Advancements in Hydrogels for Corneal Healing and Tissue Engineering
Abstract
1. Introduction
- Examine the fundamental properties of hydrogels that make them suitable for corneal tissue engineering.
- Analyze various synthesis processes and types of hydrogels, including stimuli-responsive variants, used in corneal applications.
- Evaluate current preclinical trials involving hydrogel-based corneal therapies.
- Identify the challenges and limitations to clinical translation.
- Provide insights and recommendations for future research directions to enhance the viability of hydrogels as alternatives to corneal transplants.
2. Fundamentals of Hydrogels
2.1. Definition and Properties
2.2. Types of Hydrogels
3. Cornea Anatomy and Repair Mechanisms
3.1. Corneal Structure and Function
3.2. Corneal Pathologies and Healing Process
3.3. Corneal Transplants
3.4. Bioengineered Corneas: Addressing the Donor Shortage
4. Properties Relevant to Corneal Tissue Engineering
4.1. Key Properties to Reproduce
4.2. Hydrogel Materials for Corneal Tissue Engineering
4.3. Stimuli-Responsive Hydrogels
5. Role of Hydrogels in Corneal Tissue Engineering
5.1. Hydrogel Synthesis Processes
5.2. Cell Encapsulation and Bioactive Molecule Integration
6. Clinical Applications of Hydrogel for Corneal Defects
6.1. Hydrogels for Corneal Pathologies
6.2. Hydrogels for Corneal Tissue Engineering
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, X.; Zhang, Y.; Chen, Y. Chapter 10—The Application of Natural Polymer–Based Hydrogels in Tissue Engineering. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–307. ISBN 978-0-12-816421-1. [Google Scholar]
- Zhang, Y.; Wu, B.M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Majcher, M.J.; Hoare, T. Applications of Hydrogels. In Functional Biopolymers; Jafar Mazumder, M.A., Sheardown, H., Al-Ahmed, A., Eds.; Springer International Publishing: Cham, Germany, 2019; pp. 453–490. ISBN 978-3-319-95990-0. [Google Scholar]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Maleki, B.; Kargar, P.; Ashrafi, S.; Ghani, M. Perspective Chapter: Introduction to Hydrogels—Definition, Classifications, Applications and Methods of Preparation. In Ionic Liquids—Recent Advances; IntechOpen: London, UK, 2024. [Google Scholar]
- Ghosh, S.; Ghosh, S.; Sharma, H.; Bhaskar, R.; Han, S.S.; Sinha, J.K. Harnessing the Power of Biological Macromolecules in Hydrogels for Controlled Drug Release in the Central Nervous System: A Review. Int. J. Biol. Macromol. 2024, 254, 127708. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Bryant, S.J.; Bender, R.J.; Durand, K.L.; Anseth, K.S. Encapsulating Chondrocytes in Degrading PEG Hydrogels with High Modulus: Engineering Gel Structural Changes to Facilitate Cartilaginous Tissue Production. Biotechnol. Bioeng. 2004, 86, 747–755. [Google Scholar] [CrossRef]
- Fukui, T.; Kitamura, N.; Kurokawa, T.; Yokota, M.; Kondo, E.; Gong, J.P.; Yasuda, K. Intra-Articular Administration of Hyaluronic Acid Increases the Volume of the Hyaline Cartilage Regenerated in a Large Osteochondral Defect by Implantation of a Double-Network Gel. J. Mater. Sci. Mater. Med. 2014, 25, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Xu, T.; Wang, D.; Oeser, M. The Difference in Molecular Orientation and Interphase Structure of SiO2/Shape Memory Polyurethane in Original, Programmed and Recovered States during Shape Memory Process. Polymers 2020, 12, 1994. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Dodda, J.M.; Deshmukh, K.; Bezuidenhout, D.; Yeh, Y.-C. Hydrogels: Definition, History, Classifications, Formation, Constitutive Characteristics, and Applications. In Multicomponent Hydrogels: Smart Materials for Biomedical Applications; RSC Publishing: London, UK, 2023. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic Hydrogels: Synthesis, Novel Trends, and Applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels—Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the Mechanical Enhancement of Hydrogels: Fabrication Strategies and Underlying Mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Cai, M.-H.; Chen, X.-Y.; Fu, L.-Q.; Du, W.-L.; Yang, X.; Mou, X.-Z.; Hu, P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef]
- Behera, S.; Mahanwar, P.A. Superabsorbent Polymers in Agriculture and Other Applications: A Review. Polym.-Plast. Technol. Mater. 2020, 59, 341–356. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D.; Wendroth, O. The Impacts of Bio-Based and Synthetic Hydrogels on Soil Hydraulic Properties: A Review. Polymers 2022, 14, 4721. [Google Scholar] [CrossRef] [PubMed]
- Berkovitch, Y.; Seliktar, D. Semi-Synthetic Hydrogel Composition and Stiffness Regulate Neuronal Morphogenesis. Int. J. Pharm. 2017, 523, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.J.; Dundas, C.M.; Hillsley, A.; Kasprak, D.S.; Rosales, A.M.; Keitz, B.K. Genetic Control of Radical Cross-Linking in a Semisynthetic Hydrogel. ACS Biomater. Sci. Eng. 2020, 6, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- DelMonte, D.W.; Kim, T. Anatomy and Physiology of the Cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of Cornea and Ocular Surface. Indian. J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Feizi, S.; Jafarinasab, M.R.; Karimian, F.; Hasanpour, H.; Masudi, A. Central and Peripheral Corneal Thickness Measurement in Normal and Keratoconic Eyes Using Three Corneal Pachymeters. J. Ophthalmic Vis. Res. 2014, 9, 296–304. [Google Scholar] [CrossRef]
- Rates, E.R.D.; Almeida, C.D.; de Paula Fiod Costa, E.; de Mello Farias, R.J.; Santos-Oliveira, R.; Alencar, L.M.R. Layer-by-Layer Investigation of Ultrastructures and Biomechanics of Human Cornea. Int. J. Mol. Sci. 2022, 23, 7833. [Google Scholar] [CrossRef]
- Wu, K.Y.; Akbar, D.; Giunta, M.; Kalevar, A.; Tran, S.D. Hydrogels in Ophthalmology: Novel Strategies for Overcoming Therapeutic Challenges. Materials 2024, 17, 86. [Google Scholar] [CrossRef]
- Dohlman, C.H. The Function of the Corneal Epithelium in Health and Disease. The Jonas S. Friedenwald Memorial Lecture. Investig. Ophthalmol. 1971, 10, 383–407. [Google Scholar]
- Wilson, S.E. Bowman’s Layer in the Cornea– Structure and Function and Regeneration. Exp. Eye Res. 2020, 195, 108033. [Google Scholar] [CrossRef]
- Meek, K.M.; Boote, C. The Organization of Collagen in the Corneal Stroma. Exp. Eye Res. 2004, 78, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal Structure and Transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; Wilson, S.E. Descemet’s Membrane Development, Structure, Function and Regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, L.P.; Shiju, T.M.; Hilgert, G.S.L.; de Oliveira, R.C.; DeDreu, J.; Menko, A.S.; Santhiago, M.R.; Wilson, S.E. Descemet’s Membrane Injury and Regeneration, and Posterior Corneal Fibrosis, in Rabbits. Exp. Eye Res. 2021, 213, 108803. [Google Scholar] [CrossRef]
- Ali, M.; Raghunathan, V.; Li, J.Y.; Murphy, C.J.; Thomasy, S.M. Biomechanical Relationships between the Corneal Endothelium and Descemet’s Membrane. Exp. Eye Res. 2016, 152, 57–70. [Google Scholar] [CrossRef]
- Rio-Cristobal, A.; Martin, R. Corneal Assessment Technologies: Current Status. Surv. Ophthalmol. 2014, 59, 599–614. [Google Scholar] [CrossRef]
- Murthy, K.R.; Rajagopalan, P.; Pinto, S.M.; Advani, J.; Murthy, P.R.; Goel, R.; Subbannayya, Y.; Balakrishnan, L.; Dash, M.; Anil, A.K.; et al. Proteomics of Human Aqueous Humor. OMICS 2015, 19, 283–293. [Google Scholar] [CrossRef]
- Stiefel, H.C.; Albert, D.M.; Milman, T. Pathology of the Cornea. In Albert and Jakobiec’s Principles and Practice of Ophthalmology; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 6045–6082. ISBN 978-3-030-42633-0. [Google Scholar]
- Ung, L.; Acharya, N.R.; Agarwal, T.; Alfonso, E.C.; Bagga, B.; Bispo, P.J.; Burton, M.J.; Dart, J.K.; Doan, T.; Fleiszig, S.M.; et al. Infectious Corneal Ulceration: A Proposal for Neglected Tropical Disease Status. Bull. World Health Organ. 2019, 97, 854–856. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global Causes of Blindness and Distance Vision Impairment 1990–2020: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Durand, M.L.; Barshak, M.B.; Chodosh, J. Infectious Keratitis in 2021. JAMA 2021, 326, 1319–1320. [Google Scholar] [CrossRef]
- Cabrera-Aguas, M.; Khoo, P.; Watson, S.L. Infectious Keratitis: A Review. Clin. Exp. Ophthalmol. 2022, 50, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Ung, L.; Chodosh, J. Foundational Concepts in the Biology of Bacterial Keratitis. Exp. Eye Res. 2021, 209, 108647. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, K.; ElSheikh, R.H.; Arabi, A.; Frank, C.R.; Elhusseiny, A.M.; Eleiwa, T.K.; Arami, S.; Djalilian, A.R.; Kheirkhah, A. Peripheral Ulcerative Keratitis: A Review. J. Ophthalmic Vis. Res. 2022, 17, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjögren’s Syndrome: A Systemic Autoimmune Disease. Clin. Exp. Med. 2022, 22, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Wang, J.-Y.; Xu, D.; Li, Y. Comparison of Corneal Biomechanics in Sjögren’s Syndrome and Non-Sjögren’s Syndrome Dry Eyes by Scheimpflug Based Device. Int. J. Ophthalmol. 2017, 10, 711–716. [Google Scholar] [CrossRef]
- Fusco, N.; Stead, T.G.; Lebowitz, D.; Ganti, L. Traumatic Corneal Abrasion. Cureus 2019, 11, e4396. [Google Scholar] [CrossRef]
- Sarnicola, C.; Farooq, A.V.; Colby, K. Fuchs Endothelial Corneal Dystrophy: Update on Pathogenesis and Future Directions. Eye Contact Lens 2019, 45, 1–10. [Google Scholar] [CrossRef]
- Tone, S.O.; Kocaba, V.; Böhm, M.; Wylegala, A.; White, T.L.; Jurkunas, U.V. Fuchs Endothelial Corneal Dystrophy: The Vicious Cycle of Fuchs Pathogenesis. Prog. Retin. Eye Res. 2021, 80, 100863. [Google Scholar] [CrossRef]
- Wilson, S.E.; He, Y.G.; Lloyd, S.A. EGF, EGF Receptor, Basic FGF, TGF Beta-1, and IL-1 Alpha mRNA in Human Corneal Epithelial Cells and Stromal Fibroblasts. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1756–1765. [Google Scholar]
- Wilson, S.E. Corneal Wound Healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Li, D.Q.; Lokeshwar, B.L.; Solomon, A.; Monroy, D.; Ji, Z.; Pflugfelder, S.C. Regulation of MMP-9 Production by Human Corneal Epithelial Cells. Exp. Eye Res. 2001, 73, 449–459. [Google Scholar] [CrossRef] [PubMed]
- West-Mays, J.A.; Strissel, K.J.; Sadow, P.M.; Fini, M.E. Competence for Collagenase Gene Expression by Tissue Fibroblasts Requires Activation of an Interleukin 1 Alpha Autocrine Loop. Proc. Natl. Acad. Sci. USA 1995, 92, 6768. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, X.; Zhang, Y.; Qu, Y.; Wang, L.; Ye, L. Applications of Hydrogel Materials in Different Types of Corneal Wounds. Surv. Ophthalmol. 2023, 68, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Thoft, R.A.; Friend, J. The X, Y, Z Hypothesis of Corneal Epithelial Maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Ghafar, N.A.; Jalil, N.A.A.; Kamarudin, T.A. Wound Healing of the Corneal Epithelium: A Review. Asian Biomed. (Res. Rev. News) 2021, 15, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V.; Saghizadeh, M. Progress in Corneal Wound Healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Liang, W.; Dean, D.C.; Zhang, L.; Liu, Y. Expression and Function of ZEB1 in the Cornea. Cells 2021, 10, 925. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative Capacity of the Corneal Endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef]
- Hos, D.; Matthaei, M.; Bock, F.; Maruyama, K.; Notara, M.; Clahsen, T.; Hou, Y.; Le, V.N.H.; Salabarria, A.-C.; Horstmann, J.; et al. Immune Reactions after Modern Lamellar (DALK, DSAEK, DMEK) versus Conventional Penetrating Corneal Transplantation. Prog. Retin. Eye Res. 2019, 73, 100768. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Lim, R.R.; Lakshminarayanan, R.; Mohan, R.R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277–298. [Google Scholar] [CrossRef]
- Lagali, N. Corneal Stromal Regeneration: Current Status and Future Therapeutic Potential. Curr. Eye Res. 2020, 45, 278–290. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Huang, C.-C.; Lai, J.-Y. Therapeutic Hydrogel Sheets Programmed with Multistage Drug Delivery for Effective Treatment of Corneal Abrasion. Chem. Eng. J. 2022, 429, 132409. [Google Scholar] [CrossRef]
- Li, S.; Sun, H.; Chen, L.; Fu, Y. Targeting Limbal Epithelial Stem Cells: Master Conductors of Corneal Epithelial Regeneration from the Bench to Multilevel Theranostics. J. Transl. Med. 2024, 22, 794. [Google Scholar] [CrossRef] [PubMed]
- Shigeyasu, C.; Yamada, M.; Kawashima, M.; Suwaki, K.; Uchino, M.; Hiratsuka, Y.; Yokoi, N.; Tsubota, K.; DECS-J study group. Quality of Life Measures and Health Utility Values among Dry Eye Subgroups. Health Qual. Life Outcomes 2018, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R.; Ramke, J.; Macleod, D.; Burn, H.; Lee, C.N.; Zhang, J.H.; Waldock, W.; Swenor, B.K.; Gordon, I.; Congdon, N.; et al. Association between Vision Impairment and Mortality: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2021, 9, e418–e430. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gupta, N.; Vanathi, M.; Tandon, R. Corneal Transplantation in the Modern Era. Indian J. Med. Res. 2019, 150, 7–22. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Tuli, S.S.; Steigleman, W.A.; Shah, A.A. Overview of Corneal Transplantation for the Nonophthalmologist. Transplant. Direct 2023, 9, e1434. [Google Scholar] [CrossRef]
- Ramon, C. Indications and Contraindications for Keratoplasty and Keratectomies*. Am. J. Ophthalmol. 1946, 29, 1081–1089. [Google Scholar] [CrossRef][Green Version]
- Nonpassopon, M.; Niparugs, M.; Cortina, M.S. Boston Type 1 Keratoprosthesis: Updated Perspectives. Clin. Ophthalmol. 2020, 14, 1189–1200. [Google Scholar] [CrossRef]
- Chi, M.; Yuan, B.; Xie, Z.; Hong, J. The Innovative Biomaterials and Technologies for Developing Corneal Endothelium Tissue Engineering Scaffolds: A Review and Prospect. Bioengineering 2023, 10, 1284. [Google Scholar] [CrossRef]
- Kolozsvári, L.; Nógrádi, A.; Hopp, B.; Bor, Z. UV Absorbance of the Human Cornea in the 240- to 400-Nm Range. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2165–2168. [Google Scholar]
- Lerman, S. Biophysical Aspects of Corneal and Lenticular Transparency. Curr. Eye Res. 1984, 3, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Tutchenko, L. The Refractive Index of the Human Cornea: A Review. Cont. Lens Anterior Eye 2019, 42, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Delaey, J.; De Vos, L.; Koppen, C.; Dubruel, P.; Van Vlierberghe, S.; Van den Bogerd, B. Tissue Engineered Scaffolds for Corneal Endothelial Regeneration: A Material’s Perspective. Biomater. Sci. 2022, 10, 2440–2461. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Harvey, T.; Begiristain, E.; Domínguez, C.; Sánchez-Abella, L.; Browne, M.; Cook, R.B. Measuring the Elastic Modulus of Soft Biomaterials Using Nanoindentation. J. Mech. Behav. Biomed. Mater. 2022, 133, 105329. [Google Scholar] [CrossRef]
- Blackburn, B.J.; Jenkins, M.W.; Rollins, A.M.; Dupps, W.J. A Review of Structural and Biomechanical Changes in the Cornea in Aging, Disease, and Photochemical Crosslinking. Front. Bioeng. Biotechnol. 2019, 7, 66. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Lepert, G.; Gupta, S.; Mohan, R.R.; Paterson, C.; Connon, C.J. Assessment of Corneal Substrate Biomechanics and Its Effect on Epithelial Stem Cell Maintenance and Differentiation. Nat. Commun. 2019, 10, 1496. [Google Scholar] [CrossRef]
- Rafat, M.; Li, F.; Fagerholm, P.; Lagali, N.S.; Watsky, M.A.; Munger, R.; Matsuura, T.; Griffith, M. PEG-Stabilized Carbodiimide Crosslinked Collagen–Chitosan Hydrogels for Corneal Tissue Engineering. Biomaterials 2008, 29, 3960–3972. [Google Scholar] [CrossRef]
- Harocopos, G.; Brar, V. Five Layers of the Cornea. Available online: https://www.aao.org/education/image/five-layers-of-cornea (accessed on 13 August 2024).
- Vázquez, N.; Chacón, M.; Rodríguez-Barrientos, C.A.; Merayo-Lloves, J.; Naveiras, M.; Baamonde, B.; Alfonso, J.F.; Zambrano-Andazol, I.; Riestra, A.C.; Meana, Á. Human Bone Derived Collagen for the Development of an Artificial Corneal Endothelial Graft. In Vivo Results in a Rabbit Model. PLoS ONE 2016, 11, e0167578. [Google Scholar] [CrossRef]
- Levis, H.J.; Peh, G.S.L.; Toh, K.-P.; Poh, R.; Shortt, A.J.; Drake, R.A.L.; Mehta, J.S.; Daniels, J.T. Plastic Compressed Collagen as a Novel Carrier for Expanded Human Corneal Endothelial Cells for Transplantation. PLoS ONE 2012, 7, e50993. [Google Scholar] [CrossRef]
- Niu, G.; Choi, J.-S.; Wang, Z.; Skardal, A.; Giegengack, M.; Soker, S. Heparin-Modified Gelatin Scaffolds for Human Corneal Endothelial Cell Transplantation. Biomaterials 2014, 35, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef] [PubMed]
- Huhtala, A.; Pohjonen, T.; Salminen, L.; Salminen, A.; Kaarniranta, K.; Uusitalo, H. In Vitro Biocompatibility of Degradable Biopolymers in Cell Line Cultures from Various Ocular Tissues: Extraction Studies. J. Mater. Sci. Mater. Med. 2008, 19, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, B.; Brown, K.D.; Blencowe, A.; Ladewig, K.; Stevens, G.W.; Scheerlinck, J.-P.Y.; Abberton, K.; Daniell, M.; Qiao, G.G. Biodegradable and Biocompatible Poly(Ethylene Glycol)-Based Hydrogel Films for the Regeneration of Corneal Endothelium. Adv. Healthc. Mater. 2014, 3, 1496–1507. [Google Scholar] [CrossRef]
- Luo, X.; He, X.; Zhao, H.; Ma, J.; Tao, J.; Zhao, S.; Yan, Y.; Li, Y.; Zhu, S. Research Progress of Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering. Nanomaterials 2023, 13, 1976. [Google Scholar] [CrossRef]
- Jia, S.; Yang, J.; Lau, A.D.-S.; Chen, F.; Bu, Y.; Cai, E.; Wang, H.; Chieng, H.-E.; Sun, T.; Zhou, Z.; et al. Digital Light Processing-Bioprinted Poly-NAGA-GelMA-Based Hydrogel Lenticule for Precise Refractive Errors Correction. Biofabrication 2023, 15, 035011. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, A.; Vaddavalli, P.K.; Mahapatra, N.R.; Varshney, A.; Ghosh, P. Efficient Reduction of the Scrolling of Descemet Membrane Endothelial Keratoplasty Grafts by Engineering the Medium. Acta Biomater. 2023, 171, 239–248. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, Properties, and Applications of Gelatin-Based Hydrogels (GHs) in the Environmental, Technological, and Biomedical Sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, P.; Ji, Q.; Yan, C.; Gong, A. Stimuli-Responsive Hydrogel for Disease Therapy. Polym. Bull. 2024, 81, 1981–2000. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-Responsive Hydrogels: Fabrication and Biomedical Applications. VIEW 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Downs, F.G.; Lunn, D.J.; Booth, M.J.; Sauer, J.B.; Ramsay, W.J.; Klemperer, R.G.; Hawker, C.J.; Bayley, H. Multi-Responsive Hydrogel Structures from Patterned Droplet Networks. Nat. Chem. 2020, 12, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, G.; Zare, M.; Ramakrishna, S. Intelligent Hydrogels and Their Biomedical Applications. Mater. Adv. 2022, 3, 7757–7772. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Oh, J.K. Direct Polymerization Approach to Synthesize Acid-Degradable Block Copolymers Bearing Imine Pendants for Tunable pH-Sensitivity and Enhanced Release. Macromol. Rapid Commun. 2020, 41, e2000394. [Google Scholar] [CrossRef] [PubMed]
- Vegad, U.; Patel, M.; Khunt, D.; Zupančič, O.; Chauhan, S.; Paudel, A. pH Stimuli-Responsive Hydrogels from Non-Cellulosic Biopolymers for Drug Delivery. Front. Bioeng. Biotechnol. 2023, 11, 1270364. [Google Scholar] [CrossRef]
- Schnack-Petersen, A.K.; Pápai, M.; Møller, K.B. Azobenzene Photoisomerization Dynamics: Revealing the Key Degrees of Freedom and the Long Timescale of the Trans-to-Cis Process. J. Photochem. Photobiol. A Chem. 2022, 428, 113869. [Google Scholar] [CrossRef]
- Tannock, I.F.; Rotin, D. Acid pH in Tumors and Its Potential for Therapeutic Exploitation1. Cancer Res. 1989, 49, 4373–4384. [Google Scholar]
- Liu, Y.; Ran, Y.; Ge, Y.; Raza, F.; Li, S.; Zafar, H.; Wu, Y.; Paiva-Santos, A.C.; Yu, C.; Sun, M.; et al. pH-Sensitive Peptide Hydrogels as a Combination Drug Delivery System for Cancer Treatment. Pharmaceutics 2022, 14, 652. [Google Scholar] [CrossRef]
- Ratemi, E. 5—pH-Responsive Polymers for Drug Delivery Applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, Volume 1; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2018; pp. 121–141. ISBN 978-0-08-101997-9. [Google Scholar]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting Structure-Property Relationship of pH-Responsive Polymers for Drug Delivery Applications. J. Control Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Xian, S.; Webber, M.J. Temperature-Responsive Supramolecular Hydrogels. J. Mater. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef]
- Rafael, D.; Melendres, M.M.R.; Andrade, F.; Montero, S.; Martinez-Trucharte, F.; Vilar-Hernandez, M.; Durán-Lara, E.F.; Schwartz, S.; Abasolo, I. Thermo-Responsive Hydrogels for Cancer Local Therapy: Challenges and State-of-Art. Int. J. Pharm. 2021, 606, 120954. [Google Scholar] [CrossRef] [PubMed]
- LeValley, P.J.; Sutherland, B.P.; Jaje, J.; Gibbs, S.; Jones, M.; Gala, R.; Kloxin, C.J.; Kiick, K.L.; Kloxin, A.M. On-Demand and Tunable Dual Wavelength Release of Antibody Using Light-Responsive Hydrogels. ACS Appl. Bio Mater. 2020, 3, 6944–6958. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light Responsive Hydrogels for Controlled Drug Delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Boccaccini, A.R. 3D Printing of Electrically Conductive Hydrogels for Tissue Engineering and Biosensors—A Review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dai, Z.; Sheng, X.; Xia, D.; Shao, P.; Yang, L.; Luo, X. Conducting Polymer Hydrogels as a Sustainable Platform for Advanced Energy, Biomedical and Environmental Applications. Sci. Total Environ. 2021, 786, 147430. [Google Scholar] [CrossRef] [PubMed]
- Carlini, A.S.; Gaetani, R.; Braden, R.L.; Luo, C.; Christman, K.L.; Gianneschi, N.C. Enzyme-Responsive Progelator Cyclic Peptides for Minimally Invasive Delivery to the Heart Post-Myocardial Infarction. Nat. Commun. 2019, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Meng Du, J.; Jang, M.-S.; Mo, X.W.; Sun, X.S.; Lee, D.S.; Lee, J.H.; Fu, Y. CD44-Targeted and Enzyme-Responsive Photo-Cross-Linked Nanogels with Enhanced Stability for In Vivo Protein Delivery. Biomacromolecules 2021, 22, 3590–3600. [Google Scholar] [CrossRef]
- Lin, K.; Yi, J.; Mao, X.; Wu, H.; Zhang, L.-M.; Yang, L. Glucose-Sensitive Hydrogels from Covalently Modified Carboxylated Pullulan and Concanavalin A for Smart Controlled Release of Insulin. React. Funct. Polym. 2019, 139, 112–119. [Google Scholar] [CrossRef]
- Wang, N.; Yu, H.; Wang, L.; Chen, X.; Liang, R.; Xing, Y.; Teng, L. Synthesis of Phenylboronic Acid-Based Microgels and Their Glucose-Responsive Properties. Polym. Sci. Ser. B 2021, 63, 521–530. [Google Scholar] [CrossRef]
- Aghamirsalim, M.; Mobaraki, M.; Soltani, M.; Kiani Shahvandi, M.; Jabbarvand, M.; Afzali, E.; Raahemifar, K. 3D Printed Hydrogels for Ocular Wound Healing. Biomedicines 2022, 10, 1562. [Google Scholar] [CrossRef]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable Thermoresponsive Hydrogels as Drug Delivery System for the Treatment of Central Nervous System Disorders: A Review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, Q.; Zhu, Y.; Liu, Y.; Xie, X.; Li, S.; Lin, H.; Chen, W.; Zhu, F. Co-Delivery of Metformin and Levofloxacin Hydrochloride Using Biodegradable Thermosensitive Hydrogel for the Treatment of Corneal Neovascularization. Drug Deliv. 2019, 26, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, J.; He, J.; Kan, D.; Chen, K.; Lu, J. Synthesis of Hydrogels and Their Progress in Environmental Remediation and Antimicrobial Application. Gels 2023, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.H.A.; Saad, A.P.M.; Harun, M.N.; Syahrom, A.; Ramlee, M.H.; Sulong, M.A.; Kadir, M.R.A. Developing Functionally Graded PVA Hydrogel Using Simple Freeze-Thaw Method for Artificial Glenoid Labrum. J. Mech. Behav. Biomed. Mater. 2019, 91, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Tang, L.; Qu, H.; Wu, M.; Zhou, T.; Wen, C.; Wang, P.; Xu, N.; Ruan, C. A Small-Molecule Polycationic Crosslinker Boosts Alginate-Based Bioinks for Extrusion Bioprinting. Adv. Funct. Mater. 2024, 34, 2310369. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-Based Biopolymer Hydrogels for Heavy Metal Detection and Adsorption. J. Adv. Res. 2022, 44, 53–70. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically Crosslinked Silk and Silk-Gelatin Hydrogels with Tunable Gelation Kinetics, Mechanical Properties and Bioactivity for Cell Culture and Encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and Future Scope of Hydrogels in Wound Healing: Synthesis, Materials and Evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Luo, L.-J.; Lai, J.-Y. Amination Degree of Gelatin Is Critical for Establishing Structure-Property-Function Relationships of Biodegradable Thermogels as Intracameral Drug Delivery Systems. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 897–909. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D Bioprinting of Human Tissues: Biofabrication, Bioinks, and Bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef]
- Ruiz-Alonso, S.; Villate-Beitia, I.; Gallego, I.; Lafuente-Merchan, M.; Puras, G.; Saenz-del-Burgo, L.; Pedraz, J.L. Current Insights into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics 2021, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Bu, Y.; Lau, D.-S.A.; Lin, Z.; Sun, T.; Lu, W.W.; Lu, S.; Ruan, C.; Chan, C.-H.J. Advances in 3D Bioprinting Technology for Functional Corneal Reconstruction and Regeneration. Front. Bioeng. Biotechnol. 2023, 10, 1065460. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Miller, K.; Guan, J.; Kiratitanaporn, W.; Tang, M.; Chen, S. 3D Bioprinting of Complex Tissues in Vitro: State-of-the-Art and Future Perspectives. Arch. Toxicol. 2022, 96, 691–710. [Google Scholar] [CrossRef]
- Yang, H.; Yang, K.-H.; Narayan, R.J.; Ma, S. Laser-Based Bioprinting for Multilayer Cell Patterning in Tissue Engineering and Cancer Research. Essays Biochem. 2021, 65, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Hu, Z.; Qin, Z.; Qu, Y.; Wang, F.; Huang, B.; Chen, G.; Liu, X.; Yin, L. Cell Electrospinning and Its Application in Wound Healing: Principles, Techniques and Prospects. Burn. Trauma 2023, 11, tkad028. [Google Scholar] [CrossRef]
- Madden, P.W.; Lai, J.N.X.; George, K.A.; Giovenco, T.; Harkin, D.G.; Chirila, T.V. Human Corneal Endothelial Cell Growth on a Silk Fibroin Membrane. Biomaterials 2011, 32, 4076–4084. [Google Scholar] [CrossRef]
- Kim, D.K.; Sim, B.R.; Kim, J.I.; Khang, G. Functionalized Silk Fibroin Film Scaffold Using β-Carotene for Cornea Endothelial Cell Regeneration. Colloids Surf. B Biointerfaces 2018, 164, 340–346. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]
- Deshmukh, R.; Stevenson, L.J.; Vajpayee, R. Management of Corneal Perforations: An Update. Indian J. Ophthalmol. 2020, 68, 7–14. [Google Scholar] [CrossRef]
- Jhanji, V.; Young, A.L.; Mehta, J.S.; Sharma, N.; Agarwal, T.; Vajpayee, R.B. Management of Corneal Perforation. Surv. Ophthalmol. 2011, 56, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Gopinathan, U.; Nutheti, R.; Rao, G.N. Clinical Experience with N-Butyl Cyanoacrylate Tissue Adhesive in Fungal Keratitis. Cornea 2003, 22, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, S.; Jhanji, V.; Constantinou, M.; Beltz, J.; Graue-Hernandez, E.O.; Vajpayee, R.B. Clinical Experience with N-Butyl Cyanoacrylate Tissue Adhesive in Corneal Perforations Secondary to Herpetic Keratitis. Cornea 2010, 29, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Siatiri, H.; Moghimi, S.; Malihi, M.; Khodabande, A. Use of Sealant (HFG) in Corneal Perforations. Cornea 2008, 27, 988–991. [Google Scholar] [CrossRef]
- Singh, R.B.; Zhu, S.; Yung, A.; Dohlman, T.H.; Dana, R.; Yin, J. Efficacy of Cyanoacrylate Tissue Adhesive in the Management of Corneal Thinning and Perforation Due to Microbial Keratitis. Ocul. Surf. 2020, 18, 795–800. [Google Scholar] [CrossRef]
- Uy, H.S.; Kenyon, K.R. Surgical Outcomes after Application of a Liquid Adhesive Ocular Bandage to Clear Corneal Incisions during Cataract Surgery. J. Cataract. Refract. Surg. 2013, 39, 1668–1674. [Google Scholar] [CrossRef]
- Sykakis, E.; Karim, R.; Kinsella, M.; Bhogal, M.; Patel, S.; Parmar, D.N. Study of Fluid Ingress through Clear Corneal Incisions Following Phacoemulsification with or without the Use of a Hydrogel Ocular Bandage: A Prospective Comparative Randomised Study. Acta Ophthalmol. 2014, 92, e663–e666. [Google Scholar] [CrossRef]
- Islam, M.M.; Buznyk, O.; Reddy, J.C.; Pasyechnikova, N.; Alarcon, E.I.; Hayes, S.; Lewis, P.; Fagerholm, P.; He, C.; Iakymenko, S.; et al. Biomaterials-Enabled Cornea Regeneration in Patients at High Risk for Rejection of Donor Tissue Transplantation. NPJ Regen. Med. 2018, 3, 2. [Google Scholar] [CrossRef]
- Buznyk, O.; Pasyechnikova, N.; Islam, M.M.; Iakymenko, S.; Fagerholm, P.; Griffith, M. Bioengineered Corneas Grafted as Alternatives to Human Donor Corneas in Three High-Risk Patients. Clin. Transl. Sci. 2015, 8, 558–562. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Wang, S.; Hu, H.; Zhong, S.; He, S.; Dou, Y.; Li, Z.; Cui, X. Thermoresponsive Gel for Sustained Release of BMP4 to Inhibit Corneal Neovascularization. Colloids Surf. B Biointerfaces 2020, 194, 111167. [Google Scholar] [CrossRef]
- Fagerholm, P.; Lagali, N.S.; Ong, J.A.; Merrett, K.; Jackson, W.B.; Polarek, J.W.; Suuronen, E.J.; Liu, Y.; Brunette, I.; Griffith, M. Stable Corneal Regeneration Four Years after Implantation of a Cell-Free Recombinant Human Collagen Scaffold. Biomaterials 2014, 35, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, R.; Liu, C.; Fang, H.; Yang, W.; Wang, Y.; Xian, Y.; Zhang, K.; He, Y.; Zhou, X. A “T.E.S.T.” Hydrogel Bioadhesive Assisted by Corneal Cross-Linking for in Situ Sutureless Corneal Repair. Bioact. Mater. 2023, 25, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Tiwari, A.; Chowdhury, S.K.; Vohra, M.; Gour, A.; Waghmare, N.; Bhutani, U.; Kamalnath, S.; Sangwan, B.; Rajput, J.; et al. Kuragel: A Biomimetic Hydrogel Scaffold Designed to Promote Corneal Regeneration. iScience 2024, 27, 109641. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.-W.; Seo, Y.A.; Jackson, K.J.; Jang, K.; Song, E.; Han, U.; Chen, F.; Heilshorn, S.C.; Myung, D. Photoactivated Growth Factor Release from Bio-Orthogonally Crosslinked Hydrogels for the Regeneration of Corneal Defects. Bioact. Mater. 2024, 40, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, S.; Zhao, X.; Han, J.; Chen, J.; Rao, Z.; Zhang, K.; Quan, D.; Yuan, J.; Bai, Y. Dual-Crosslinked Regenerative Hydrogel for Sutureless Long-Term Repair of Corneal Defect. Bioact. Mater. 2022, 20, 434–448. [Google Scholar] [CrossRef]
- Yoon, C.H.; Choi, H.J.; Kim, M.K. Corneal Xenotransplantation: Where Are We Standing? Prog. Retin. Eye Res. 2021, 80, 100876. [Google Scholar] [CrossRef]
- Borouman, S.; Sigaroodi, F.; Ahmadi Tafti, S.M.; Khoshmaram, K.; Soleimani, M.; Khani, M.-M. ECM-Based Bioadhesive Hydrogel for Sutureless Repair of Deep Anterior Corneal Defects. Biomater. Sci. 2024, 12, 2356–2368. [Google Scholar] [CrossRef]
- Li, L.; Lu, C.; Wang, L.; Chen, M.; White, J.; Hao, X.; McLean, K.M.; Chen, H.; Hughes, T.C. Gelatin-Based Photocurable Hydrogels for Corneal Wound Repair. ACS Appl. Mater. Interfaces 2018, 10, 13283–13292. [Google Scholar] [CrossRef]
- Gurnani, B.; Czyz, C.N.; Mahabadi, N.; Havens, S.J. Corneal Graft Rejection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the Clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Clegg, J.R.; Adebowale, K.; Zhao, Z.; Mitragotri, S. Hydrogels in the Clinic: An Update. Bioeng. Transl. Med. 2024, 9, e10680. [Google Scholar] [CrossRef]
- Vijayan, V.; Kiran, M.S. Hybrid Nanostructured Gadolinium Oxide-Collagen-Dextran Polymeric Hydrogel for Corneal Repair and Regeneration. Int. J. Biol. Macromol. 2023, 224, 1423–1438. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, Y.; Gu, R.; Zhang, Z.; Liu, X.; Hu, Y.; Li, X.; Lin, D.; Bao, Z. Nanoparticle-Hydrogel Composite as Dual-Drug Delivery System for the Potential Application of Corneal Graft Rejection. Eur. J. Pharm. Biopharm. 2024, 201, 114351. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, C.D.; Simpson, F.C.; Haagdorens, M.; Samarawickrama, C.; Hunter, D.; Buznyk, O.; Fagerholm, P.; Ljunggren, M.K.; Lewis, P.; Pintelon, I.; et al. LiQD Cornea: Pro-Regeneration Collagen Mimetics as Patches and Alternatives to Corneal Transplantation. Sci. Adv. 2020, 6, eaba2187. [Google Scholar] [CrossRef] [PubMed]
- Barroso, I.A.; Man, K.; Robinson, T.E.; Cox, S.C.; Ghag, A.K. Photocurable GelMA Adhesives for Corneal Perforations. Bioengineering 2022, 9, 53. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]

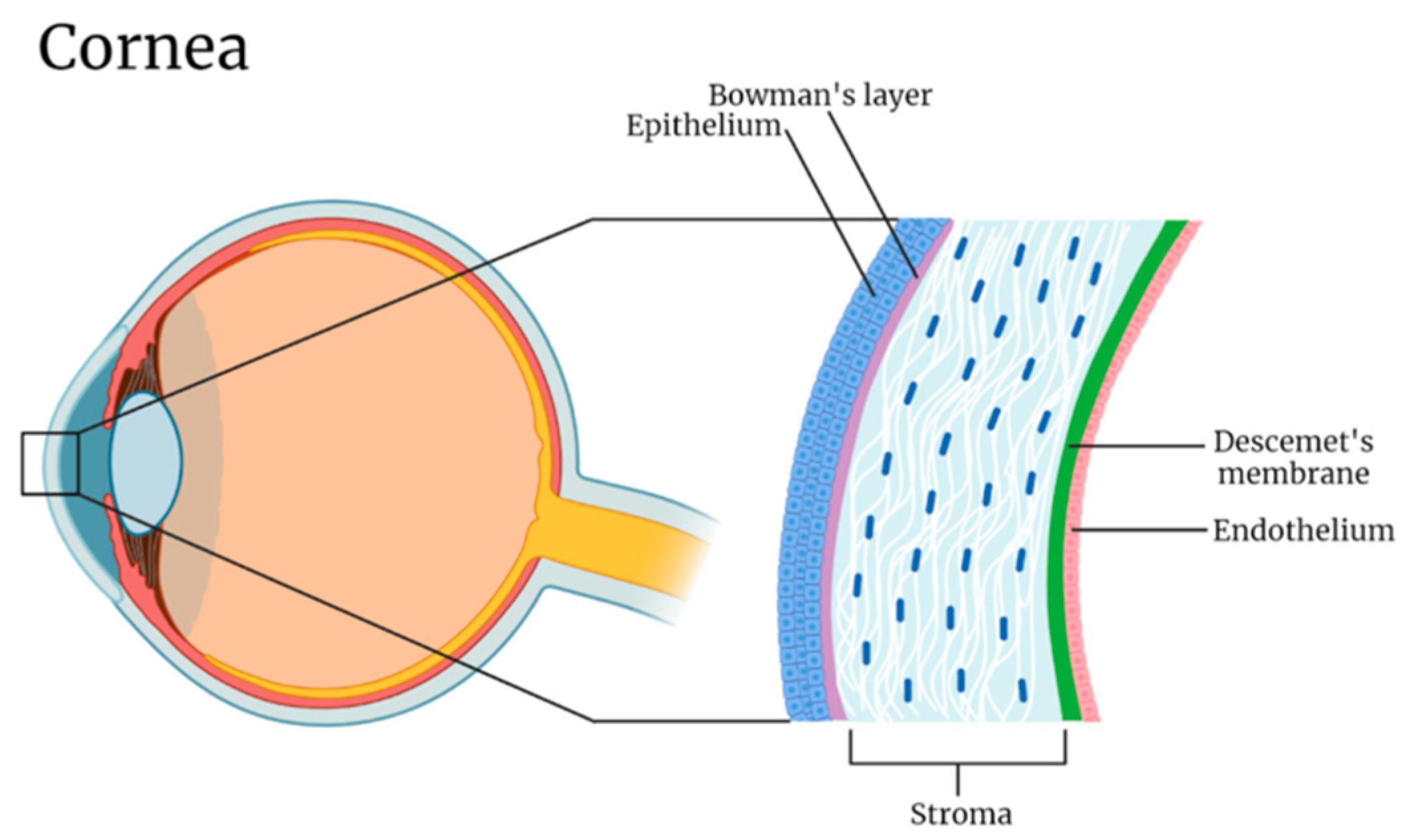


| Layer | Thickness (μm) | Refractive Index | Elastic Modulus (kPa) |
|---|---|---|---|
| Epithelium | 40–50 | 1.400 | 0.57 |
| Bowman’s layer | 8–15 | 1.380 | 109.8 |
| Stroma | 470–500 | 1.369 | 33.1 |
| Descemet’s membrane | 10–12 | Not reported | 50 |
| Endothelium | 4–6 | 1.373 | 4.1 |
| References | [84] | [78] | [81] |
| Source | Examples | Strengths | Limitations | References |
|---|---|---|---|---|
| Natural | Collagen Gelatin Hyaluronic acid Chitosan | Good transparency Biocompatible Biodegradable Cost-effective | Insufficient mechanical strength Fragile Low stability | [6,85,88,94] |
| Synthetic | PLGA PEG PVDF | Good mechanical strength Greater stability Greater water absorption Chemically inert | Lower biocompatibility No reports on biodegradation Greater risk of foreign body response | [13,32,75] |
| Hybrid | GelMA PEG + chitosan | Good mechanical strength Good transparency Biodegradable | No in vivo studies Underdeveloped Complex and more expensive production process | [83,92,93] |
| Stimuli | Examples | Mechanism of Action | References |
|---|---|---|---|
| pH | Guar gum succinate PEI PVA | pH variations induce changes in the acidic or basic functional groups’ ionization state, leading to swelling or shrinking. | [105,106] |
| Temperature | Poloxamer PNIPA Glycerophosphate | Temperature changes disrupt the equilibrium state between hydrophobic segments, hydrophilic segments, and water, therefore inducing sol-gel transformations. | [107,108] |
| Light | Azobenzene Black phosphorus o-nitrobenzyl ester | Photosensitive functional groups (chromophores) undergo photoisomerization when exposed to visible or UV light, thus modifying the gel’s properties. | [109,110] |
| Electric field | Agarose Carbomer Calcium alginate | Electrostatic forces generated by the electric field induces positional changes and ion movements in charged polymer chains. | [111,112] |
| Enzyme | Hyaluronidase Cinnamyloxy groups | The presence of enzymes will degrade its corresponding specific linkage, causing morphological changes that subsequently modify the hydrogel’s physical properties and activity level. | [113,114] |
| Glucose | Concanavalin A Phenylboronic acid | Hydrogels containing these substances can detect and react to blood glucose levels, of which higher concentrations would increase the binding of glucose to the hydrogel. This would induce conformational changes to stimulate insulin secretion. | [115,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Qian, S.Y.; Faucher, A.; Tran, S.D. Advancements in Hydrogels for Corneal Healing and Tissue Engineering. Gels 2024, 10, 662. https://doi.org/10.3390/gels10100662
Wu KY, Qian SY, Faucher A, Tran SD. Advancements in Hydrogels for Corneal Healing and Tissue Engineering. Gels. 2024; 10(10):662. https://doi.org/10.3390/gels10100662
Chicago/Turabian StyleWu, Kevin Y., Shu Yu Qian, Anne Faucher, and Simon D. Tran. 2024. "Advancements in Hydrogels for Corneal Healing and Tissue Engineering" Gels 10, no. 10: 662. https://doi.org/10.3390/gels10100662
APA StyleWu, K. Y., Qian, S. Y., Faucher, A., & Tran, S. D. (2024). Advancements in Hydrogels for Corneal Healing and Tissue Engineering. Gels, 10(10), 662. https://doi.org/10.3390/gels10100662







