Geopolymer Composites—In Environmentally Friendly Aspects
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthetic Geopolymer Synthesis
2.2. Synthesis of Geopolymer Composites from Industrial Wastes
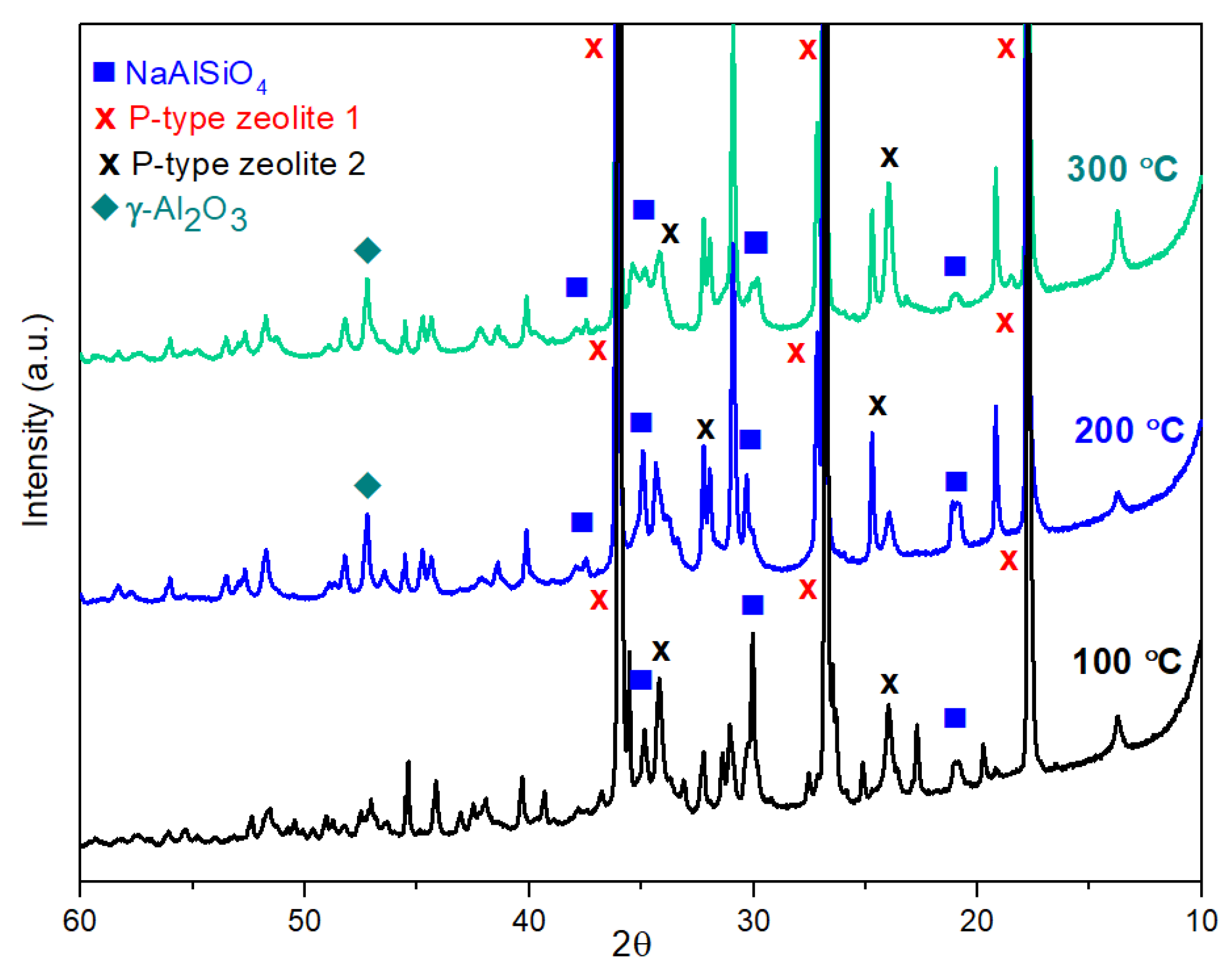
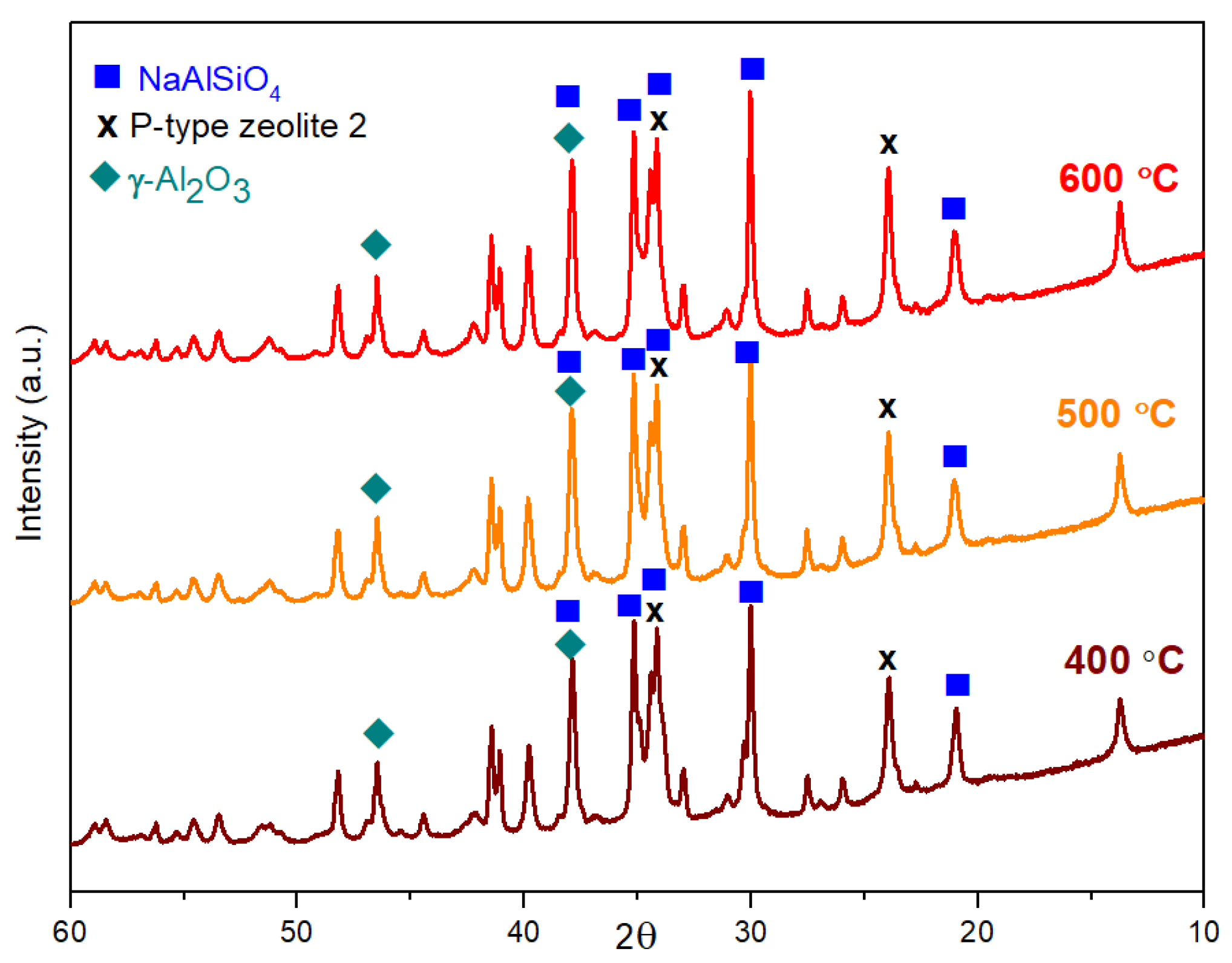
2.3. Structures of Geopolymer Composites
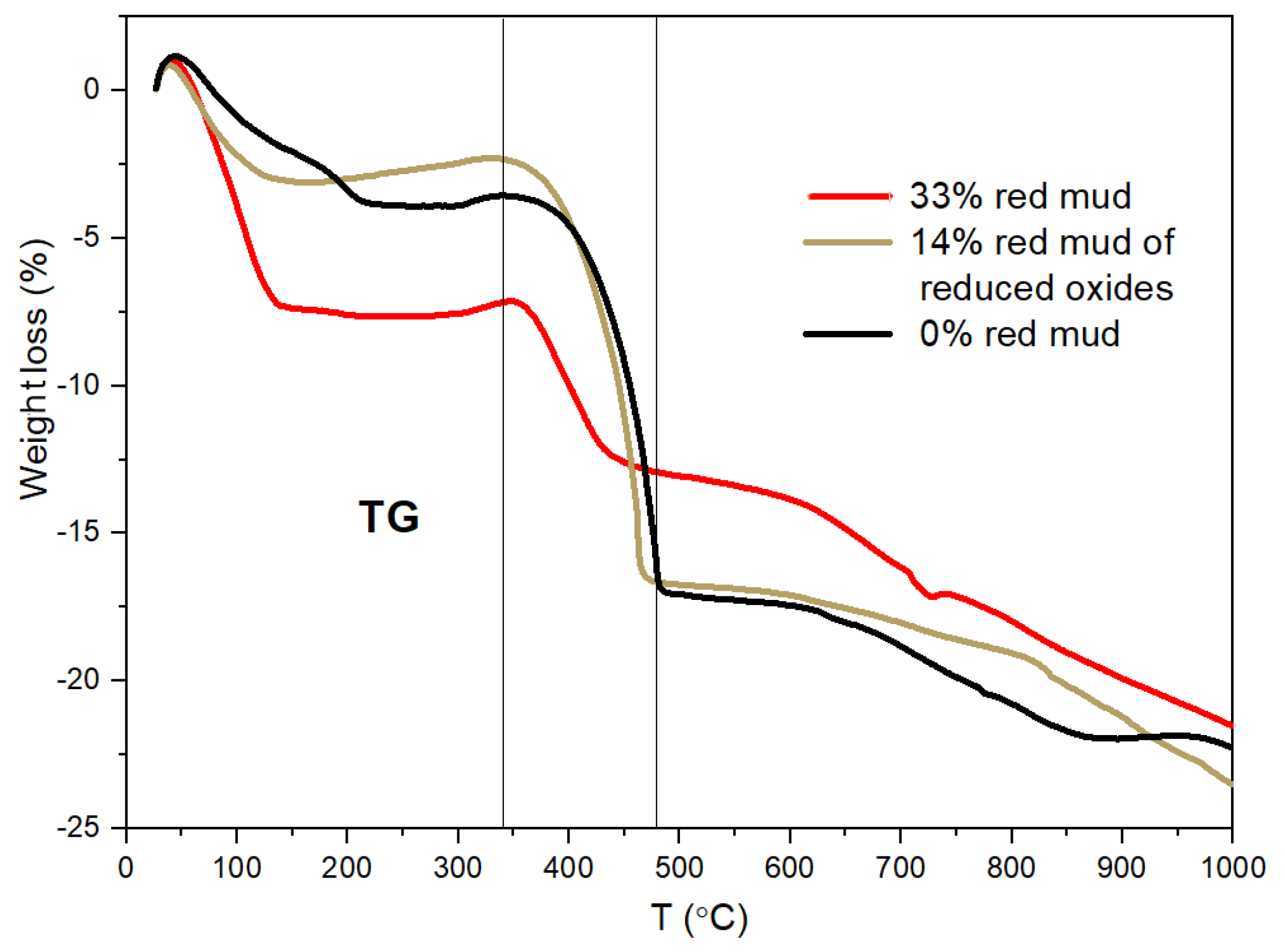
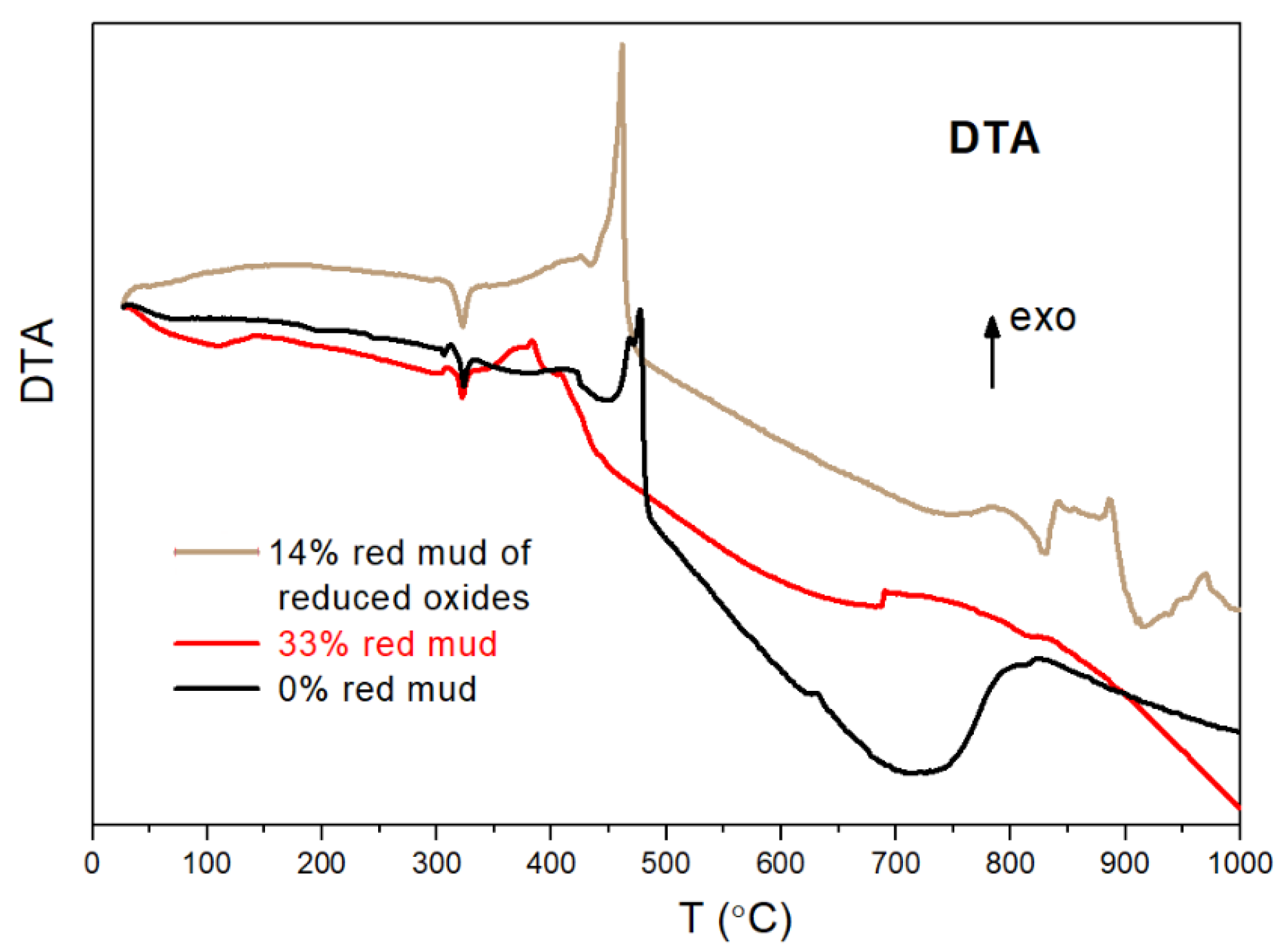
2.4. Characterization of Geopolymer Composites
3. Conclusions
4. Experimental Part
4.1. Synthesis
4.1.1. Synthesis of Synthetic Geopolymers
4.1.2. Synthesis of Geopolymer Composites from Industrial Wastes
4.2. Investigation Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavel, K. Alkali Activated Cements Versus Geopolymers. Civ. Eng. Res. J. 2017, 1, 555574. [Google Scholar]
- Davidovits, J. Geopolymers: Inorganic polymerie new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The effect of composition and temperature on the properties of fly ash- and kaolinite-based geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Sridhar Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.-H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Cheng, T.-W.; Chiu, J.-W. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Waste glass in the geopolymer preparation. Mechanical and microstructural characterisation. J. Clean. Prod. 2015, 90, 397–408. [Google Scholar] [CrossRef]
- Sun, S.; Lin, J.; Zhang, P.; Fang, L.; Ma, R.; Quan, Z.; Song, X. Geopolymer synthetized from sludge residue pretreated by the wet alkalinizing method: Compressive strength and immobilization efficiency of heavy metal. Constr. Build. Mater. 2018, 170, 619–626. [Google Scholar] [CrossRef]
- Kaya, K.; Soyer-Uzun, S. Evolution of structural characteristics and compressive strength in red mud–metakaolin based geopolymer systems. Ceram. Int. 2016, 42, 7406–7413. [Google Scholar] [CrossRef]
- He, J.; Zhang, G. Geopolymerization of red mud and fly ash for civil infrastructure applications. Geotech. Spec. Publ. 2011, 1287–1296. [Google Scholar]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in construction—recent developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Yi, Z.; Feipeng, D.; Zhang, Y. The properties and latest application of geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 472, 012029. [Google Scholar] [CrossRef]
- Lyon, R.E.; Balagaru, P.N.; Foden, A.; Davidovits, J. Fire-resistant Aluminosilicate Composites. Fire-Resist. Aluminosilicate Compos. 1997, 21, 67–73. [Google Scholar] [CrossRef]
- Wanjari, S.-P.; Agrawal, U.-S.; Naresh, D.-N. Geopolymer Sand as a replacement to Natural Sand in concrete. IOP Conf. Ser. Mater. Sci. Eng. 2018, 431, 1–7. [Google Scholar] [CrossRef]
- Khan, H.; Purohit, D.; Bagara, D.; Pahadiya, H.-S. Geopolymer Concrete with Replacement of Cement. Int. J. Eng. Res. Technol. 2016, 4, 1–3. [Google Scholar]
- Tran, H.; Nguyen, H. A Review on Immobilisation of Toxic Wastes Using Geopolymer Technique. In Proceedings of the 4th Congrès International de Géotechnique-Ouvrages-Structures, Ho Chi Minh City, Vietnam, 26–27 October 2018; pp. 299–309. [Google Scholar]
- Garces, J.I.T.; Tigue, A.A.S.; Promentilla, M.A.B. A Systematic Mapping Study of Geopolymers for Radioactive Waste Management. Chem. Eng. Trans. 2022, 94, 1345–1350. [Google Scholar]
- Ameer Radhi, H.A.; Abdulaziz Ahmad, M. Biological Test of Porous Geopolymer as a Bone Substitute. J. Med. Chem. Sci. 2023, 6, 710–719. [Google Scholar]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Xing, X.; Wei, J.; Xu, W.; Wang, B.; Luo, S.; Yu, Q. Effect of Organic Polymers on Mechanical Property and Toughening Mechanism of Slag Geopolymer Matrix. Polymers 2022, 14, 4214. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dong, S.S.; Kim, G.J.; Lee, J.K. Effect of Water Glass on Compressive Strength of Aluminosilicate-Based Geopolymer. Adv. Mater. Res. 2010, 97–101, 2273–2276. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Ming, L.Y.; Yong, H.C.; Tahir, M.F.M. Clay-Based Materials in Geopolymer Technology. In Cement Based Materials; IntechOpn: London, UK, 2018; pp. 239–264. [Google Scholar]
- Yi, M.; Wang, K.; Wei, H.; Wei, D.; Wei, X.; Shao, L.; Fujita, T.; Cui, X. Efficient preparation of red mud-based geopolymer microspheres (RM@GMs) and adsorption of fluoride ions in wastewater. J. Hazard. Mater. 2023, 442, 130027. [Google Scholar] [CrossRef]
- Abdul Rahim, R.H.; Rahmiati, T.; Azizli, K.A.; Man, Z.; Nuruddin, M.F.; Ismail, L. Comparison of Using NaOH and KOH Activated Fly Ash-Based Geopolymer on the Mechanical Properties. Mater. Sci. Forum 2015, 803, 179–184. [Google Scholar] [CrossRef]
- Hua, X.; van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium-polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Zheng, G.; Cui, X.; Huang, D.; Pang, J.; Mo, G.; Shujuan, Y.; Tong, Z. Alkali-activation reactivity of chemosynthetic Al2O3-2SiO2 powders and their 27Al and 29Si magic-angle spinning nuclear magnetic resonance spectra. Particuology 2015, 22, 151–156. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, C.; Zhuang, K.; Shuai, L.; Li, D.; Long, W.; Xing, F.; Fang, Y. Insights into the microstructure of hydrothermal synthesized nanoscale K2O-Al2O3-SiO2-H2O particles. Nanomaterials 2020, 10, 63. [Google Scholar] [CrossRef]
- He, Y.; Liu, L.; He, L.; Cui, X. Characterization of chemosynthetic H3PO4-Al2O3-2SiO2 geopolymers. Ceram. Int. 2016, 42, 10908–10912. [Google Scholar] [CrossRef]
- Cui, X.; Liu, L.; Zheng, G.; Wang, R.; Jianping, L. Characterization of chemosynthetic Al2O3-2SiO2 geopolymers. J. Non-Cryst. Solids 2010, 356, 72–76. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Q.; Li, C.; He, Y.; Cui, X. Preparation of NaA zeolite spheres from geopolymer gels using a one-step method in silicone oil. Int. J. Appl. Ceram. Technol. 2017, 14, 982–986. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Dell’Era, A.; Ciprioti, S.V. Pure Al2O3-2SiO2 synthesized via a sol-gel technique as a raw material to replace metakaolin: Chemical and structural characterization and thermal behavior. Ceram. Int. 2016, 42, 16303–16309. [Google Scholar] [CrossRef]
- Michelina, C.; Flavia, B.; Alice, S.C.; Piercarlo, M. Al2O3-2SiO2 powders synthesized via sol-gel as pure raw material in geopolymer preparation. J. Am. Ceram. Soc. 2017, 100, 1919–1927. [Google Scholar]
- Chen, S.; Wang, K.; Wei, E.; Muhammad, Y.; Yi, M.; Wei, Y.; Fujita, T. Preparation of Al2O3-2SiO2/geopolymer powder by hydrolytic sol-gel method and its activity characterization and research on the reaction mechanism. Powder Technol. 2022, 397, 117026. [Google Scholar] [CrossRef]
- Zheng, G.; Cui, X.; Zhang, W.; Tong, Z. Preparation of geopolymer precursors by sol–gel method and their characterization. J. Mater. Sci. 2009, 44, 3991–3996. [Google Scholar] [CrossRef]
- Espejel, K.R.; Bueno, J.P.; López, C.M.; López, M.L.M.; Siller, M.A.; Araiza, J.L.R.; Manzano-Ramírez, A.; Hernández, J.M. Geopolymeric Composite Materials Made of Sol-Gel Silica and Agroindustrial Wastes of Rice, Barley, and Coffee Husks with Wood-Like Finishing. Sustainability 2022, 14, 16689. [Google Scholar] [CrossRef]
- Sinkó, K. Synthesis of aluminosilicate monolithic system by a novel fast ambient drying process. Ceram. Int. 2016, 42, 5100–5106. [Google Scholar] [CrossRef]
- Mazur, A.; Tolstoy, P.; Sotiriadis, K. 13C, 27Al and 29Si NMR Investigation of the Hydration Kinetics of Portland-Limestone Cement Pastes Containing CH3-COO−-R+ (R=H or Na) Additives. Materials 2022, 15, 2004. [Google Scholar] [CrossRef]
- Xu, S.; Jaegers, N.R.; Hu, W.; Kwak, J.H.; Bao, X.; Sun, J.; Wang, Y.; Hu, J.Z. High-field one-dimensional and two-dimensional 27Al magic-angle spinning nuclear magnetic resonance study of θ-, δ-, and γ-Al2O3 dominated aluminum oxides: Toward understanding the Al sites in γ-Al2O3. ACS Omega 2021, 6, 4090–4099. [Google Scholar] [CrossRef] [PubMed]
- Dressler, M.; Nofz, M.; Malz, F.; Pauli, J.; Jaeger, C.; Reinsch, S.; Scholz, G. Aluminum speciation and thermal evolution of aluminas resulting from modified Yoldas sols. J. Solid State Chem. 2007, 180, 2409–2419. [Google Scholar] [CrossRef]
- Kureti, S.; Weisweiler, W. A novel sol–gel method for the synthesis of γ-aluminium oxide: Development of the sol–gel transformation and characterization of the xerogel. J. Non-Cryst. Solids 2002, 303, 253–261. [Google Scholar] [CrossRef]
- Murakami, Y.; Iijima, A.; Ward, J.W. New Developments in Zeolite Science and Technology; Elsevier: Amsterdam, The Netherlands, 1986; Volume 28. [Google Scholar]
- Neuville, D.R.; Cormier, L.; Massiot, D. Al coordination and speciation in calcium aluminosilicate glasses: Effects of composition determined by 27Al MQ-MAS NMR and Raman spectroscopy. Chem. Geol. 2006, 229, 173–185. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; Marthala, V.R.R.; Thomas, B.; Romanova, E.; Hunger, M. Characterization and Acidic Properties of Aluminum-Exchanged Zeolites X and Y. J. Phys. Chem. C 2008, 112, 3811–3818. [Google Scholar] [CrossRef]
- Paglia, G.; Buckley, C.E.; Rohl, A.L.; Hart, R.D.; Winter, K.; Studer, A.J.; Hunter, B.A.; Hanna, J.V. Boehmite derived γ-alumina system. 1. Structural evolution with temperature, with the identification and structural determination of a new transition phase, γ‘-alumina. Chem. Mater. 2004, 16, 220–236. [Google Scholar] [CrossRef]
- O’Dell, L.A.; Savin, S.L.P.; Chadwick, A.V.; Smith, M.E. 27Al MAS NMR study of a sol-gel produced alumina: Identification of the NMR parameters of th θ-Al2O3 transition aluminum phase. Solid State Nucl. Magn. Reson. 2007, 31, 169–173. [Google Scholar] [CrossRef]
- Pena, P.; Mercury, J.M.R.; de Aza, A.H.; Turrillas, X.; Sobrados, I.; Sanz, J. Solid-state 27Al and 29Si NMR characterization of hydrates formed in calcium aluminate–silica fume mixtures. J. Solid State Chem. 2008, 181, 1744–1752. [Google Scholar] [CrossRef]
- Skibsted, J.; Henderson, E.; Jakobsen, H.J. Characterization of calcium aluminate phases in cements by aluminum-27 MAS NMR spectroscopy. Inorg. Chem. 1993, 32, 777–1058. [Google Scholar] [CrossRef]




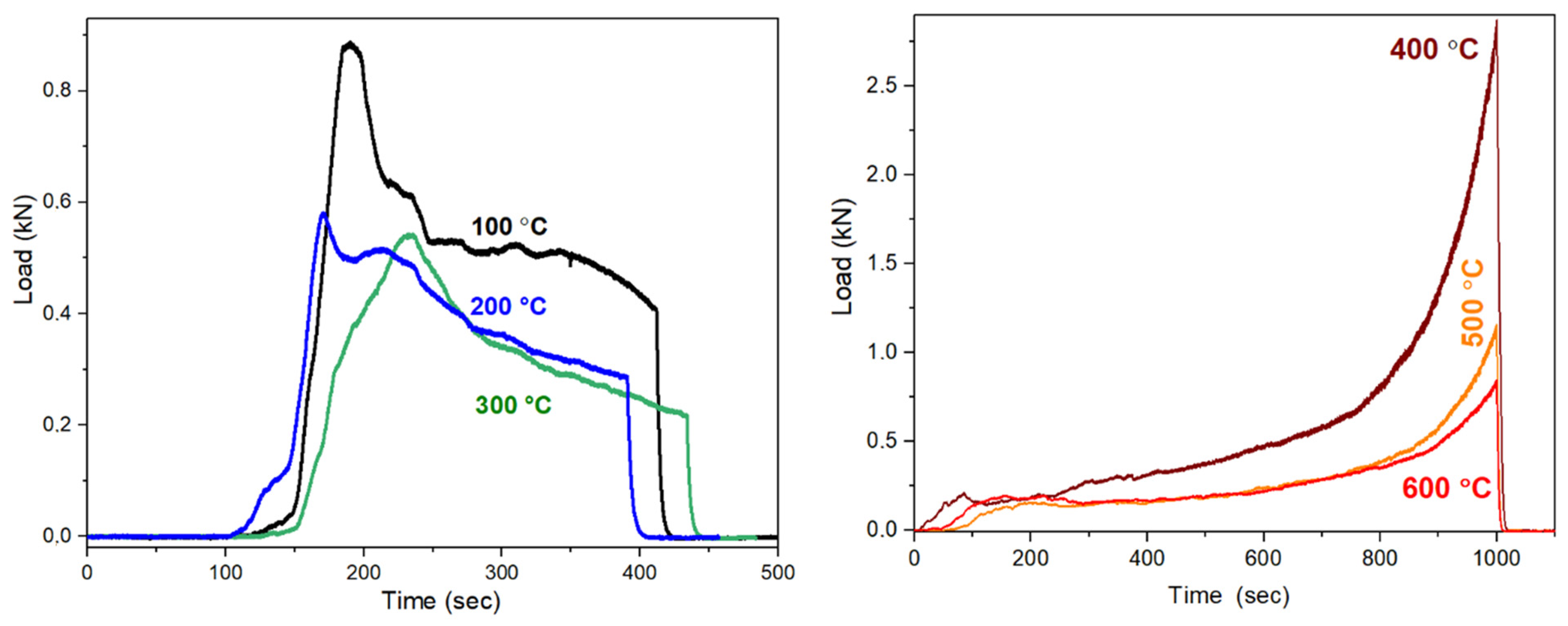

| Temperature of Heat Treatment (°C) | Yield Point Parameters of Synthetic Geopolymer Composites | Yield Point Parameters of Red Mud-Containing Composites | ||
|---|---|---|---|---|
| Maximal Load/kN | Compressive Strength/kPa | Maximal Load /kN | Compressive Strength/kPa | |
| 100 | 0.89 | 2.826 | 1.18 | 3.78 |
| 200 | 0.58 | 1.835 | 0.48 | 1.53 |
| 300 | 0.54 | 1.729 | 1.01 | 3.20 |
| 400 | 2.87 | 9.140 | 7.59 | 24.16 |
| 500 | 1.16 | 3.680 | 7.17 | 22.83 |
| 600 | 0.84 | 2.657 | 2.17 | 6.92 |
| Ions | Monitored Isotope | m (cps dm3 μg−1) | Concentration (mg/dm3) ± SD | |
|---|---|---|---|---|
| Red Mud Sample | Synthetic Sample | |||
| Na | 23 | 2380 | 5636 ± 22 | 5261 ± 37 |
| Al | 27 | 508 | 262 ± 3 | 151 ± 2 |
| K | 39 | 956 | 3.20 ± 0.02 | 2.03 ± 0.6 |
| V | 51 | 22.182 | 1.93 ± 0.03 | n.d. |
| Cr | 52 | 34,523 | 0.74 ± 0.02 | n.d. |
| Ca | 44 | 437 | <1.0 | <1.0 |
| Sc | 45 | 656 | <1.0 | <1.0 |
| Si | 28 | 7689 | <1.0 | <1.0 |
| Sb | 121 | 27,577 | <1.0 | <1.0 |
| Co | 59 | 60,437 | <0.5 | <0.5 |
| Mo | 98 | 46,792 | <0.5 | <0.5 |
| Sn | 120 | 54,537 | <0.5 | <0.5 |
| Cu | 63 | 40,678 | <0.3 | <0.3 |
| Rb | 85 | 14,409 | <0.3 | <0.3 |
| Sr | 88 | 19,450 | <0.3 | <0.3 |
| Ni | 58 | 32,922 | <0.1 | <0.1 |
| Fe | 56 | 24,712 | n.d. | n.d. |
| Main Components | Red Mud (w/w%) | Red Mud with Reduced Oxides |
|---|---|---|
| Fe2O3 α-Fe2O3, FeO(OH) | 32–37 | 11–12 |
| Al2O3 γ-Al(OH)3, AlO(OH) | 15–18 | 10–11 |
| TiO2 rutile | 5–8 | 5–6 |
| SiO2 Na/Al/Ca mixed silicates | 10–15 | 63–66 |
| Na2O Na silicates | 7–10 | 1–2 |
| CaO CaCO3 (calcite) | 4–7 | 4–4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kóth, J.; Sinkó, K. Geopolymer Composites—In Environmentally Friendly Aspects. Gels 2023, 9, 196. https://doi.org/10.3390/gels9030196
Kóth J, Sinkó K. Geopolymer Composites—In Environmentally Friendly Aspects. Gels. 2023; 9(3):196. https://doi.org/10.3390/gels9030196
Chicago/Turabian StyleKóth, János, and Katalin Sinkó. 2023. "Geopolymer Composites—In Environmentally Friendly Aspects" Gels 9, no. 3: 196. https://doi.org/10.3390/gels9030196
APA StyleKóth, J., & Sinkó, K. (2023). Geopolymer Composites—In Environmentally Friendly Aspects. Gels, 9(3), 196. https://doi.org/10.3390/gels9030196






