The Effect of Temperature on the Mechanical Properties of Alginate Gels in Water/Alcohol Solutions
Abstract
1. Introduction
2. Results and Discussion
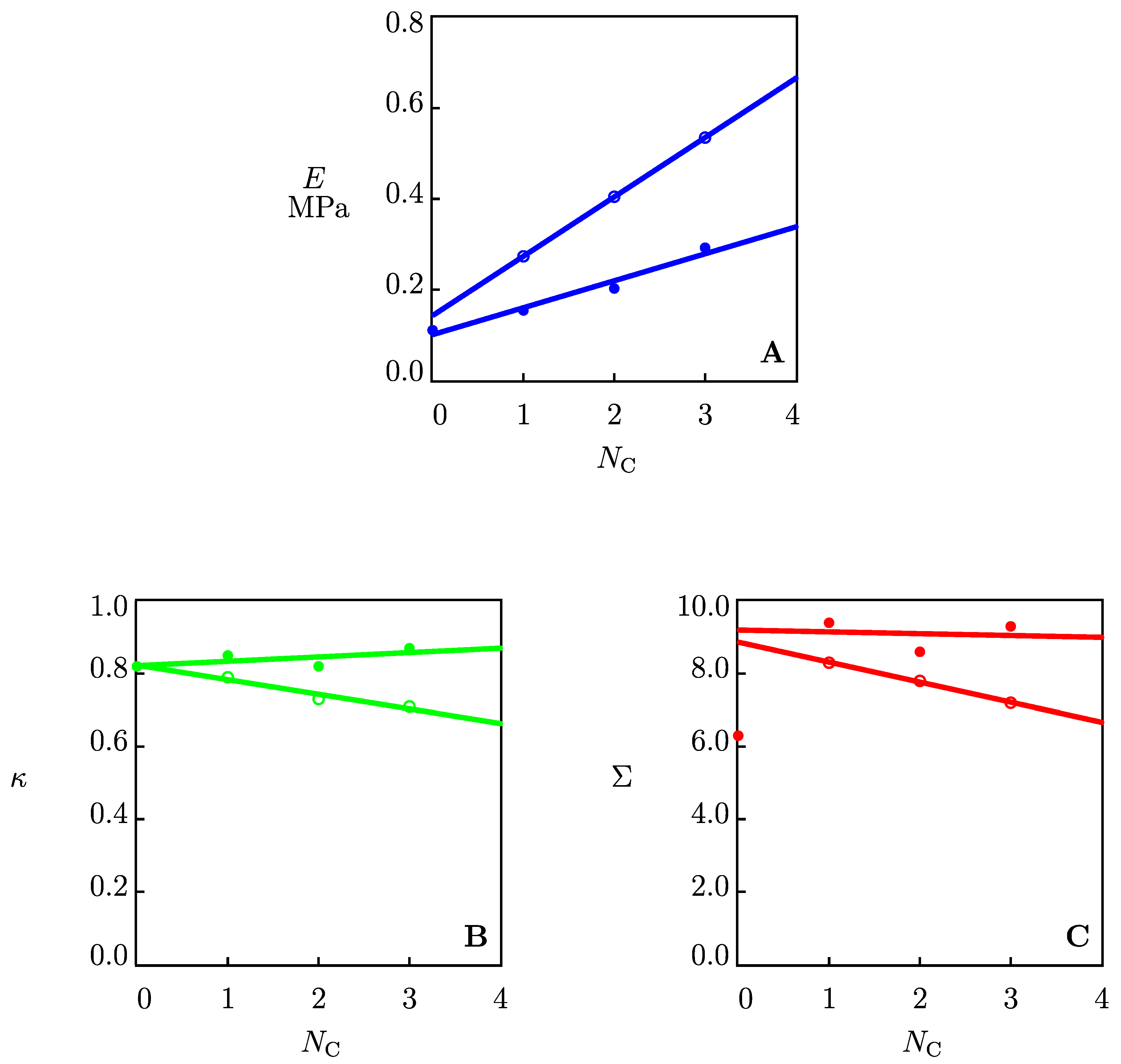
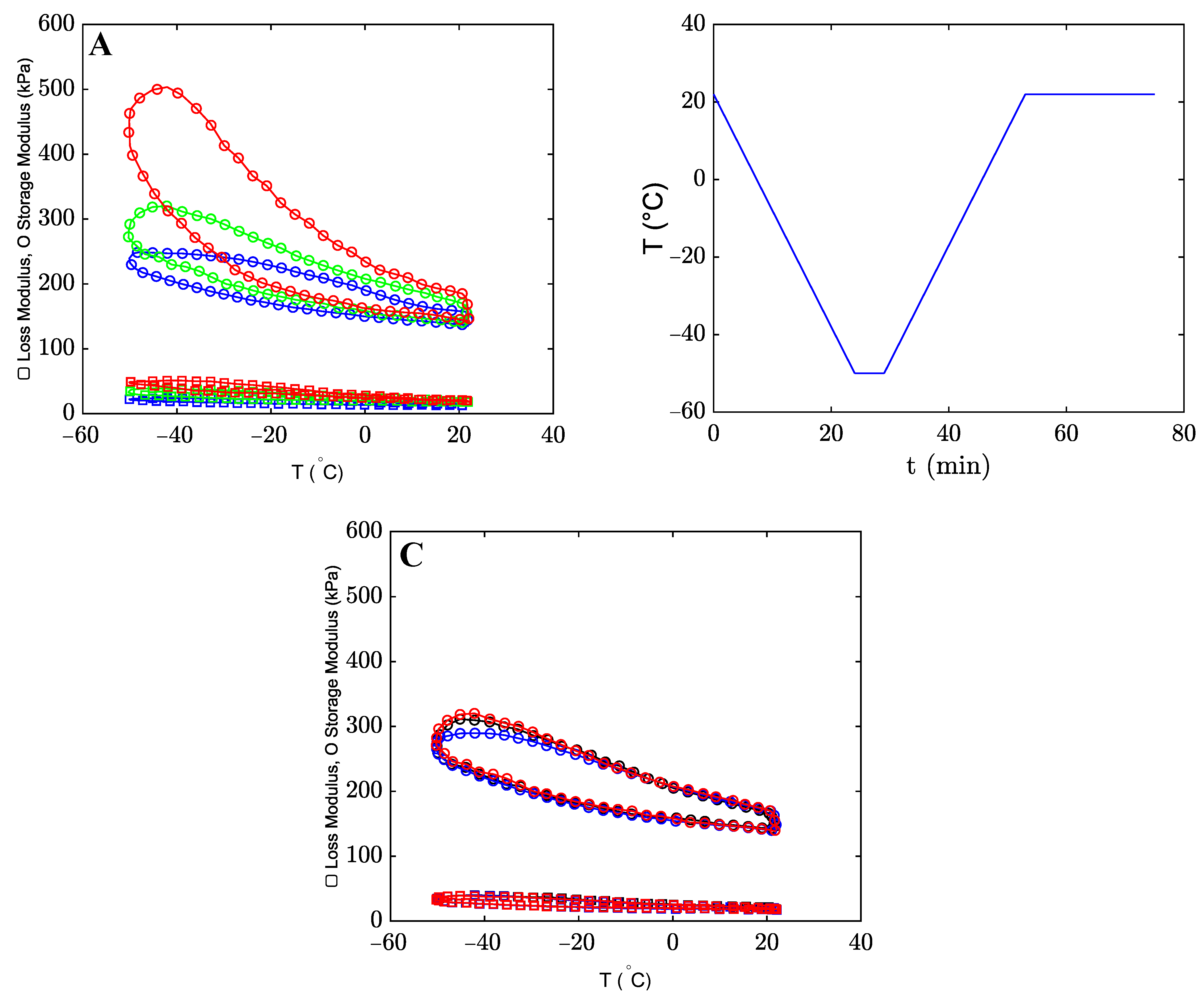
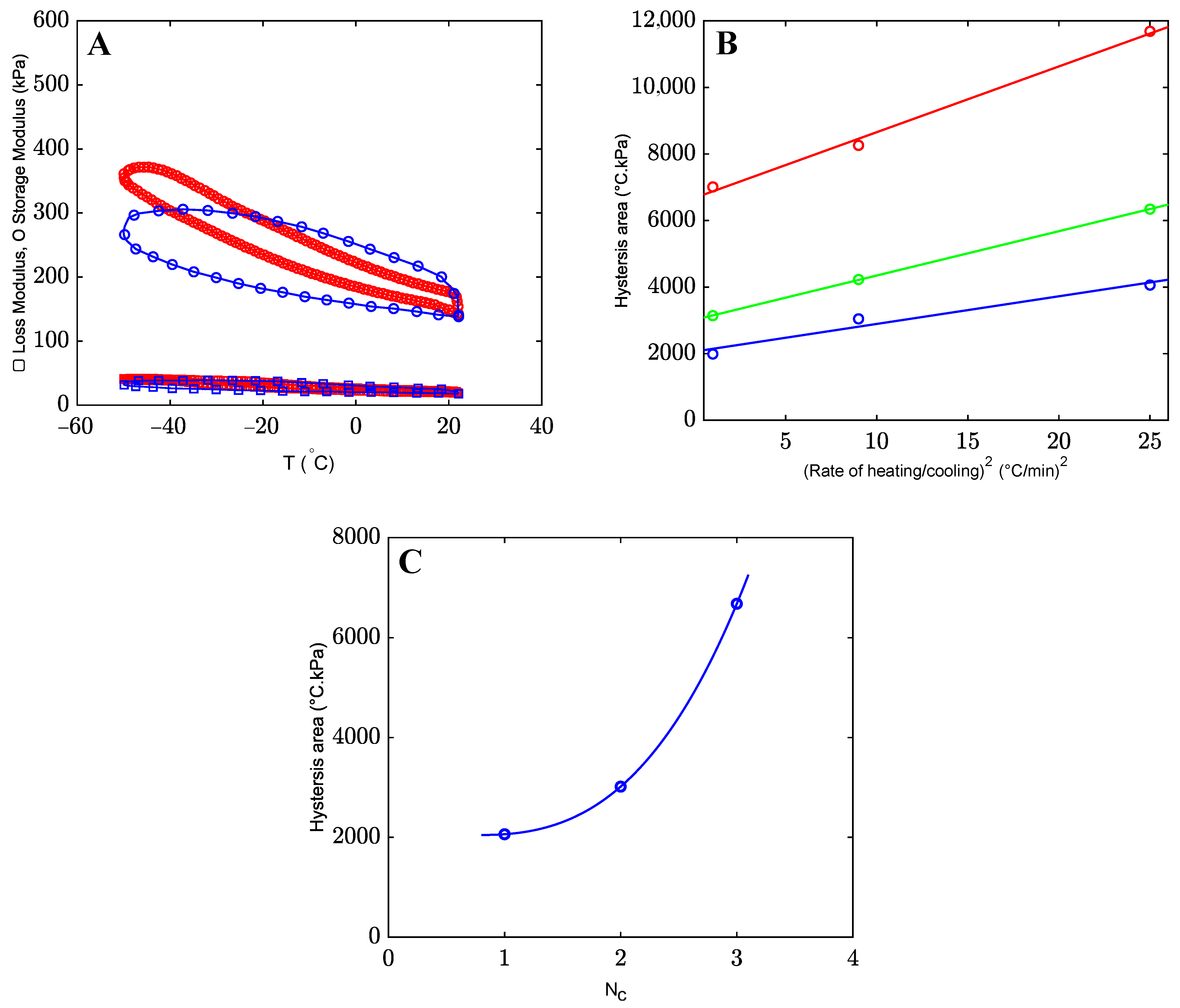
2.1. Results
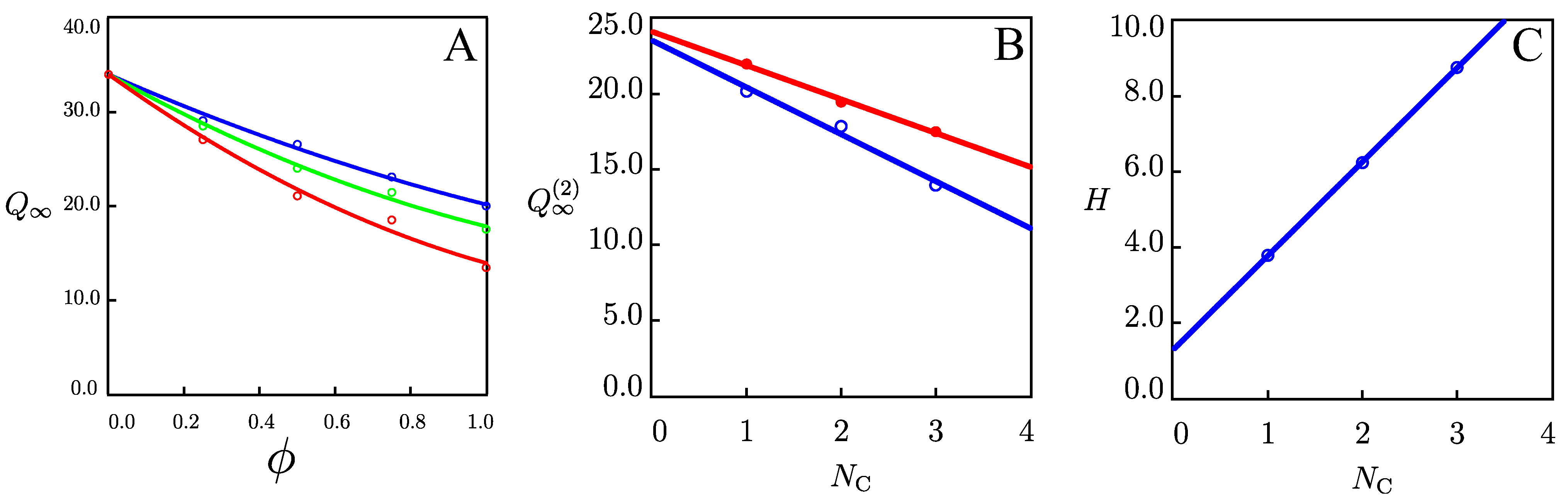
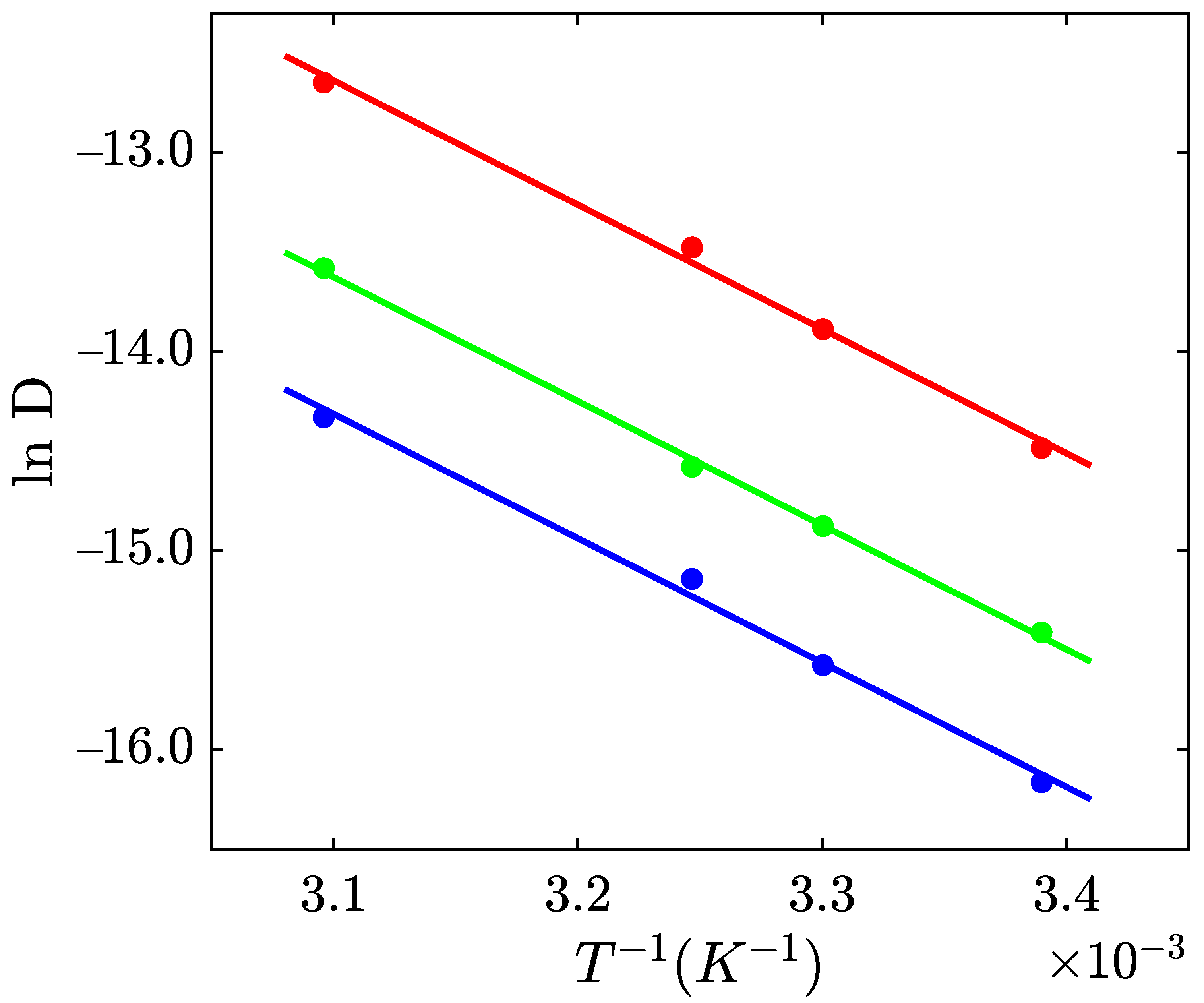
2.1.1. Swelling of Alginate Hydrogels in Solutions with Various Volume Fraction of Alcohols
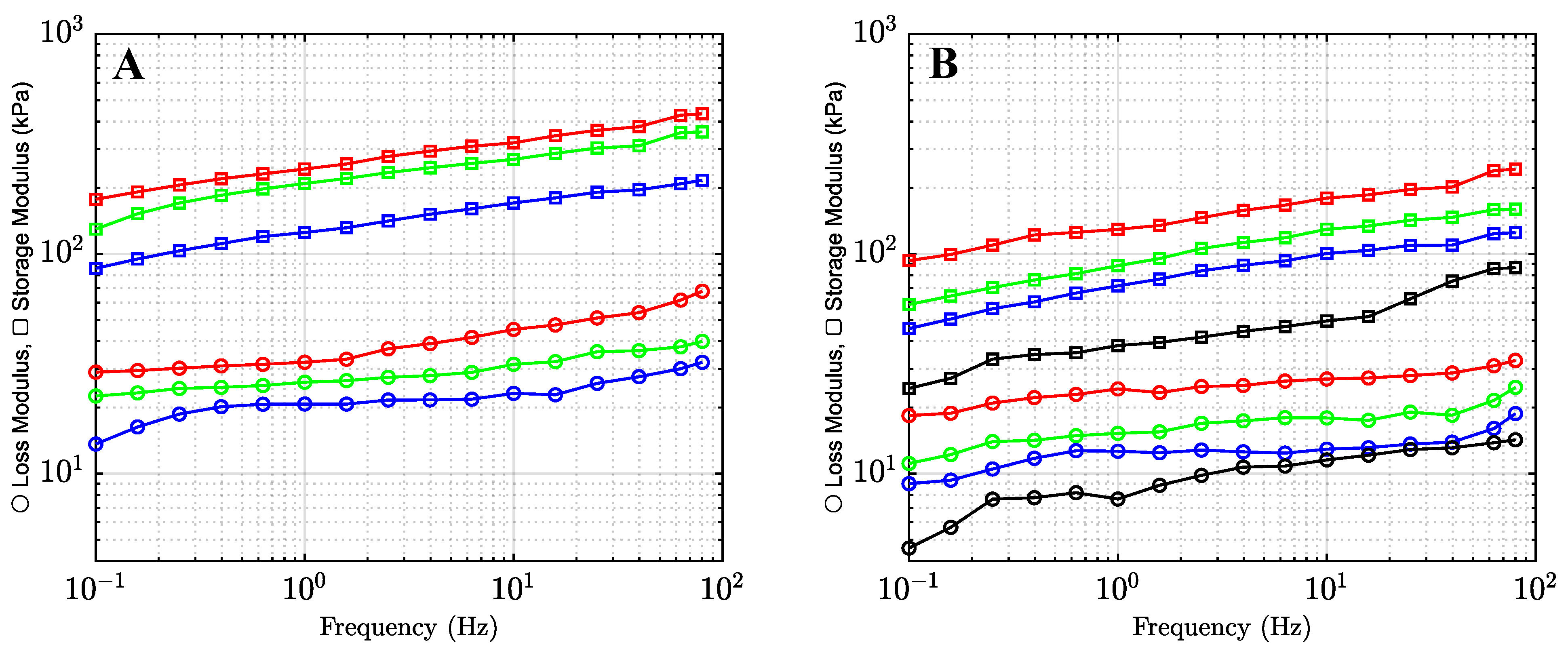
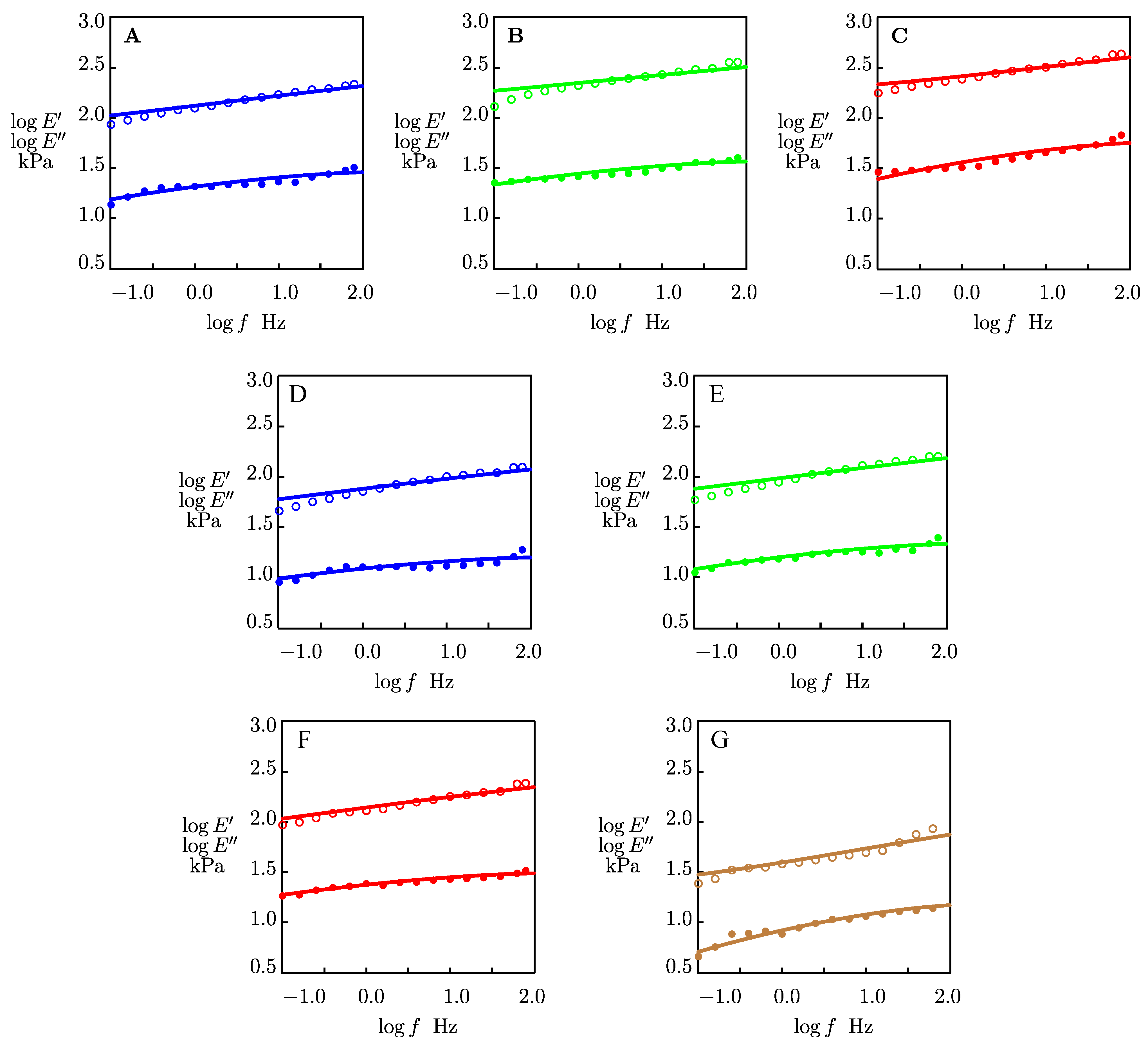
2.1.2. The Effects of Co-Solvents on Mechanical Properties of Organohydrogels
2.2. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Organohydrogels
4.3. Swelling Tests
4.4. Mechanical Tests
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Manthiram, A. An Outlook on Lithium Ion Battery Technology. ACS Central Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Cui, W.; Liu, X.; Ding, Y.; Wang, Y. In situ preparation of gel polymer electrolyte for lithium batteries: Progress and perspectives. Infomat 2022, 4, e12232. [Google Scholar] [CrossRef]
- Huang, S.; Hou, L.; Li, T.; Jiao, Y.; Wu, P. Antifreezing Hydrogel Electrolyte with Ternary Hydrogen Bonding for High-Performance Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2110140. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, W.; Wang, A.; Huang, A.; Chen, J.; Xu, J.; Wong, C.-P. Anti-freezing flexible aqueous Zn–MnO2 batteries working at− 35 C enabled by a borax-crosslinked polyvinyl alcohol/glycerol gel electrolyte. J. Mater. Chem. A 2020, 8, 6828–6841. [Google Scholar] [CrossRef]
- Bohnke, O.; Rousselot, C.; Gillet, P.A.; Truche, C. Gel Electrolyte for Solid-State Electrochromic Cell. J. Electrochem. Soc. 1992, 139, 1862–1865. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Yang, X.; Lu, J.; Zhang, X.; Yuan, D.; Zhang, Y. Water in Gel Electrolyte for Zinc Ion Battery. Chem. Asian J. 2023, 18, e202201280. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Z.; Sun, C.; Li, F.; Chen, K.; Zhang, Z.; Hou, G.; Cheng, H.M.; Li, F. Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature. Adv. Funct. Mater. 2020, 30, 2007172. [Google Scholar] [CrossRef]
- Ling, W.; Mo, F.; Wang, J.; Liu, Q.; Liu, Y.; Yang, Q.; Qiu, Y.; Huang, Y. Self-healable hydrogel electrolyte for dendrite-free and self-healable zinc-based aqueous batteries. Mater. Today Phys. 2021, 20, 100458. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, J.; Wang, Y. Quasi-Solid-State Zinc Ion Rechargeable Batteries for Subzero Temperature Applications. ACS Appl. Energy Mater. 2020, 3, 9058–9065. [Google Scholar] [CrossRef]
- Huang, X.; Wang, D.; Yuan, Z.; Xie, W.; Wu, Y.; Li, R.; Zhao, Y.; Luo, D.; Cen, L.; Chen, B.; et al. A Fully Biodegradable Battery for Self-Powered Transient Implants. Small 2018, 14, e1800994. [Google Scholar] [CrossRef]
- Xu, T.; Liu, K.; Sheng, N.; Zhang, M.; Liu, W.; Liu, H.; Dai, L.; Zhang, X.; Si, C.; Du, H.; et al. Biopolymer-based hydrogel electrolytes for advanced energy storage/conversion devices: Properties, applications, and perspectives. Energy Storage Mater. 2022, 48, 244–262. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Cheng, L.; Liu, Q.; Wei, J.; Huang, Y. Anti-Freezing Strategies of Electrolyte and their Application in Electro-chemical Energy Devices. Chem. Rec. 2022, 22, e202200068. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Kubo, A. Structural functions of antimicrobial long-chain alcohols and phenols. Bioorganic Med. Chem. 1995, 3, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; He, J.; Cao, Y.; Wang, J.; Qiao, Z.; Jiang, X.; Hou, L.; Zhang, J. Tissue-adhesive and highly mechanical double-network hydrogel for cryopreservation and sustained release of anti-cancer drugs. Smart Mater. Med. 2021, 2, 229–236. [Google Scholar] [CrossRef]
- Chen, J.; Shi, D.; Yang, Z.; Dong, K.; Kaneko, D.; Chen, M. Hand-extended noodle inspired physical conjoined-network or-ganohydrogels with anti-freezing, high stiffness and toughness properties. J. Mater. Sci. 2021, 56, 8887–8899. [Google Scholar] [CrossRef]
- Tu, Y.; Chen, Q.; Liang, S.; Zhao, Q.; Zhou, X.; Huang, W.; Huang, X.; Zhang, L. Antifreezing Heat-Resistant Hollow Hydrogel Tubes. ACS Appl. Mater. Interfaces 2019, 11, 18746–18754. [Google Scholar] [CrossRef]
- Hu, O.; Chen, G.; Gu, J.; Lu, J.; Zhang, J.; Zhang, X.; Hou, L.; Jiang, X. A facile preparation method for anti-freezing, tough, transparent, conductive and thermoplastic poly (vinyl alcohol)/sodium alginate/glycerol organohydrogel electrolyte. Int. J. Biol. Macromol. 2020, 164, 2512–2523. [Google Scholar] [CrossRef]
- Liu, X.; Qin, J.; Wang, J.; Chen, Y.; Miao, G.; Qi, P.; Qu, J.; Zheng, J.; Liu, X. Robust conductive organohydrogel strain sensors with wide range linear sensing, UV filtering, anti-freezing and water-retention properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 632, 127823. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, D.; Wang, J.; Li, T.; Zhou, X.; Gan, T.; Handschuh-Wang, S.; Zhou, X. Rational fabrication of anti-freezing, non-drying tough organohydrogels by one-pot solvent displacement. Angew. Chem. 2018, 130, 6678–6681. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Z.; Wei, Y.; Ding, H.; Li, Z.; Tao, K.; Wu, J. Anti-Freezing and Anti-Drying Organohydrogel Coated with Graphene for Highly Sensitive and Ultrastretchable Strain Sensing. In Proceedings of the 2021 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Orlando, FL, USA, 20–24 June 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1231–1234. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Huang, W.; Yang, X.; Liang, Y.; Tao, K.; Yang, B.-R.; Shi, W.; Xie, X. Stretchable, Stable, and Room-Temperature Gas Sensors Based on Self-Healing and Transparent Organohydrogels. ACS Appl. Mater. Interfaces 2020, 12, 52070–52081. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, H.; Ding, Q.; Wu, Z.; Zhang, H.; Tao, K.; Xie, X.; Wu, J. Hydrogel-and organohydrogel-based stretchable, ul-trasensitive, transparent, room-temperature and real-time NO2 sensors and the mechanism. Mater. Horiz. 2022, 9, 1921–1934. [Google Scholar] [CrossRef]
- Jian, Y.; Wu, B.; Le, X.; Liang, Y.; Zhang, Y.; Zhang, D.; Zhang, L.; Lu, W.; Zhang, J.; Chen, T. Antifreezing and Stretchable Organohydrogels as Soft Actuators. Research 2019, 2019, 2384347. [Google Scholar] [CrossRef] [PubMed]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2019, 27, 101072. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Chen, Y.; Rehman, H.U.; Guo, Y.; Li, H.; Liu, H. Ionic Conductive Organohydrogels with Dynamic Pattern Behavior and Multi-Environmental Stability. Adv. Funct. Mater. 2021, 31, 2101464. [Google Scholar] [CrossRef]
- Ding, Q.; Wu, Z.; Tao, K.; Wei, Y.; Wang, W.; Yang, B.-R.; Xie, X.; Wu, J. Environment tolerant, adaptable and stretchable organohydrogels: Preparation, optimization, and applications. Mater. Horiz. 2022, 9, 1356–1386. [Google Scholar] [CrossRef] [PubMed]
- Malektaj, H.; Drozdov, A.D.; Christiansen, J.D. Swelling of Homogeneous Alginate Gels with Multi-Stimuli Sensitivity. Int. J. Mol. Sci. 2023, 24, 5064. [Google Scholar] [CrossRef]
- Moe, S.T.; Draget, K.I.; Skjåk-Bræk, G.; Simdsrød, O. Temperature dependence of the elastic modulus of alginate gels. Carbohydr. Polym. 1992, 19, 279–284. [Google Scholar] [CrossRef]
- Dastgerdi, J.N.; Marquis, G.; Salimi, M. The effect of nanotubes waviness on mechanical properties of CNT/SMP composites. Compos. Sci. Technol. 2013, 86, 164–169. [Google Scholar] [CrossRef]
- Miki, K.; Westh, P.; Koga, Y. Hydrophobicity vs Hydrophilicity: Effects of Poly(ethylene glycol) and tert-Butyl Alcohol on H2O as Probed by 1-Propanol. J. Phys. Chem. B 2005, 109, 19536–19541. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Caccavo, D. An overview on the mathematical modeling of hydrogels’ behavior for drug delivery systems. Int. J. Pharm. 2019, 560, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Luo, L.; Fan, Y.; Du, Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2021, 238, 121652. [Google Scholar] [CrossRef]
- Ahn, D.; Jeon, I.; Jang, S.; Park, S.; Lee, S.; Cheong, W. Hydrogen Bonding in Aromatic Alcohol-Water Clusters: A Brief Review. Bull. Korean Chem. Soc. 2003, 24, 695–702. [Google Scholar] [CrossRef]
- Drozdov, A.D.; Christiansen, J.D. Tuning the viscoelastic response of hydrogel scaffolds with covalent and dynamic bonds. J. Mech. Behav. Biomed. Mater. 2022, 130, 105179. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Mechanical Properties of Alginate Hydrogels Cross-Linked with Multivalent Cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. Role of Water/Methanol Clustering Dynamics on Thermosensitivity of Poly(N-isopropylacrylamide) from Spectral and Calorimetric Insights. Macromolecules 2010, 43, 9501–9510. [Google Scholar] [CrossRef]
- Kumar, A.; Lee, Y.; Kim, D.; Rao, K.M.; Kim, J.; Park, S.; Haider, A.; Han, S.S. Effect of crosslinking functionality on micro-structure, mechanical properties, and in vitro cytocompatibility of cellulose nanocrystals reinforced poly (vinyl alcohol)/sodium alginate hybrid scaffolds. Int. J. Biol. Macromol. 2017, 95, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lou, D.; Wang, H.; Sun, X.; Li, J.; Liu, Y.N. Flexible supercapacitor based on organohydrogel electrolyte with long-term anti-freezing and anti-drying property. Adv. Funct. Mater. 2020, 30, 2007291. [Google Scholar] [CrossRef]
- Nguyen, Q.; Favre, E.; Ping, Z.; Néel, J. Clustering of solvents in membranes and its influence on membrane transport prop-erties. J. Membr. Sci. 1996, 113, 137–150. [Google Scholar] [CrossRef]
- Takaizumi, K.; Wakabayashi, T. The freezing process in methanol-, ethanol-, and propanol-water systems as revealed by differential scanning calorimetry. J. Solut. Chem. 1997, 26, 927–939. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Guo, Y.Z.; Nonoyama, T.; Nakajima, T.; Kurokawa, T.; Gong, J.P. A facile method to fabricate anisotropic hydrogels with perfectly aligned hierarchical fibrous structures. Adv. Mater. 2018, 30, 1704937. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Q.; Shi, D.; Yang, Z.; Dong, K.; Kaneko, D.; Dong, W.; Chen, M. Tough and antifreezing organohydrogel elec-trolyte for flexible supercapacitors with wide temperature stability. ACS Appl. Energy Mater. 2021, 4, 9353–9361. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. The Effect of Temperature on the Mechanical Properties of Alginate Gels in Water/Alcohol Solutions. Gels 2023, 9, 579. https://doi.org/10.3390/gels9070579
Malektaj H, Drozdov AD, deClaville Christiansen J. The Effect of Temperature on the Mechanical Properties of Alginate Gels in Water/Alcohol Solutions. Gels. 2023; 9(7):579. https://doi.org/10.3390/gels9070579
Chicago/Turabian StyleMalektaj, Haniyeh, Aleksey D. Drozdov, and Jesper deClaville Christiansen. 2023. "The Effect of Temperature on the Mechanical Properties of Alginate Gels in Water/Alcohol Solutions" Gels 9, no. 7: 579. https://doi.org/10.3390/gels9070579
APA StyleMalektaj, H., Drozdov, A. D., & deClaville Christiansen, J. (2023). The Effect of Temperature on the Mechanical Properties of Alginate Gels in Water/Alcohol Solutions. Gels, 9(7), 579. https://doi.org/10.3390/gels9070579






