Synthesis and Characterization of Starch-Based Acid- and Alkali-Resistant Hydrogels Optimized by Box–Behnken Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formation Mechanism of Hydrogels
2.2. Effect of Different Component Concentrations on the Swelling Ratio of Hydrogels
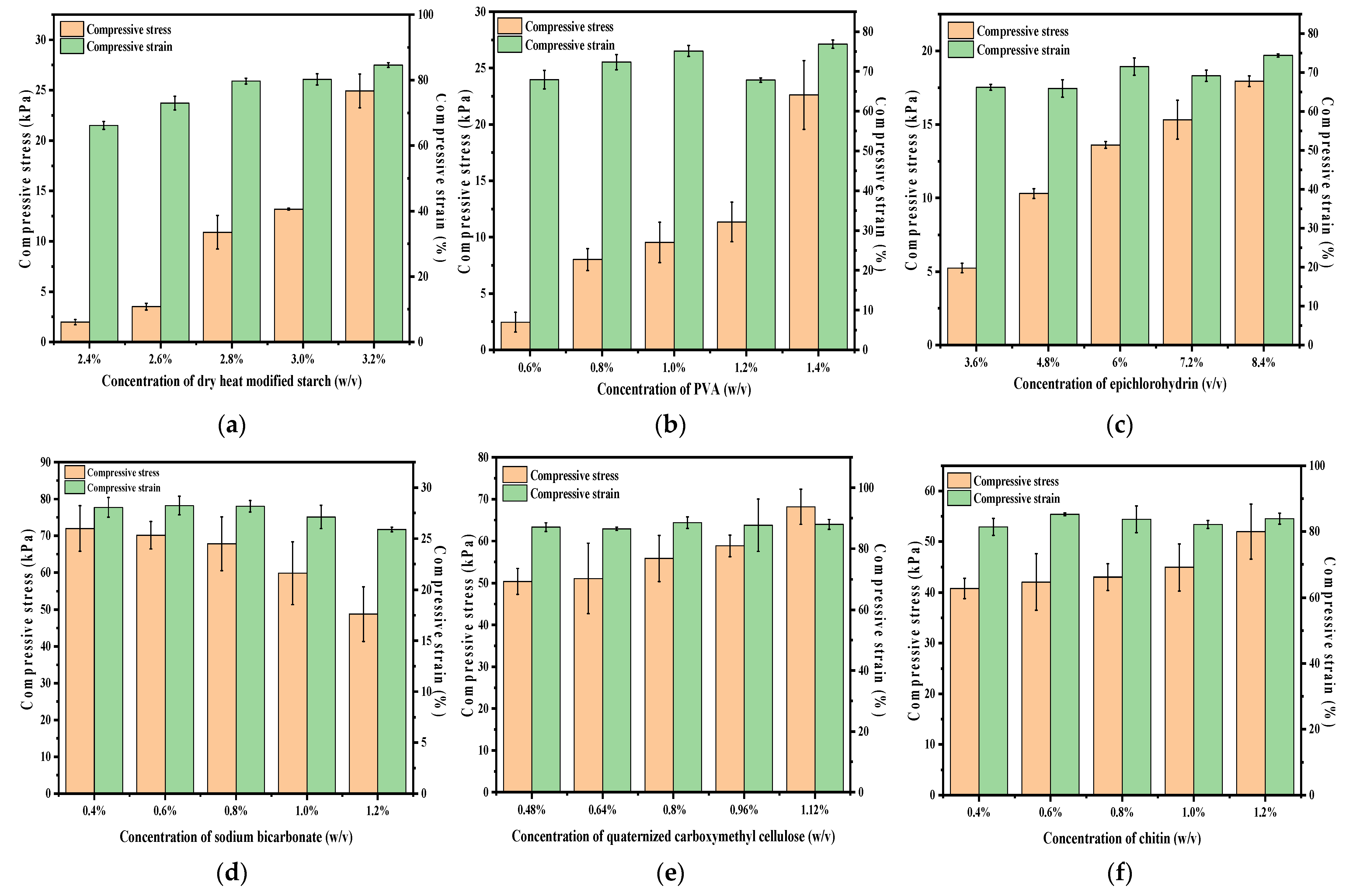
2.3. Mechanical Test Analysis
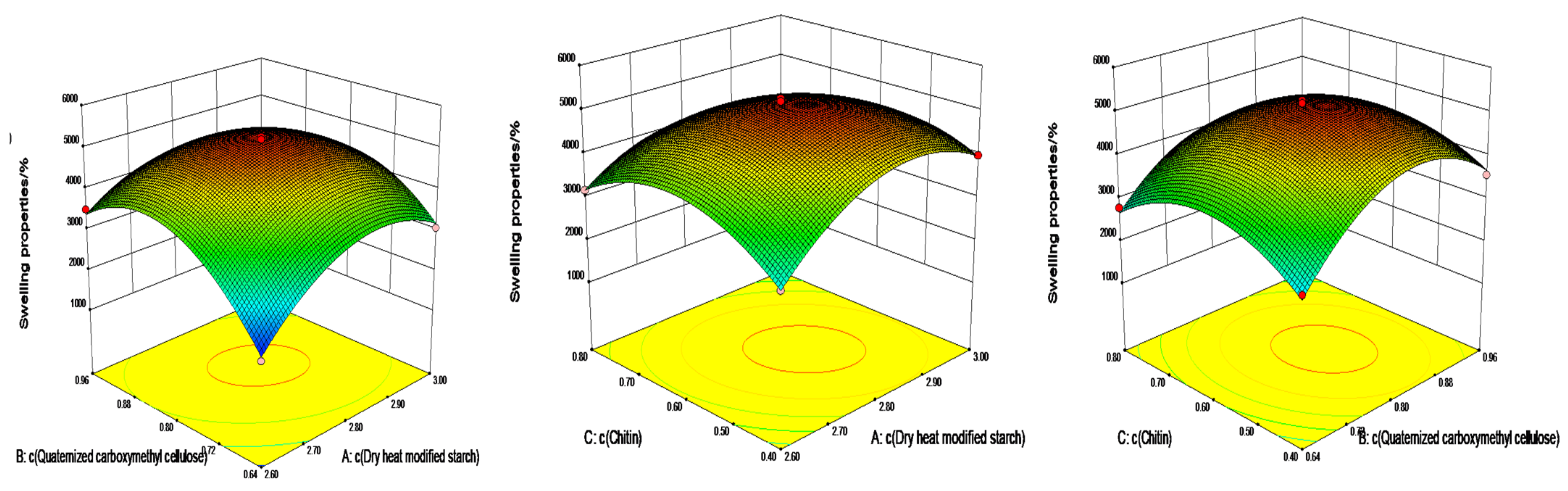
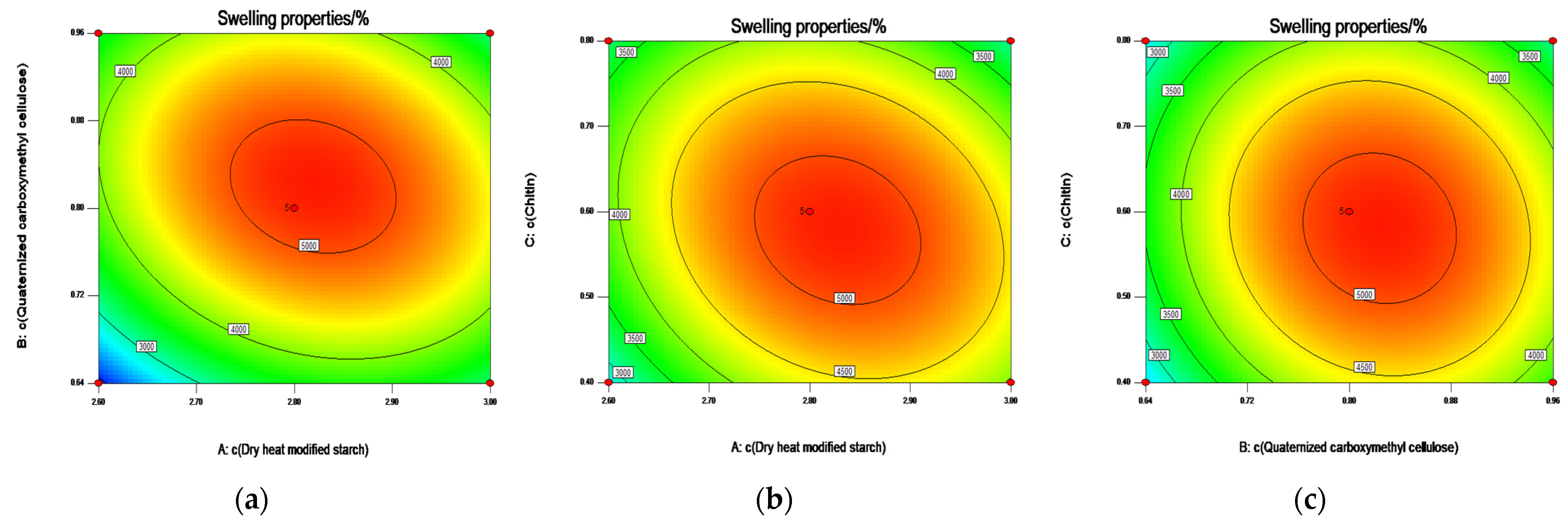
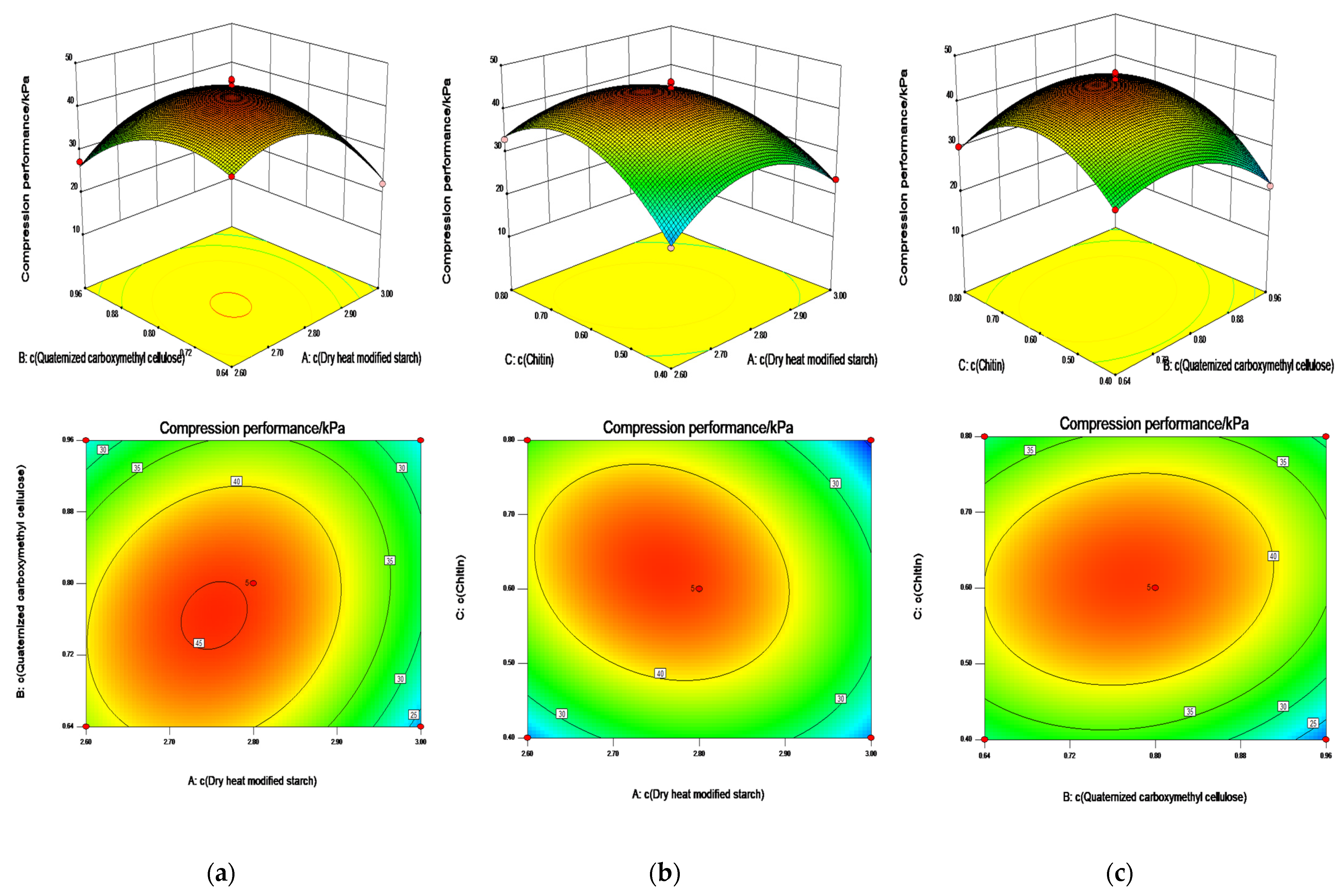
2.4. Box—Behnken Design Optimization of Swelling and Compression Performance
2.4.1. Analysis of Variance (ANOVA) with Swelling Properties as the Response Value
2.4.2. ANOVA with Compression Performance as Response Value
2.5. Swelling Kinetics
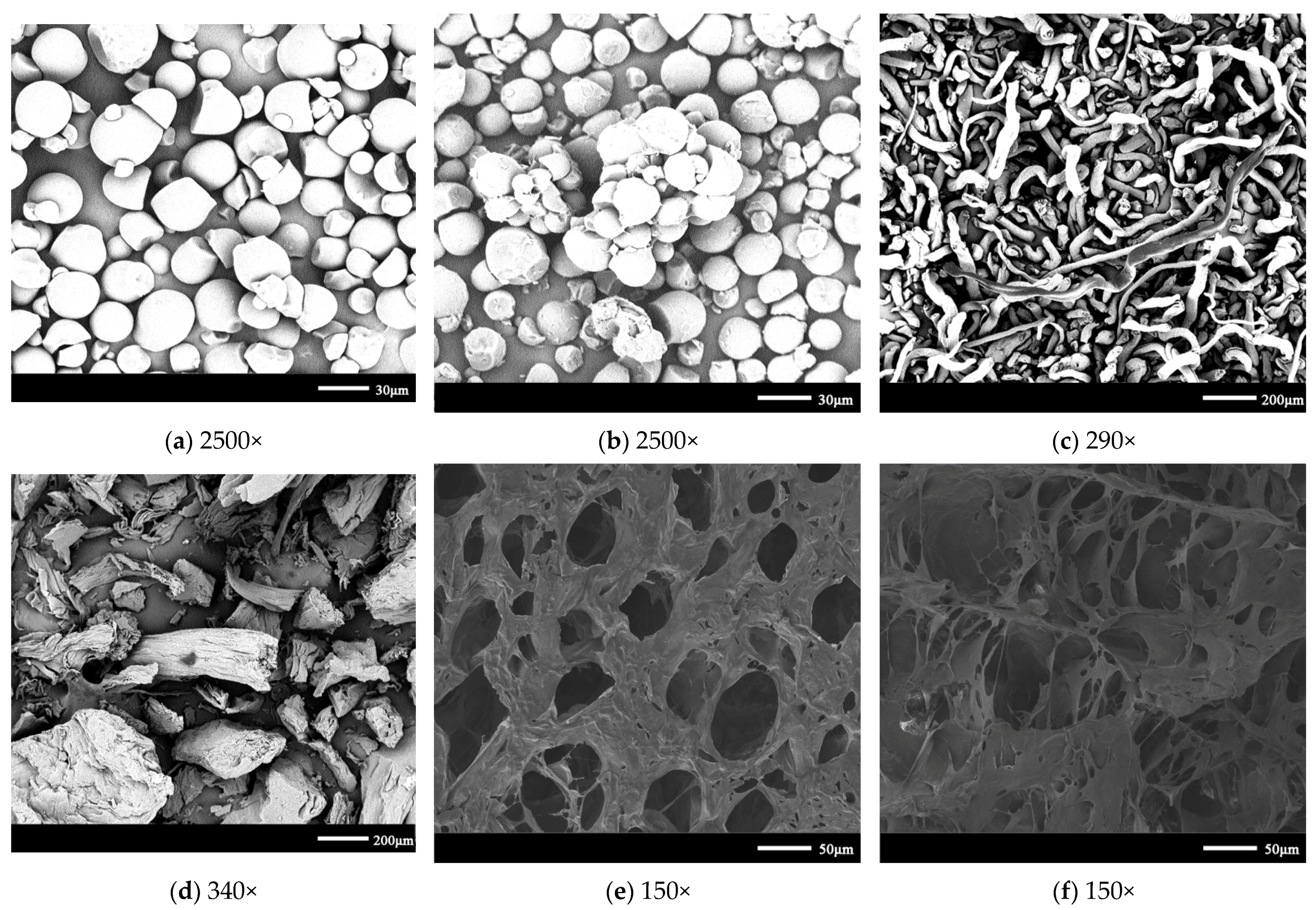
2.6. Scanning Electron Microscopy
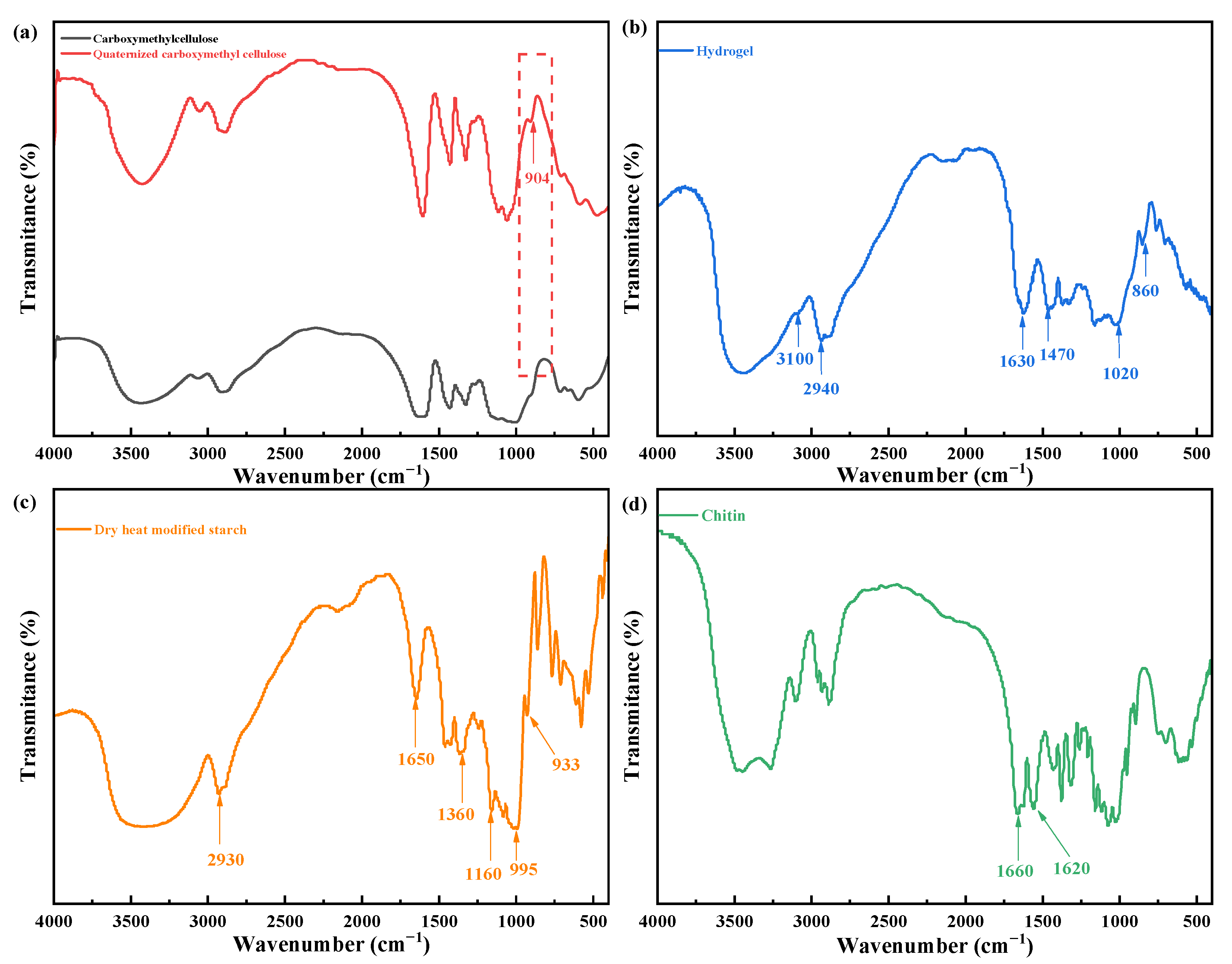
2.7. FTIR Analysis
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Hsu, S.H. Synthesis and biomedical applications of self-healing hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef]
- Xu, Y.S.; Li, Y.S.; Chen, Q.M.; Fu, L.H.; Tao, L.; Wei, Y. Injectable and self-Healing chitosan hydrogel based on imine bonds: Design and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 2198. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, H.; Xu, H.; Qi, M.; Yi, T.; Huang, C.; Wang, S.; An, S.; Li, C. Kinetic model of a carboxymethylcellulose-agar hydrogel for long-acting and slow-release of chlorine dioxide with a modification of fick’s diffusion law. Bioresources 2019, 14, 8821–8834. [Google Scholar] [CrossRef]
- Athab, Z.H.; Halbus, A.F.; Abbas, A.S.; Salman, J.M.; Atiyah, A.J. Enhanced macroporous cationic chitosan hydrogel by freezing and thawing method with superadsorption capacity for anionic dyes. J. Polym. Environ. 2022, 30, 3815–3831. [Google Scholar] [CrossRef]
- Sohail, M.; Mudassir; Minhas, M.U.; Khan, S.; Hussain, Z.; de Matas, M.; Shah, S.A.; Khan, S.; Kousar, M.; Ullah, K. Natural and synthetic polymer-based smart biomaterials for management of ulcerative colitis: A review of recent developments and future prospects. Drug Deliv. Transl. Res. 2019, 9, 595–614. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K. Synthesis, characterization, properties of N-succinyl chitosan-g-poly (methacrylic acid) hydrogels and in vitro release of theophylline. Polymer 2016, 92, 36–49. [Google Scholar] [CrossRef]
- Diaz, A.; Dini, C.; Vina, S.Z.; Alejandra Garcia, M. Technological properties of sour cassava starches: Effect of fermentation and drying processes. LWT-Food. Sci. Technol. 2018, 93, 116–123. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Lima, D.C.; Matta Junior, M.D.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Hydrogels based on ozonated cassava starch: Effect of ozone processing and gelatinization conditions on enhancing 3D-printing applications. Int. J. Biol. Macromol. 2019, 138, 1087–1097. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel biodegradable hydrogel based on natural polymers: Synthesis, characterization, swelling/reswelling and biodegradability. Eur. Polym. J. 2019, 112, 678–687. [Google Scholar] [CrossRef]
- Vudjung, C.; Saengsuwan, S. Biodegradable IPN hydrogels based on pre-vulcanized natural rubber and cassava starch as coating membrane for environment-friendly slow-release urea fertilizer. J. Polym. Environ. 2018, 26, 3967–3980. [Google Scholar] [CrossRef]
- Junlapong, K.; Maijan, P.; Chaibundit, C.; Chantarak, S. Effective adsorption of methylene blue by biodegradable superabsorbent cassava starch-based hydrogel. Int. J. Biol. Macromol. 2020, 158, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Chia, C.H.; Jaafar, S.N.S.; Amran, U.A. Chemically crosslinked hydrogel and its driving force towards superabsorbent behaviour. Int. J. Biol. Macromol. 2018, 118, 1422–1430. [Google Scholar] [CrossRef]
- Lewicka, K.; Siemion, P.; Kurcok, P. Chemical Modifications of Starch: Microwave Effect. Int. J. Polym. Sci. 2015, 2015, 867697. [Google Scholar] [CrossRef]
- Qi, X.; Liu, M.; Chen, Z.; Zhang, F.; Zhao, L. Preparation and properties of macroporous superabsorbent composite. Polym. Adv. Technol. 2010, 21, 196–204. [Google Scholar] [CrossRef]
- Maiti, S.; Khillar, P.S.; Mishra, D.; Nambiraj, N.A.; Jaiswal, A.K. Physical and self-crosslinking mechanism and characterization of chitosan-gelatin-oxidized guar gum hydrogel. Polym. Test. 2021, 97, 107155. [Google Scholar] [CrossRef]
- Thongsuksaengcharoen, S.; Samosorn, S.; Songsrirote, K. A facile synthesis of self-catalytic hydrogel films and their application as a wound dressing material coupled with natural active compounds. Acs Omega 2020, 5, 25973–25983. [Google Scholar] [CrossRef]
- Akar, E.; Altinisik, A.; Seki, Y. Preparation of pH- and ionic-strength responsive biodegradable fumaric acid crosslinked carboxymethyl cellulose. Carbohydr. Polym. 2012, 90, 1634–1641. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takacs, E.; Wojnarovits, L. Synthesis of cellulose derivative based superabsorbent hydrogels by radiation induced crosslinking. Cellulose 2014, 21, 4157–4165. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Chen, D. Preparation and characterization of chitosan based injectable hydrogels enhanced by chitin nano-whiskers. J. Mech. Behav. Biomed. 2017, 65, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Du, Y.M.; Chen, L.Y.; Huang, R.H.; Chen, X. The synthesis of carboxymethylchitosan hydrogel and the application in drug controlled release systems. Acta. Polym. Sin. 2004, 2, 191–195. [Google Scholar]
- Qin, Y.; Wang, J.; Qiu, C.; Xu, X.; Jin, Z. A Dual Cross-linked strategy to construct moldable hydrogels with high stretchability, good self-recovery, and self-healing capability. J. Agric. Food Chem. 2019, 67, 3966–3980. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, P.; Zhao, B.; Wang, S.; Zhong, J.; Cao, Z.; Liu, C.; Gong, F.; Matsuyama, H. Swelling resistance and mechanical performance of physical crosslink-based poly(Vinyl Alcohol) hydrogel film with various molecular weight. J. Polym. Sci. Pol. Phys. 2019, 57, 1673–1683. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, C.; Peng, X.; Zhang, K.; Yu, X. A self-healing and injectable oxidized quaternized guar gum/carboxymethyl chitosan hydrogel with efficient hemostatic and antibacterial properties for wound dressing. Colloids Surface. B 2022, 209, 112207. [Google Scholar] [CrossRef]
- Gazori, T.; Khoshayand, M.R.; Azizi, E.; Yazdizade, P.; Nomanie, A.; Haririan, I. Evaluation of alginate/chitosan nanoparticles as antisense delivery vector: Formulation, optimization and in vitro characterization. Carbohydr. Polym. 2009, 77, 599–606. [Google Scholar] [CrossRef]
- Tripathi, P.; Srivastava, V.C.; Kumar, A. Optimization of an azo dye batch adsorption parameters using Box-Behnken design. Desalination 2009, 249, 1273–1279. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zhao, L.; Meng, G.; Wu, J.; Liu, Z. Cellulose-based porous adsorbents with high capacity for methylene blue adsorption from aqueous solutions. Fibers Polym. 2017, 18, 891–899. [Google Scholar] [CrossRef]
- Eris, S.; Bashiri, H. Kinetic study of the adsorption of dyes onto activated carbon. Prog. React. Kinet. Mech. 2016, 41, 109–119. [Google Scholar] [CrossRef]
- Zhang, M.; Wan, Y.; Wen, Y.X.; Li, C.T.; Kanwal, A. A novel Poly(vinyl alcohol)/carboxymethyl cellulose / yeast double degradable hydrogel with yeast foaming and double degradable property. Ecotox. Environ. Safe. 2020, 187, 109765. [Google Scholar] [CrossRef]
- Zhao, L.W.; Li, Q.; Xu, X.; Kong, W.J.; Li, X.D.; Su, Y.; Yue, Q.Y.; Gao, B.Y. A novel Enteromorpha based hydrogel optimized with Box-Behnken response surface method: Synthesis, characterization and swelling behaviors. Chem. Eng. J. 2016, 287, 537–544. [Google Scholar] [CrossRef]
- Liu, X.; Luan, S.; Li, W. Utilization of waste hemicelluloses lye for superabsorbent hydrogel synthesis. Int. J. Biol. Macromol. 2019, 132, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yao, W.; Gao, F.; Zheng, D.; Wang, Q.; Cao, J.; Tan, H.; Zhang, Y. Physicochemical properties comparative analysis of corn starch and cassava starch, and comparative analysis as adhesive. J. Renew. Mater. 2021, 9, 979–992. [Google Scholar] [CrossRef]
- Chandanasree, D.; Gul, K.; Riar, C.S. Effect of hydrocolloids and dry heat modification on physicochemical, thermal, pasting and morphological characteristics of cassava (Manihot esculenta) starch. Food Hydrocoll. 2016, 52, 175–182. [Google Scholar] [CrossRef]
- Mondal, M.I.H.; Yeasmin, M.S.; Rahman, M.S. Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int. J. Biol. Macromol. 2015, 79, 144–150. [Google Scholar] [CrossRef]
- Li, X.; Hu, A.; Ye, L. Structure and property of porous polyvinylalcohol hydrogels for microorganism immobilization. J. Polym. Environ. 2011, 19, 398–404. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Li, J.; Zhou, C. Chitin-natural clay nanotubes hybrid hydrogel. Int. J. Biol. Macromol. 2013, 58, 23–30. [Google Scholar] [CrossRef]
- Fan, X.; Liu, H.; Wang, J.; Tang, K. Investigation of double network hydrogel with controllable swelling behavior by response surface methodology. J. Appl. Polym. Sci. 2020, 137, 48805. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Adsorption of Reactive blue 21 onto functionalized cellulose under ultrasonic pretreatment: Kinetic and equilibrium study. J. Taiwan Inst. Chem. Eng. 2015, 50, 229–235. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Double network hydrophobic starch based amphoteric hydrogel as an effective adsorbent for both cationic and anionic dyes. Carbohydr. Polym. 2020, 242, 116320. [Google Scholar] [CrossRef]
- Wang, K.; Gao, Q.Y. Study on Dry-heat Modification of Starch and Xanthan Gum Blend. Sci. Technol. Food Ind. 2011, 32, 75–78. [Google Scholar]
- Zhao, Q. Preparation and Properties of Quaternized Carboxymethyl Chitosan as a Polymer Hemostatic Coating. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2020. [Google Scholar]
- Chen, C.; Li, D.; Yano, H.; Abe, K. Dissolution and gelation of alpha-chitin nanofibers using a simple NaOH treatment at low temperatures. Cellulose 2014, 21, 3339–3346. [Google Scholar] [CrossRef]




| Serial Number | c(Dry Heat-Modified Starch) | c(Quaternized Carboxymethyl Cellulose) | c(Chitin) | Swelling Properties /% | Compression Performance /kPa |
|---|---|---|---|---|---|
| 1 | 2.8% | 0.8% | 0.6% | 5032 | 43.785 |
| 2 | 2.8% | 0.96% | 0.4% | 3560 | 21.410 |
| 3 | 2.8% | 0.8% | 0.6% | 5094 | 44.784 |
| 4 | 2.8% | 0.64% | 0.4% | 2690 | 30.744 |
| 5 | 2.6% | 0.8% | 0.8% | 3170 | 33.110 |
| 6 | 2.6% | 0.96% | 0.6% | 3509 | 27.390 |
| 7 | 3% | 0.8% | 0.8% | 2964 | 19.360 |
| 8 | 2.6% | 0.8% | 0.4% | 2765 | 23.140 |
| 9 | 2.6% | 0.64% | 0.6% | 1794 | 37.555 |
| 10 | 3% | 0.96% | 0.6% | 3182 | 25.230 |
| 11 | 2.8% | 0.64% | 0.8% | 2790 | 30.175 |
| 12 | 2.8% | 0.8% | 0.6% | 5258 | 45.980 |
| 13 | 2.8% | 0.8% | 0.6% | 5194 | 42.445 |
| 14 | 3% | 0.64% | 0.6% | 3055 | 22.195 |
| 15 | 2.8% | 0.96% | 0.8% | 2895 | 28.465 |
| 16 | 2.8% | 0.8% | 0.6% | 5134 | 46.325 |
| 17 | 3% | 0.8% | 0.4% | 3976 | 23.729 |
| Source of Variance | Sum of Square | Degree of Freedom | Mean Square | F Value | p Value | Salience |
|---|---|---|---|---|---|---|
| Model | 18,910,000 | 9 | 2,101,000 | 117.24 | <0.0001 | ** |
| A-c(Dry heat modified starch) | 470,000 | 1 | 470,000 | 26.23 | 0.0014 | ** |
| B-c(Quaternized carboxymethyl cellulose) | 991,900 | 1 | 991,900 | 55.35 | 0.0001 | ** |
| C-c(Chitin) | 171,700 | 1 | 171,700 | 9.58 | 0.0174 | * |
| AB | 630,400 | 1 | 630,400 | 35.18 | 0.0006 | ** |
| AC | 502,000 | 1 | 502,000 | 28.01 | 0.0011 | ** |
| BC | 146,300 | 1 | 146,300 | 8.16 | 0.0244 | * |
| A2 | 4,305,000 | 1 | 4,305,000 | 240.26 | <0.0001 | ** |
| B2 | 6,539,000 | 1 | 6,539,000 | 364.90 | <0.0001 | ** |
| C2 | 3,506,000 | 1 | 3,506,000 | 195.62 | <0.0001 | ** |
| Residual | 125,400 | 7 | 17,919.85 | |||
| Lack of fit | 94,811.75 | 3 | 31,603.92 | 4.13 | 0.1022 | Not significant |
| Pure error | 30,627.20 | 4 | 7656.80 | |||
| Total difference | 19,030,000 | 16 |
| Source of Variance | Sum of Square | Degrees of Freedom | Mean Square | F Value | p Value | Salience |
|---|---|---|---|---|---|---|
| Model | 1428.82 | 9 | 158.76 | 76.17 | <0.0001 | ** |
| A-c(Dry heat modified starch) | 117.67 | 1 | 117.67 | 56.45 | 0.0001 | ** |
| B-c(Quaternized carboxymethyl cellulose) | 41.29 | 1 | 41.29 | 19.81 | 0.0030 | ** |
| C-c(Chitin) | 18.26 | 1 | 18.26 | 8.76 | 0.0211 | * |
| AB | 43.56 | 1 | 43.56 | 20.90 | 0.0026 | ** |
| AC | 51.40 | 1 | 51.40 | 24.66 | 0.0016 | ** |
| BC | 14.53 | 1 | 14.53 | 6.97 | 0.0334 | * |
| A2 | 397.60 | 1 | 397.60 | 190.77 | <0.0001 | ** |
| B2 | 197.79 | 1 | 197.79 | 94.90 | <0.0001 | ** |
| C2 | 430.50 | 1 | 430.50 | 206.55 | <0.0001 | ** |
| Residual | 14.59 | 7 | 2.08 | |||
| Lack of fit | 4.39 | 3 | 1.46 | 0.57 | 0.6621 | Not significant |
| Pure error | 10.20 | 4 | 2.55 | |||
| Total difference | 1443.41 | 16 |
| Model | Equation | R2 | k | q |
|---|---|---|---|---|
| pseudo first-order kinetic model | Qe − exp(ln(Qe) − k1 × t) | 0.989 | 0.10 ± 0.006 | 8.09 ± 0.15 |
| pseudo second-order kinetic model | t/((1/(k × Qe2)) + t/Qe) | 0.997 | 0.009 ± 0.0005 | 10.88 ± 0.18 |
| Range and Levels (Coded) | ||||
|---|---|---|---|---|
| Factors | Variable | −1 | 0 | 1 |
| c(Dry heat modified starch) | A | 2.6% | 2.8% | 3% |
| c(Quaternized carboxymethylcellulose) | B | 0.64% | 0.8% | 0.96% |
| c(Chitin) | C | 0.4% | 0.6% | 0.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Huang, L.; Mo, Q.; Wei, Z.; Wang, Y.; Li, Y.; Huang, C.; Duan, Q.; Wei, Y. Synthesis and Characterization of Starch-Based Acid- and Alkali-Resistant Hydrogels Optimized by Box–Behnken Response Surface Methodology. Gels 2022, 8, 585. https://doi.org/10.3390/gels8090585
Han X, Huang L, Mo Q, Wei Z, Wang Y, Li Y, Huang C, Duan Q, Wei Y. Synthesis and Characterization of Starch-Based Acid- and Alkali-Resistant Hydrogels Optimized by Box–Behnken Response Surface Methodology. Gels. 2022; 8(9):585. https://doi.org/10.3390/gels8090585
Chicago/Turabian StyleHan, Xiaoxue, Lijie Huang, Qi Mo, Zhehao Wei, Yanan Wang, Yishan Li, Chongxing Huang, Qingshan Duan, and Yingnan Wei. 2022. "Synthesis and Characterization of Starch-Based Acid- and Alkali-Resistant Hydrogels Optimized by Box–Behnken Response Surface Methodology" Gels 8, no. 9: 585. https://doi.org/10.3390/gels8090585
APA StyleHan, X., Huang, L., Mo, Q., Wei, Z., Wang, Y., Li, Y., Huang, C., Duan, Q., & Wei, Y. (2022). Synthesis and Characterization of Starch-Based Acid- and Alkali-Resistant Hydrogels Optimized by Box–Behnken Response Surface Methodology. Gels, 8(9), 585. https://doi.org/10.3390/gels8090585







