Nanofabrication Technologies to Control Cell and Tissue Function in Three-Dimension
Abstract
1. Introduction
2. Cell-Patterning Techniques
2.1. 2D Cell Patterning
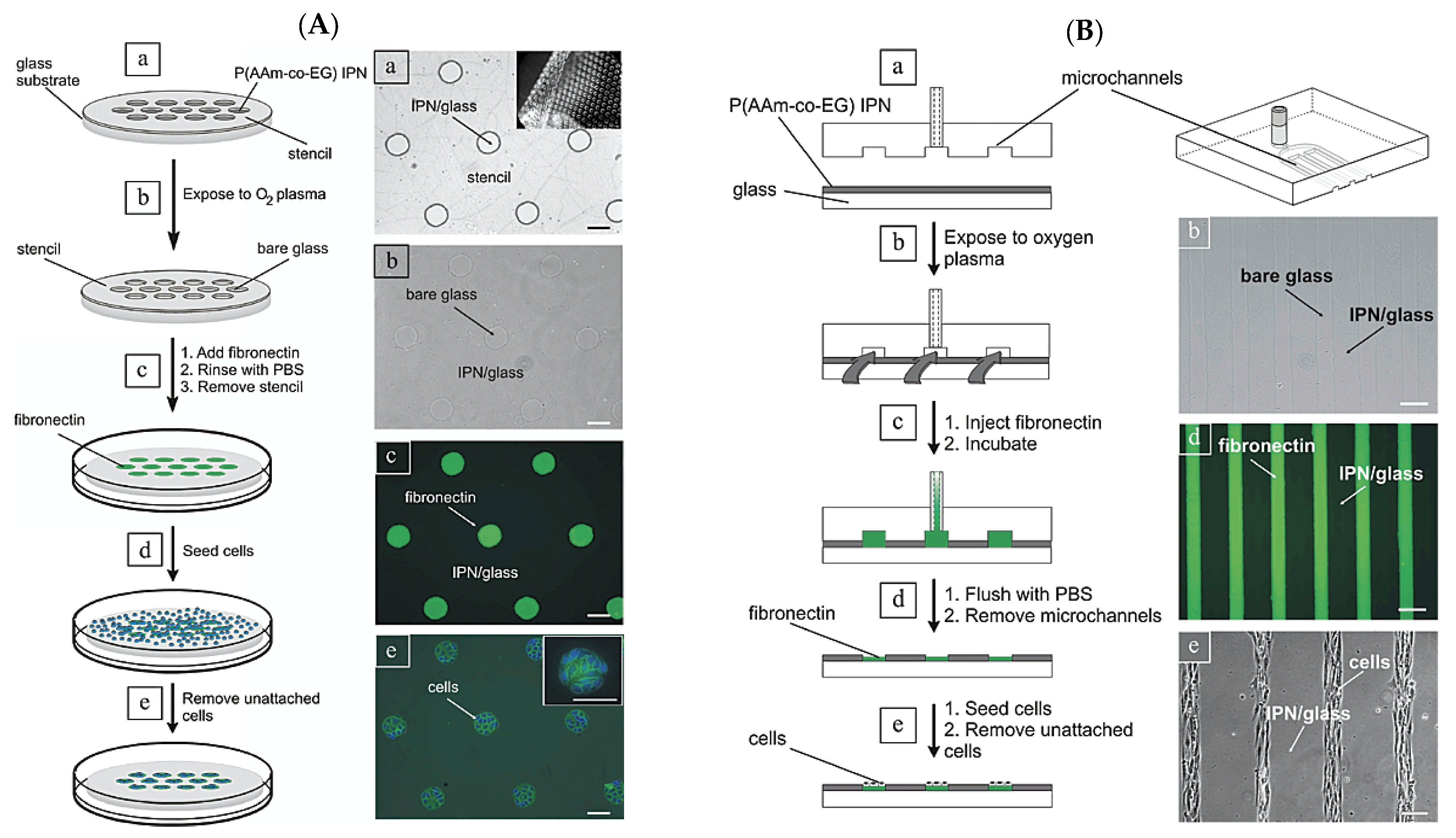
2.2. Dry Etching (Plasma Etching)
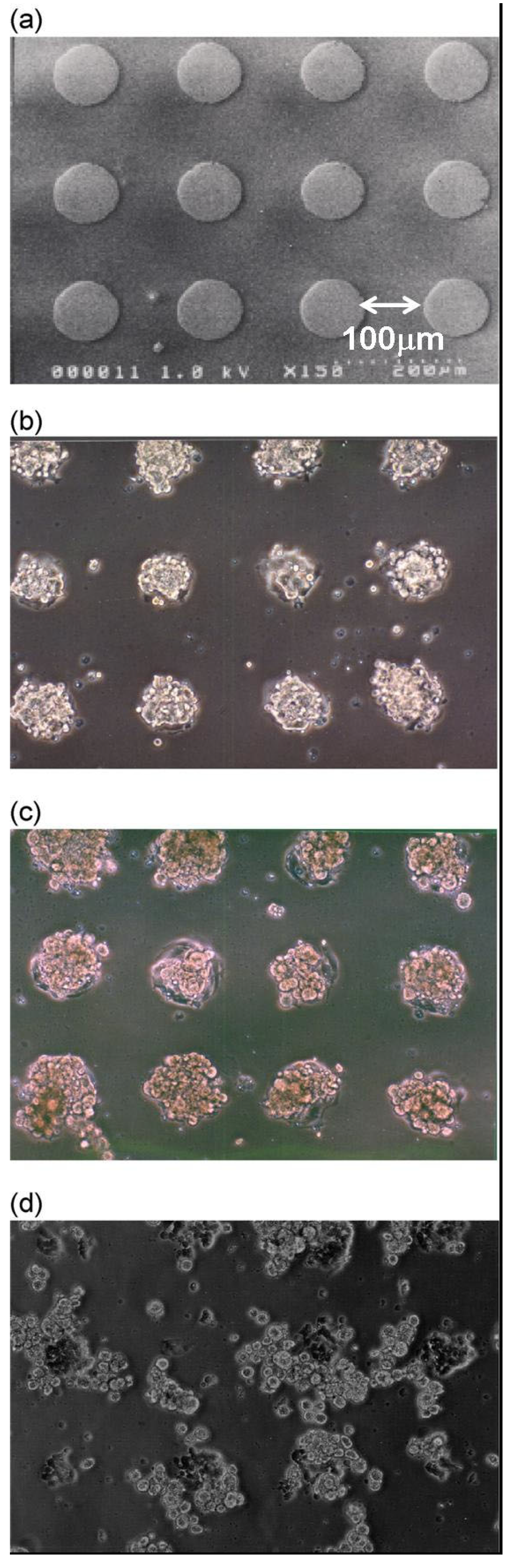
2.3. Patterning of Spheroids as 3D-Microorganized Cells
2.4. Cell Aggregates for Tissue Engineering
3. Hydrogels as Three-Dimensional Cell Scaffolds
3.1. Synthetic Polymers
3.2. Biopolymers
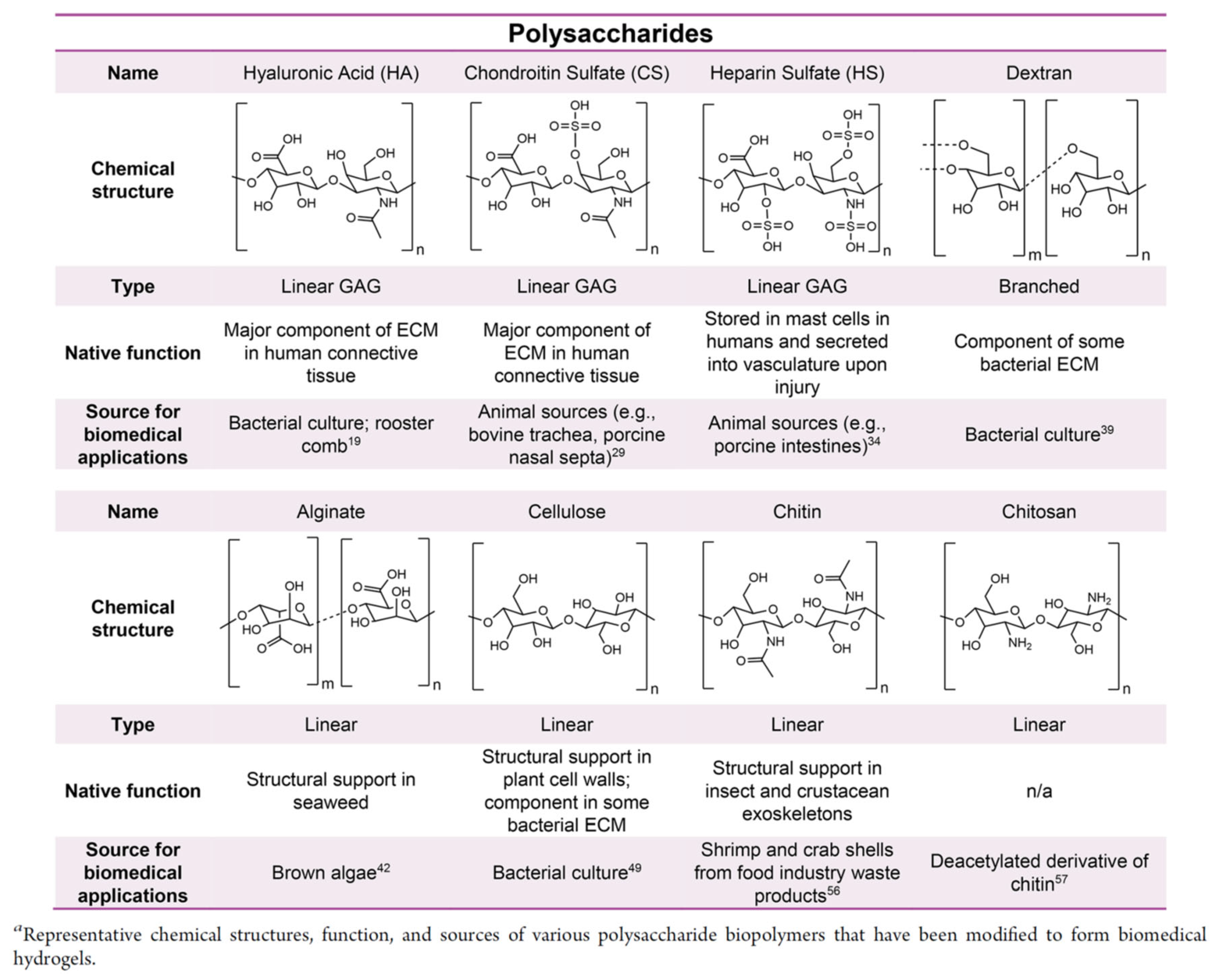
3.2.1. Polysaccharides
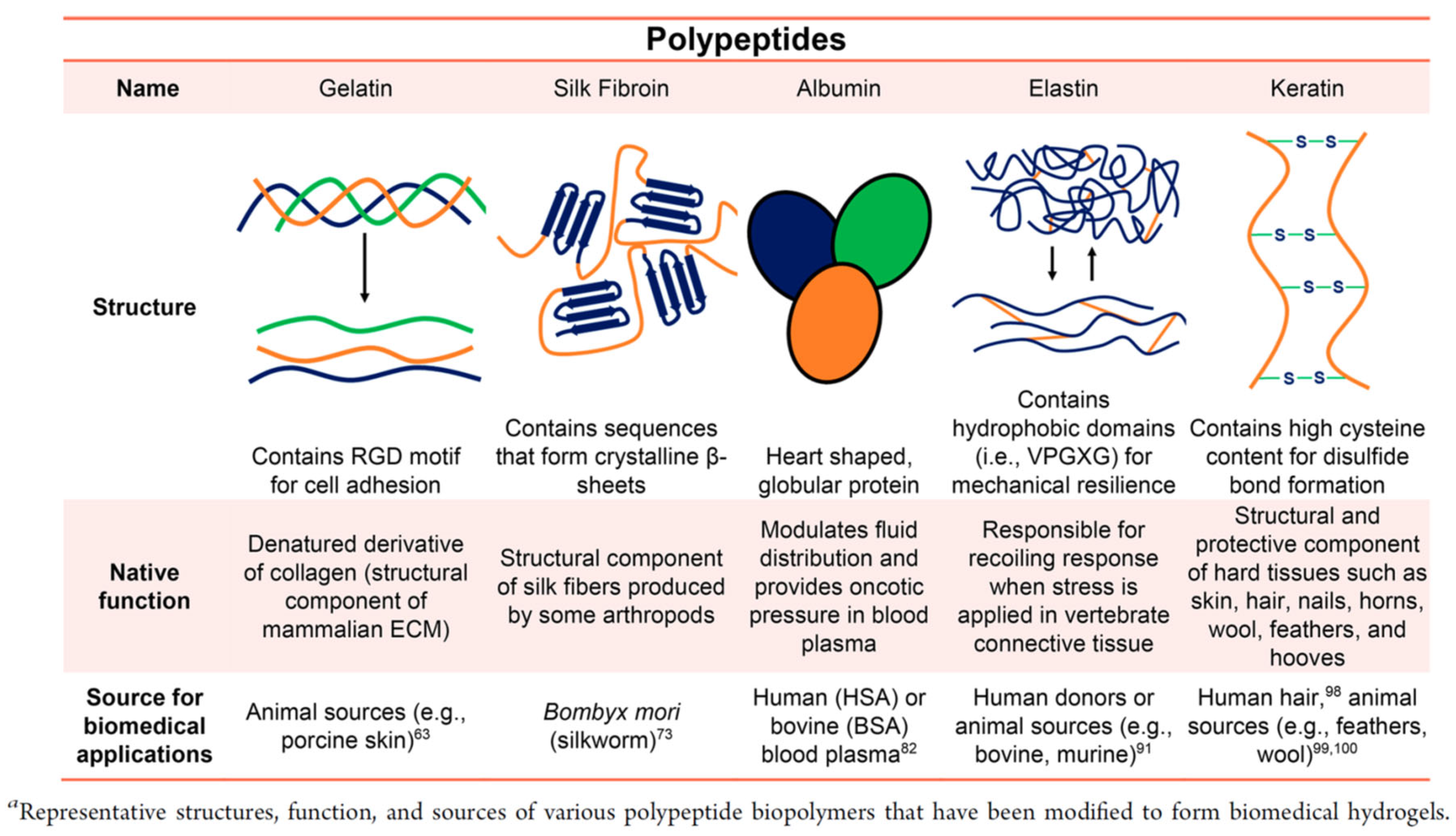
3.2.2. Polypeptide
3.3. Methodologies of Cross-Linking
3.3.1. Chemical Cross-Linking
Click Chemistry
Active-Ester Reaction
Schiff Bases
3.3.2. Physical Cross-Linking
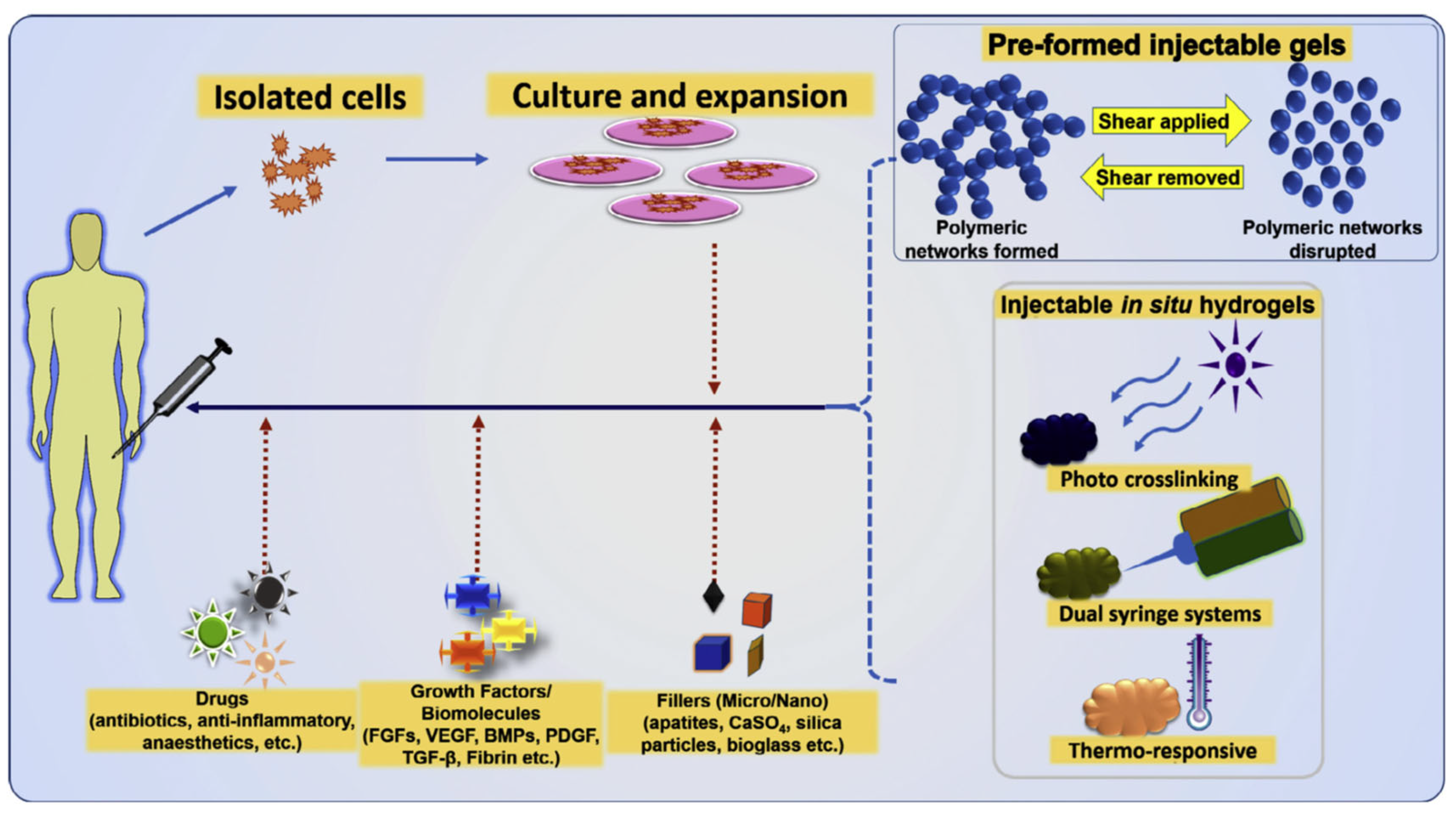
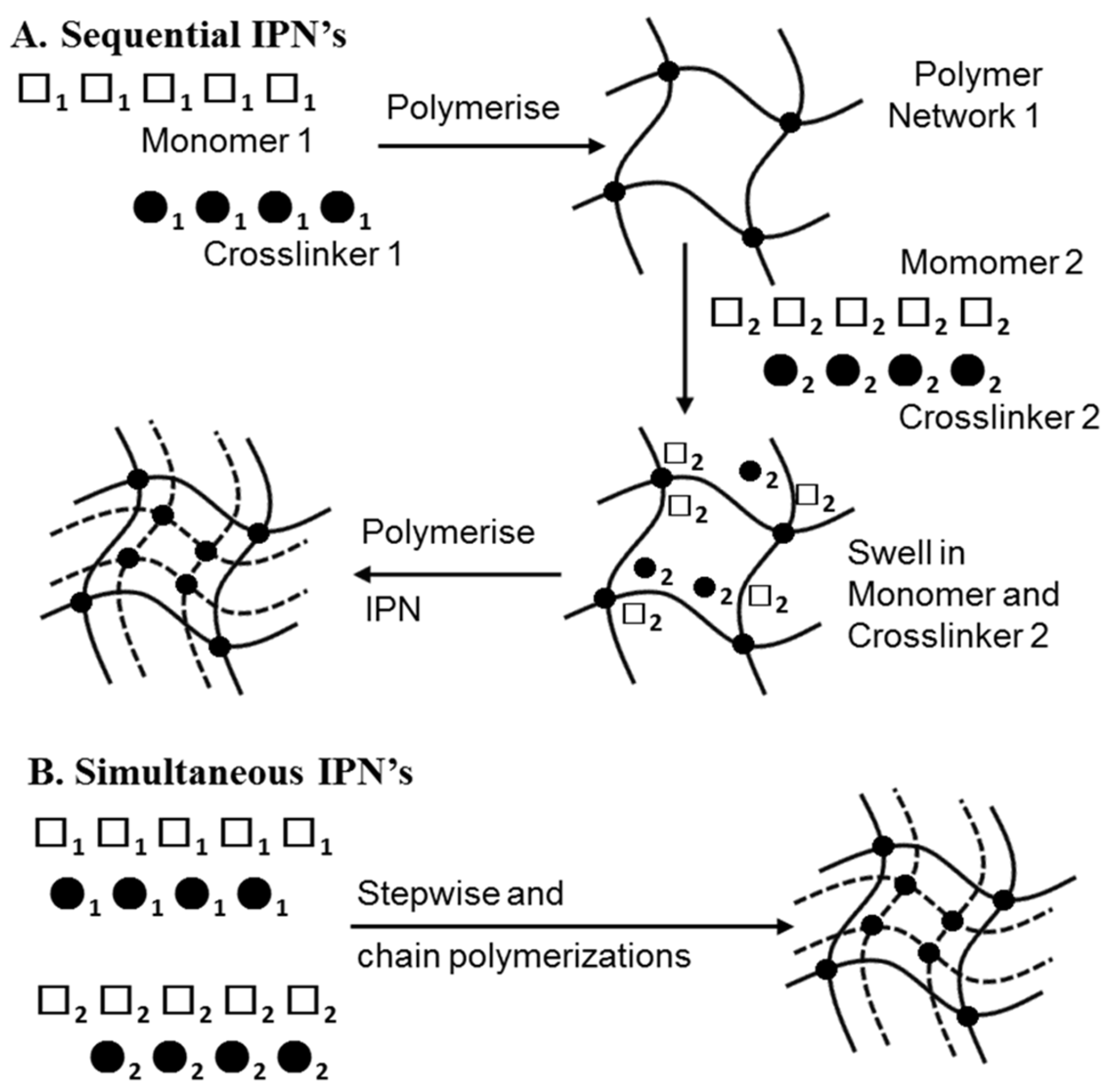
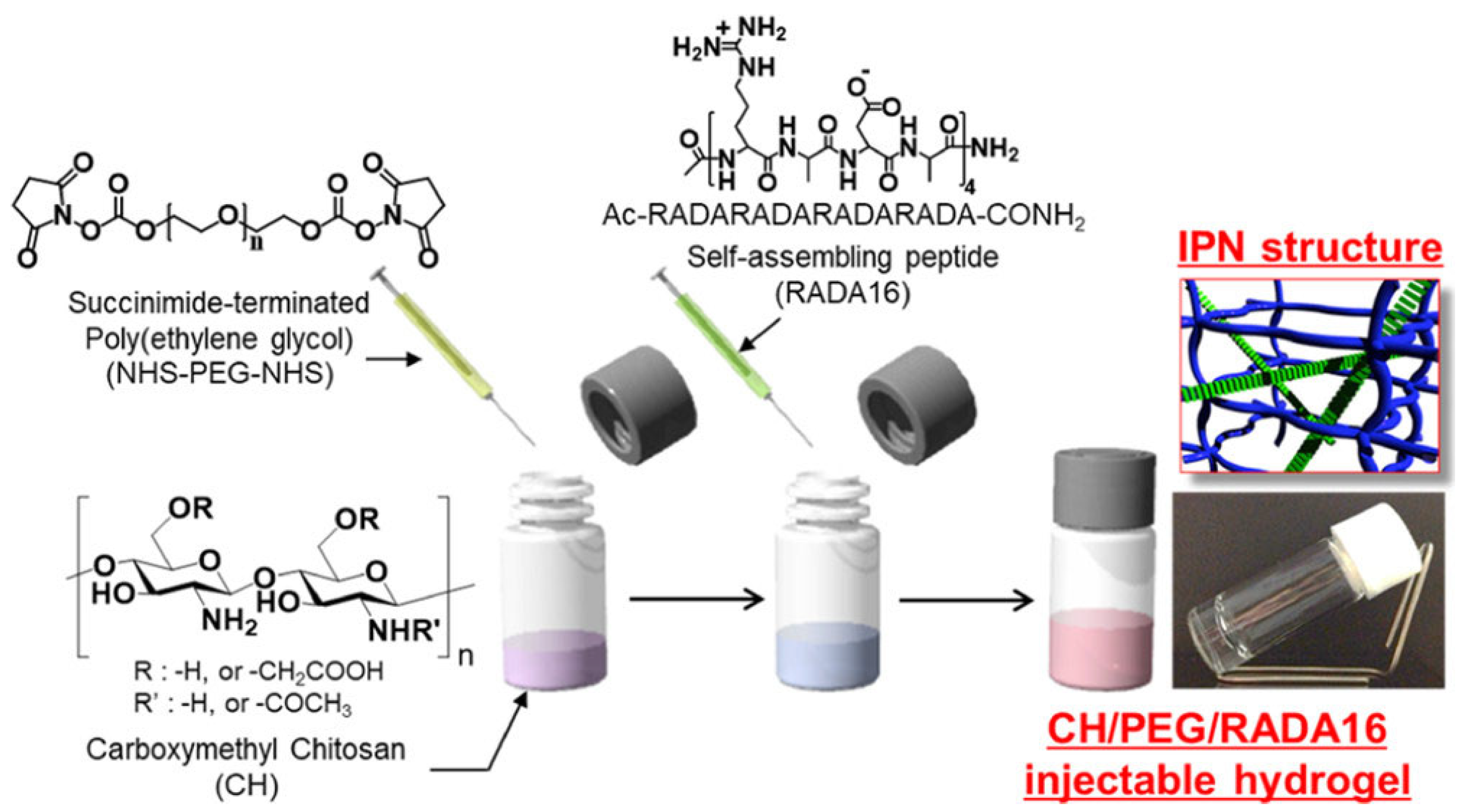
3.3.3. Injectable IPN Gels as New and Minimally Invasive Applications
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sivashanmugam, A.; Kumar, R.A.; Priya, M.V.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Jeon, N.L.; Baskaran, H.; Detringer, S.K.W.; Whitesides, G.M.; Water, L.V.; Toner, M. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat. Biotechnol. 2002, 20, 826–830. [Google Scholar] [CrossRef]
- Britland, S.; Clark, P.; Connolly, P.; Moores, G. Micropatterned substratum adhesiveness: A model for morphogenetic cues controlling cell behavior. Ex. Cell Res. 1992, 198, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Healy, K.E.; Lom, B.; Hockberger, P.E. Spatial distribution of mammalian cells dictated by material surface chemistry. Biotechnol. Bioeng. 1994, 43, 792–800. [Google Scholar] [CrossRef]
- Hickman, J.J.; Bhatia, S.K.; Quong, J.N.; Shoen, P.; Stenger, D.A.; Pike, C.J.; Cotman, C.W. Rational pattern design for in-vitro cellular networks using surface photochemistry. J. Vac. Sci. Technol. A 1994, 12, 607–616. [Google Scholar] [CrossRef]
- Bekos, E.J.; Ranien, J.P.; Aebischer, P.; Gardella, J.A.; Bnght, F.V. Structural Changes of Bovine Serum Albumin upon Adsorption to Modified Fluoropolymer Substrates Used for Neural Cell Attachment Studies. Langmuir 1995, 11, 984–989. [Google Scholar] [CrossRef]
- Singhvi, R.; Kumar, A.; Lopez, G.P.; Stehanopoulos, G.N.; Wang, D.I.C.; Whitesides, G.M.; Ingber, D.E. Engineering Cell Shape and Function. Science 1994, 264, 696–698. [Google Scholar] [CrossRef]
- Spargo, B.J.; Testoff, M.A.; Nielsen, T.B.; Stenger, D.A.; Hickman, J.J.; Rudolph, A.A. Spatially controlled adhesion, spreading, and differentiation of endothelial cells on self-assembled molecular monolayers. Proc. Natl. Acad. Sci. USA 1994, 91, 11070–11074. [Google Scholar] [CrossRef] [PubMed]
- Stenger, D.A.; Georger, J.H.; Dulcey, C.S.; Hickman, J.J.; Rudolph, A.S.; Nielsen, T.B.; McCort, S.M.; Calvert, J.M. Coplanar molecular assemblies of amino- and perfluorinated alkylsilanes: Characterization and geometric definition of mammalian cell adhesion and growth. J. Am. Chem. Soc. 1992, 114, 8435–8442. [Google Scholar] [CrossRef]
- van den Berg, A.; Lammerink, T.S.J. Micro Total Analysis Systems: Microfluidic Aspects, Integration Concept and Applications. In Microsystem Technology in Chemistry and Life Science. Topics in Current Chemistry; Manz, A., Becker, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 194, pp. 21–49. [Google Scholar]
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuat. B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Balis, U.J.; Yarmush, M.L.; Toner, M. Effect of cell-cell interactions in preservation of cellular phenotype: Cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999, 13, 1883–1900. [Google Scholar] [CrossRef] [PubMed]
- Folch, A.; Toner, M. Microengineering of cellular interactions. Annu. Rev. Biomed. Eng. 2000, 2, 227–256. [Google Scholar] [CrossRef]
- Tsang, V.L.; Bhatia, S.N. Three-dimensional tissue engineering. Adv. Drug. Deliv. Rev. 2004, 56, 1635–1647. [Google Scholar] [CrossRef]
- Wang, W.; Itaka, K.; Ohba, S.; Nishiyam, N.; Chung, U.; Yamasaki, Y.; Kataoka, K. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials 2009, 30, 2705–2715. [Google Scholar] [CrossRef]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric Control of Cell Life and Death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Zamir, E.; Katz, B.Z.; Aota, K.M.; Yamada, K.M.; Geiger, B.; Kam, Z. Molecular diversity of cell-matrix adhesions. J. Cell Sci. 1999, 112, 1655. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, R.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between t he extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef]
- Chou, L.; Firth, J.D.; Uitto, V.J.; Brunette, D.M. Substratum surface topography alters cell shape and regulates fibronectin mRNA level, mRNA stability, secretion and assembly in human fibroblasts. J. Cell. Sci. 1995, 108, 1563–1573. [Google Scholar] [CrossRef]
- Clark, P.; Connolly, P.; Curtis, A.S.G.; Dow, J.A.T.; Wilkinson, C.D.W. Topographical control of cell behaviour: II. Multiple grooved substrata. Development 1990, 108, 635–644. [Google Scholar] [CrossRef]
- Stenger, D.A.; Gross, G.W.; Keefer, E.W.; Shaffer, K.M.; Andreadis, J.D.; Ma, W.; Pancrazio, J.J. Detection of physiologically active compounds using cell-based biosensors. Trends Biotechnol. 2001, 19, 304–309. [Google Scholar] [CrossRef]
- Kononen, J.; Bubendorf, L.; Kallioniemi, A.; Barlund, M.; Schraml, P.; Leighton, S.; Torhorst, J.; Mihatsch, M.J.; Sauter, G.; Kallioniemi, O.P. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med. 1998, 4, 844–847. [Google Scholar] [CrossRef]
- Ziauddin, J.; Sabatini, D.M. Microarrays of cells expressing defined cDNAs. Nature 2001, 411, 107–110. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; DeFrances, M.C. Liver regeneration. Science 1997, 276, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.G.; Levenberg, S.; Langer, R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 2004, 22, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Revzin, A.; Tompkins, R.G.; Toner, M. Surface Engineering with Poly(ethylene glycol) Photolithography to Create High-Density Cell Arrays on Glass. Langmuir 2003, 19, 9855–9862. [Google Scholar] [CrossRef]
- Thielecke, H.; Mack, A.; Robitzki, A. A multicellular spheroid-based sensor for anti-cancer therapeutics. Biosen. Bioelect. 2001, 16, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mack, A.R.; Thielecke, H.; Robitzki, A.A. 3D-biohybrid systems: Applications in drug screening. Trends Biotechnol. 2002, 20, 56–61. [Google Scholar] [CrossRef]
- Otsuka, H.; Hirano, A.; Nagasaki, Y.; Okano, T.; Horiike, Y.; Kataoka, K. Two-dimensional multiarray formation of hepatoytes sphroids on a microfabricated PEG-brush surface. ChemBioChem 2004, 5, 850–855. [Google Scholar] [CrossRef]
- Koudan, E.V.; Bulanova, E.A.; Pereira, F.D.A.S.; Parfenov, V.A.; Kasyanov, V.A.; Hesuani, Y.D.; Mironov, V.A. Patterning of tissue spheroids biofabricated from human fibroblasts on the surface of electrospun polyurethane matrix using 3D bioprinter. Int. J. Bioprinting 2016, 2, 45–52. [Google Scholar]
- Ko, J.; Ahn, J.; Kim, S.; Lee, Y.; Lee, J.; Park, D.; Jeon, N.L. Tumor spheroid-on-a-chip: A standardized microfluidic culture platform for investigating tumor angiogenesis. Lab Chip 2019, 19, 2822–2833. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Hsieh, F.Y.; Hsu, S.H. Self-patterning of adipose-derived mesenchymal stem cells and chondrocytes cocultured on hyaluronan-grafted chitosan surface. Biointerphases 2016, 11, 11011. [Google Scholar] [CrossRef] [PubMed]
- Berg, I.C.; Mohagheghian, E.; Habing, K.; Wang, N.; Underhill, G.H. Microtissue Geometry and Cell-Generated Forces Drive Patterning of Liver Progenitor Cell Differentiation in 3D. Adv. Healthcare Mater. 2021, 10, 2100223. [Google Scholar] [CrossRef]
- Su, C.; Chuah, Y.J.; Ong, H.B.; Tay, H.M.; Dalan, R.; Hou, H.W. A facile and scalable hydrogel patterning method for microfluidic 3D cell culture and spheroid-in-gel culture array. Biosensors 2021, 11, 509. [Google Scholar] [CrossRef]
- Heo, D.N.; Hospodiuk, M.; Ozbolat, I.T. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019, 95, 348–356. [Google Scholar] [CrossRef]
- Ho, S.S.; Keown, A.T.; Addison, B.; Leach, J.K. Cell Migration and Bone Formation from Mesenchymal Stem Cell Spheroids in Alginate Hydrogels Are Regulated by Adhesive Ligand Density. Biomacromolecules 2017, 18, 4331–4340. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; He, S.; Su, Z.; Yang, Z.; Liang, X.; Wu, Y. Thermosensitive Injectable Chitosan/Collagen/β-Glycerophosphate Composite Hydrogels for Enhancing Wound Healing by Encapsulating Mesenchymal Stem Cell Spheroids. ACS Omega 2020, 5, 21015–21023. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, E.M.; Yamamoto, M.; Park, H.; Shin, H. Engineering Multi-Cellular Spheroids for Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2020, 9, 2000608. [Google Scholar] [CrossRef]
- Baptista, L.S.; Kronemberger, G.S.; Côrtes, I.; Charelli, L.E.; Matsui, R.A.M.; Palhares, T.N.; Sohier, J.; Rossi, A.M.; Granjeiro, J.M. Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.C.; Wong, C.W.; Hsieh, F.Y.; Hsu, S.H. Biomaterial Substrate-Mediated Multicellular Spheroid Formation and Their Applications in Tissue Engineering. Biotechnol. J. 2017, 12, 1700064. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Murphy, K.C.; Binder, B.Y.K.; Vissers, C.B.; Leach, J.K. Increased Survival and Function of Mesenchymal Stem Cell Spheroids Entrapped in Instructive Alginate Hydrogels. Stem Cells Transl. Med. 2016, 5, 773–781. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Yazdi, M.K.; Ghavami, S.B.; Farokhimanesh, S.; Amirabad, L.M.; Zarrintaj, P.; Saeb, M.; Hamblin, M.R.; Zare, M.; Mozafari, M. Mesenchymal Stem Cell Spheroids Embedded in an Injectable Thermosensitive Hydrogel: An In Situ Drug Formation Platform for Accelerated Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5096–5109. [Google Scholar] [CrossRef]
- Gionet-Gonzales, M.A.; Leach, J.K. Engineering principles for guiding spheroid function in the regeneration of bone, cartilage, and skin. Biomed. Mater. 2018, 13, 034109. [Google Scholar] [CrossRef] [PubMed]
- Griffin, K.H.; Fok, S.W.; Leach, J.K. Strategies to capitalize on cell spheroid therapeutic potential for tissue repair and disease modeling. NPJ Regen. Med. 2022, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Hoch, A.I.; Mittal, V.; Mitra, D.; Vollmer, N.; Zikry, C.A.; Leach, J.K. Cell-secreted matrices perpetuate the bone-forming phenotype of differentiated mesenchymal stem cells. Biomaterials 2016, 74, 178–187. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Zhou, X.; Han, J.; Huang, C.Y.; Nashed, A.; Khatri, S.; Mattson, G.; Fukunishi, T.; Zhang, H.; Hibino, N. In vivo therapeutic applications of cell spheroids. Biotechnol. Adv. 2018, 36, 494–505. [Google Scholar] [CrossRef]
- Shen, J.X.; Youhanna, S.; Shafagh, R.Z.; Kele, J.; Lauschke, V.M. Organotypic and Microphysiological Models of Liver, Gut, and Kidney for Studies of Drug Metabolism, Pharmacokinetics, and Toxicity. Chem. Res. Toxicol. 2020, 33, 38–60. [Google Scholar] [CrossRef]
- Rodrigues, T.; Kundu, B.; Correia, J.S.; Kundu, S.C.; Oliveira, J.M.; Reis, R.L.; Correlo, V.M. Emerging tumor spheroids technologies for 3D in vitro cancer modeling. Pharmacol. Ther. 2018, 184, 201–211. [Google Scholar] [CrossRef]
- Park, J.; Choe, G.; Oh, S.; Lee, J.Y. In Situ Formation of Proangiogenic Mesenchymal Stem Cell Spheroids in Hyaluronic Acid/Alginate Core–Shell Microcapsules. ACS Biomater. Sci. Eng. 2020, 6, 6938–6948. [Google Scholar] [CrossRef]
- Whitehead, J.; Griffin, K.H.; Gionet-Gonzales, M.; Vorwald, C.E.; Cinque, S.E.; Leach, J.K. Hydrogel mechanics are a key driver of bone formation by mesenchymal stromal cell spheroids. Biomaterials 2021, 269, 120607. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Byun, H.; Lee, J.; Kumar, S.; Perikamana, M.; Shin, Y.M.; Kim, E.M.; Shin, H. Stem cell spheroids incorporating fibers coated with adenosine and polydopamine as a modular building blocks for bone tissue engineering. Biomaterials 2020, 230, 119652. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Choi, J.H.; Lee, S.; Song, J.E.; Khang, G. Fabrication and characterization of silk fibroin microfiberincorporated bone marrow stem cell spheroids to promote cell-cell interaction and osteogenesis. ACS Omega 2020, 5, 18021–18027. [Google Scholar] [CrossRef]
- Ahmad, T.; Shin, H.J.; Lee, J.; Shin, Y.M.; Perikamana, S.K.M.; Park, S.Y.; Jung, H.S.; Shin, H. Fabrication of in vitro 3D mineralized tissue by fusion of composite spheroids incorporating biomineral-coated nanofibers and human adipose-derived stem cells. Acta Biomater. 2018, 74, 464–477. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Ahmad, T.; Perikamana, S.K.M.; Lee, J.; Kim, E.M.; Shin, H. Human adiposederived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials 2020, 255, 120192. [Google Scholar] [CrossRef]
- Lee, N.-H.; Bayaraa, O.; Zechu, Z.; Kim, H.S. Biomaterials-assisted spheroid engineering for regenerative therapy. BMB Rep. 2021, 54, 356–367. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, J.; Fang, H.; Li, G.; Yan, S.; Zhang, K.; Wang, C.; Yin, J. All-in-one hydrogel realizing adipose-derived stem cell spheroid production and in vivo injection via “Gel-Sol” transition for angiogenesis in hind limb ischemia. ACS Appl. Mater. Interfaces 2020, 12, 11375–11387. [Google Scholar] [CrossRef]
- Ran, P.; Chen, W.; Wei, J.; Qiu, B.; Chen, M.; Xie, S.; Li, X. Macrophage spheroids with chronological phenotype shifting to promote therapeutic angiogenesis in critical limb ischemia. ACS Appl. Bio Mater. 2020, 3, 3707–3717. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yoshitomi, H.; Rossant, J.; Zaret, K.S. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 2001, 294, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Zaret, K.S. Regulatory phases of early liver development: Paradigms of organogenesis. Nat. Rev. Genet. 2002, 3, 499–512. [Google Scholar] [CrossRef]
- LeCouter, J.; Moritz, D.R.; Li, B.; Phillips, G.L.; Liang, H.; Gerber, H.P.; Hillan, K.J.; Ferrara, N. Angiogenesis-independent endothelial protection of liver: Role of VEGFR-1. Science 2003, 299, 890–893. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Cheri, Y.L.; Kelly, R.S.; Robert, E.S.; Brian, S.A.; Joanne, H.H.; Sangeeta, N.B. Micropatterned Cell–Cell Interactions Enable Functional Encapsulation of Primary Hepatocytes in Hydrogel Microtissues. Tissue Eng. Part A 2014, 20, 2200–2212. [Google Scholar]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Lu, H.F.; Chua, K.N.; Zhang, P.C.; Lim, W.S.; Ramakrishna, S.; Leong, K.W.; Mao, H.Q. Three-dimensional co-culture of rat hepatocyte spheroids and NIH/3T3 fibroblasts enhances hepatocyte functional maintenance. Acta. Biomater. 2005, 1, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Underhill, G.H.; Chen, A.A.; Albrecht, D.R.; Bhatia, S.N. Assessment of hepatocellular function within PEG hydrogels. Biomaterials 2007, 28, 256–270. [Google Scholar] [CrossRef]
- Chen, A.A.; Thomas, D.K.; Ong, L.L.; Schwartz, R.E.; Golub, T.R.; Bhatia, S.N. Humanized mice with ectopic artificial liver tissues. Proc. Natl. Acad. Sci. USA 2021, 108, 11842–11847. [Google Scholar] [CrossRef]
- Hui, E.E.; Bhatia, S.N. Micromechanical control of cell-cell interactions. Proc. Natl. Acad. Sci. USA 2007, 104, 5722–5726. [Google Scholar] [CrossRef]
- Miyoshi, H.; Stappenbeck, T.S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 2013, 8, 2471–2482. [Google Scholar] [CrossRef]
- Kim, H.J.; Alam, Z.; Hwang, J.W.; Hwang, Y.H.; Kim, M.J.; Yoon, S.; Byun, Y.; Lee, D.Y. Optimal formation of genetically modified and functional pancreatic islet spheroids by using hanging-drop strategy. Transplant. Proc. 2013, 45, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.J.; Choi, H.; Chang, Y.; Park, H.; Shin, J.; Kim, J.; Lengner, C.J.; Lee, Y.K.; Kim, J. Modeling G2019S-LRRK2 sporadic parkinson’s disease in 3D midbrain organoids. Stem Cell Rep. 2019, 12, 518–531. [Google Scholar] [CrossRef]
- Schwank, G.; Koo, B.K.; Sasselli, V.; Dekkers, J.F.; Heo, I.; Demircan, T.; Sasaki, N.; Boymans, S.; Cuppen, E.; van der Ent, C.K.; et al. Functional repair of CFTR by CRISPR/cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 2013, 13, 653–658. [Google Scholar] [CrossRef]
- Geurts, M.H.; de Poel, E.; Amatngalim, G.D.; Oka, R.; Meijers, F.M.; Kruisselbrink, E.; van Mourik, P.; Berkers, G.; De Winter-de Groot, K.M.; Michel, S.; et al. CRISPR-based adenine editors correct nonsense mutations in a cystic fibrosis organoid biobank. Cell Stem Cell 2020, 26, 503–510.e7. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, C.; Im, J.; Kim, J.; Kim, J.; Kim, S.; Cho, Y.; Kim, E.; Kim, Y.; Ryu, J.; et al. Targeted suicide gene therapy for liver cancer based on ribozyme-mediated RNA replacement through post-transcriptional regulation. Mol. Ther. Nucl. Acid. 2021, 23, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Malek-Khatabi, A.; Javar, H.; Dashtimoghadam, E.; Ansari, S.; Hasani-Sadrabadi, M.; Moshaverinia, A. In situ bone tissue engineering using gene delivery nanocomplexes. Acta Biomater. 2020, 108, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Luo, Y.; Lv, Y. Mesenchymal stem cell-derived microvesicles mediate BMP2 gene delivery and enhance bone regeneration. J. Mater. Chem. B 2020, 8, 6378–6389. [Google Scholar] [CrossRef] [PubMed]
- Loozen, L.; Kruyt, M.; Kragten, A.; Schoenfeldt, T.; Croes, M.; Oner, C.; Dhert, W.; Alblas, J. BMP-2 gene delivery in cell-loaded and cell-free constructs for bone regeneration. PLoS ONE 2019, 14, e0220028. [Google Scholar] [CrossRef] [PubMed]
- Curtin, C.; Tierney, E.; McSorley, K.; Cryan, S.; Duffy, G.; O’Brien, F. Combinatorial gene therapy accelerates bone regeneration: Non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold. Adv. Health. Mater. 2015, 4, 223–227. [Google Scholar] [CrossRef]
- Celik, N.; Kim, M.H.; Hayes, D.J.; Ozbolat, I.T. miRNA induced co-differentiation and cross-talk of adipose tissue-derived progenitor cells for 3D heterotypic prevascularized bone formation. Biofabrication 2021, 13, 044107. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Kim, M.H.; Yeo, M.; Kamal, F.; Hayes, D.J.; Ozbolat, I.T. miRNA induced 3D bioprinted-heterotypic osteochondral interface. Biofabrication 2022, 14, 044104. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Li, P.; Diao, H.; Zhu, S.; Dong, L.; Wang, R.; Guo, T.; Zhao, J.; Zhang, J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials 2011, 32, 4793–4805. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shang, H.; Katz, A.; Li, X. A modified aggregate culture for chondrogenesis of human adipose-derived stem cells genetically modified with growth and differentiation factor 5. Biores. Open Access 2013, 2, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Joo, M.; Mok, H.; Lee, M.; Hwang, K.; Kim, S.; Jeong, J.; Choi, D.; Kim, S. MSC-based VEGF gene therapy in rat myocardial infarction model using facial amphipathic bile acid-conjugated polyethyleneimine. Biomaterials 2014, 35, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Chen, W.; Xie, L.; Zhao, Z.; Yang, J.; Chen, Y.; Lei, W.; Shen, Z. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res. Ther. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ebner-Peking, P.; Krisch, L.; Wolf, M.; Hochmann, S.; Hoog, A.; V’ari, B.; Muigg, K.; Poupardin, R.; Scharler, C.; Schmidhuber, S.; et al. Self-assembly of differentiated progenitor cells facilitates spheroid human skin organoid formation and planar skin regeneration. Theranostics 2021, 11, 8430–8447. [Google Scholar] [CrossRef] [PubMed]
- Guye, P.; Ebrahimkhani, M.R.; Kipniss, N.; Velazquez, J.J.; Schoenfeld, E.; Kiani, S.; Griffith, L.G.; Weiss, R. Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using gata6. Nat. Commun. 2016, 7, 10243. [Google Scholar] [CrossRef]
- Kelm, J.M.; Fussenegger, M. Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol. 2004, 22, 195–202. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 2003, 24, 2309–2316. [Google Scholar] [CrossRef]
- L’Heureux, N.; Paquet, S.; Labbe, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56. [Google Scholar]
- Fukuda, J.; Mizumoto, H.; Nakazawa, K.; Kajiwara, T.; Funatsu, K. Hepatocyte organoid culture in elliptic hollow fibers to develop a hybrid artificial liver. Int. J. Artif. Organs 2004, 27, 1091–1099. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Yeh, J.; Jon, S.; Eng, G.; Suh, K.Y.; Burdick, J.A.; Langer, R. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab. Chip 2004, 4, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Putnam, A.J.; Mooney, D.J. Tissue engineering using synthetic extracellular matrices. Nat. Med. 1996, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Marler, J.J.; Upton, J.; Langer, R.; Vacanti, J.P. Transplantation of cells in matrices for tissue regeneration. Adv. Drug Deliv. Rev. 1998, 33, 165. [Google Scholar] [CrossRef]
- Alberts, B.; Bray, D.; Lewis, J.; Raff, M.; Roberts, K.; Watson, J.D. Molecular Biology of the Cell; Garland Publishing: New York, NY, USA, 1994; p. 971. [Google Scholar]
- Kim, B.S.; Mooney, D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998, 16, 224. [Google Scholar] [CrossRef]
- Jhon, M.S.; Andrade, J.D. Water and hydrogels. J. Biomed. Mater. Res. 1973, 7, 509. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Chen, X. Hydrogels based on pH-responsive reversible carbon-nitrogen double-bond linkages for biomedical applications. Master Chem. Front. 2018, 2, 1765–1778. [Google Scholar] [CrossRef]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2020, 121, 10908–10949. [Google Scholar] [CrossRef] [PubMed]
- IUPAC. Glossary of basic terms in polymer science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Sperling, L.H.; Mishra, V. The current status of interpenetrating polymer networks. Polym. Adv. Technol. 1996, 7, 197–208. [Google Scholar] [CrossRef]
- Sperling, L.H. Interpenetrating Polymer Networks; Klempner, D., Sperling, L.H., Utracki, L.A., Eds.; American Chemical Society: Washington, DC, USA, 1994; pp. 3–38. [Google Scholar]
- Sperling, L.H. Encyclopedia of Polymer Science and Technology; Mark, H.F., Ed.; John Wiley & Sons: New York, NY, USA, 2005; Volume 10, pp. 272–311. [Google Scholar]
- Ishikawa, S.; Iijima, K.; Matsukuma, D.; Asawa, Y.; Hoshi, K.; Osawa, S.; Otsuka, H. Interpenetrating Polymer Network Hydrogels via a One-Pot and in Situ Gelation System Based on Peptide Self-Assembly and Orthogonal Cross-Linking for Tissue Regeneration. Chem. Mater. 2020, 32, 2353–2364. [Google Scholar] [CrossRef]
- Jensen, K.F. Microchemical systems: Status, challenges, and opportunities. AIChE J. 1999, 45, 2051–2054. [Google Scholar] [CrossRef]
- Stone, H.A.; Kim, S. Microfluidics: Basic issues, applications, and challenges. AIChE J. 2001, 47, 1250–1254. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, H. Nanofabrication Technologies to Control Cell and Tissue Function in Three-Dimension. Gels 2023, 9, 203. https://doi.org/10.3390/gels9030203
Otsuka H. Nanofabrication Technologies to Control Cell and Tissue Function in Three-Dimension. Gels. 2023; 9(3):203. https://doi.org/10.3390/gels9030203
Chicago/Turabian StyleOtsuka, Hidenori. 2023. "Nanofabrication Technologies to Control Cell and Tissue Function in Three-Dimension" Gels 9, no. 3: 203. https://doi.org/10.3390/gels9030203
APA StyleOtsuka, H. (2023). Nanofabrication Technologies to Control Cell and Tissue Function in Three-Dimension. Gels, 9(3), 203. https://doi.org/10.3390/gels9030203






