Effects of the Amylose/Amylopectin Content and Storage Conditions on Corn Starch Hydrogels Produced by High-Pressure Processing (HPP)
Abstract
1. Introduction
2. Results and Discussion
2.1. Gel Formation
2.2. Physical Characterization
2.2.1. Organoleptic Properties
2.2.2. Shrinkage Index and Weight Loss
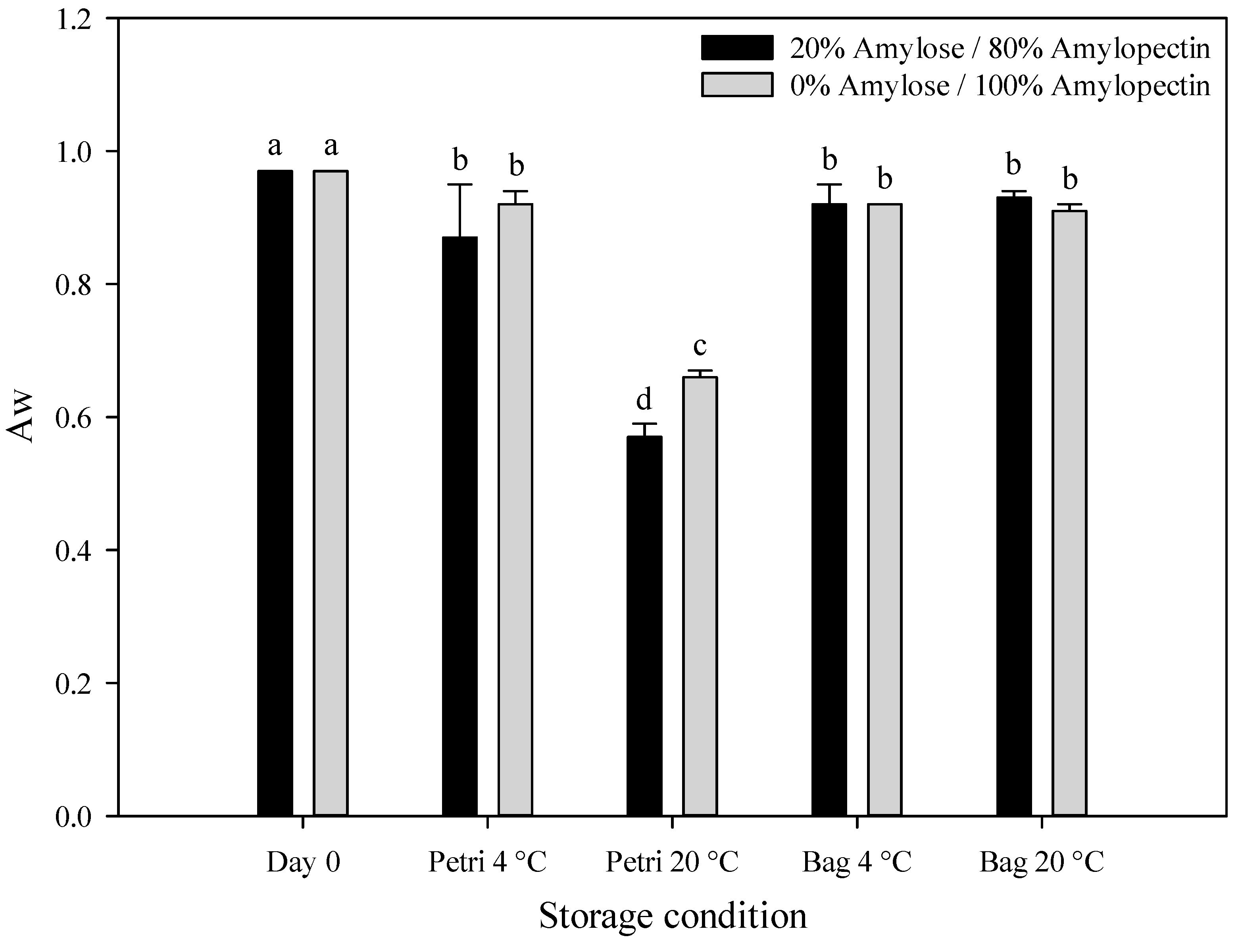
2.2.3. Water Activity (Aw)
2.3. Rheological Properties
3. Conclusions
4. Materials and Methods
4.1. Starch-Based Hydrogels Preparation via High-Pressure Processing (HPP)
4.1.1. Materials
4.1.2. Samples Preparation
4.1.3. High-Pressure Processing (HPP) Treatments
4.2. Experimental Protocol
4.3. Samples Analysis
4.3.1. Optical Measurements
4.3.2. Shrinkage Index
4.3.3. Weight Loss
4.3.4. Visual Observation
4.3.5. Determination of Water Activity (Aw)
4.3.6. Rheology
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; El Halal, S.L.D.M.; Lim, L.T.; Dias, Á.R.G.; da Rosa Zavareze, E. Starch Hydrogels: The Influence of the Amylose Content and Gelatinization Method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel Wound Dressings for Bioactive Treatment of Acute and Chronic Wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Kamath, K.R.; Park, K. Biodegradable Hydrogels in Drug Delivery. Adv. Drug Deliv. Rev. 1993, 11, 59–84. [Google Scholar] [CrossRef]
- Chang, D.; Park, K.; Famili, A. Hydrogels for Sustained Delivery of Biologics to the Back of the Eye. Drug Discov. Today 2019, 24, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan Cross-Linked Poly(Acrylic Acid) Hydrogels: Drug Release Control and Mechanism. Colloids Surf. B Biointerfaces 2017, 152, 252–259. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as Intelligent Materials: A Brief Review of Synthesis, Properties and Applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Xiao, Y.-Y.; Gong, X.-L.; Kang, Y.; Jiang, Z.-C.; Zhang, S.; Li, B.-J. Light-, PH- and Thermal-Responsive Hydrogels with the Triple-Shape Memory Effect. Chem. Commun. 2016, 52, 10609–10612. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Qian, K.; Chen, J.; Li, S.; Lee, P.S. Extremely Stretchable Strain Sensors Based on Conductive Self-Healing Dynamic Cross-Links Hydrogels for Human-Motion Detection. Adv. Sci. 2017, 4, 1600190. [Google Scholar] [CrossRef] [PubMed]
- Bashari, A.; Rouhani Shirvan, A.; Shakeri, M. Cellulose-Based Hydrogels for Personal Care Products. Polym. Adv. Technol. 2018, 29, 2853–2867. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of Hydrogel-Based Scaffolds for Tissue Engineering Applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Mirvakili, S.M.; Hunter, I.W. Artificial Muscles: Mechanisms, Applications, and Challenges. Adv. Mater. 2018, 30, 1704407. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Maleki, H.; Larrañeta, E.; Fajardo, A.R.; Nik, A.B.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Govindh, P.; et al. Status and Future Scope of Plant-Based Green Hydrogels in Biomedical Engineering. Appl. Mater. Today 2019, 16, 213–246. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.; Salamon, A.; Adam, S.; Dubruel, P.; Van Vlierberghe, S.; Peters, K. Gelatin- and Starch-Based Hydrogels. Part B: In Vitro Mesenchymal Stem Cell Behavior on the Hydrogels. Carbohydr. Polym. 2017, 161, 295–305. [Google Scholar] [CrossRef]
- McClements, D.J. Recent Progress in Hydrogel Delivery Systems for Improving Nutraceutical Bioavailability. Food Hydrocoll. 2017, 68, 238–245. [Google Scholar] [CrossRef]
- Mun, S.; Kim, Y.R.; McClements, D.J. Control of β-Carotene Bioaccessibility Using Starch-Based Filled Hydrogels. Food Chem. 2015, 173, 454–461. [Google Scholar] [CrossRef]
- García-Astrain, C.; Avérous, L. Synthesis and Evaluation of Functional Alginate Hydrogels Based on Click Chemistry for Drug Delivery Applications. Carbohydr. Polym. 2018, 190, 271–280. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H.; Ji, Z.; Chen, L. One-Step Method to Prepare Starch-Based Superabsorbent Polymer for Slow Release of Fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Li, J.; Su, T.; Pan, X.; Zuo, G.; Zhang, J.; Dong, W. Design of Salecan-Containing Semi-IPN Hydrogel for Amoxicillin Delivery. Mater. Sci. Eng. C 2017, 75, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Larrea-Wachtendorff, D.; Sousa, I.; Ferrari, G. Starch-Based Hydrogels Produced by High-Pressure Processing (HPP): Effect of the Starch Source and Processing Time. Food Eng. Rev. 2020, 13, 622–633. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Di Nobile, G.; Ferrari, G. Effects of Processing Conditions and Glycerol Concentration on Rheological and Texture Properties of Starch-Based Hydrogels Produced by High Pressure Processing (HPP). Int. J. Biol. Macromol. 2020, 159, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Larrea-Wachtendorff, D.; Tabilo-Munizaga, G.; Ferrari, G. Potato Starch Hydrogels Produced by High Hydrostatic Pressure (HHP): A First Approach. Polymers 2019, 11, 1673. [Google Scholar] [CrossRef]
- Stute, R.; Klingler, R.W.; Boguslawski, S.; Eshtiaghi, M.N.; Knorr, D. Effects of High Pressures Treatment on Starches. Starch/Staerke 1996, 48, 399–408. [Google Scholar] [CrossRef]
- Katopo, H.; Song, Y.; Jane, J.L. Effect and Mechanism of Ultrahigh Hydrostatic Pressure on the Structure and Properties of Starches. Carbohydr. Polym. 2002, 47, 233–244. [Google Scholar] [CrossRef]
- Bauer, B.A.; Knorr, D. The Impact of Pressure, Temperature and Treatment Time on Starches: Pressure-Induced Starch Gelatinisation as Pressure Time Temperature Indicator for High Hydrostatic Pressure Processing. J. Food Eng. 2005, 68, 329–334. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Liu, P.; Bai, Y.; Gao, L.; Shen, Q. Effect of High Hydrostatic Pressure on Physicochemical, Thermal and Morphological Properties of Mung Bean (Vigna radiata L.) Starch. J. Food Eng. 2011, 103, 388–393. [Google Scholar] [CrossRef]
- Błaszczak, W.; Fornal, J.; Valverde, S.; Garrido, L. Pressure-Induced Changes in the Structure of Corn Starches with Different Amylose Content. Carbohydr. Polym. 2005, 61, 132–140. [Google Scholar] [CrossRef]
- Li, W.; Bai, Y.; Mousaa, S.A.S.S.; Zhang, Q.; Shen, Q. Effect of High Hydrostatic Pressure on Physicochemical and Structural Properties of Rice Starch. Food Bioprocess Technol. 2012, 5, 2233–2241. [Google Scholar] [CrossRef]
- Ji, Z.; Yu, L.; Liu, H.; Bao, X.; Wang, Y.; Chen, L. Effect of Pressure with Shear Stress on Gelatinization of Starches with Different Amylose/Amylopectin Ratios. Food Hydrocoll. 2017, 72, 331–337. [Google Scholar] [CrossRef]
- Xie, F.; Yu, L.; Su, B.; Liu, P.; Wang, J.; Liu, H.; Chen, L. Rheological Properties of Starches with Different Amylose/Amylopectin Ratios. J. Cereal Sci. 2009, 49, 371–377. [Google Scholar] [CrossRef]
- Yang, Z.; Swedlund, P.; Gu, Q.; Hemar, Y.; Chaieb, S. Retrogradation of Maize Starch after High Hydrostatic Pressure Gelation: Effect of Amylose Content and Depressurization Rate. PLoS ONE 2016, 11, e0156061. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Swedlund, P.; Hemar, Y.; Mo, G.; Wei, Y.; Li, Z.; Wu, Z. Effect of High Hydrostatic Pressure on the Supramolecular Structure of Corn Starch with Different Amylose Contents. Int. J. Biol. Macromol. 2016, 85, 604–614. [Google Scholar] [CrossRef]
- Szwengiel, A.; Lewandowicz, G.; Górecki, A.R.; Błaszczak, W. The Effect of High Hydrostatic Pressure Treatment on the Molecular Structure of Starches with Different Amylose Content. Food Chem. 2018, 240, 51–58. [Google Scholar] [CrossRef]
- Hu, X.; Xu, X.; Jin, Z.; Tian, Y.; Bai, Y.; Xie, Z. Retrogradation Properties of Rice Starch Gelatinized by Heat and High Hydrostatic Pressure (HHP). J. Food Eng. 2011, 106, 262–266. [Google Scholar] [CrossRef]
- Stolt, M.; Oinonen, S.; Autio, K. Effect of High Pressure on the Physical Properties of Barley Starch. Innov. Food Sci. Emerg. Technol. 2000, 1, 167–175. [Google Scholar] [CrossRef]
- Oh, H.E.; Pinder, D.N.; Hemar, Y.; Anema, S.G.; Wong, M. Effect of High-Pressure Treatment on Various Starch-in-Water Suspensions. Food Hydrocoll. 2008, 22, 150–155. [Google Scholar] [CrossRef]
- Buckow, R.; Jankowiak, L.; Knorr, D.; Versteeg, C. Pressure−Temperature Phase Diagrams of Maize Starches with Different Amylose Contents. J. Agric. Food Chem. 2009, 57, 11510–11516. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, Q.; Hemar, Y. In Situ Study of Maize Starch Gelatinization under Ultra-High Hydrostatic Pressure Using X-Ray Diffraction. Carbohydr. Polym. 2013, 97, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, F. Effect of High Pressure on Rheological and Thermal Properties of Quinoa and Maize Starches. Food Chem. 2018, 241, 380–386. [Google Scholar] [CrossRef]
- Knorr, D.; Heinz, V.; Buckow, R. High Pressure Application for Food Biopolymers. Biochim. Biophys. Acta—Proteins Proteom. 2006, 1764, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Szepes, A.; Makai, Z.; Blümer, C.; Mäder, K.; Kása, P.; Szabó-Révész, P. Characterization and Drug Delivery Behaviour of Starch-Based Hydrogels Prepared via Isostatic Ultrahigh Pressure. Carbohydr. Polym. 2008, 72, 571–578. [Google Scholar] [CrossRef]
- Błaszczak, W.; Fornal, J.; Kiseleva, V.I.; Yuryev, V.P.; Sergeev, A.I.; Sadowska, J. Effect of High Pressure on Thermal, Structural and Osmotic Properties of Waxy Maize and Hylon VII Starch Blends. Carbohydr. Polym. 2007, 68, 387–396. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists, 3rd ed.; Woodhead Publishing and AACC International Press: London, UK, 2018; ISBN 9780128120699. [Google Scholar]
- Doona, C.J.; Feeherry, F.E.; Baik, M.Y. Water Dynamics and Retrogradation of Ultrahigh Pressurized Wheat Starch. J. Agric. Food Chem. 2006, 54, 6719–6724. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chaib, S.; Gu, Q.; Hemar, Y. Impact of Pressure on Physicochemical Properties of Starch Dispersions. Food Hydrocoll. 2017, 68, 164–177. [Google Scholar] [CrossRef]
- BeMiller, J.N. Preface to the Third Edition. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2009; pp. xvii–xviii. ISBN 978-0-12-746275-2. [Google Scholar]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Li, C.; Hamaker, B.R. Effects of Different Storage Temperatures on the Intra- and Intermolecular Retrogradation and Digestibility of Sago Starch. Int. J. Biol. Macromol. 2021, 182, 65–71. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Jensen, C.T.B.; Kristensen, P.G. Experimental and Modelling Studies of the Flow Properties of Maize and Waxy Maize Starch Pastes. Chem. Eng. J. 1998, 70, 165–171. [Google Scholar] [CrossRef]
- Nurul, M.; Azemi, B.M.; Manan, D.M.A. Rheological Behaviour of Sago (Metroxylon sagu) Starch Paste. Food Chem. 1999, 64, 501–505. [Google Scholar] [CrossRef]
- Lapasin, R. Rheological Characterization of Hydrogels. In Polysaccharide Hydrogels; Matricardi, P., Alhaique, F., Coviello, T., Eds.; Jenny Stanford Publishing: New York, NY, USA, 2016; pp. 99–154. ISBN 9780429069338. [Google Scholar]
- Douglas, J. Weak and Strong Gels and the Emergence of the Amorphous Solid State. Gels 2018, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N.; Whistler, R. Starch: Chemistry and Technology, 3rd ed.; BeMiller, J.N., Whistler, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; ISBN 9780127462752. [Google Scholar]
- Larrea-Wachtendorff, D.; Del Grosso, V.; Ferrari, G. Evaluation of the Physical Stability of Starch-Based Hydrogels Produced by High-Pressure Processing (HPP). Gels 2022, 8, 152. [Google Scholar] [CrossRef] [PubMed]
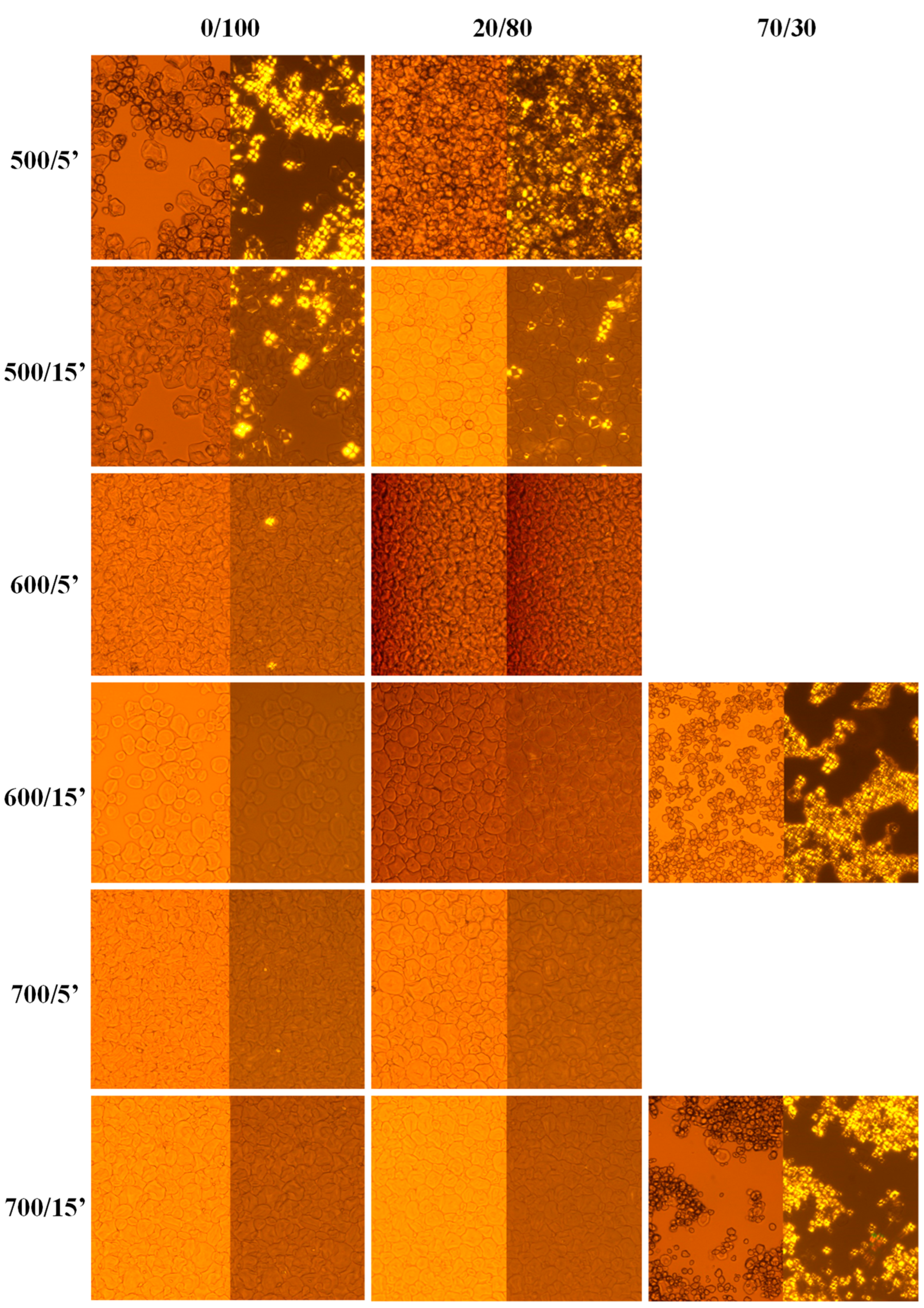




| Amylose Content (%) | Water/ Starch (% w/w) | Applied Pressures (MPa) | Treatment Time (min) |
|---|---|---|---|
| 0 | 80/20 | 500 | 5 |
| 20 | 600 | ||
| 15 | |||
| 70 | 700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulgarín, O.; Larrea-Wachtendorff, D.; Ferrari, G. Effects of the Amylose/Amylopectin Content and Storage Conditions on Corn Starch Hydrogels Produced by High-Pressure Processing (HPP). Gels 2023, 9, 87. https://doi.org/10.3390/gels9020087
Pulgarín O, Larrea-Wachtendorff D, Ferrari G. Effects of the Amylose/Amylopectin Content and Storage Conditions on Corn Starch Hydrogels Produced by High-Pressure Processing (HPP). Gels. 2023; 9(2):87. https://doi.org/10.3390/gels9020087
Chicago/Turabian StylePulgarín, Oscar, Dominique Larrea-Wachtendorff, and Giovanna Ferrari. 2023. "Effects of the Amylose/Amylopectin Content and Storage Conditions on Corn Starch Hydrogels Produced by High-Pressure Processing (HPP)" Gels 9, no. 2: 87. https://doi.org/10.3390/gels9020087
APA StylePulgarín, O., Larrea-Wachtendorff, D., & Ferrari, G. (2023). Effects of the Amylose/Amylopectin Content and Storage Conditions on Corn Starch Hydrogels Produced by High-Pressure Processing (HPP). Gels, 9(2), 87. https://doi.org/10.3390/gels9020087








