Development of Novel pH-Sensitive Eudragit Coated Beads Containing Curcumin-Mesalamine Combination for Colon-Specific Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phase-Solubility Analysis
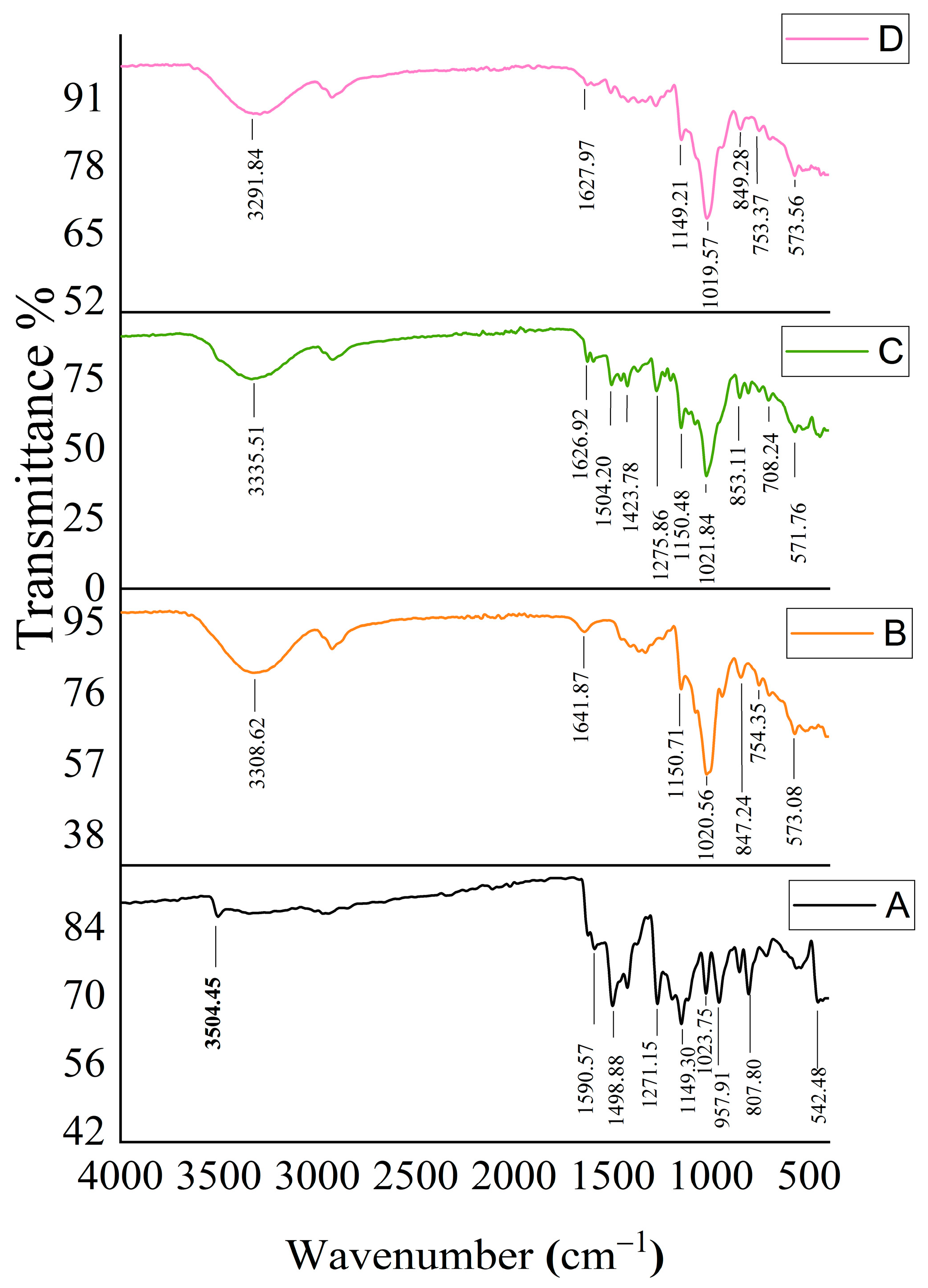
2.2. Characterization of MSZ/HP-β-CD Inclusion Complexes
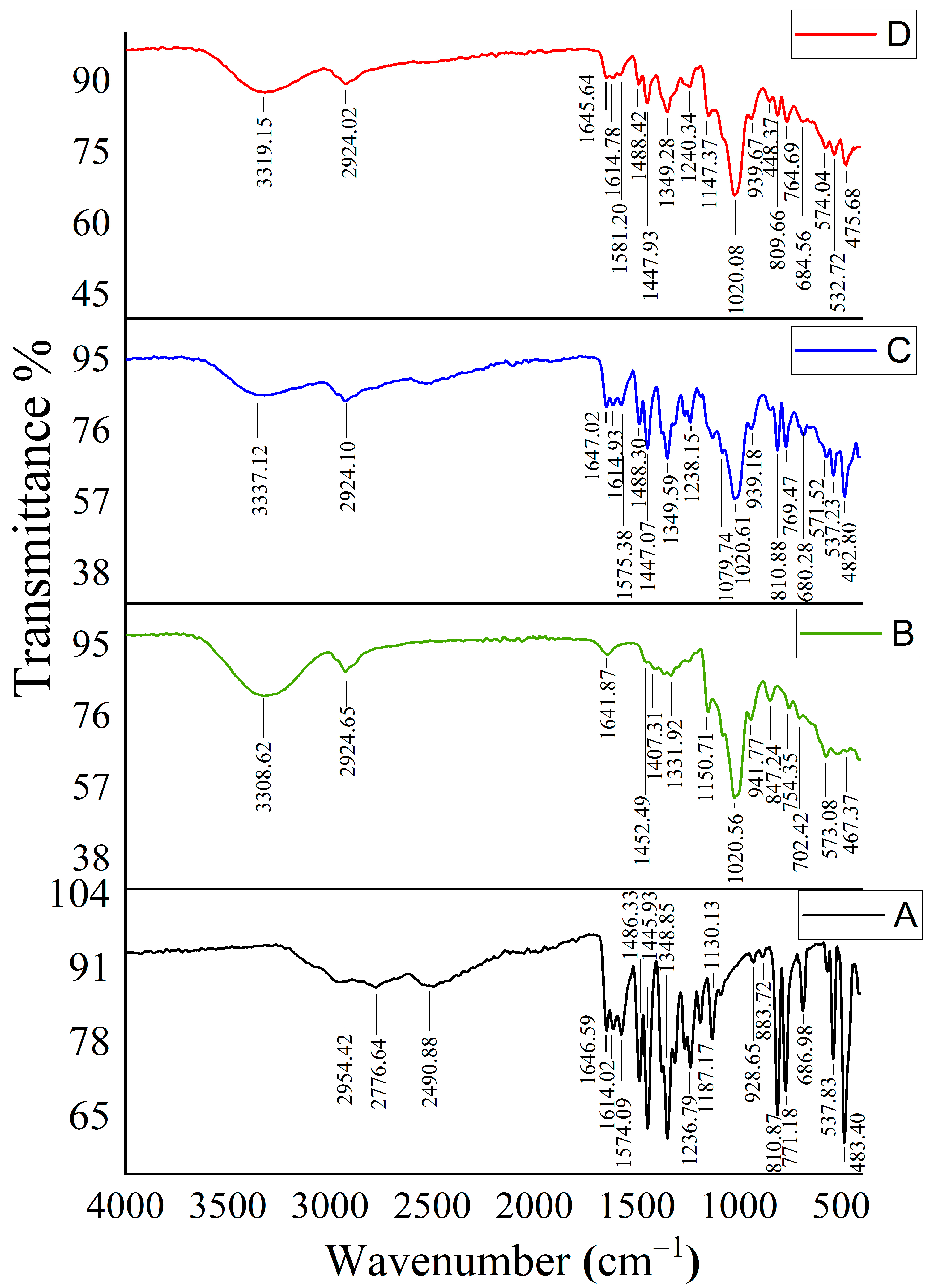
2.3. Characterization of CUR/HP-β-CD Inclusion Complexes
2.4. Formulation Optimization Using D-Optimal Design
2.4.1. Bead Diameter
2.4.2. Swelling (%)
2.4.3. Drug Entrapment Efficiency (%)
| Run | SALG (%) | CaCl2 (%) | CS (%) | Diameter (mm) | Swelling (%) | Entrapment (%) |
|---|---|---|---|---|---|---|
| F1 | 1 | 4 | 0.5 | 1.6 ± 0.20 * | 455.55 ± 63.83 * | 93.03 ± 2.02 |
| F2 | 3 | 2 | 0.5 | 2.5 ± 0.17 | 800 ± 40.4 | 92.37 ± 0.26 |
| F3 | 2 | 3 | 0.3 | 2.3 ± 0.44 | 507.47 ± 59.95 * | 92.35 ± 0.02 |
| F4 | 1 | 2 | 0.5 | 1.6 ± 0.26 * | 488.23 ± 7.12 * | 87.49 ± 1.17 |
| F5 | 3 | 3 | 0.1 | 2.6 ± 0.30 | 738.09 ± 34.34 | 93.35 ± 1.65 |
| F6 | 3 | 4 | 0.1 | 2.8 ± 0.26 | 743.13 ± 54.05 | 95.23 ± 1.10 |
| F7 | 1 | 3 | 0.1 | 1.7 ± 0.17 * | 470 ± 62.5 * | 89.57 ± 1.94 |
| F8 | 2 | 3 | 0.3 | 2.3 ± 0.20 | 529.63 ± 54.68 * | 91.85 ± 1.60 |
| F9 | 1 | 4 | 0.1 | 1.9 ± 0.20 | 472.72 ± 45.16 * | 94.36 ± 2.00 |
| F10 | 2 | 2 | 0.1 | 2.6 ± 0.36 | 500 ± 19.77 * | 92.43 ± 1.12 |
| F11 | 1 | 2 | 0.3 | 1.9 ± 0.44 | 409.80 ± 9.49 * | 88.49 ± 1.08 |
| F12 | 2 | 3 | 0.3 | 2.4 ± 0.26 | 507.84 ± 27.82 * | 92.39 ± 0.64 |
| F13 | 3 | 4 | 0.5 | 2.7 ± 0.26 | 890.19 ± 16.99 | 97.89 ± 1.61 |
| F14 | 3 | 4 | 0.5 | 2.7 ± 0.50 | 900 ± 26.97 | 97.68 ± 0.38 |
| F15 | 2 | 4 | 0.1 | 2.4 ± 0.46 | 517.64 ± 5.06 * | 97.64 ± 1.61 |
| F16 | 2 | 2 | 0.1 | 2.6 ± 0.17 | 505.76 ± 30.93 * | 91.88 ± 0.89 |
| F17 | 3 | 2 | 0.1 | 2.7 ± 0.56 | 757.14 ± 20.67 | 96.05 ± 0.66 |
| F18 | 1 | 4 | 0.5 | 1.6 ± 0.17 * | 457.69 ± 20.5 * | 93.28 ± 1.01 |
| F19 | 1 | 2 | 0.5 | 1.6 ± 0.17 * | 490 ± 19.8 * | 87.50 ± 0.65 |
| F20 | 3 | 2 | 0.5 | 2.5 ± 0.36 | 792.15 ± 29.31 | 92.68 ± 1.61 |
| F21 | 3 | 2 | 0.3 | 2.8 ± 0.61 | 834.57 ± 30.52 | 93.11 ± 0.4 |
| F22 | 1 | 4 | 0.3 | 1.6 ± 0.30 * | 423.81 ± 30.98 * | 92.15 ± 0.34 |
2.5. Developing Optimization Models
2.5.1. Model Fit Report for the Effect of Sodium Alginate
2.5.2. The Effect of Calcium Chloride
2.5.3. The Impact of Chitosan on Diameter and Entrapment Efficacy
2.6. Selection of the Optimized Formulation
2.7. Morphology of Optimized Formula-Coated Beads
2.8. FTIR for Optimized Formula-Coated Beads
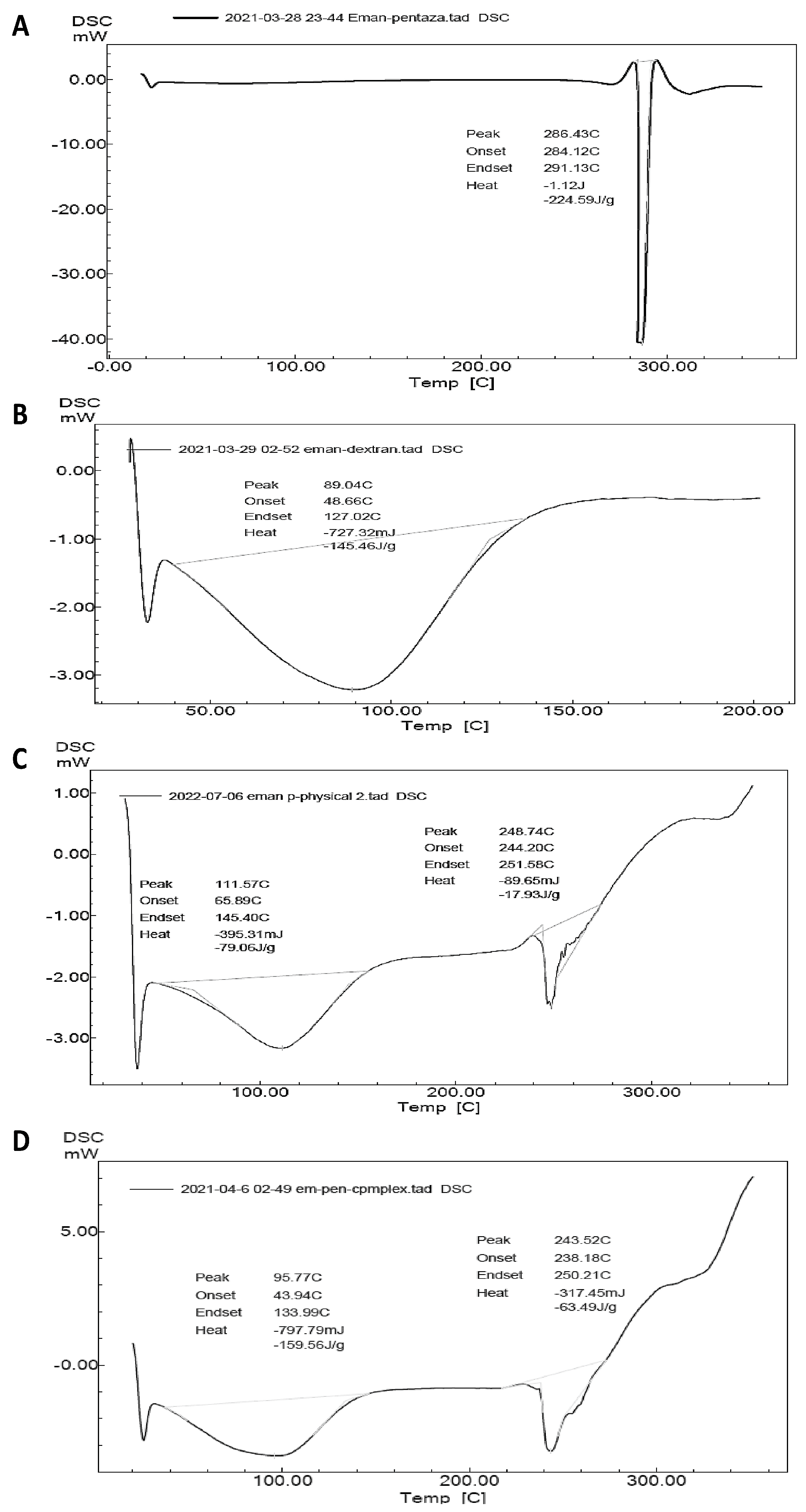
2.9. DSC for Optimized Formula-Coated Beads
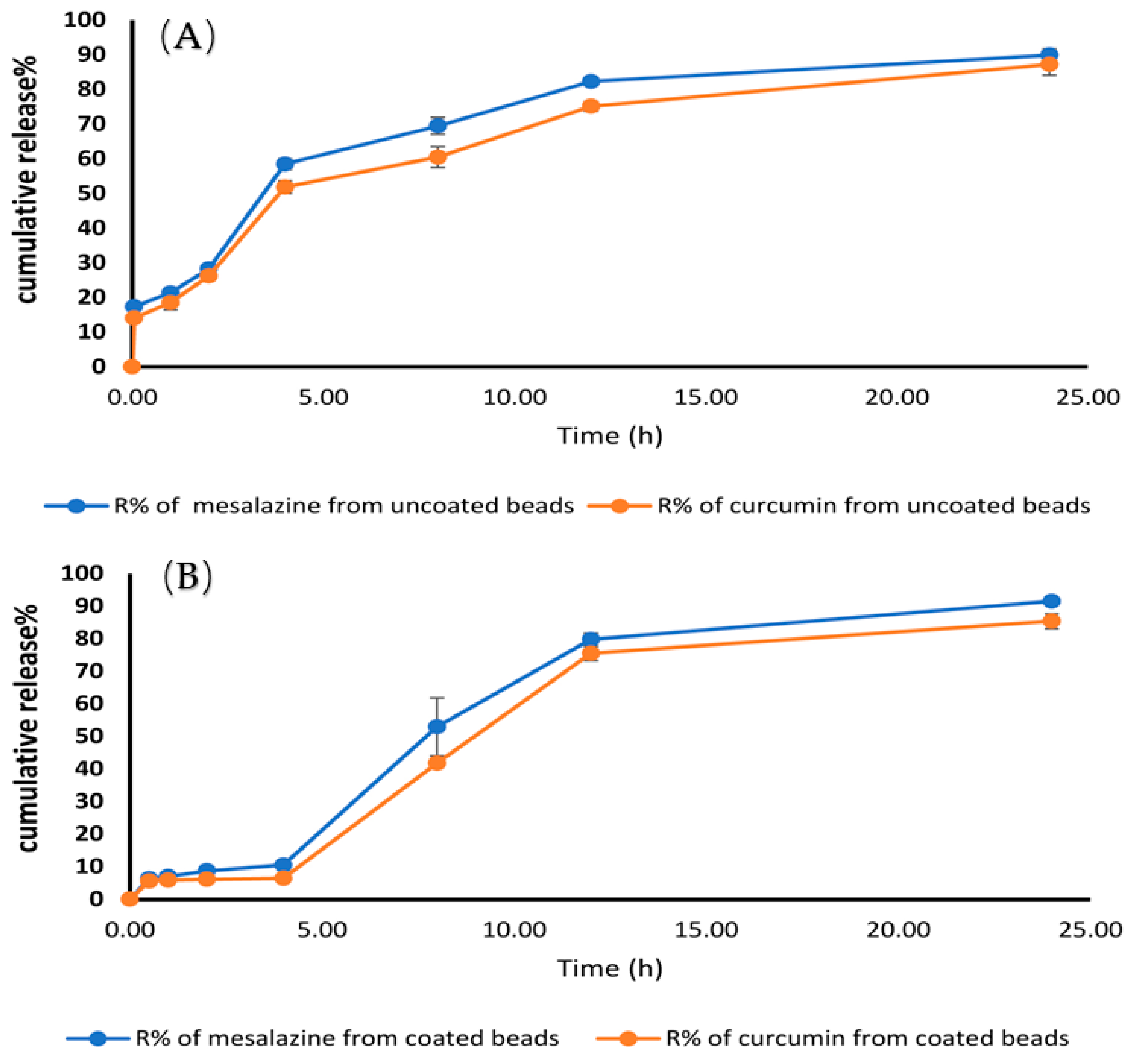
2.10. In-Vitro Release and Release Kinetic Study
2.11. Stability Study
| Week No. | Diameter (mm) |
|---|---|
| Week 1 | 0.406 ± 0.011 |
| Week 2 | 0.406 ± 0.005 |
| Week 3 | 0.403 ± 0.006 |
| Week 4 | 0.406 ± 0.005 |
| Week 5 | 0.403 ± 0.005 |
2.12. Colon/Body Weight (C/B) Ratio
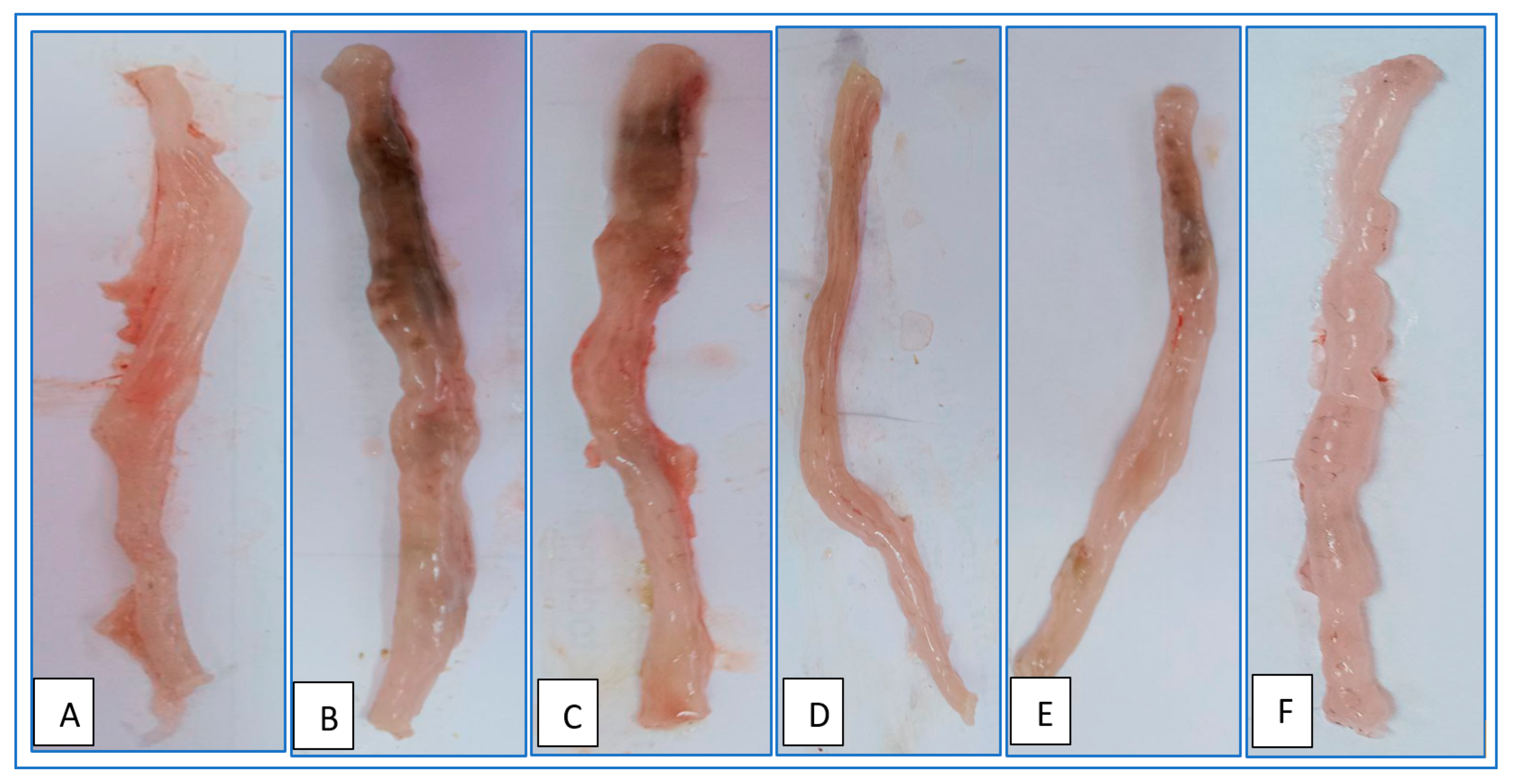
2.13. Evaluation of Colonic Macroscopic Injury
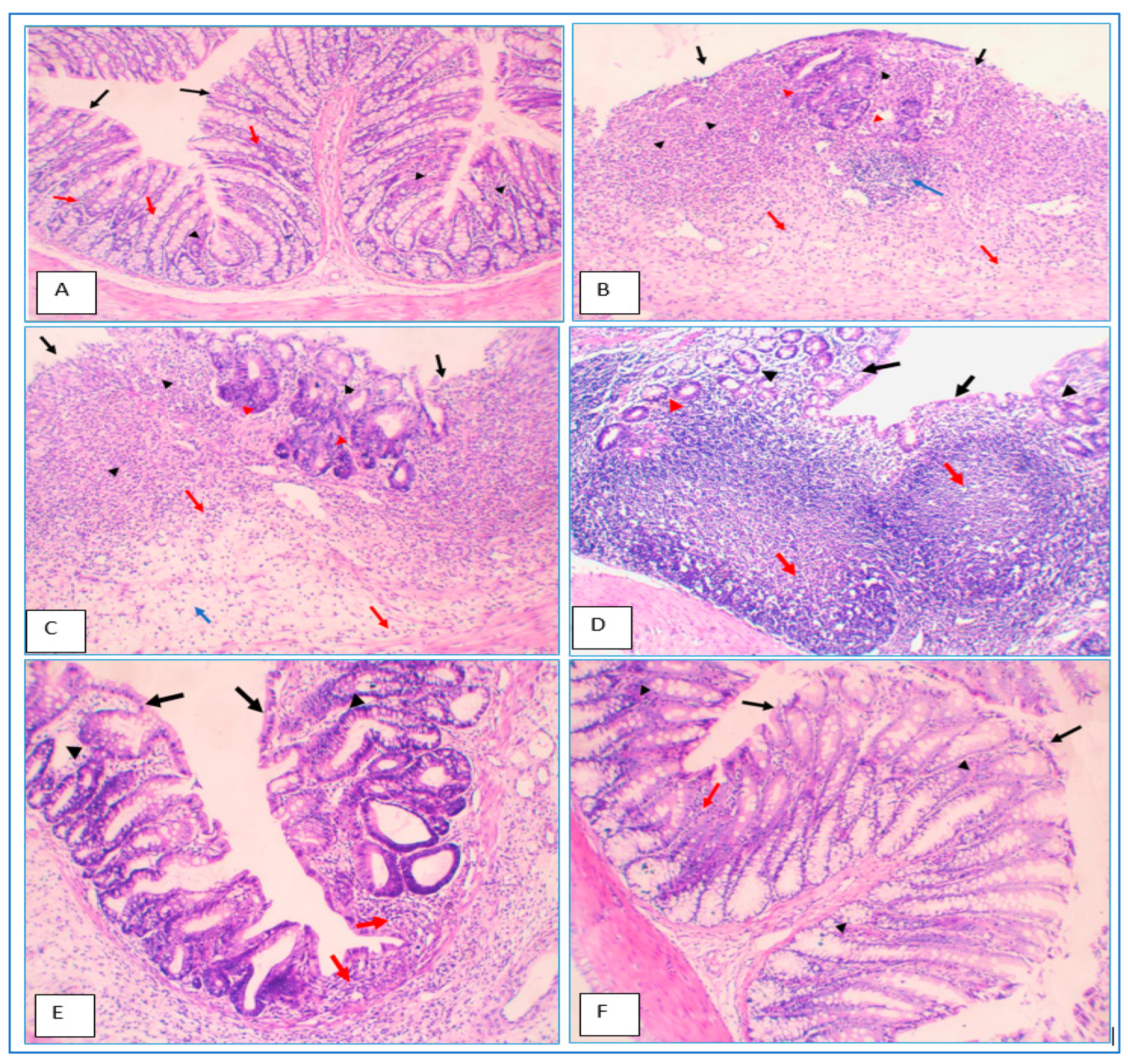
2.14. Histopathological Evaluation of Colon Specimens Stained with Haematoxylin and Eosin
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Phase Solubility Study
4.3. Preparing and Characterizing the Drug/HP-β-CD Inclusion Complexes
4.4. Formulation of CS/SALG Hydrogel Beads
4.4.1. Optimization of Hydrogel Bead Formulation Variables
4.4.2. Characteristics of the Hydrogel Beads
Bead Diameter Analysis
Swelling Experiments
Encapsulation Efficiency (EE%)
4.4.3. Optimization Employing the D-Optimal Design
4.5. Enteric Coating of the Selected Optimal Hydrogel Bead Formula #13
4.5.1. Scanning Electron Microscopy Analysis of the Optimized Formula #13 of the Coated Beads
4.5.2. FTIR Analysis of the Optimized Formula-Coated Beads
4.5.3. DSC Studies for the Optimized Formula of the Coated Beads
4.5.4. In-Vitro Drug Release from the Optimized Formula of the Coated Beads
4.6. Stability Study
4.7. In-Vivo Study of the Protective Effect of the Optimized Formula-Coated Beads
4.7.1. Induction of Ulcerative Colitis
4.7.2. Experimental Animal Groups
4.7.3. Scarification of Rats and Collection of Colon Specimens
4.7.4. Calculation of the Colon/Body Weight Ratio
4.7.5. Histological Procedures and Assessment of the Colon Specimens
4.7.6. Statistical Analysis for the Results
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Göke, K.; Lorenz, T.; Repanas, A.; Schneider, F.; Steiner, D.; Baumann, K.; Bunjes, H.; Dietzel, A.; Finke, J.H.; Glasmacher, B.; et al. Novel strategies for the formulation and processing of poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2018, 126, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Sookkasem, A.; Chatpun, S.; Yuenyongsawad, S.; Wiwattanapatapee, R. Alginate beads for colon specific delivery of self-emulsifying curcumin. J. Drug Deliv. Sci. Technol. 2015, 29, 159–166. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Ahuja, V.; Midha, V.; Sinha, S.K.; Pai, C.G.; Kedia, S.; Mehta, V.; Bopanna, S.; Abraham, P.; Banerjee, R.; et al. Colitis and Crohn’s Foundation (India) consensus statements on use of 5-aminosalicylic acid in inflammatory bowel disease. Intest. Res. 2020, 18, 355. [Google Scholar] [CrossRef]
- Deshmukh, R.; Harwansh, R.K.; Das Paul, S.; Shukla, R. Controlled release of sulfasalazine loaded amidated pectin microparticles through Eudragit S 100 coated capsule for management of inflammatory bowel disease. J. Drug. Deliv. Sci. Technol. 2020, 55, 101495. [Google Scholar] [CrossRef]
- Deshmukh, R.; Harwansh, R.K. Preformulation Considerations Development and Evaluation of Mesalamine Loaded Polysaccharide-Based Complex Mucoadhesive Beads for Colon Targeting. Indian J. Pharm. Educ. Res. 2021, 55, 95–106. [Google Scholar] [CrossRef]
- Seenan, J.P.; Lafferty, H. Gastroenterologic Problems; Elsevier: London, UK, 2019; Volume 109. [Google Scholar]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef] [Green Version]
- Chifiriuc, M.C.; Mihai Grumezescu, A.; Grumezescu, V.; Bezirtzoglou, E.; Lazar, V.; Bolocan, A. Biomedical applications of natural polymers for drug delivery. Curr. Org. Chem. 2014, 18, 152–164. [Google Scholar] [CrossRef]
- Wu, T.; Yu, S.; Lin, D.; Wu, Z.; Xu, J.; Zhang, J.; Ding, Z.; Miao, Y.; Liu, T.; Chen, T.; et al. Preparation, characterization, and release behavior of doxorubicin hydrochloride from dual cross-linked chitosan/alginate hydrogel beads. ACS Appl. Bio Mater. 2020, 3, 3057–3065. [Google Scholar] [CrossRef]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, É.; Mangin, D.; Elaissari, A. pH-sensitive polymers: Classification and some fine potential applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Lu, Z.; Cheng, B.; Hu, Y.; Zhang, Y.; Zou, G. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Elnashar, M.M.; Yassin, M.A.; Moneim, A.E.-F.A.; Bary, E.M.A. Surprising performance of alginate beads for the release of low-molecular-weight drugs. J. Appl. Polym. Sci. 2010, 116, 3021–3026. [Google Scholar] [CrossRef]
- Dai, Y.N.; Li, P.; Zhang, J.P.; Wang, A.Q.; Wei, Q. Swelling characteristics and drug delivery properties of nifedipine-loaded pH sensitive alginate—Chitosan hydrogel beads. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Malakar, J.; Nayak, A.K. Formulation and statistical optimization of multiple-unit ibuprofen-loaded buoyant system using 23-factorial design. Chem. Eng. Res. Des. 2012, 90, 1834–1846. [Google Scholar] [CrossRef]
- Kerdsakundee, N.; Mahattanadul, S.; Wiwattanapatapee, R. Development and evaluation of gastroretentive raft forming systems incorporating curcumin-Eudragit®EPO solid dispersions for gastric ulcer treatment. Eur. J. Pharm. Biopharm. 2015, 94, 513–520. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Zhang, Z. Calcium-carboxymethyl chitosan hydrogel beads for protein drug delivery system. J. Appl. Polym. Sci. 2007, 103, 3164–3168. [Google Scholar] [CrossRef]
- Mennini, N.; Furlanetto, S.; Cirri, M.; Mura, P. Quality by design approach for developing chitosan-Ca-alginate microspheres for colon delivery of celecoxib-hydroxypropyl-β-cyclodextrin-PVP complex. Eur. J. Pharm. Biopharm. 2012, 80, 67–75. [Google Scholar] [CrossRef]
- Anal, A.K.; Stevens, W.F. Chitosan—Alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm. 2005, 290, 45–54. [Google Scholar] [CrossRef]
- Sinha, P.; Udhumansha, U.; Rathnam, G.; Ganesh, M.; Jang, H.T. Capecitabine encapsulated chitosan succinate-sodium alginate macromolecular complex beads for colon cancer targeted delivery: In vitro evaluation. Int. J. Biol. Macromol. 2018, 117, 840–850. [Google Scholar] [CrossRef]
- Mi, Y.; Su, R.; Fan, D.-D.; Zhu, X.-L.; Zhang, W.-N. Preparation of N, O-carboxymethyl chitosan coated alginate microcapsules and their application to Bifidobacterium longum BIOMA 5920. Mater. Sci. Eng. C 2013, 33, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Bueno, P.V.; Almeida, E.A.; Rodrigues, F.H.; Rubira, A.F.; Muniz, E.C. Characterization of N-trimethyl chitosan/alginate complexes and curcumin release. Int. J. Biol. Macromol. 2013, 57, 174–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakral, N.K.; Ray, A.R.; Majumdar, D.K. Eudragit S-100 entrapped chitosan microspheres of valdecoxib for colon cancer. J. Mater. Sci. Mater. Med. 2010, 21, 2691–2699. [Google Scholar] [CrossRef]
- Jantarat, C.; Sirathanarun, P.; Ratanapongsai, S.; Watcharakan, P.; Sunyapong, S.; Wadu, A. Curcumin-hydroxypropyl-β-cyclodextrin inclusion complex preparation methods: Effect of common solvent evaporation, freeze drying, and pH shift on solubility and stability of curcumin. Trop. J. Pharm. Res. 2014, 13, 1215–1223. [Google Scholar] [CrossRef]
- Tang, P.; Sun, Q.; Zhao, L.; Pu, H.; Yang, H.; Zhang, S.; Gan, R.; Gan, N.; Li, H. Mesalazine/hydroxypropyl-$β$-cyclodextrin/chitosan nanoparticles with sustained release and enhanced anti-inflammation activity. Carbohydr. Polym. 2018, 198, 418–425. [Google Scholar] [CrossRef]
- Tang, P.; Ma, X.; Wu, D.; Li, S.; Xu, K.; Tang, B.; Li, H. Posaconazole/hydroxypropyl-β-cyclodextrin host—Guest system: Improving dissolution while maintaining antifungal activity. Carbohydr. Polym. 2016, 142, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Alves, N.M.; Mano, J.F. Chitosan coated alginate beads containing poly (N-isopropylacrylamide) for dual-stimuli-responsive drug release. J. Biomed. Mater. Res. Part B 2008, 84, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Karakas, C.Y.; Ordu, H.R.; Bozkurt, F.; Karadag, A. Electrosprayed chitosan-coated alginate—Pectin beads as potential system for colon-targeted delivery of ellagic acid. J. Sci. Food Agric. 2022, 102, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Padhmavathi, V.; Shruthy, R.; Preetha, R. Chitosan coated skim milk-alginate microspheres for better survival of probiotics during gastrointestinal transit. J. Food. Sci. Technol. 2021, 60, 889–895. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.M.; Yang, L.Y.; Ouyang, X.K. Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 132, 487–494. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Chatzitaki, A.T.; Karavasili, C.; Katsamenis, O.L.; Tzetzis, D.; Mystiridou, E.; Bouropoulos, N.; Fatouros, D.G. Controlled release of 5-fluorouracil from alginate beads encapsulated in 3D printed pH-responsive solid dosage forms. AAPS PharmSciTech 2018, 19, 3362–3375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeeb, B.; Saberi, A.H.; Weiss, J.; McClements, D.J. Retention and release of oil-in-water emulsions from filled hydrogel beads composed of calcium alginate: Impact of emulsifier type and pH. Soft Matter. 2015, 11, 2228–2236. [Google Scholar] [CrossRef]
- Omer, A.M.; Taher, M.A.; Hamed, A.M.; Ali, A.M.; Tamer, T.M.; Eldin, M.S.M. Development of smart alginate/chitosan grafted microcapsules for colon site-specific drug delivery. Egypt J. Chem. 2019, 62, 1037–1045. [Google Scholar] [CrossRef]
- Umaredkar, A.A.; Dangre, P.V.; Mahapatra, D.K.; Dhabarde, D.M. Fabrication of chitosan-alginate polyelectrolyte complexed hydrogel for controlled release of cilnidipine: A statistical design approach. Mater. Technol. 2020, 35, 697–707. [Google Scholar] [CrossRef]
- Rehman, S.; Ranjha, N.M.; Raza, M.R.; Hanif, M.; Majed, A.; Ameer, N. Enteric-coated Ca-alginate hydrogel beads: A promising tool for colon targeted drug delivery system. Polym. Bull. 2021, 78, 5103–5117. [Google Scholar] [CrossRef]
- Othman, S.F.C.; Samsudin, M.N.; Abdullah, N.I.W. Assessing the Stability of Essential Oil Encapsulated in Hydrogel Beads: Assessing the Stability of Essential Oil Encapsulated in Hydrogel. J. Trop. Life Sci. 2023, 13, 51–60. [Google Scholar]
- Sawarkar, S.P.; Deshpande, S.G.; Bajaj, A.N.; Nikam, V.S. In vivo evaluation of 5-ASA colon-specific tablets using experimental-induced colitis rat animal model. AAPS PharmSciTech 2015, 16, 1445–1454. [Google Scholar] [CrossRef]
- Singh, A.; Mandal, U.K.; Narang, R.K. Development and characterization of enteric coated pectin pellets containing mesalamine and Saccharomyces boulardii for specific inflamed colon: In vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2021, 62, 102393. [Google Scholar] [CrossRef]
- Nidhi; Rashid, M.; Kaur, V.; Hallan, S.S.; Sharma, S.; Mishra, N. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis: A brief review. Saudi Pharm. J. 2016, 24, 458–472. [Google Scholar] [CrossRef] [Green Version]
- Desai, N.; Momin, M. Colon targeted bioadhesive pellets of curcumin and cyclosporine for improved management of inflammatory bowel disease. Drug Deliv. Transl. Res. 2020, 10, 1288–1301. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Zhu, Y.; Ma, J.; Ma, H.; Zhang, H. Probiotic mixture protects dextran sulfate sodium-induced colitis by altering tight junction protein expressions and increasing Tregs. Mediat. Inflamm. 2018, 2018, 9416391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
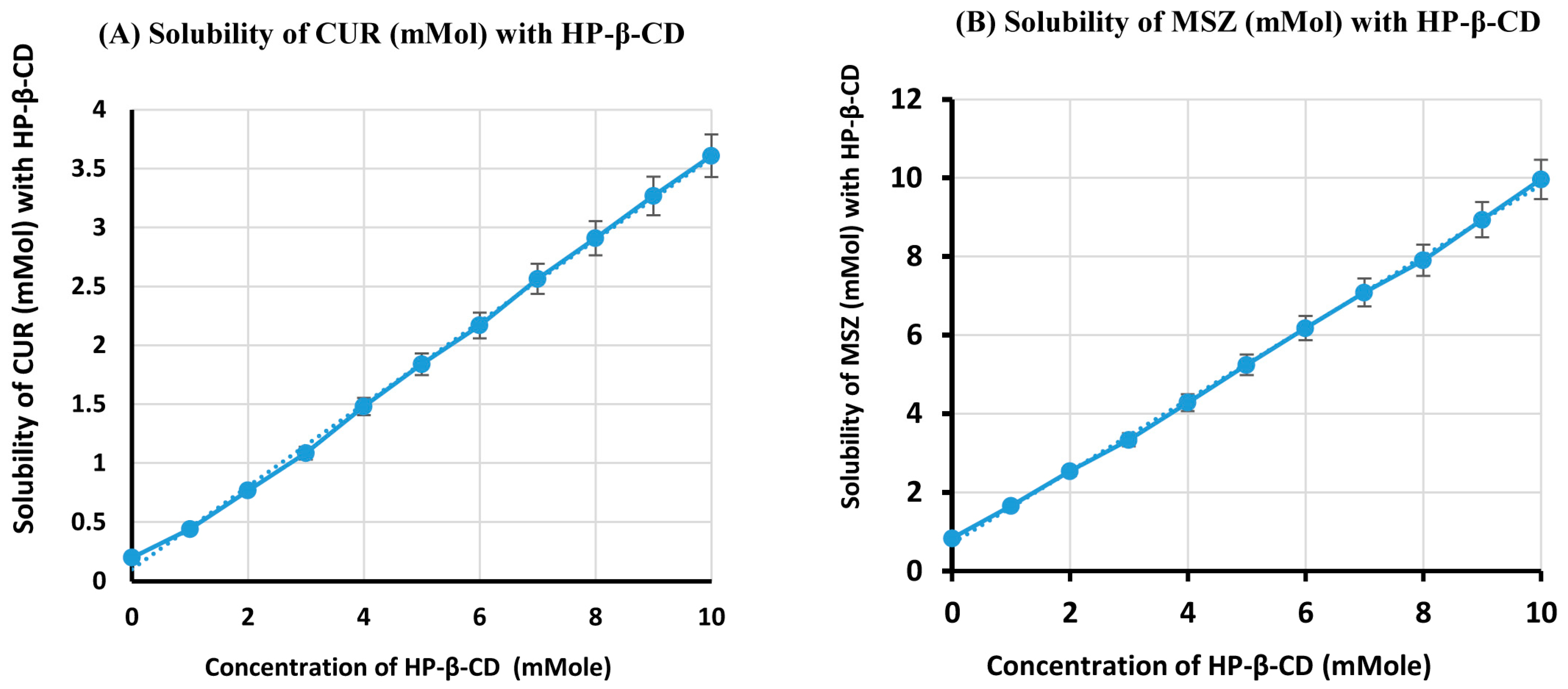
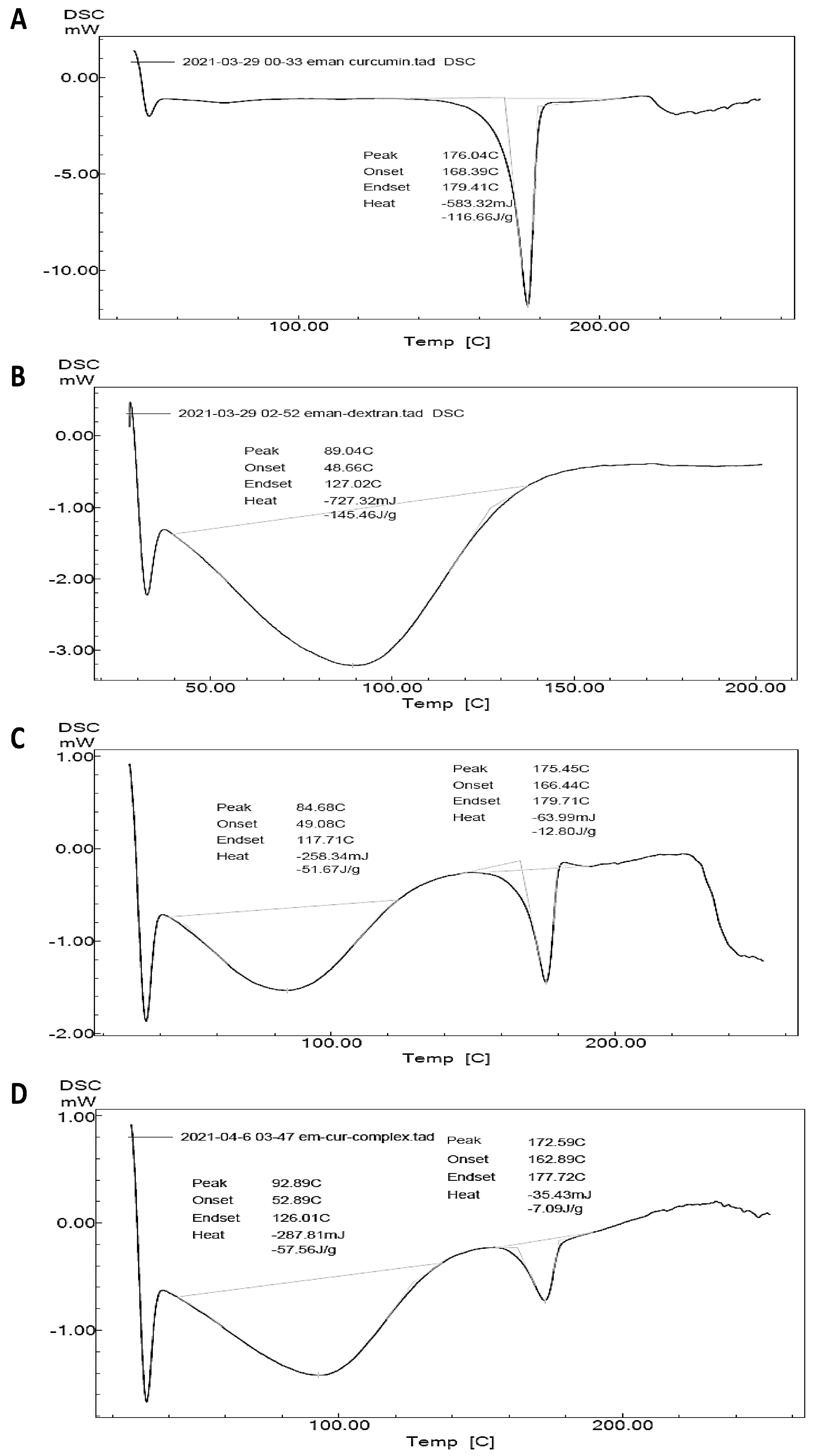
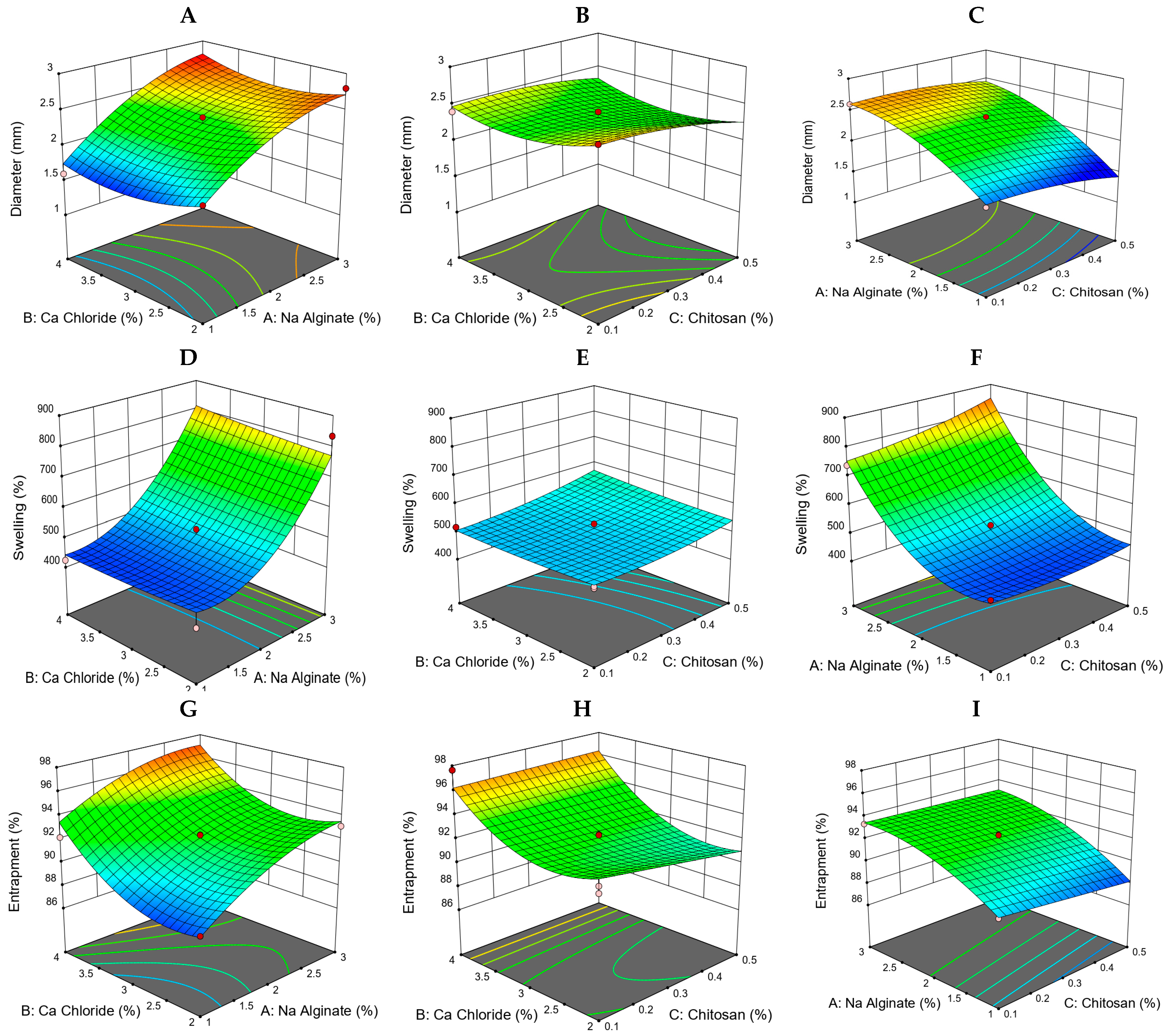
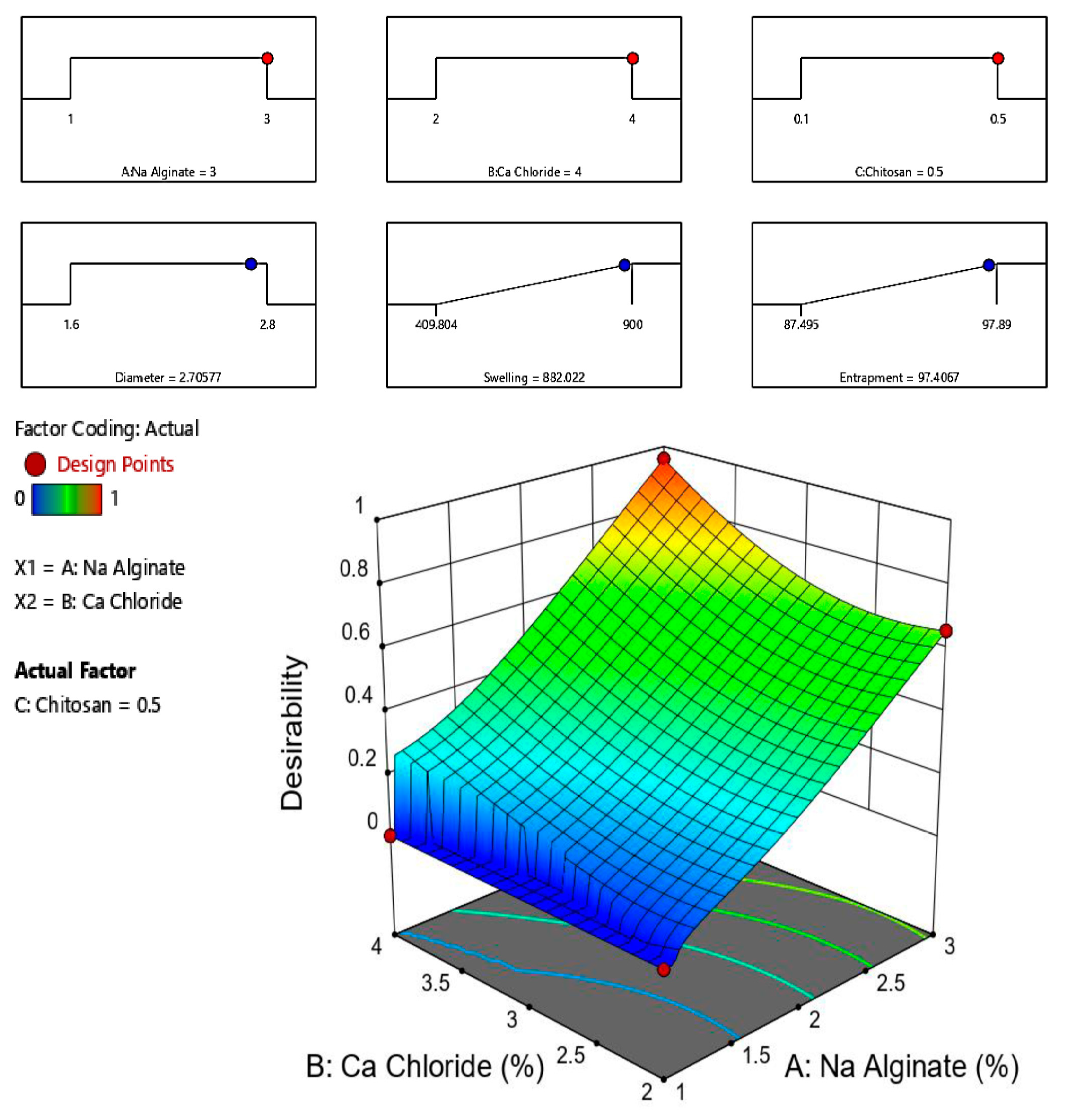
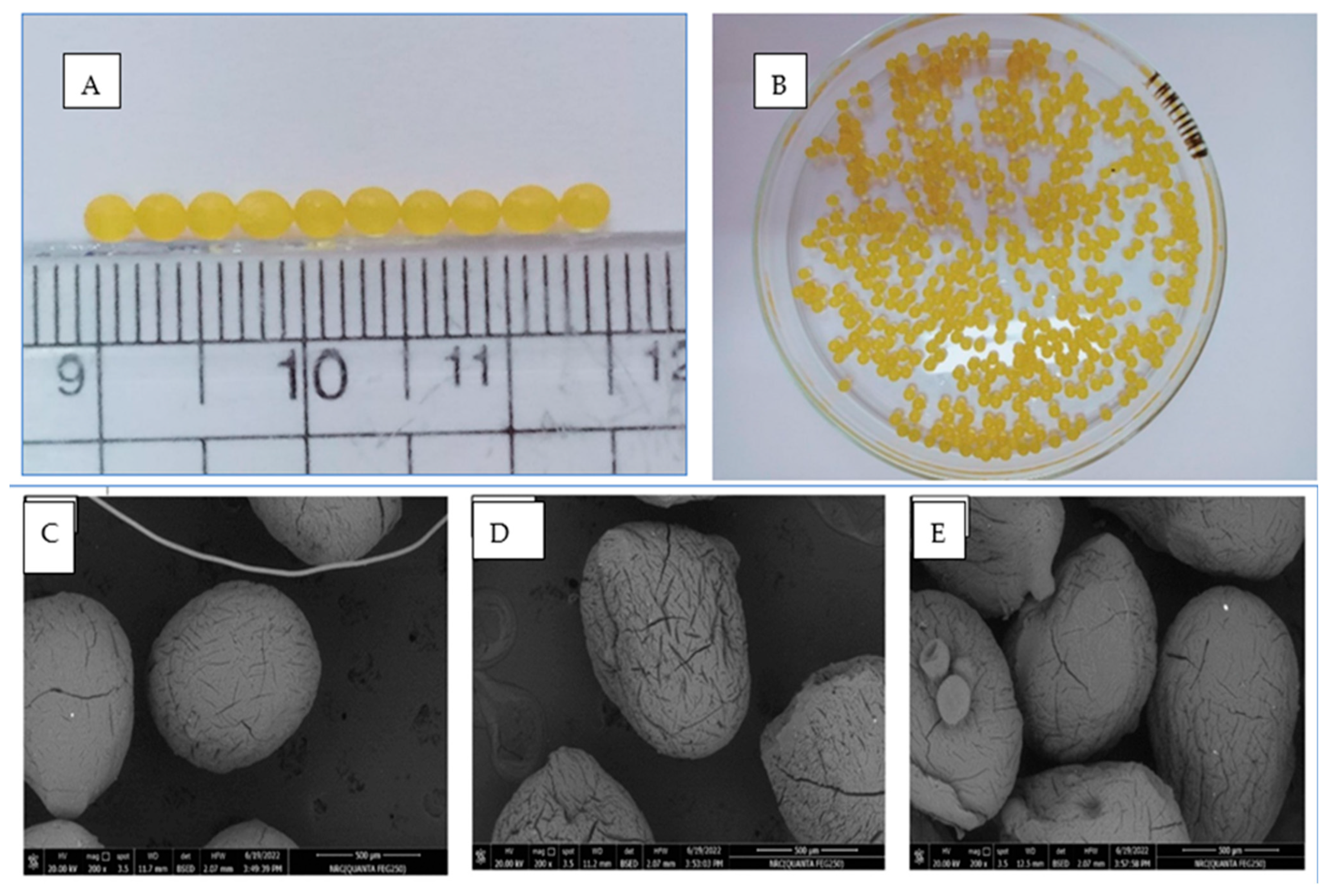

| Concentration of Cyclodextrin (mMol) | Solubility of CUR (mMol) with HP-β-CD | Solubility of MSZ (mMol) with HP-β-CD |
|---|---|---|
| 0 | 0.196 ± 0.013 | 0.833 ± 0.045 |
| 1 | 0.442 ± 0.022 | 1.856 ± 0.081 |
| 2 | 0.768 ± 0.041 | 2.538 ± 0.095 |
| 3 | 1.074 ± 0.053 | 3.333 ± 0.137 |
| 4 | 1.420 ± 0.068 | 4.280 ± 0.231 |
| 5 | 1.739 ± 0.077 | 5.038 ± 0.256 |
| 6 | 2.158 ± 0.091 | 6.174 ± 0.343 |
| 7 | 2.564 ± 0.097 | 7.083 ± 0.375 |
| 8 | 2.910 ± 0.136 | 7.803 ± 0.387 |
| 9 | 3.269 ± 0.142 | 8.636 ± 0.401 |
| 10 | 3.609 ± 0.145 | 9.962 ± 0.434 |
| Group | Histopathology Scoring |
|---|---|
| Saline control | Inflammation (1). Score = 1 |
| UC control (untreated) | Inflammation (3), Inflamed area (3), Crypt damage (3), %involvement (3). Score = 12 * |
| UC + blank beads | Inflammation (3), Inflamed area (3), Crypt damage (3), %involvement (3). Score = 12 * |
| UC + free MSZ | Inflammation (2), Inflamed area (2), Crypt damage (2), %involvement (2). Score = 8 * |
| UC + coated MSZ-loaded beads | Inflammation (1), Inflamed area (1), Crypt damage (1), %involvement (1). Score = 4 # |
| UC + coated CUR/MSZ-loaded beads | Inflammation (1), Inflamed area/extent (1). Score = 2 # |
| Factors | Independent Variables | Low (−1) | Medium (0) | High (+1) | |
|---|---|---|---|---|---|
| X1 | Na Alginate (SALG) | % | 1 | 2 | 3 |
| X2 | Ca Chloride | % | 2 | 3 | 4 |
| X3 | Chitosan (CS) | % | 0.1 | 0.3 | 0.5 |
| Response | Dependent Variables | ||||
| Y1 | Diameter | Mm | In range | ||
| Y2 | Swelling | % | Maximize | ||
| Y3 | Entrapment Efficiency | % | Maximize | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heikal, E.J.; Kaoud, R.M.; Gad, S.; Mokhtar, H.I.; Alattar, A.; Alshaman, R.; Zaitone, S.A.; Moustafa, Y.M.; Hammady, T.M. Development of Novel pH-Sensitive Eudragit Coated Beads Containing Curcumin-Mesalamine Combination for Colon-Specific Drug Delivery. Gels 2023, 9, 264. https://doi.org/10.3390/gels9040264
Heikal EJ, Kaoud RM, Gad S, Mokhtar HI, Alattar A, Alshaman R, Zaitone SA, Moustafa YM, Hammady TM. Development of Novel pH-Sensitive Eudragit Coated Beads Containing Curcumin-Mesalamine Combination for Colon-Specific Drug Delivery. Gels. 2023; 9(4):264. https://doi.org/10.3390/gels9040264
Chicago/Turabian StyleHeikal, Eman J., Rashad M. Kaoud, Shadeed Gad, Hatem I. Mokhtar, Abdullah Alattar, Reem Alshaman, Sawsan A. Zaitone, Yasser M. Moustafa, and Taha M. Hammady. 2023. "Development of Novel pH-Sensitive Eudragit Coated Beads Containing Curcumin-Mesalamine Combination for Colon-Specific Drug Delivery" Gels 9, no. 4: 264. https://doi.org/10.3390/gels9040264
APA StyleHeikal, E. J., Kaoud, R. M., Gad, S., Mokhtar, H. I., Alattar, A., Alshaman, R., Zaitone, S. A., Moustafa, Y. M., & Hammady, T. M. (2023). Development of Novel pH-Sensitive Eudragit Coated Beads Containing Curcumin-Mesalamine Combination for Colon-Specific Drug Delivery. Gels, 9(4), 264. https://doi.org/10.3390/gels9040264







