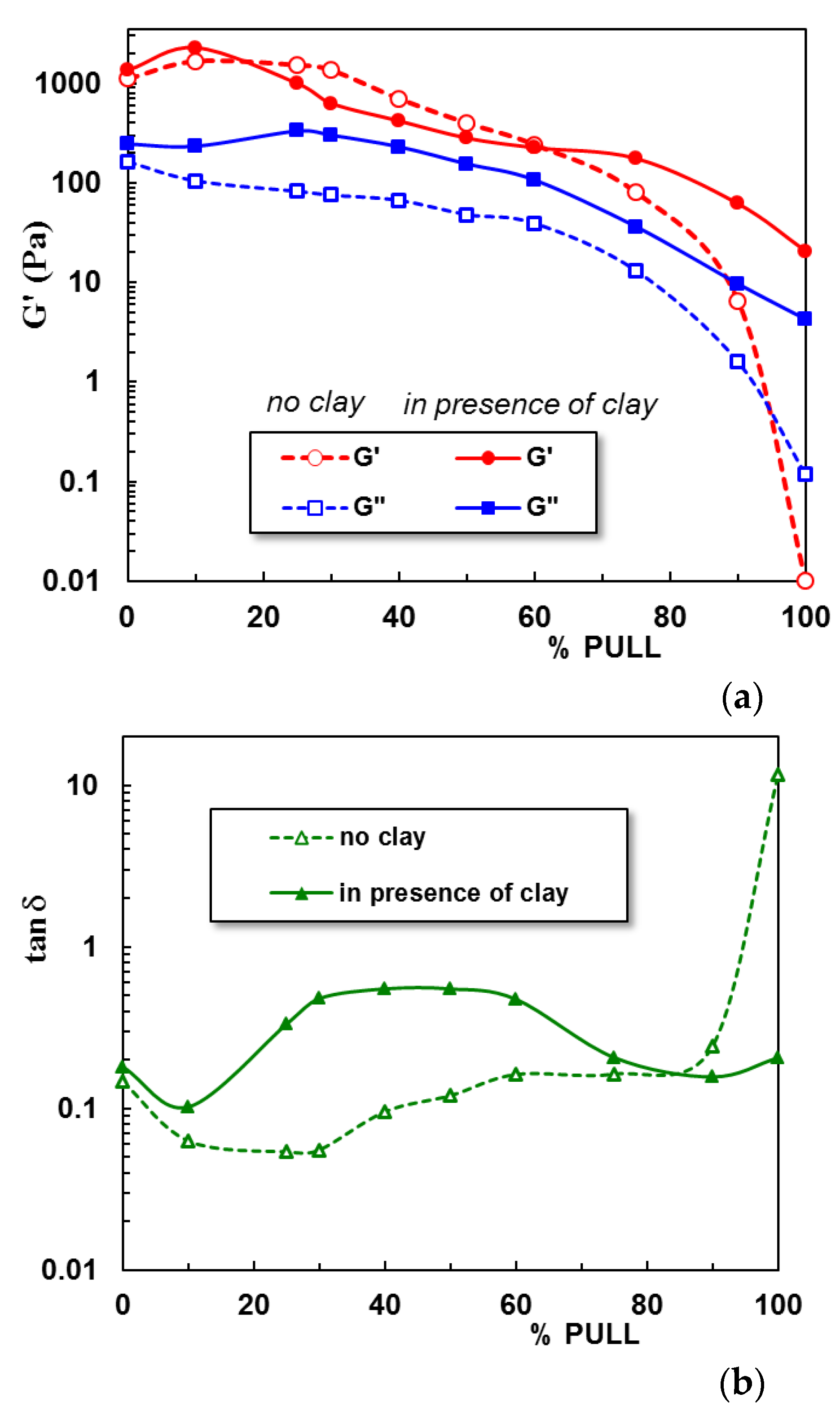
2.2. Viscoelastic Behavior of Hydrogels
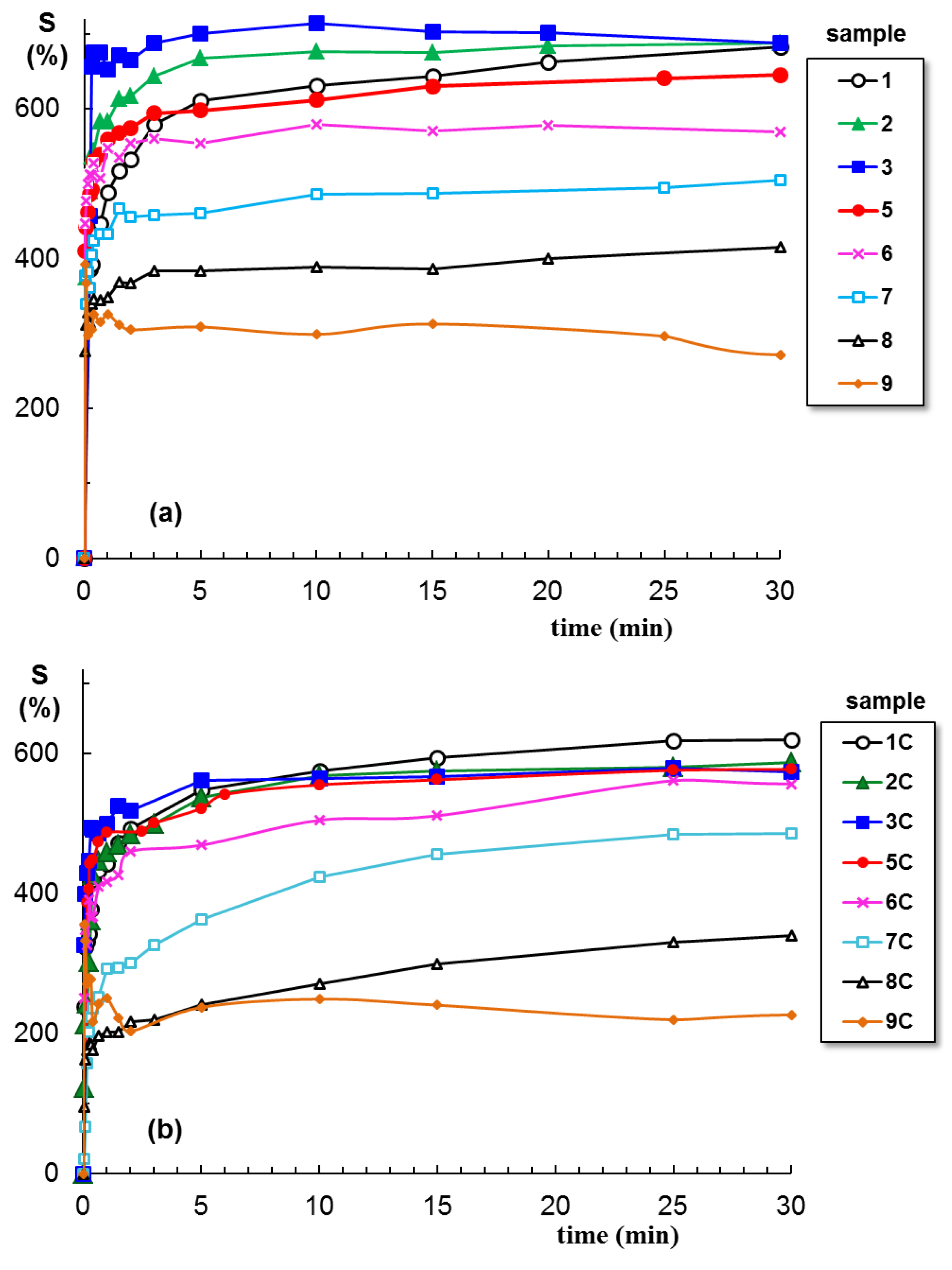
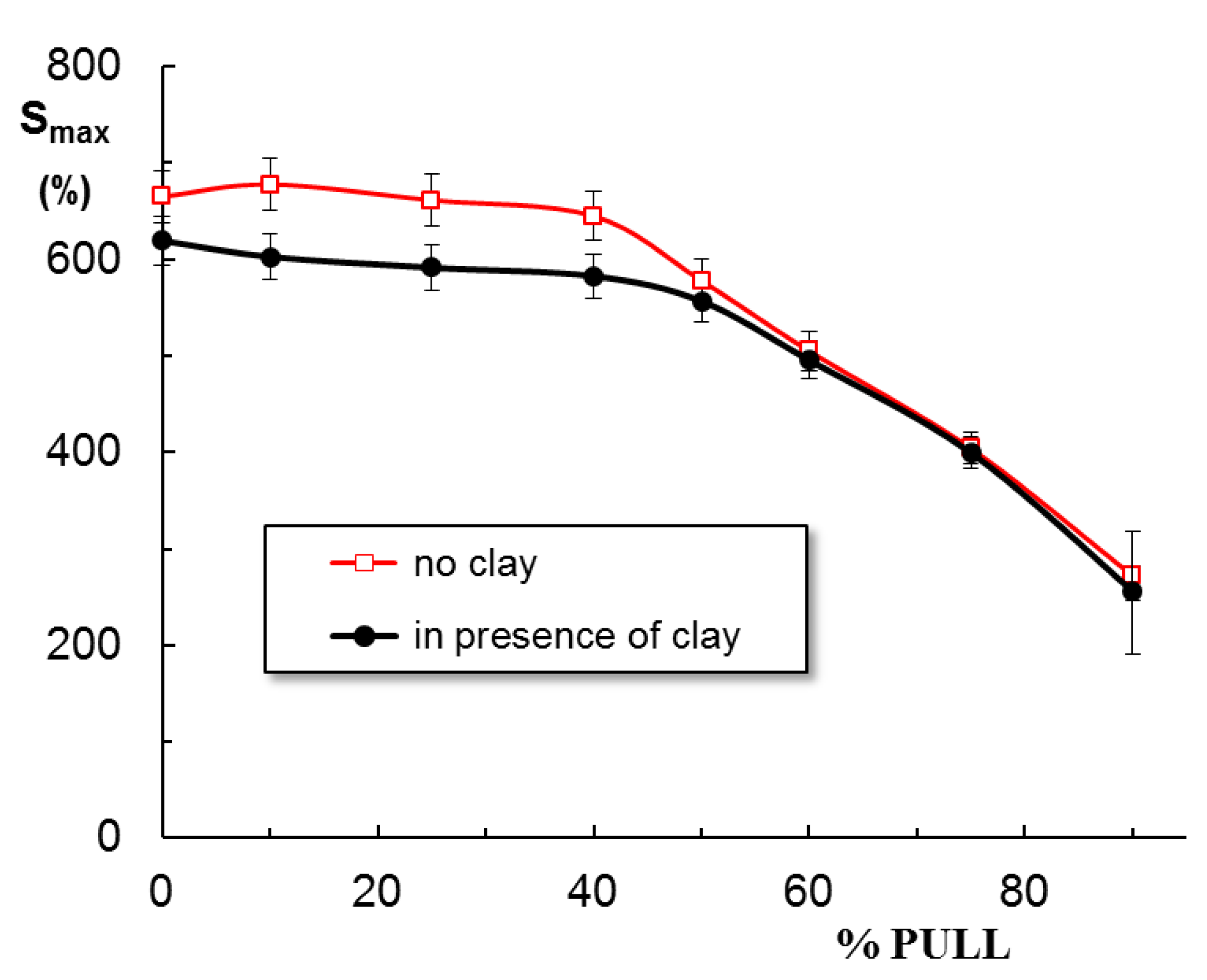
2.3. Swelling Behavior
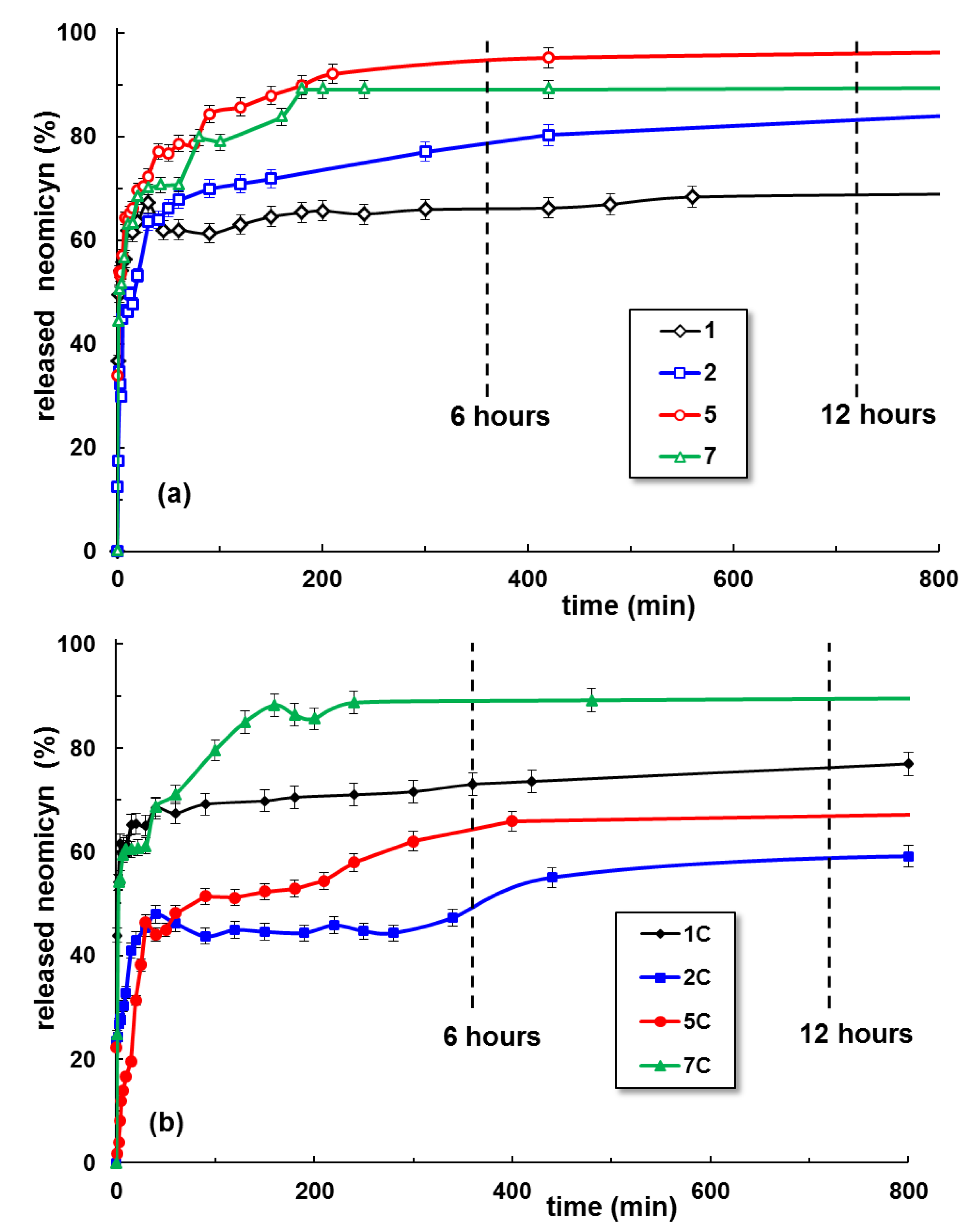
2.4. Neomycin Delivery
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Composite Hydrogels
4.3. Scanning Electron Microscopy Studies
4.4. Rheological Investigations
4.5. Swelling Behavior
4.6. In Vitro Drug Release Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, B.; Li, G.; Cao, E.; Luo, J.; Zhao, X.; Huang, H. Recent progress of antibacterial hydrogels in wound dressings. Mater. Today Bio 2023, 19, 100582. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, Y.; Li, Q.; Yang, K.; Sun, L.; Xu, H.; Wang, R. Intelligent design and medical applications of antimicrobial hydrogels. Colloids Interface Sci. Commun. 2023, 53, 100696. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S. Polysaccharide-based multifunctional hydrogel bio-adhesives for wound healing: A review. Gels 2023, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Deng, X.; Guo, Y.; Xu, P. Engineering functional natural polymer-based nanocomposite hydrogels for wound healing. Nanoscale Adv. 2023, 5, 27–45. [Google Scholar] [CrossRef]
- Bercea, M. Bioinspired Hydrogels as platforms for life-science applications: Challenges and opportunities. Polymers 2022, 14, 2365. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of hydrogels: From synthetic strategies, classification, and properties to biomedical applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Bercea, M.; Plugariu, I.-A.; Gradinaru, L.M.; Avadanei, M.; Doroftei, F.; Gradinaru, V.R. Hybrid hydrogels for neomycin delivery: Synergistic effects of natural/synthetic polymers and proteins. Polymers 2023, 15, 630. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current understanding of hydrogel for drug release and tissue engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Polyakova, V.O.; Krasichkov, A.; Yablonskiy, P.K.; Uspenskaya, M.V. Pullulan-based hydrogels in wound healing and skin tissue engineering applications: A review. Int. J. Mol. Sci. 2023, 24, 4962. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G.; Kontogiorgos, V. Molecular weight effects on solution rheology of pullulan and mechanical properties of its films. Carbohydr. Polym. 2003, 52, 151–166. [Google Scholar] [CrossRef]
- Grigoras, A.G. Drug delivery systems using pullulan, a biocompatible polysaccharide produced by fungal fermentation of starch. Environ. Chem. Lett. 2019, 17, 1209–1223. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Han, Q. Modulation of swelling of PVA hydrogel by polymer and crosslinking agent concentration. Polym. Bull. 2023, 80, 1303–1320. [Google Scholar] [CrossRef]
- Feldman, D. Poly(vinyl alcohol) recent contributions to engineering and medicine. J. Compos. Sci. 2020, 4, 175. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Teodorescu, M. Rheological investigation of poly(vinyl alcohol)/poly(N-vinyl pyrrolidone) mixtures in aqueous solution and hydrogel state. J. Polym. Res. 2016, 23, 142. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Rusu, D. In-situ gelation of aqueous solutions of entangled poly(vinyl alcohol). Soft Matter 2013, 9, 1244–1253. [Google Scholar] [CrossRef]
- Bercea, M. Self-healing behavior of polymer/protein hybrid hydrogels. Polymers 2022, 14, 130. [Google Scholar] [CrossRef]
- Teramoto, N.; Saitoh, M.; Kuroiwa, J.; Shibata, M.; Yosomiya, R. Morphology and mechanical properties of pullulan/poly(vinyl alcohol) blends crosslinked with glyoxal. J. Appl. Polym. Sci. 2001, 82, 2273–2280. [Google Scholar] [CrossRef]
- Samoila, I.; Dinescu, S.; Gradisteanu Pircalabioru, G.; Marutescu, L.; Fundueanu, G.; Aflori, M.; Constantin, M. Pullulan/poly(vinyl alcohol) composite hydrogels for adipose tissue engineering. Materials 2019, 12, 3220. [Google Scholar] [CrossRef]
- Soni, S.R.; Ghosh, A. Exploring pullulan-poly(vinyl alcohol) interpenetrating network microspheres as controlled release drug delivery device. Carbohydr. Polym. 2017, 174, 812–822. [Google Scholar] [CrossRef]
- Islam, M.I.; Rabbani, M.M.; Yang, S.B.; Choi, W.S.; Choi, J.H.; Oh, W.; Shin, J.C.; Lee, J.T.; Yeum, J.H. Poly(vinyl alcohol)/pullulan blend nanofibres prepared from aqueous solutions using electrospinning method. Polym. Polym. Compos. 2014, 22, 779–786. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahaman, M.S.; Yeum, J.H. Electrospun novel super-absorbent based on polysaccharide-polyvinyl alcohol-montmorillonite clay nanocomposites. Carbohydr. Polym. 2015, 115, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Baigorria, E.; Souza dos Santos, S.; de Moura, M.R.; Fraceto, L.F. Nanocomposite hydrogels 3D printed for application in water remediation. Mater. Today Chem. 2023, 30, 101559. [Google Scholar] [CrossRef]
- Ipate, A.M.; Hamciuc, C.; Kalvachev, Y.; Gherman, S.; Ochiuz, L. New cryogels based on polymers and zeolite L for controlled Enalapril maleate release. J. Drug Deliv. Sci. Technol. 2018, 44, 505–512. [Google Scholar] [CrossRef]
- Bercea, M.; Biliuta, G.; Avadanei, M.; Baron, R.I.; Butnaru, M.; Coseri, S. Self-healing hydrogels of oxidized pullulan and poly(vinyl alcohol). Carbohydr. Polym. 2019, 206, 210–219. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Shah, S.A.; Mahmood, A.; Kousar, M.; Jabeen, N. Bioactive and multifunctional keratin-pullulan based hydrogel membranes facilitate re-epithelization in diabetic model. Int. J. Biol. Macromol. 2022, 209, 1826–1836. [Google Scholar] [CrossRef]
- Shitole, A.A.; Raut, P.W.; Khandwekar, A.; Sharma, N.; Baruah, M. Design and engineering of polyvinyl alcohol based biomimetic hydrogels for wound healing and repair. J. Polym. Res. 2019, 26, 201. [Google Scholar] [CrossRef]
- Tabasum, S.; Noreen, A.; Maqsood, M.F.; Umar, H.; Akrama, N.; Nazli, Z.H.; Chatha, S.A.S.; Khalid Mahmood Zia, K.M. A review on versatile applications of blends and composites of pullulan with natural and synthetic polymers. Int. J. Biol. Macromol. 2018, 120, 603–632. [Google Scholar] [CrossRef]
- Villalba-Rodríguez, A.M.; Martínez-González, S.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Nanoclay/polymer-based hydrogels and enzyme-loaded nanostructures for wound healing applications. Gels 2021, 7, 59. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Niem, J.; Wang, J. Carboxyl-modified poly(vinyl alcohol)-cross-linked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural polymer-based antimicrobial hydrogels without synthetic antibiotics as wound dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, C.; Wang, T.; Xu, J. Advances in functional hydrogel wound dressings: A review. Polymers 2023, 15, 2000. [Google Scholar] [CrossRef] [PubMed]
- Psarrou, M.; Mitraki, A.; Vamvakaki, M.; Kokotidou, C. Stimuli-responsive polysaccharide hydrogels and their composites for wound healing applications. Polymers 2023, 15, 986. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Y.; Liu, Q.; Liu, M.; Yang, F.; Wang, C.; Pan, P.; Wang, L.; Chen, L.; Chen, J. Real-time monitoring flexible hydrogels based on dual physically cross-linked network for promoting wound healing. Chin. Chem. Lett. 2023, 108262. [Google Scholar] [CrossRef]
- Gowda, B.H.J.; Mohanto, S.; Singh, A.; Bhunia, A.; Abdelgawad, M.A.; Ghosh, S.; Ansari, M.J.; Pramanik, S. Nanoparticle-based therapeutic approaches for wound healing: A review of the state-of-the-art. Mater. Today Chem. 2023, 27, 101319. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Ciardelli, G.; Chiono, V. Wound dressing products: A translational investigation from the bench to the market. Eng. Regen. 2022, 3, 182–200. [Google Scholar] [CrossRef]
- Morariu, S.; Bercea, M.; Gradinaru, L.M.; Rosca, I.; Avadanei, M. Versatile poly(vinyl alcohol)/clay physical hydrogels with tailorable structure as potential candidates for wound healing application. Mater. Sci. Eng. C 2020, 109, 110395. [Google Scholar] [CrossRef]
- Boulet, C.C.S.; Brown, A.; Formstone, C.; Aarts, D.G.A.L. Mixture design applied to the rheology of clay gel mixtures. Rheol. Acta 2022, 61, 811–825. [Google Scholar] [CrossRef]
- Blanco-López, M.; González-Garcinuño, Á.; Tabernero, A.; Martín Del Valle, E.M. Steady and oscillatory shear flow behavior of different polysaccharides with Laponite®. Polymers 2021, 13, 966. [Google Scholar] [CrossRef]
- Lapasin, R.; Abrami, M.; Grassi, M.; Šebenik, U. Rheology of Laponite-scleroglucan hydrogels. Carbohydr. Polym. 2017, 168, 290–300. [Google Scholar] [CrossRef]
- Šebenik, U.; Lapasin, R.; Krajnc, M. Rheology of aqueous dispersions of Laponite and TEMPO-oxidized nanofibrillated cellulose. Carbohydr. Polym. 2020, 240, 116330. [Google Scholar] [CrossRef]
- Sheikhi, A.; Afewerki, S.; Oklu, R.; Gaharwar, A.K.; Khademhosseini, A. Effect of ionic strength on shear-thinning nanoclay-polymer composite hydrogels. Biomater. Sci. 2018, 6, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Yeum, J.H. Electrospun pullulan/poly(vinyl alcohol)/silver hybrid nanofibers: Preparation and property characterization for antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 286. [Google Scholar] [CrossRef]
- Zafalon, A.T.; Dos Santos, V.J.; Lugão, A.B.; Rangari, V.; Temesgen, S.; Parra, D.F. Stability of the neomycin antibiotic in irradiated polymeric biomaterials. Eur. J. Biomed. Pharm. Sci. 2018, 5, 49–57. [Google Scholar]
- Wan, Y.C.; Liu, Y.; Liu, C.; Ma, H.; Yu, H.; Kang, J.; Gao, C.; Wu, Z.; Zheng, D.; Lu, B. Rapid determination of neomycin in biological samples using fluorescent sensor based on quantum dots with doubly selective binding sites. J. Pharm. Biomed. Anal. 2018, 154, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gumy, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Stypulkowska, K.; Blazewicz, A.; Fijalek, K.; Warowna-Grzeskiewicz, M.; Srebrzynska, K. Determination of neomycin and related substances in pharmaceutical preparations by reversed-phase high performance liquid chromatography with mass spectrometry and charged aerosol detection. J. Pharm. Biomed. Anal. 2013, 76, 207–214. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable device. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Brazel, C.S.; Huang, X. The cost of optimal drug delivery: Reducing and preventing the burst effect in matrix systems. ACS Symp. Ser. 2004, 879, 267–282. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. Analysis of burst release of proxyphylline from poly(vinyl alcohol) hydrogels. Chem. Eng. Commun. 2003, 190, 519–532. [Google Scholar] [CrossRef]
- Gopinathan, N.; Yang, B.; Lowe, J.P.; Edler, K.J.; Rigby, S.P. NMR cryoporometry characterisation studies of the relation between drug release profile and pore structural evolution of polymeric nanoparticles. Int. J. Pharm. 2014, 469, 146–158. [Google Scholar] [CrossRef]
- Bercea, M.; Wolf, B.A. Intrinsic viscosities of polymer blends: Sensitive probes of specific interactions between the counterions of polyelectrolytes and uncharged macromolecules. Macromolecules 2018, 51, 7483–7490. [Google Scholar] [CrossRef]
- Napoli, S.; Carbone, G.M.; Catapano, C.V.; Shaw, N.; Arya, D.P. Neomycin improves cationic lipid-mediated transfection of DNA in human cells. Bioorg. Med. Chem. Lett. 2005, 15, 3467–3469. [Google Scholar] [CrossRef]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biophacodyn. 1978, 6, 165–175. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Ambalangodage, C.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
| Sample Code | PVA (% wt) | PULL (% wt.) | Clay (% wt.) | tan δ | |
|---|---|---|---|---|---|
| 1 | 100 | 0 | 0 | 0.1465 | 0.1002 |
| 2 | 90 | 10 | 0 | 0.0629 | 0.1137 |
| 3 | 75 | 25 | 0 | 0.0539 | 0.0822 |
| 4 | 70 | 30 | 0 | 0.0555 | 0.0937 |
| 5 | 60 | 40 | 0 | 0.0956 | 0.0811 |
| 6 | 50 | 50 | 0 | 0.1203 | 0.0521 |
| 7 | 40 | 60 | 0 | 0.1624 | 0.0882 |
| 8 | 25 | 75 | 0 | 0.1634 | 0.0349 |
| 9 | 10 | 90 | 0 | 0.2435 | 0.0258 |
| 10 | 0 | 100 | 0 | 11.7000 | - |
| 1C | 100 | 0 | 0.5 | 0.1809 | 0.218 |
| 2C | 90 | 10 | 0.5 | 0.1022 | 0.1894 |
| 3C | 75 | 25 | 0.5 | 0.3330 | 0.1454 |
| 4C | 70 | 30 | 0.5 | 0.4794 | 0.1297 |
| 5C | 60 | 40 | 0.5 | 0.5500 | 0.1012 |
| 6C | 50 | 50 | 0.5 | 0.5495 | 0.1210 |
| 7C | 40 | 60 | 0.5 | 0.4735 | 0.1993 |
| 8C | 25 | 75 | 0.5 | 0.2063 | 0.4367 |
| 9C | 10 | 90 | 0.5 | 0.1568 | 0.4129 |
| 10C | 0 | 100 | 0.5 | 0.2063 | - |
| Sample Code | Korsmeyer–Peppas Equation (2) | Peppas–Sahlin Equation (3) | ||||
|---|---|---|---|---|---|---|
| n | AIC | k1 (min−m) | k2 (min−2m) | m | AIC | |
| 1 | 0.1339 | −79.2907 | −0.1481 | 0.3787 | 0.1602 | −77.3351 |
| 2 | 0.3161 | −32.5148 | −3.2568 | 1.9673 | 0.2034 | −68.2583 |
| 5 | 0.1661 | −50.1921 | −3.7674 | 2.3748 | 0.1216 | −62.5688 |
| 7 | 0.2021 | −69.0031 | −3.3078 | 2.0284 | 0.1805 | −62.8957 |
| 1C | 0.0873 | −82.4993 | −0.0585 | 0.1397 | 0.1712 | −71.4268 |
| 2C | 0.1335 | −73.3387 | −4.9473 | 3.1669 | 0.1069 | −67.7979 |
| 5C | 0.2089 | −60.4088 | −2.7178 | 1.6331 | 0.2459 | −56.6507 |
| 7C | 0.1788 | −70.5489 | −2.6381 | 0.5584 | 0.2036 | −57.4339 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plugariu, I.-A.; Bercea, M.; Gradinaru, L.M.; Rusu, D.; Lupu, A. Poly(vinyl alcohol)/Pullulan Composite Hydrogels as a Potential Platform for Wound Dressing Applications. Gels 2023, 9, 580. https://doi.org/10.3390/gels9070580
Plugariu I-A, Bercea M, Gradinaru LM, Rusu D, Lupu A. Poly(vinyl alcohol)/Pullulan Composite Hydrogels as a Potential Platform for Wound Dressing Applications. Gels. 2023; 9(7):580. https://doi.org/10.3390/gels9070580
Chicago/Turabian StylePlugariu, Ioana-Alexandra, Maria Bercea, Luiza Madalina Gradinaru, Daniela Rusu, and Alexandra Lupu. 2023. "Poly(vinyl alcohol)/Pullulan Composite Hydrogels as a Potential Platform for Wound Dressing Applications" Gels 9, no. 7: 580. https://doi.org/10.3390/gels9070580
APA StylePlugariu, I.-A., Bercea, M., Gradinaru, L. M., Rusu, D., & Lupu, A. (2023). Poly(vinyl alcohol)/Pullulan Composite Hydrogels as a Potential Platform for Wound Dressing Applications. Gels, 9(7), 580. https://doi.org/10.3390/gels9070580









