Abstract
TiO2 aerogels doped with Ni, Co, Cu, and Fe were prepared, and their structure and photocatalytic activity during the decomposition of a model pollutant, acid orange (AO7), were studied. After calcination at 500 °C and 900 °C, the structure and composition of the doped aerogels were evaluated and analyzed. XRD analysis revealed the presence of anatase/brookite and rutile phases in the aerogels along with other oxide phases from the dopants. SEM and TEM microscopy showed the nanostructure of the aerogels, and BET analysis showed their mesoporosity and high specific surface area of 130 to 160 m2·g−1. SEM–EDS, STEM–EDS, XPS, EPR methods and FTIR analysis evaluated the presence of dopants and their chemical state. The concentration of doped metals in aerogels varied from 1 to 5 wt.%. The photocatalytic activity was evaluated using UV spectrophotometry and photodegradation of the AO7 pollutant. Ni–TiO2 and Cu–TiO2 aerogels calcined at 500 °C showed higher photoactivity coefficients (kaap) than aerogels calcined at 900 °C, which were ten times less active due to the transformation of anatase and brookite to the rutile phase and the loss of textural properties of the aerogels.
1. Introduction
TiO2 aerogels have promising applications in environmental remediation, especially by photodegradation of pollutants [1,2]. However, their narrow bandgap and high recombination rate of charge carriers (e−/h+) are issues that need to be addressed [3]. Creating more surfactant sites, heterojunctions, oxygen vacancies or defects can improve the kinetics of redox reactions on the catalyst surface and accelerate the cleavage of H–OH bonds, leading to improved photocatalytic activity [4,5,6].
Previous studies have shown that biphasic TiO2 of anatase/rutile and anatase/brookite has better visible light photocatalytic activity [7,8,9], attributed to the delayed charge recombination effect initiated by the electron-hole separation effect. However, design rules are still needed to develop more efficient neutral catalysts [10]. Improving the crystallinity of TiO2 can increase the probability that charge carriers (e−/h+) reach the photocatalyst surface without being trapped by crystal defects [11], thus preventing their recombination and allowing subsequent redox reactions [12].
Therefore, doping non-metal and metal ions is an effective strategy to tune the band gap by introducing new energy levels between them, which improves the photocatalytic properties of TiO2 [13]. In this regard, cationic dopants served to prolong visible light absorption and enriched the high-temperature stability of the anatase phase of TiO2 [14,15]. Various transition metals such as Fe, Cu, Ni, Cr, and Co have been successfully doped on TiO2 to minimize charge transfer at the interface [16,17,18]. The scavenger of photogenerated holes in oxidized forms reacts with surface-adsorbed hydroxyl ions to form hydroxyl radicals and O2 in the TiO2 surface lattice [19,20,21]. Entrapment experiments have also been studied mainly for the photodegradation of pollutants using various doped TiO2 aerogels [1,22].
Using Cu2+ as a TiO2 catalyst dopant showed that the photocatalytic activity of Cu-TiO2 increased in acid orange 7 (AO7) degradation. The reason was that Cu2+ acted as an electron scavenger to form Cu+, thereby increasing the oxidation of the substrate [2,23]. In addition, cobalt-doped TiO2 [24] has been studied in various oxidation applications, such as the degradation of methylene blue and methyl orange [25,26]. Another study revealed new defect states in anatase-TiO2 and enhanced Ni dopants such as oxygen vacancies [27]. In addition, photoluminescence (PL) studies reported a region of transparency of doped Ni–TiO2 due to an increase in defect density and the transformation of brookite into an amorphous phase. Nickel cations induce the release of carbon and oxygen atoms, resulting in a significant oxygen deficiency [28]. In addition, oxygen vacancies can change the surface properties of TiO2 and affect the adsorption of organic pollutants on its surface. The presence of oxygen vacancies can create surface defects that can provide more active sites for the adsorption of organic pollutants, leading to better photocatalytic activity [29].
Synthesis conditions, dopants, and complexes present in the precursors significantly affect the photoactivity of TiO2 aerogels [16]. Acid-catalyzed sol-gel synthesis is a simple and effective way to prepare stable, mesoporous TiO2 aerogels with a large specific surface area [30,31]. The –COOH and –OH ligand substituents, provided by the organic complexes, offer proper gel networks and photoinduced structures that increase active surface sites and heterojunction defects, thus improving the photocatalytic activity of the material. The optimal formation of crystalline phases depends on the nature of the complexing agent coordinated with the central titanium [32]. Transition metal–organic salts are cheap and easy to handle and release metal complexes in basic suspensions, making them a promising source of dopants for metal-doped TiO2 aerogels [33].
A promising method for synthesizing mesoporous networks of TiO2 aerogels doped with transition metals is sol-gel synthesis using acetate salts as a source of dopants [34,35].
The aim of the research is to evaluate the effect of metal ions nickel (Ni2+), cobalt (Co2+), copper (Cu2+), and iron (Fe3+) on the structure and photocatalytic activity of metal-doped TiO2 aerogels prepared by sol-gel acid-catalyzed synthesis of Ti alkoxide in the presence of transition metals acetate salts.
2. Results and Discussion
2.1. Crystallographic Analysis of Ni, Co, Cu, and Fe-Doped TiO2 Aerogels: Synthesis, Nanostructure, and Calcination Effects
Figure 1a–c shows a schematic of the synthesis process of metal-doped TiO2 aerogels using Ni, Co, Cu, and Fe precursors. The process involves several steps, starting with the preparation of alcogels, which are then subjected to gel aging and solvent exchange using acetone. The final step involves supercritical drying used to form metal-doped TiO2 aerogels. Prior to supercritical drying, the metal-doped TiO2 aerogels were immersed in acetone. The weak binding of transient ions in the alcohol gel structure caused them to diffuse into the acetone solution, resulting in incomplete retention of Ni2+, Co2+, Cu2+, and Fe3+ ions in the mesoporous network of aerogels, as shown in Figure 1b. The presence of these ions in the mesoporous network of aerogels can induce surface defects and increase the surface area of aerogels, increasing its photoactivity. TiO2 aerogels after supercritical drying were amorphous (Figure S1) and not active in photocatalytic tests. During calcination at temperatures of 500 °C and 900 °C, crystalline phases were formed that were photocatalytically active. It was assumed that in particular, calcination at high temperature (900 °C) would lead to the formation of crystalline TiO2 phases doped with Ni, Co, Cu, and Fe.

Figure 1.
(a) The process of synthesis of the metal-doped TiO2 aerogels prepared with the metal acetate precursor of Ni2+, Co2+, Cu2+, Fe3+ ions. (b) Involving the gelation of the alcogels followed by solvent exchange with acetone. (c) Finally, supercritical drying to obtain aerogel samples.
TiO2 occurs in studied samples in three primary phases, rutile (tetragonal, P42/mnm), anatase (tetragonal, I41/amd), and brookite (orthorhombic, Pbca). The XRD patterns show anatase (011) and brookite (210) peaks at 25.3° and 25.6°, respectively (Figure 2a). Brookite (211) was found at 30.82° in 2θ for all metal-doped TiO2 aerogel samples calcined at 500 °C [36]. After calcination at 900 °C, all metal-doped aerogels were transformed from anatase to the rutile phase (110) [35]; see Table 1. Ni-TiO2 and Cu-TiO2 aerogel samples calcined at 500 °C contained the most brookite and the least anatase [36], while the undoped TiO2 sample mainly contained anatase and some rutile. At a higher calcination temperature (900 °C), the rutile content increased from 87 to 98 wt.% for all doped aerogels; the undoped sample contained only the rutile phase. The anatase crystallite size was 4–7 nm at 500 °C, less than the undoped TiO2 aerogel. The size of brookite crystallites was in the range of 2–3 nm [37]. At the calcination temperature of 900 °C, the rutile crystallites’ size increased to 66–78 nm in all samples.
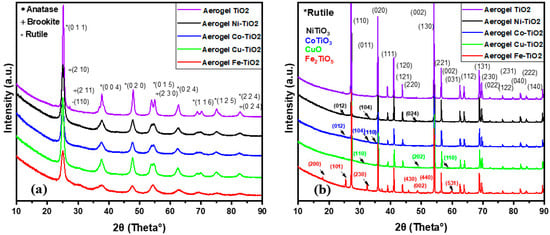
Figure 2.
XRD patterns of metal-doped TiO2 aerogels calcined at (a) 500 °C and (b) 900 °C.

Table 1.
The phase composition and structural characteristics of the doped TiO2 aerogels calcined at 500 °C and 900 °C.
For samples calcined at 500 °C, Ni–TiO2 had the smallest crystallite size (2–5 nm), while Co–TiO2 showed the largest crystallite size (2–7.1 nm). Co–TiO2 showed the highest strain (0.8–2.5%). On the other hand, TiO2 aerogel showed the lowest strain (0.4%), which confirms the absence of dopants and well-crystalline phases. Fe–TiO2 had the most FeTiO3 phase (2 wt%), while the other samples had only a small amount of the doped phase. The TiO2 aerogel had the most anatase phase (99.08%), while the other samples contained a mixture of different TiO2 phases, including doped ones. Overall, the samples had similar lattice parameters and crystallite sizes but differed in strain and phase with the dopants present in the aerogels.
2.1.1. The Influence of Lattice Strain on the Crystalline Size and Photocatalytic Activity of Metal-Doped TiO2 Aerogels
Lattice strain can affect the electronic properties of a material and its crystal structure. Therefore, it can affect its photocatalytic activity [38]. In the case of the brookite phase, the stress affected its crystal structure and caused peak broadening in the XRD patterns. Strain can come from various sources, such as vacancies, point defects, and dislocations. Brookite strain ranged from 1.24% to 2.5% for Fe–TiO2 and Co–TiO2 samples. The FeTiO3 phase was one of the highest in contrast to that of Co3O4, which was 0.52%. Looking at Table 1, we can see that doped samples generally have smaller crystallite sizes than undoped TiO2 at 500 °C. This difference may be due to the incorporation of dopant ions that could hinder the growth of TiO2 crystals. Based on the data provided, it can be seen that samples calcined at 900 °C generally have larger crystallite sizes than samples calcined at 500 °C. For example, in the Ni–TiO2 aerogel, the NiTiO3 phase has a larger crystallite size (3.2 ± 12 nm vs. 15.3 ± 3 nm) and a lower strain (1.3% vs. 0.35%) after calcination at 900 °C compared with 500 °C. A smaller crystal size leads to a larger surface area and better photocatalytic activity [39].
Regarding the correlation between stress and crystal size, smaller crystal sizes can result in higher stress due to the presence of defects and dislocations. Stress can hinder crystal growth, which can also affect crystal size. It follows that the stress in different crystalline phases can play a non-negligible role in the photocatalytic activity of TiO2. The smaller crystal sizes of the doped samples could be the reason that contributes to their higher photocatalytic activity, apart from the doping ions themselves. The crystallite sizes determined by XRD analysis (see Table 1) are consistent with the TEM micrographs in Figure 3. These show that the particle size of the doped aerogels ranged from 2 nm to 7 nm for the samples calcined at 500 °C, with the doped Cu–TiO2 aerogel having the largest nanoparticle size [14]. The observed increase in particle size may be attributed to the larger crystallite size of the dopant phase present in the Cu-TiO2 aerogel in comparison with the other dopant phases from Ni, Co, and Fe.

Figure 3.
TEM images of the anatase/brookite metal-doped TiO2 by Ni, Co, Cu, and Fe calcined at 500 °C.
2.1.2. The Role of Dopants on the Crystal Structure and Phase Composition of TiO2
As can be seen in the table, the undoped TiO2 sample calcined at 500 °C contains both anatase and rutile phases, which is a common composition after the transformation of part of the anatase to rutile at high temperature. In contrast, samples of doped TiO2 calcined at 500 °C mainly contain anatase and brookite phases. This fact could be due to dopant ions that affected the crystal structure and growth of TiO2 particles. For example, Ni2+ ions have been reported to stabilize the anatase phase [40], while Cu2+ and Fe3+ ions promote the formation of the brookite phase. Co2+ ions did not interact with TiO2, resulting in a mixture of the TiO2 phase and a small percentage of cobalt oxide Co3O4 [41]. Co3+-O and Co2+-O bonds existing in Co3O4 formed aggregates on the TiO2 surface [42]. The fact that the dopant precursors were acetate salts may have contributed to stabilizing specific phases, as the acetate ions could act as chelating agents and affect crystal growth and TiO2 particle formation. In addition, the presence of dopants could affect the nucleation and growth of TiO2 crystals, leading to different phase compositions. In summary, the presence of dopants in the TiO2 samples affected the crystal structure and phase composition, forming anatase and brookite phases rather than rutile. Dopant ions and their precursor forms may have played a role in determining the final phase composition of TiO2 aerogel samples [1,43], such as NiTiO3 [44,45], CoTiO3, CuO, and Fe2TiO5 (pseudobrookite) [46,47]. These new phases may have different properties than the original TiO2 phase and may affect the photoactivity of the samples [48].
2.2. Effect of Metal-Doped TiO2 Aerogels on the Textural Parameters and Electronic Properties
The textural parameters of the metal-doped TiO2 samples calcined at 500 °C were studied using the nitrogen adsorption/desorption isotherms shown in Figure 4a. The pore size distributions (DVP) calculated from the desorption branches of the isotherm are shown in Figure 4b. Following IUPAC [49], all samples showed similar physisorption isotherms. These are typical solids with a mesoporous network and macropores that do not contain pore condensate. The textural parameter values (BET SSA specific surface area, BJH pore size, and pore volume) are shown in Table 2. Increasing the calcination temperature from 500 °C to 900 °C caused an extreme decrease in the specific surface area (SSA) of TiO2 samples doped with metal from 160 m2·g−1 to <5 m2·g−1. The mesoporous structure of the metal-doped TiO2 aerogels can be seen in Figure 5 for the aerogels calcined at 500 °C and the decrease in the BET SSA value of the sintered samples at 900 °C. Sintering at a temperature of 900 °C led to an excessive reduction of the surface area of photocatalytic aerogels [3,37]. For samples calcined at 500 °C, the Fe–TiO2 doped aerogel had the highest absorption volume and smaller than average pore size. The metal-doped TiO2 aerogels were found to have an average pore size ranging from 12 nm to 18 nm. A TiO2 aerogel sample calcined at 500 °C shows that increasing the pore size decreased the cumulative pore volume and area (Table 2) [50].
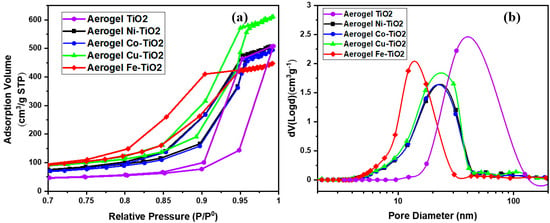
Figure 4.
(a) BET–N2 absorption-desorption and (b) BJH pore size distribution for the metal-doped aerogel samples calcined at 500 °C.

Table 2.
Physicochemical and photocatalytic properties of the doped TiO2 aerogels with Ni, Co, Cu, and Fe calcined at 500 °C.
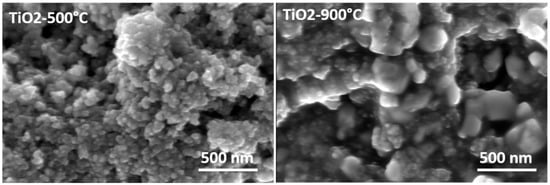
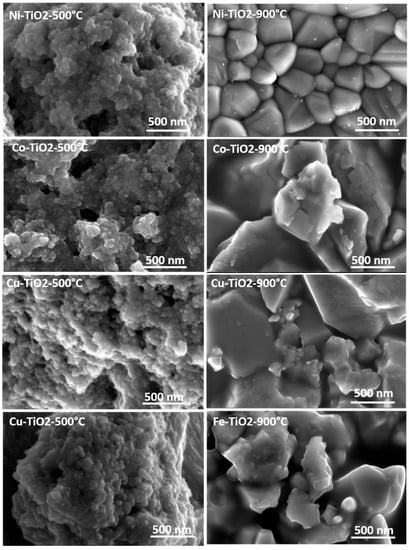
Figure 5.
SEM images of the TiO2 and metal-doped TiO2 aerogels calcined at 500 °C and 900 °C.
The specific surface area of TiO2 significantly affects the photocatalytic activity [51], Table 2 shows that the undoped TiO2 aerogels have an SSA of 86.48 m2.g−1, and its value increased by doping Ni, Co, Cu, and Fe. The highest BET SSA value was observed for Fe–TiO2 (158.1 m2·g−1), followed by Cu–TiO2 (157.6 m2·g−1), Ni–TiO2 (129.3 m2·g−1), and Co–TiO2 (115.7 m2·g−1). The BJH pore size, which refers to the average pore size in the material, was 32.1 nm for the TiO2 aerogel and was lower for the doped samples. The smallest pore size was observed for Fe–TiO2 (12.2 nm), followed by Ni–TiO2, Co–TiO2, and Cu–TiO2, all with pore sizes of about 17 nm. The total pore volume in the material remained similar for Co–TiO2 (0.80 cm3/g−1) and Ni–TiO2 (0.82 cm3/g−1), followed by Cu–TiO2 (0.98 cm3/g−1) [52]. The lowest pore volume value was observed for Fe–TiO2 (0.73 cm3/g−1). Thus, the presented data showed that doping the TiO2 aerogel with Ni, Co, Cu, and Fe ions significantly increased the surface area but decreased the pore size and had negligible influence on the pore volume. Among the doped samples, Cu–TiO2 had the highest BET SSA and BJH pore volume, while Fe–TiO2 had the smallest pore size. These physicochemical properties are fundamental factors in determining the photocatalytic activity of materials. In the case of TiO2-doped aerogels, it was observed that the BET SSA decreased with increasing brookite to anatase ratio (B/A), and the pore size and pore volume also decreased.
The band gap in TiO2 aerogels doped with Ni, Co, Cu, and Fe and in the undoped sample calcined at 500 °C was calculated using indirect α1/2 transitions, as shown in Figure 6. The relationship between surface area (SSA) and the size of the pores of the materials and their band gaps are presented in this work The TiO2 aerogel had the largest band gap (3.0 eV), the smallest specific surface area, and the most significant pore size of all aerogels. This result is consistent with the relationship between the higher band gap and the lower surface area [52]. However, all doped aerogels have a smaller band gap than the undoped TiO2 aerogel. This result is probably due to the introduction of dopant atoms that create defects, leading to the narrowing of the band gap. Table 2 shows that the Fe–TiO2 aerogel has the smallest bandgap (1.7 eV) and the most significant BET SSA [21]. A similar relationship between B/A ratio, pore size, and band gap was observed for Ni–TiO2, Co–TiO2, and Cu–TiO2, which showed similar B/A ratios, pore size, and band gap with slightly higher values than Fe–TiO2.
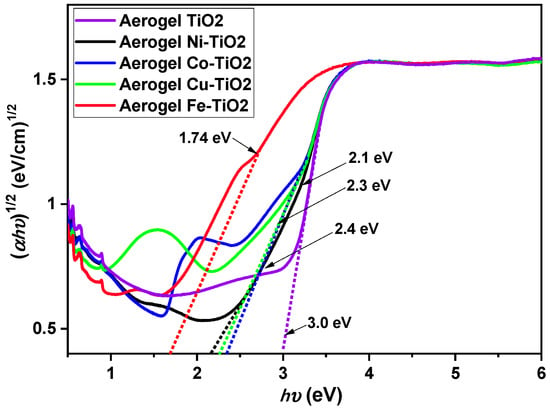
Figure 6.
Indirect bandgap measurements of Ni-, Co-, Cu-, and Fe-doped TiO2 aerogels calcined at 500 °C.
Overall, the results of this study indicate that the band gap of TiO2 aerogel and doped TiO2 aerogels is influenced by the presence of dopants and the material composition, surface area, and pore size, which may have implications for their photocatalytic activity.
2.3. Chemical Composition of the Metal-Doped TiO2 Aerogels: Ni, Co, Cu, Fe
Energy dispersive X-ray spectroscopic analysis (EDS) was used to analyze the chemical composition of the synthesized metal-doped TiO2 aerogels calcined at 500 °C. Table 3 shows that TiO2 doped aerogels contained 0.7 wt.% Ni, 0.7 wt.% Co, 1.9 wt.% Cu, and 4.9 wt.% Fe [52]. The doped aerogels mainly contained TiO2, as evidenced by peaks associated with titanium and oxygen (Figure S2). EDS elemental maps of TiO2 aerogels doped with Ni, Co, Cu, and Fe, shown in Figure 7, show a homogeneous distribution of elements without agglomeration [53].

Table 3.
SEM–EDS elemental composition of the Ni-, Co-, Cu-, Fe-doped TiO2 aerogels calcined at 500 °C.
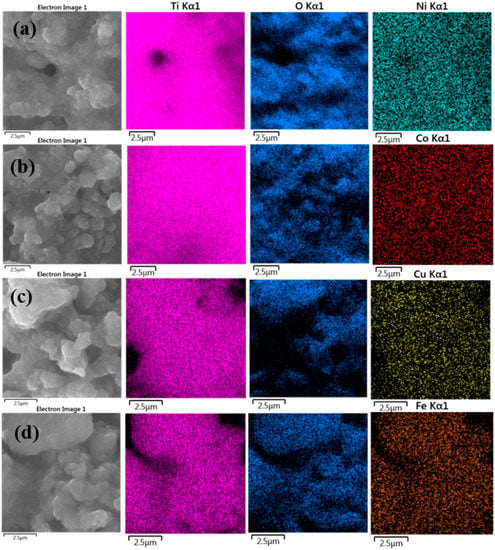
Figure 7.
EDS chemical mapping spectra of the metal-doped TiO2 aerogels (a) Ni, (b) Co, (c) Cu, (d) Fe calcined at 500 °C.
X-ray photoelectron spectroscopy (XPS) was used to analyze the elemental surface composition of metal-doped TiO2 aerogels. Figure 8 shows wide, high-resolution XPS scans of Ti 2p, O 1s, and C 1s peaks, along with the additional metals Ni 2p [54], Co 2p [25], Cu 2p, and Fe 2p [55], of TiO2 aerogels calcined at 500 °C. The two Ti 2p peaks in the XPS spectra are specified in Table 4 and have binding energies of 464.5 and 458.7 eV, corresponding to Ti4+ 2p3/2 and Ti4+ 2p1/2 [56]. The carbon content of the sample is related to the trace organic residue bound as Ti–OCH(CH3)2 [9]. Quantifications in mass and atomic concentrations provide satisfactory fitting (Table S1) and are consistent with SEM–EDS results.
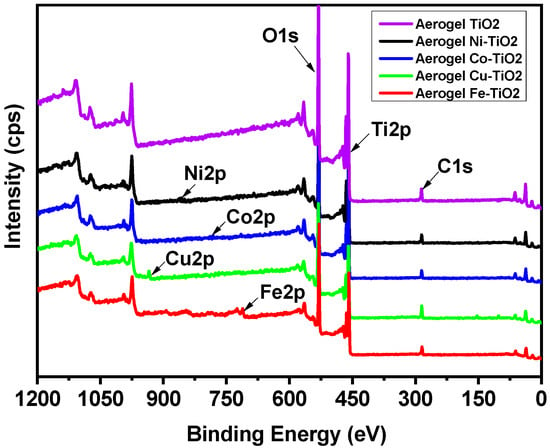
Figure 8.
XPS chemical composition spectra of the doped TiO2 aerogels by Ni, Co, Cu, and Fe calcined at 500 °C.

Table 4.
XPS chemical composition and quantification of the doped TiO2 aerogels by Ni, Co, Cu, and Fe calcined at 500 °C.
Chemical analysis (Table 3 and Table 4) shows that the dopant concentration affected the surface area and physicochemical properties of the TiO2 aerogel samples (Table 1). It was found that metal ion admixtures such as Cu and Fe increased the surface area of TiO2 aerogel [57]. The specific metal ion used as a dopant can also affect the physicochemical properties of the material, such as band gap energy and redox properties with different oxidation states. The effect of dopant concentration also depends on the specific type of dopant used and the experimental conditions of TiO2 preparation [48].
The presence of metal ions in the samples was further verified using electron paramagnetic resonance (EPR) at low temperature (77K). Figure 9a–e shows the EPR spectra of the undoped TiO2 aerogel and metal-doped TiO2 aerogels. Cu2+ [13,58], Fe3+, Ni3+, and Co2+ ions [25] can be seen. TiO2 aerogel and metal-doped TiO2 aerogels exhibit additional paramagnetic species potentially associated with forming oxygen vacancies and defects [51] with a g factor ranging from 1.99 to 2.00 [59]. The g factors obtained for Ni3+, Co2+, and Cu2+ were 2.01 [60], 2.00 [61], and 2.13 [62], respectively, in agreement with the literature. Ni2+ was partially oxidized to Ni3+, which decreased the simultaneous broadening of the EPR signal [60]. The presence of two resonance peaks at g = 2.00 and at g = 4.12, respectively, were attributed to Fe3+ ions substituted in the anatase TiO2 structure. The study showed the presence of isolated octahedral Fe3+ ions in anatase surrounded by Ti4+ ions [46]. These observations can be explained by the diffusion of iron ions from the TiO2 surface into the oxide lattice at g~2.0 and at g~4.0. These species are attributed to high-spin Fe3+ ions (spin S = 5/2) in the rhombic states of the ligand field, in this case with a distorted rhombic environment in the anatase phase, or to iron cations in the orthorhombic brookite structure [63]. The signal at g = 1.99, recorded in Fe-TiO2, was attributed to Ti4+-substituted Fe3+ ions in the TiO2 lattice. The presence of Fe3+ ions observed at g~2.0 with EPR suggests that the dopants are located in small iron oxide-type clusters or tiny nanoparticles within the TiO2 anatase framework. Figure 10 displays STEM-EDS maps indicating the presence of Fe3+ ions in the TiO2 aerogel and Fe clusters segregated on the surface of TiO2 nanoparticles. Additionally, the signal detected at g~4.0 was associated with the presence of oxygen vacancies caused by isolated rhombic Fe3+ ions [46]. The EPR parameters of the Cu2+ ion were reported in the range of g = 2.05–2.10. In this range, it is assumed that oxides containing metal ions such as Ti4+ or Ti3+ and Cu2+ were produced during the calcination process of the material. As a result of this process, Cu2+ can be distributed in the TiO2 ground structure [43].
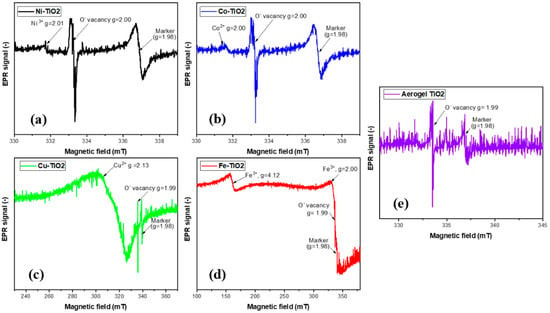
Figure 9.
EPR spectra of (a) Ni-, (b) Co-, (c) Cu-, (d) Fe-doped TiO2, (e) aerogels calcined at 500 °C.
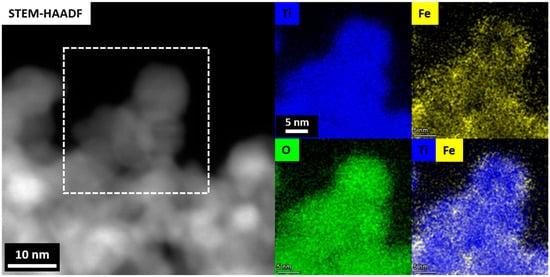
Figure 10.
STEM–HAADF and corresponding STEM–EDS maps of the Fe–TiO2 aerogel sample showing the Fe3+ ions in the TiO2 aerogel matrix and the segregated Fe phase clusters on the surface of the TiO2 nanoparticles.
Figure 11 shows the FTIR spectra of metal-doped TiO2 aerogels calcined at 500 °C. Ti–O–Ti, Ni–O–Ti, Co–O–Ti, Cu–O–Ti and Fe–O–Ti bonding was presented at vibrational frequencies of 500–1200 cm−1 [55]. The band at 738 cm−1 is attributed to stretching vibrations of the Ti–O titanium backbone. The O-H stretching vibrations of adsorbed water and the surface O-H bending group are the causes of the band of two-phase metal-doped TiO2 aerogels in the region of 3200–3800 cm−1 and in the band of 1400–1600 cm−1, respectively. Metal-doped TiO2 aerogels developed two-phase bands at 3679 and 3797 cm−1 due to hydrogen bonding between water and carbonate anion dispersed on the surface [36]. These results are consistent with previous studies that indicated that adsorbed water could facilitate photocatalytic H–OH bond cleavage [64]. Asymmetric carboxylate segments have 1442 cm−1 and symmetric carboxylate segments have 1689 cm−1, corresponding to monodentate bridging in heterojunctions [9,65]. Moreover, the one at 1519.69 cm−1 is due to the stretching band of the carbon–carbon double bond. After calcination of metal-doped two-phase TiO2 aerogels at 500 °C, the carboxyl group was oxidized and cleaved in CO2. This process facilitated the transformation of brookite to anatase and contributed to a slight increase in the proportion of the anatase phase in the metal-doped TiO2 aerogels (Table 1). Ni–TiO2 and Cu–TiO2 doped aerogels showed significant intensities for Ti–O–C stretching vibrations with frequencies ranging from 948.93 to 1080.08 cm−1 [66], followed by Fe–TiO2 and Co–TiO2. These results are consistent with the deconvoluted peaks and C1s binding energies obtained by XPS analysis for C–O–C bonds (see Figure S3 and Table S2).
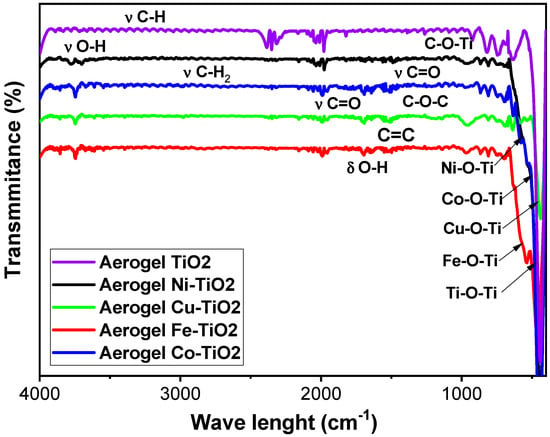
Figure 11.
FTIR spectra of Ni-, Co-, Cu-, Fe-doped TiO2 aerogels calcined at 500 °C.
The combination of different parameters, such as the brookite to anatase ratio (B/A) and the dopant composition, can affect the photocatalytic activity of the material. The nature of the dopant can also affect the physicochemical properties of the aerogel samples, such as its surface area, pore size, pore volume, and adsorption volume, which in turn can affect the material’s electronic properties, such as the band gap. The band gap is an essential factor in determining the photocatalytic activity of a material because it resolves the absorbed light energy required to form electron-hole pairs. In addition, the presence of bonded ligands such as –OH and –COOH on the aerogel surface, as well as residual carbon bonded as Ti–O–C in the crystal lattice of the samples, can promote the formation of additional defects and oxygen vacancies that can serve as active sites to enhance the photocatalytic material performance [9,12,66].
2.4. Photoactivity of the Metal-Doped TiO2 Aerogels by Ni, Co, Cu, Fe
In order to investigate the photocatalytic activity of metal-doped TiO2 aerogels calcined at 500 °C, AO7 degradation was tested. The Langmuir–Hinshelwood kinetics [67,68,69] was used to characterize the degradation of AO7 photocatalyzed by TiO2 aerogels, which is expressed by Equation (1).
−dC/dt = kapp Co
Integrating Equation (1) gives Equation (2)
−ln (C/Co)= kapp t
Co is the initial concentration, “C” is the concentration at any time “t”, and “kapp” is the apparent rate constant for pseudo-first-order kinetic reaction [70,71] whose values are presented in Table 2. As shown in Figure 12a, the AO7 concentration decreased linearly with the AO7 degradation time for all metal-doped TiO2 aerogels. The dependences of ln (C/Co) on time are linear, presented in Figure 12b, indicating that the photocatalytic degradation of AO7 confirms the pseudo-first-order reaction kinetics.
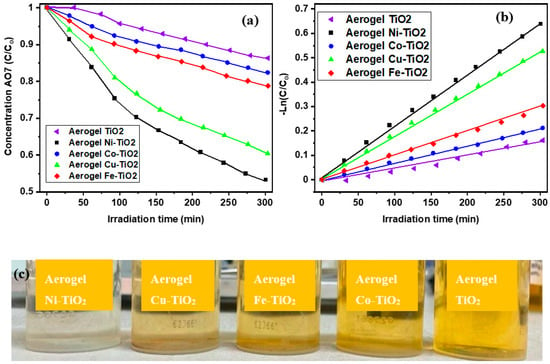
Figure 12.
Photocatalytic performance of the Ni-, Co-, Cu-, Fe-doped TiO2 aerogels: (a) time change of AO7 concentration during photocatalytic degradation (b) fitting the kinetic reaction curves ln(C/Co) = kapp t, where kapp refers to the apparent rate of photoactivity for the samples calcined at 500 °C, (c) AO7-Acid Orange 7 solution photodegradated by the metal-doped TiO2 aerogels.
The highest photocatalytic activity was determined for TiO2 aerogels doped with nickel (Ni2+) and copper (Cu2+) ions and calcined at 500 °C (Figure 12a,b). TiO2 aerogels doped with cobalt (Co2+) and iron (Fe2+) ions had lower activity than the previous two samples (Table 5). The apparent rate constant kapp decreased with increasing calcination temperature of doped aerogels from 2.0 × 10−3 min−1 (500 °C) to 1.0 × 10−4 min−1 (900 °C) (Figure S4). The decrease in photoactivity of the samples calcined at 900 °C was caused by the phase changes of the samples and the reduction of their specific surface area [36,54,72].

Table 5.
Photocatalytic properties of the metal-doped TiO2 aerogels by Ni, Co, Cu, F calcined at 500 °C.
2.4.1. Effect of Strain, Cristallynity, and Band Gab over Photocatalytic Performance
The strain in the TiO2-doped samples could have influenced their photoactivity. Strain can affect the electronic band structure of TiO2, which in turn can affect the absorption and utilization of light for the photocatalytic reaction. Based on the strain values in Table 1, the samples with the highest photoactivity, Ni–TiO2 and Cu–TiO2, have a relatively low strain value (0.6% and 0.4%) for the anatase phase. The doped sample with the lowest photoactivity, Co–TiO2, has a higher strain value (0.8%). This finding suggests that higher strain may have reduced the photocatalytic activity of the samples, although other factors, such as crystal phase and dopant concentration, also affect the activity. This factor is evident in the Ni–TiO2 sample with two crystal phases (anatase and brookite), calcined at a lower temperature (500 °C), which had higher photocatalytic activity due to a higher B/A ratio.
The band gap of the studied materials is an essential factor that affects their photocatalytic efficiency. The surface area (SSA) and pore size of a material also play a role in determining its photocatalytic activity. The Fe–TiO2 aerogel form indeed has a large surface area and a suitable bandgap for photocatalytic degradation with 5 wt.% of Fe3+ in agreement with previous reports [21], but several factors may limit its efficiency in degrading AO7. Factors that can negatively affect the photocatalytic activity of the Fe–TiO2 aerogel may include less dye adsorption on the Fe–TiO2 surface or the pH of the solution. AO7 has an acidic pH in the range of 3–5; at this pH, Fe–TiO2 may not be fully effective. The formation of partial dopant clusters on the TiO2 surface can also limit its photocatalytic activity. There are several reasons why Ni–TiO2 and Cu–TiO2 aerogels can be more active in the degradation of AO7 than Fe–TiO2 aerogel, despite their lower surface area and smaller bandgap energy. Ni–TiO2 and Cu–TiO2 aerogels show higher surface electron density of states than Fe–TiO2 aerogel, which may increase the photocatalytic activity by improving the separation of photo-generated electron-hole pairs [73]. The chemical structure of AO7 may be more suitable for adsorption and degradation by Ni–TiO2 aerogels and Cu–TiO2 than by using Fe–TiO2 aerogel.
2.4.2. Chemical Interaction of the Metal-Doped Aerogels during the Photodegradation of Acid Orange 7
The chemical structure of AO7 may be more suitable for degradation by Ni–TiO2 and Cu–TiO2 aerogels than Fe–TiO2 aerogel. Some functional groups present in AO7, such as azo (–N=N–) and sulfonic acid (–SO3H) groups [74], can promote the adsorption of AO7 on Ni–TiO2 and Cu–TiO2 aerogels more effectively than on Fe–TiO2 aerogel. These functional groups can also facilitate electron transfer and increase the efficiency of the photocatalytic reaction. Ni–TiO2 and Cu–TiO2 can form metal–organic complexes with functional groups present in AO7. These complexes can increase the adsorption of AO7 on the surface of Ni–TiO2 and Cu–TiO2 aerogels and thus promote the degradation of AO7. Under UV radiation, Ni–TiO2 and Cu–TiO2 aerogels can be photoreduced to form NiO and CuO, which can act as TiO2 cocatalysts and promote the separation of photogenerated electron-hole pairs [16,75].
2.4.3. The Impact of Dopant Concentration and Oxygen Vacancies on the Photocatalytic Efficiency of Metal-Doped Aerogel
The reason for the highest photoactivity of Ni–TiO2 can be seen in Figure 13, which shows the STEM-EDS analysis of Ni–TiO2. The Figure shows a homogeneous distribution of Ni in the nanostructure of the TiO2 aerogel, with a concentration of 0.8 wt.% (Table S3), which is consistent with SEM–EDS and XPS analysis. A low content of Ni dopant, which did not lead to the formation of inclusions and clusters, which can act as recombination centers for photogenerated electron-hole pairs and reduce the photocatalytic activity, could have contributed to the homogeneous distribution of Ni in TiO2. Oxygen vacancies can also positively affect the photocatalytic process. The presence of oxygen vacancies in the EPR spectrum of the Ni–TiO2 aerogel could create surface states that act as electron traps, improving the separation of photogenerated electron-hole pairs and enhancing photocatalytic activity. It has been reported that the Ni dopant enhanced new defect states in anatase-TiO2 as oxygen vacancies [29]. Oxygen vacancies can also create surface Ti3+ species that can act as electron donors and promote the generation of reactive oxygen species (ROS) such as hydroxyl radicals (•OH), superoxide radicals (•O2−), and hydrogen peroxide (H2O2). These ROS can react with organic pollutants adsorbed on the surface of TiO2 aerogels and promote their degradation [74].
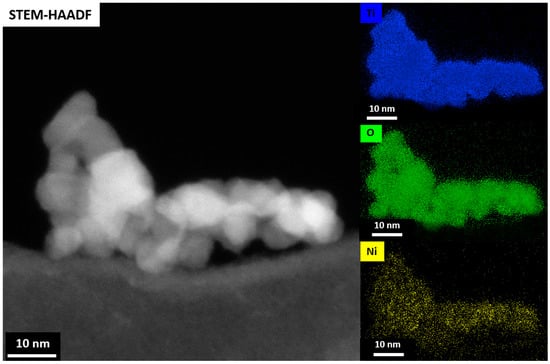
Figure 13.
STEM–HAADF and corresponding STEM–EDS maps of the Ni–TiO2 aerogel sample showing homogeneous distribution of Ni element whithin the TiO2 aerogel nanoparticles and without presence of agregates from a secondary nickel phase.
3. Conclusions
An acid-catalyzed sol-gel process and subsequent supercritical drying prepared TiO2 aerogels doped with Ni, Co, Cu and Fe. After calcination at 500 °C, the catalysts had a mesoporous structure, a significant specific surface area (SSA) ranging from 116 to 158 m2·g−1, a pore size of 12.2–17.5 nm, and a pore volume of 0.73–0.98 cm3.g−1. EDS and XPS analysis confirmed the presence of Ni, Co, Cu, and Fe in TiO2 aerogels with dopant concentrations ranging from 1 to 5 wt.%. Doped TiO2 aerogels calcined at 500 °C contained 76–82 wt.% anatase and 16–24 wt.% brookite. The crystal size of anatase was 4–7 nm, and brookite was 2–3 nm. After calcination at 900 °C, anatase and brookite transformed into rutile, the crystals became suspended, and the mesoporous structure disappeared. Metal doping affected the composition of the crystalline phases, brookite and anatase, and the formation of minor phases containing Ni, Co, Cu, or Fe.
The highest amount of brookite was found in the Ni–TiO2 sample, which contained 1 wt.% Ni and had the highest photocatalytic activity in the degradation of AO7. In addition to the anatase/brookite ratio, other factors such as crystal structure and morphology, energy band gap, and surface defects influenced photocatalytic activity. The homogeneity of the distribution in the TiO2 aerogels was analyzed by STEM–EDS. Seated Fe clusters on the surface of Fe–TiO2 aerogel nanoparticles were the leading cause of the lowest photocatalytic activity of all doped aerogels.
Conversely, Ni–TiO2 with homogeneously distributed Ni in TiO2 nanoparticles had the highest photocatalytic activity. The high photocatalytic activity of Ni–TiO2 and Cu–TiO2 during the photodegradation of AO7 could also be supported by the formation of metal–AO7 complexes and cocatalyst effects. These interactions could enhance the photocatalytic activity of Ni–TiO2 and Cu–TiO2 aerogels for AO7 degradation. The presence of oxygen vacancies in doped TiO2 detected by EPR increased their photocatalytic activity by creating surface states that act as electron traps and activate the TiO2 surface for better adsorption of AO7.
4. Materials and Methods
4.1. Reagents
Nickel (II) acetate tetrahydrate (Ni(OCOCH3)2 4H2O), 98%, Sigma-Aldrich (St.Louis, MO, USA), cobalt (II) acetate tetrahydrate ((CH3COO)2Co·4 H2O), p.a., Fluka, copper (II) acetate monohydrate ((CH3COO)2Cu·H2O), ≥98%, Sigma-Aldrich, iron (III) acetylacetonate (Fe(C5H7O2)3), 97%, Sigma-Aldrich, tetrapropyl orthotitanate ((CH3CH2CH2O)4Ti) and Tetraethyl ortho-titanate, ((C₂H₅O)₄Ti), 95%, Merck, for synthesis, Merck, ethanol (CH3CH2OH), 99.8%, ISO reagent, Lachner, nitric acid (HNO3), Pnta, water (H2O), CEITEC-demi.
4.2. Synthesis of the Metal-Doped TiO2 Aerogels by Ni, Co, Cu, Fe
A solution (A) with the M-acetate tetrahydrate and a solution (B) was prepared by mixing ethanol and demi water in a 250 mL beaker. The metal salt was weighed, and 7 mL of ethanol was added. Immediately, nitric acid was added. During the stirring process, the weigh-in was dissolved after 2 min. Tetrapropyl ortho-titanate was added to the reaction mixture under stirring.
The stirring speed was raised to 350 rpm, followed by dissolution after 5 min. Solution B (in one batch) was added to solution A, increasing the stirring speed to 600 rpm and mixing for 30 s. Subsequently, the reaction mixture was poured into a plastic vial for setting, resulting in fast gelation (1 min). In a couple of minutes, an adequate amount of acetone was added to the surface of the gel to avoid its evaporation, followed by its ageing/strengthening overnight. Transfer the sample into the jar with acetone was provided. The gel was allowed to continuously exchange its solvent with acetone for the next five days. Finally, the wet aged gel was dried under supercritical conditions with supercritical CO2 using the medium temperature supercritical drying technique in a typical drying procedure. The autoclave is pressurized with liquid CO2 to 5.8 MPa and then heated to 80 °C while maintaining a pressure of 18 MPa. With 2 bars/min of depressurization, the system slowly returns to atmospheric pressure after three hours in a supercritical state [76]. The metal-doped TiO2 aerogels are obtained after cooling the autoclave to room temperature.
4.3. Characterization of the Metal-Doped TiO2 Aerogels
X-ray powder diffraction patterns (XRD) were obtained using a Smart Lab diffractometer (Rigaku, Japan). The crystalline phases of the prepared TiO2 were measured in the reflection mode (Bragg-Brentano geometry) using a Cu lamp using X-ray diffraction (XRD—Rigaku SmartLab 3 kW, Cu Kα radiation) (Rigaku, Japan). The crystallite size and proportions of individual phases were calculated according to the Rietveld analysis and the Halder-Wagner (HW) method using the PDXL evaluation software v.3. The microstructure of the TiO2 products was studied using a high-resolution scanning electron microscope 460L Verios (Thermo Fisher Scientific, Eindhoven, the Netherlands). TEM (transmission electron microscopy) LVEM 25 (Delong Ins.), provided high contrast and magnification images in the range of 15–25 Kv from the TiO2 aerogels nanostructure. The specific surface area (SBET) measurement was obtained as nitrogen physisorption isotherms at 77 K using Autosorb iQ (Quantachrome Instruments). SBET was calculated using five points according to the classical BET (Brunauer–Emmet–Teller) method for a P/P0 range of 0.1–0.3, assuming cylindrical shape and mutually non-intersecting pores. The pore size distribution of samples was determined from adsorption and desorption isotherms between the analyzed pressures P/P0 = 0.35–1.0, using the BJH (Barrett–Joyner–Halenda) method. The band gap calculation was made using the UV/Vis/NIR Spectrophotometer Jasco V-770S with a 60 mm integrating spheres with barium sulfate coating providing the reflectance measurement for solid samples with a powder sample holder, the diameter of the measuring area was 16 mm, sample thickness 0.5–6 mm and the indirect transitions allowed α1/2 method based on Kubelka–Munk function. A scanning electron microscope Mira 3 (Tescan, Shanghai, China, CZ) equipped with energy-dispersive spectroscope (Oxford Instruments, Abingdon, UK) was used for chemical composition analysis. To collect and evaluate spectra, Aztec software (OXFORD Ins, UK) was used. Additionally, a transmission electron microscopy (TEM) with a CS aberration image corrected microscope Titan Themis 60–300 Cubed (Thermo Fisher Scientific, Waltham, MA, USA) performed STEM energy dispersive X-ray (EDX) elemental mapping by using SuperX spectrometer (Thermo Fisher Scientific, USA) to reveal the presence and distribution of iron and Ni within the TiO2 aerogel. EDX elemental maps were collected as a spectrum image using the Velox v.2.14 software (Thermo Fisher Scientific, USA). XPS analysis used the Axis Supra (Kratos Analytical, UK) with monochromatic Kα radiation, 60 W emission power, magnetic lens, and the charge compensation turned on. The survey and detailed elemental spectra were measured using pass energies of 160 and 20 eV, respectively. The spectra were evaluated using the XPS Kratos ESCAPE data processing using Lorentzian–Gaussian function with a G/L ratio of 0.3. The electron paramagnetic resonance (EPR) experiments were conducted using a Magnettech X-band EPR spectrometer. The measurement was performed at 77 K using a Nitrogen Dewar Flask. The samples (weight approximately 10 mg) were prepared by locating and encapsulating them in 5 mm diameter quartz tubes with a Cr4+ marker (g = 1.98) for further g factor calculation. The spectra were recorded using a microwave power of 10 mW and a modulation amplitude of 0.27 mT at 100 kHz.
4.4. Photoactivity Assessment of the Metal-Doped TiO2 Aerogels by Using AO7 as a Model Probe Molecule
The photocatalytic test was conducted using the photoreactors under irradiation by UV–Vis light. A xenon lamp with a UV filter (03LWPG02, Barleword Scientific) was the source of radiation. The radiation wavelength range was between 250–750 nm, while the radiation intensity was 2.50 mW cm−2. A total of 40 mL of the acid orange (AO7) water solution with an initial concentration of 1.7 × 10 −7 mol. L −1 was magnetically stirred in a 1.5 cm2 photoreactor under UV–Vis light. Next, 20 mg of the nanoparticle sample was dispersed in the solution. The UV–Vis spectrometer Red Tide USB650UV (Ocean optics) measured the photoactivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gels9050357/s1, Figure S1: XRD spectra of the metal-doped TiO2 aerogels after supercritical drying process. Figure S2: XRD spectra of the metal-doped TiO2 aerogels after calcined at 500 °C. Figure S3: Deconvoluted binding energies and atomic concentrations of C 1s obtained from the primary XPS spectra from metal-doped TiO2 aerogels calcined at 500 °C. Figure S4: Photocatalytic performance of the metal-doped TiO2 aerogels: Ni, Co, Cu, Fe fitting the kinetic reaction curves—ln(C/Co) = kapp t, where kapp refers to the apparent rate of photoactivity for the samples calcined at 900 °C. Table S1: Chemical quantification from the wide XPS spectra of the metal-doped TiO2 aerogels. Table S2: Quantification of deconvoluted binding energies and atomic concentrations of C 1s obtained from the primary XPS spectra from metal-doped TiO2 aerogels calcined at 500 °C. Table S3: STEM–EDS elemental quantification of the Ni, Ti, and O presented in the Ni–TiO2 aerogel lattice after calcined at 500 °C.
Author Contributions
Conceptualization, C.J. and T.N.L.K.; Methodology, T.N.L.K., C.J. and B.V.; software, T.N.L.K.; validation, T.N.L.K. and K.J.; formal analysis, T.N.L.K. and K.J.; investigation, T.N.L.K. and C.J.; resources T.N.L.K., C.J. and B.V.; data curation, T.N.L.K.; writing—T.N.L.K.; writing—review and editing, T.N.L.K., C.J. and B.V.; visualization, T.N.L.K.; supervision, C.J.; project administration, C.J.; funding acquisition, C.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic under grant no. LTC20019 and the COST Action CA18125 “Advanced Engineering and Research of Aerogels for Environment and Life Sciences” (AERoGELS), and funded by the European Commission and the CzechNanoLab Research Infrastructure supported by MEYS CR (LM2018110).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was conducted within the framework of the doctoral program “In Advance Material” of the Brno University of Technology, Central European Institute of Technology (CEITEC) by T.N.L.K. The authors acknowledge the support of the Ministry of Education, Youth and Sports of the Czech Republic under grant no. LTC20019. We also acknowledge CzechNanoLab Research Infrastructure supported by MEYS CR (LM2018110) and the specialists Kateřina Tmejová, Jan Michalička, Pavla Roupcová, Tkachenko Serhii, and the head of the research group, Martin Trunec. Moreover, the CEITEC MOTES lab-Magneto-Optical, THz Spectroscopy and the specialist Vinicius Tadeu Santana, as well as Delong Instruments in Brno.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hasanpour, M.; Hatami, M. Photocatalytic Performance of Aerogels for Organic Dyes Removal from Wastewaters: Review Study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic Hydrogen Production Using Metal Doped TiO2: A Review of Recent Advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Dorraj, M.; Goh, B.T.; Sairi, N.A.; Woi, P.M.; Basirun, W.J. Improved Visible-Light Photocatalytic Activity of TiO2 Co-Doped with Copper and Iodine. Appl. Surf. Sci. 2018, 439, 999–1009. [Google Scholar] [CrossRef]
- Khan, H.; Berk, D. Effect of a Chelating Agent on the Physicochemical Properties of TiO2: Characterization and Photocatalytic Activity. Catal. Letters 2014, 144, 890–904. [Google Scholar] [CrossRef]
- Mandari, K.K.; Police, A.K.R.; Do, J.Y.; Kang, M.; Byon, C. Rare Earth Metal Gd Influenced Defect Sites in N Doped TiO2: Defect Mediated Improved Charge Transfer for Enhanced Photocatalytic Hydrogen Production. Int. J. Hydrog. Energy 2018, 43, 2073–2082. [Google Scholar] [CrossRef]
- Cihlar, J.; Tinoco Navarro, L.K.; Kasparek, V.; Michalicka, J.; Kastyl, J.; Castkova, K.; Celko, L. Influence of LA/Ti Molar Ratio on the Complex Synthesis of Anatase/Brookite Nanoparticles and Their Hydrogen Production. Int. J. Hydrog. Energy 2021, 46, 8578–8593. [Google Scholar] [CrossRef]
- Reza Gholipour, M.; Dinh, C.T.; Béland, F.; Do, T.O. Nanocomposite Heterojunctions as Sunlight-Driven Photocatalysts for Hydrogen Production from Water Splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef]
- Cho, H.-W.; Liao, K.-L.; Yang, J.-S.; Wu, J.-J. Revelation of Rutile Phase by Raman Scattering for Enhanced Photoelectrochemical Performance of Hydrothermally-Grown Anatase TiO2 Film. Appl. Surf. Sci. 2018, 440, 125–132. [Google Scholar] [CrossRef]
- Cihlar, J.; Navarro, L.K.T.; Cihlar, J.; Kasparek, V.; Michalicka, J.; Castkova, K.; Lazar, I.; Kastyl, J.; Celko, L.; Vesely, M.; et al. Influence of Substituted Acetic Acids on “Bridge” Synthesis of Highly Photocatalytic Active Heterophase TiO2 in Hydrogen Production. J. Sol.-Gel. Sci. Technol. 2022, 105, 471–488. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Cao, Y.; Xiang, J.; Gao, H. Enhanced Photocatalytic Activities of Low-Bandgap TiO2-Reduced Graphene Oxide Nanocomposites. J. Nanoparticle Res. 2017, 19, 200. [Google Scholar] [CrossRef]
- Hippargi, G.; Mangrulkar, P.; Chilkalwar, A.; Labhsetwar, N.; Rayalu, S. Chloride Ion: A Promising Hole Scavenger for Photocatalytic Hydrogen Generation. Int. J. Hydrog. Energy 2018, 43, 6815–6823. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic Degradation for Environmental Applications—A Review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Vargas Hernández, J.; Coste, S.; García Murillo, A.; Carrillo Romo, F.; Kassiba, A. Effects of Metal Doping (Cu, Ag, Eu) on the Electronic and Optical Behavior of Nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Yasuda, M.; Matsumoto, T.; Yamashita, T. Sacrificial Hydrogen Production over TiO2-Based Photocatalysts: Polyols, Carboxylic Acids, and Saccharides. Renew. Sustain. Energy Rev. 2018, 81, 1627–1635. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A.; Grabowska, E.; Marchelek, M.; Paszkiewicz-Gawron, M.; Zaleska-Medynska, A. Metal Oxide Photocatalysts. In Metal Oxide-Based Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 51–209. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, X.; Wang, L.; Wu, F.; Liu, S.; Chang, C.; Luo, X. A Simple Strategy to Design 3-Layered Au-TiO2 Dual Nanoparticles Immobilized Cellulose Membranes with Enhanced Photocatalytic Activity. Carbohydr. Polym. 2020, 231, 115694. [Google Scholar] [CrossRef]
- Cihlar, J.; Cihlar, J.; Bartonickova, E. Low-Temperature Sol-Gel Synthesis of Anatase Nanoparticles Modified by Au, Pd and Pt and Activity of TiO2/Au, Pd, Pt Photocatalysts in Water Splitting. J. Sol.-Gel. Sci. Technol. 2013, 65, 430–442. [Google Scholar] [CrossRef]
- Cihlar, J.; Vrba, R.; Castkova, K.; Cihlar, J. Effect of Transition Metal on Stability and Activity of La-Ca-M-(Al)-O (M=Co, Cr, Fe and Mn) Perovskite Oxides during Partial Oxidation of Methane. Int. J. Hydrog. Energy 2017, 42, 19920–19934. [Google Scholar] [CrossRef]
- Moradi, V.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Significant Improvement in Visible Light Photocatalytic Activity of Fe Doped TiO2 Using an Acid Treatment Process. Appl. Surf. Sci. 2018, 427, 791–799. [Google Scholar] [CrossRef]
- Ganesh, I.; Kumar, P.P.; Gupta, A.K.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and Characterization of Fe-Doped TiO2 Powders for Solar Light Response and Photocatalytic Applications. Process. Appl. Ceram. 2012, 6, 21–36. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review Doped-TiO2: A Review. Recent Patents Eng. 2014, 2, 157–164. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Li, J.; Yang, X. Photocatalytic Hydrogen Production from Methanol Aqueous Solution under Visible-Light Using Cu/S–TiO2 Prepared by Electroless Plating Method. Catal. Commun. 2015, 59, 189–194. [Google Scholar] [CrossRef]
- Mugundan, S.; Rajamannan, B.; Viruthagiri, G.; Shanmugam, N.; Gobi, R.; Praveen, P. Synthesis and Characterization of Undoped and Cobalt-Doped TiO2 Nanoparticles via Sol–Gel Technique. Appl. Nanosci. 2015, 5, 449–456. [Google Scholar] [CrossRef]
- Sadanandam, G.; Lalitha, K.; Kumari, V.D.; Shankar, M.V.; Subrahmanyam, M. Cobalt Doped TiO2: A Stable and Efficient Photocatalyst for Continuous Hydrogen Production from Glycerol: Water Mixtures under Solar Light Irradiation. Int. J. Hydrog. Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Seadira, T.; Sadanandam, G.; Ntho, T.A.; Lu, X.; Masuku, C.M.; Scurrell, M. Hydrogen Production from Glycerol Reforming: Conventional and Green Production. Rev. Chem. Eng. 2018, 34, 695–726. [Google Scholar] [CrossRef]
- Franceschini, E.A.; Gomez, M.J.; Lacconi, G.I. One Step Synthesis of High Efficiency Nickel/Mesoporous TiO2 Hybrid Catalyst for Hydrogen Evolution Reaction. J. Energy Chem. 2019, 29, 79–87. [Google Scholar] [CrossRef]
- Lamouchi, W.; Ben Slama, S.; Saadallah, F.; Bouaicha, M. Nickel Doping Induced Amorphization of Brookite TiO2: Photoluminescence Enhancement. Optik 2021, 227, 166123. [Google Scholar] [CrossRef]
- Ali, H.; Vandevyvere, T.; Lauwaert, J.; Kansal, S.K.; Saravanamurugan, S.; Thybaut, J.W. Impact of Oxygen Vacancies in Ni Supported Mixed Oxide Catalysts on Anisole Hydrodeoxygenation. Catal. Commun. 2022, 164, 106436. [Google Scholar] [CrossRef]
- Moussaoui, R.; Elghniji, K.; ben Mosbah, M.; Elaloui, E.; Moussaoui, Y. Sol–Gel Synthesis of Highly TiO2 Aerogel Photocatalyst via High Temperature Supercritical Drying. J. Saudi Chem. Soc. 2017, 21, 751–760. [Google Scholar] [CrossRef]
- Budtova, T.; Aguilera, D.A.; Beluns, S.; Berglund, L.; Chartier, C.; Espinosa, E.; Gaidukovs, S.; Klimek-kopyra, A.; Kmita, A.; Lachowicz, D.; et al. Biorefinery Approach for Aerogels. Polymers 2020, 12, 2779. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Shi, Z.; Zhi, M.; Hong, Z. The Investigation of an Organic Acid Assisted Sol-Gel Method for Preparing Monolithic Zirconia Aerogels. RSC Adv. 2018, 8, 8011–8020. [Google Scholar] [CrossRef] [PubMed]
- Vorsina, I.; Grigorieva, T.; Barinova, A.; Lyakhov, N. Mechanochemical Interaction of Silicon Dioxide With Organic Acids. Chem. Sustain. Dev. 2011, 19, 236–247. [Google Scholar]
- Cihlar, J.; Kasparek, V.; Kralova, M.; Castkova, K. Biphasic Anatase-Brookite Nanoparticles Prepared by Sol-Gel Complex Synthesis and Their Photocatalytic Activity in Hydrogen Production. Int. J. Hydrog. Energy 2015, 40, 2950–2962. [Google Scholar] [CrossRef]
- Mahy, J.G.; Cerfontaine, V.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Heinrichs, B.; Lambert, S.D. Highly Efficient Low-Temperature N-Doped TiO2 Catalysts for Visible Light Photocatalytic Applications. Materials 2018, 11, 584. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Ram, C. Calcination Temperature Effect on Titanium Oxide (TiO2) Nanoparticles Synthesis. Optik 2021, 241, 166934. [Google Scholar] [CrossRef]
- Kim, M.G.; Kang, J.M.; Lee, J.E.; Kim, K.S.; Kim, K.H.; Cho, M.; Lee, S.G. Effects of Calcination Temperature on the Phase Composition, Photocatalytic Degradation, and Virucidal Activities of TiO2Nanoparticles. ACS Omega 2021, 6, 10668–10678. [Google Scholar] [CrossRef]
- Kim, G.H.; Jeong, S. Change of Electronic Structures by Dopant-Induced Local Strain. Sci. Rep. 2015, 5, 11227. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Z.; Chen, J.; Sheng, W.; Sun, L.; Xiang, J. Effect of Calcination Temperature on the Activity and Structure of MnOx/TiO2 Adsorbent for Hg0 Removal. Fuel Process. Technol. 2015, 135, 25–33. [Google Scholar] [CrossRef]
- Espinos, J.P.; Gonz, A.R.; Caballero, A.; GARCfA, J.; Munuera, G. The State of Nickel in Ni/SiO2 and Ni/TiO2-Calcined Catalysts. J. Catal. 1992, 136, 415–422. [Google Scholar] [CrossRef]
- Tyo, E.C.; Yin, C.; Di Vece, M.; Qian, Q.; Kwon, G.; Lee, S.; Lee, B.; Debartolo, J.E.; Seifert, S.; Winans, R.E.; et al. Oxidative Dehydrogenation of Cyclohexane on Cobalt Oxide (Co3O4) Nanoparticles: The Effect of Particle Size on Activity and Selectivity. ACS Catal. 2012, 2, 2409–2423. [Google Scholar] [CrossRef]
- Habibi, M.H.; Shojaee, E. Synthesis of a Heterojunction CoTiO3/Co3O4 Nano-Composite Thin Film with Superior Photocatalytic Activity and Reusability: Effect of Calcination Temperature on Phase Transformation and Effect of Oxidants on Enhanced Degradation of Indo Light Blue Dye. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 229, 117796. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi Miyamoto, N.; Miyamoto, R.; Giamello, E.; Kurisaki, T.; Wakita, H. Evaluation of Coexistent Metal Ions with TiO2: An EPR Approach. Res. Chem. Intermed. 2018, 44, 4563–4575. [Google Scholar] [CrossRef]
- Phani, A.R.; Santucci, S. Structural Characterization of Nickel Titanium Oxide Synthesized by Sol-Gel Spin Coating Technique. Thin Solid Films 2001, 396, 1–4. [Google Scholar] [CrossRef]
- Gabal, M.A.E.F.; Al Angari, Y.M.; Obaid, A.Y. Structural Characterization and Activation Energy of NiTiO3 Nanopowders Prepared by the Co-Precipitation and Impregnation with Calcinations. Comptes Rendus Chim. 2013, 16, 704–711. [Google Scholar] [CrossRef]
- Kokorin, A.I.; Amal, R.; Teoh, W.Y.; Kulak, A.I. Studies of Nanosized Iron-Doped TiO2 Photocatalysts by Spectroscopic Methods. Appl. Magn. Reson. 2017, 48, 447–459. [Google Scholar] [CrossRef]
- Fernando, N.; Swaminathan, J.; Robles Hernandez, F.C.; Priyadarshana, G.; Sandaruwan, C.; Yang, W.; Karunaratne, V.; Wang, Z.; Amaratunga, G.A.J.; Kottegoda, N.; et al. Pseudobrookite Based Heterostructures for Efficient Electrocatalytic Hydrogen Evolution. Mater. Rep. Energy 2021, 1, 100020. [Google Scholar] [CrossRef]
- Dubey, R.S.; Jadkar, S.R.; Bhorde, A.B. Synthesis and Characterization of Various Doped TiO2 Nanocrystals for Dye-Sensitized Solar Cells. ACS Omega 2021, 6, 3470–3482. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications. Available online: https://books.google.cz/books?hl=es&lr=&id=UOE-ZscCYncC&oi=fnd&pg=PP1&ots=0T__DEslnv&sig=jF4A7rVRX5_aDPQtMEamWcE7XvM&redir_esc=y#v=onepage&q&f=false (accessed on 9 November 2022).
- Yu, J.; Yu, H.; Cheng, B.; Trapalis, C. Effects of Calcination Temperature on the Microstructures and Photocatalytic Activity of Titanate Nanotubes. J. Mol. Catal. A Chem. 2006, 249, 135–142. [Google Scholar] [CrossRef]
- Wu, S.; Ishisone, K.; Sheng, Y.; Manuputty, M.Y.; Kraft, M.; Xu, R. TiO2 with Controllable Oxygen Vacancies for Efficient Isopropanol Degradation: Photoactivity and Reaction Mechanism. Catal. Sci. Technol. 2021, 11, 4060–4071. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. Synthesis and Characterization of Solar Photoactive TiO2 Nanoparticles with Enhanced Structural and Optical Properties. Adv. Powder Technol. 2018, 29, 211–219. [Google Scholar] [CrossRef]
- Rahul, T.K.; Mohan, M.; Sandhyarani, N. Enhanced Solar Hydrogen Evolution over in Situ Gold-Platinum Bimetallic Nanoparticle-Loaded Ti3+ Self-Doped Titania Photocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 3049–3059. [Google Scholar] [CrossRef]
- Qiu, C.; Lin, J.; Shen, J.; Liu, D.; Zhang, Z.; Lin, H.; Wang, X. Regulation of the Rutile/Anatase TiO2 heterophase Interface by Ni12P5to Improve Photocatalytic Hydrogen Evolution. Catal. Sci. Technol. 2020, 10, 3709–3719. [Google Scholar] [CrossRef]
- Liu, J.; Hodes, G.; Yan, J.; Liu, S. (Frank) Metal-Doped Mo2C (Metal = Fe, Co, Ni, Cu) as Catalysts on TiO2 for Photocatalytic Hydrogen Evolution in Neutral Solution. Chinese J. Catal. 2020, 42, 205–216. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, srep32355. [Google Scholar] [CrossRef] [PubMed]
- Lyson-Sypien, B.; Czapla, A.; Zakrzewska, K.; Swierczek, K.; Radecka, M.; Rekas, M.; Michalow, K.; Graule, T. Influence of Grain Size on Gas Sensing Properties of TiO2 Nanopowders. Procedia Eng. 2012, 47, 1057–1060. [Google Scholar] [CrossRef]
- Godiksen, A.; Stappen, F.N.; Vennestrøm, P.N.R.; Giordanino, F.; Birk Rasmussen, S.; Lundegaard, L.F.; Mossin, S.; Topsøe, H.A. Coordination Environment of Copper Sites in Cu-CHA Zeolite Investigated by Electron Paramagnetic Resonance. J. Phys. Chem. C 2014, 118, 23126–23138. [Google Scholar] [CrossRef]
- Zhou, S.; Cižmár, E.; Potzger, K.; Krause, M.; Talut, G.; Helm, M.; Fassbender, J.; Zvyagin, S.A.; Wosnitza, J.; Schmidt, H. Origin of Magnetic Moments in Defective TiO2 Single Crystals. Phys. Rev. B 2009, 79, 113201. [Google Scholar] [CrossRef]
- Niemöller, A.; Jakes, P.; Eurich, S.; Paulus, A.; Kungl, H.; Eichel, R.-A.; Granwehr, J.; Niemöller, N.; Eichel, R.-A. Monitoring Local Redox Processes in LiNi0.5Mn1.5O4 Battery Cathode Material by in Operando EPR Spectroscopy. Cit. J. Chem. Phys. 2018, 148, 14705. [Google Scholar] [CrossRef]
- Savoyant, A.; Alnoor, H.; Bertaina, S.; Nur, O.; Willander, M. EPR Investigation of Pure and Co-Doped ZnO Oriented Nanocrystals. Nanotechnology 2017, 28, 035705. [Google Scholar] [CrossRef]
- Bennett, B.; Kowalski, J.M. EPR Methods for Biological Cu(II): L-Band CW and NARS. Methods Enzymol. 2015, 563, 341. [Google Scholar] [CrossRef]
- Kumar, C.P.; Gopal, N.O.; Wang, T.C.; Wong, M.S.; Ke, S.C. EPR Investigation of TiO2 Nanoparticles with Temperature-Dependent Properties. J. Phys. Chem. B 2006, 110, 5223–5229. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Shahwar, D.; Khatoon, B. Trans-Generational Response of TiO2 Nanoparticles in Inducing Variability and Changes in Biochemical Pool of Lentil F2 Progenies. J. Biosci. 2022, 47, 35. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.A.; Narasimharao, K.; Ali, T.T.; Khalil, K.M.S. Acidic Peptizing Agent Effect on Anatase-Rutile Ratio and Photocatalytic Performance of TiO2 Nanoparticles. Nanoscale Res. Lett. 2018, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Chelbi, S.; Djouadi, D.; Chelouche, A.; Hammiche, L.; Touam, T.; Doghmane, A. Effects of Ti-Precursor Concentration and Annealing Temperature on Structural and Morphological Properties of TiO2 Nano-Aerogels Synthesized in Supercritical Ethanol. SN Appl. Sci. 2020, 2, 872. [Google Scholar] [CrossRef]
- Asenjo, N.G.; Santamaría, R.; Blanco, C.; Granda, M.; Álvarez, P.; Menéndez, R. Correct Use of the Langmuir–Hinshelwood Equation for Proving the Absence of a Synergy Effect in the Photocatalytic Degradation of Phenol on a Suspended Mixture of Titania and Activated Carbon. Carbon 2013, 55, 62–69. [Google Scholar] [CrossRef]
- Qourzal, S.; Barka, N.; Tamimi, M.; Assabbane, A.; Ait-Ichou, Y. Photodegradation of 2-Naphthol in Water by Artificial Light Illumination Using TiO2 Photocatalyst: Identification of Intermediates and the Reaction Pathway. Appl. Catal. A Gen. 2008, 334, 386–393. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band Gap Engineered TiO2 Nanoparticles for Visible Light Induced Photoelectrochemical and Photocatalytic Studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Jafarikojour, M.; Dabir, B.; Sohrabi, M.; Royaee, S.J. Evaluation and Optimization of a New Design Photocatalytic Reactor Using Impinging Jet Stream on a TiO2 Coated Disc. Chem. Eng. Process. Process. Intensif. 2017, 121, 215–223. [Google Scholar] [CrossRef]
- Phromma, S.; Wutikhun, T.; Kasamechonchung, P.; Eksangsri, T.; Sapcharoenkun, C. Effect of Calcination Temperature on Photocatalytic Activity of Synthesized TiO2 Nanoparticles via Wet Ball Milling Sol-Gel Method. Appl. Sci. 2020, 10, 993. [Google Scholar] [CrossRef]
- Muthee, D.K.; Dejene, B.F. Effect of Annealing Temperature on Structural, Optical, and Photocatalytic Properties of Titanium Dioxide Nanoparticles. Heliyon 2021, 7, e07269. [Google Scholar] [CrossRef]
- Ganesh, I.; Gupta, A.K.; Kumar, P.P.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and Characterization of Ni-Doped TiO2 Materials for Photocurrent and Photocatalytic Applications. Sci. World J. 2012, 2012, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, D.D.; Li, G.; Wang, Y. Investigation of the Roles of Active Oxygen Species in Photodegradation of Azo Dye AO7 in TiO2 Photocatalysis Illuminated by Microwave Electrodeless Lamp. J. Photochem. Photobiol. A Chem. 2008, 199, 311–315. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, e1807660. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodríguez, J.; Kalmár, J.; Menelaou, M.; Čelko, L.; Dvořak, K.; Cihlář, J.; Cihlař, J.; Kaiser, J.; Győri, E.; Veres, P.; et al. Heat Treatment Induced Phase Transformations in Zirconia and Yttria-Stabilized Zirconia Monolithic Aerogels. J. Supercrit. Fluids 2019, 149, 54–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).