Specific Alcohol-Responsive Photonic Crystal Sensors Based on Host-Guest Recognition
Abstract
1. Introduction
2. Results and Discussion
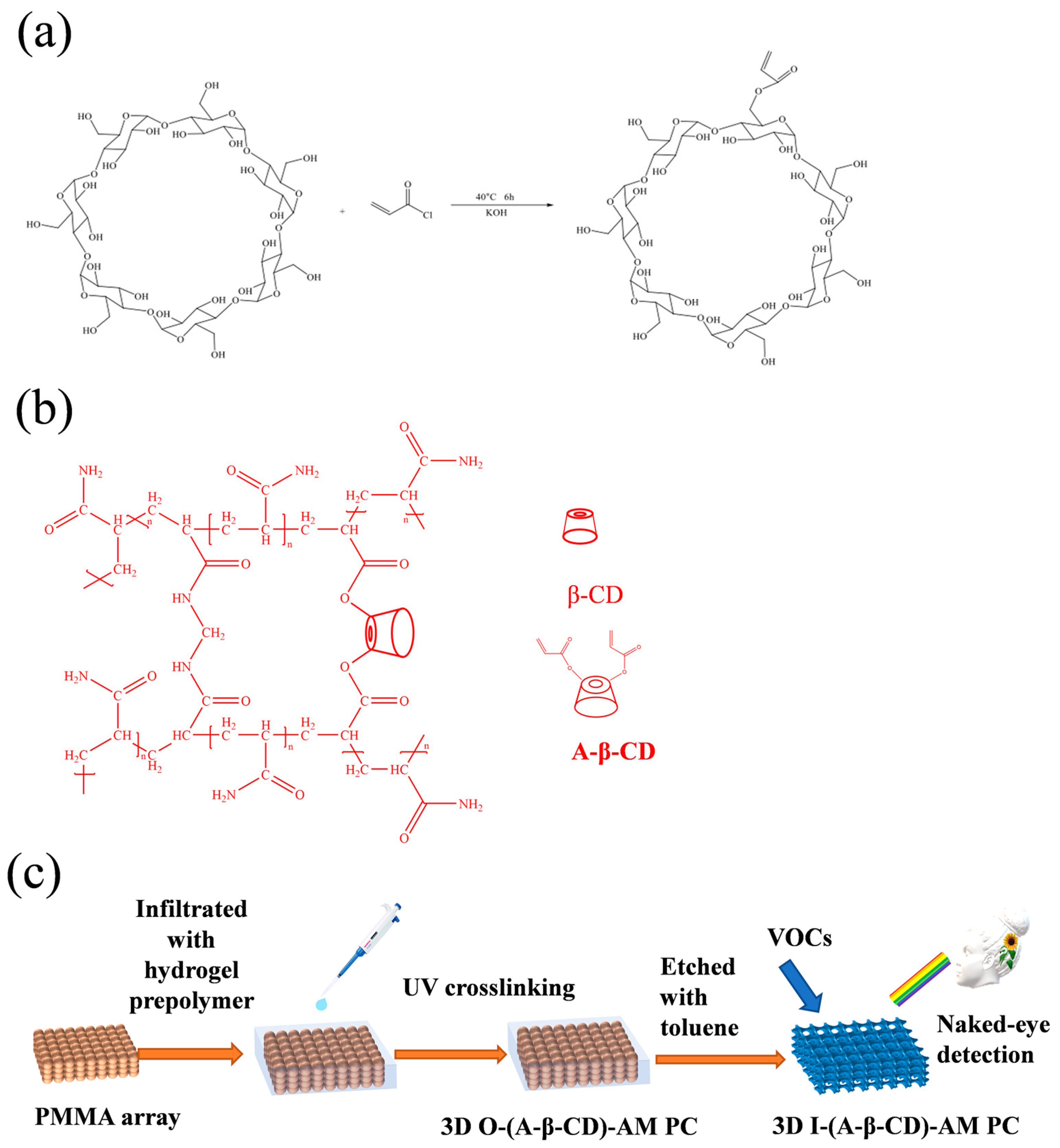
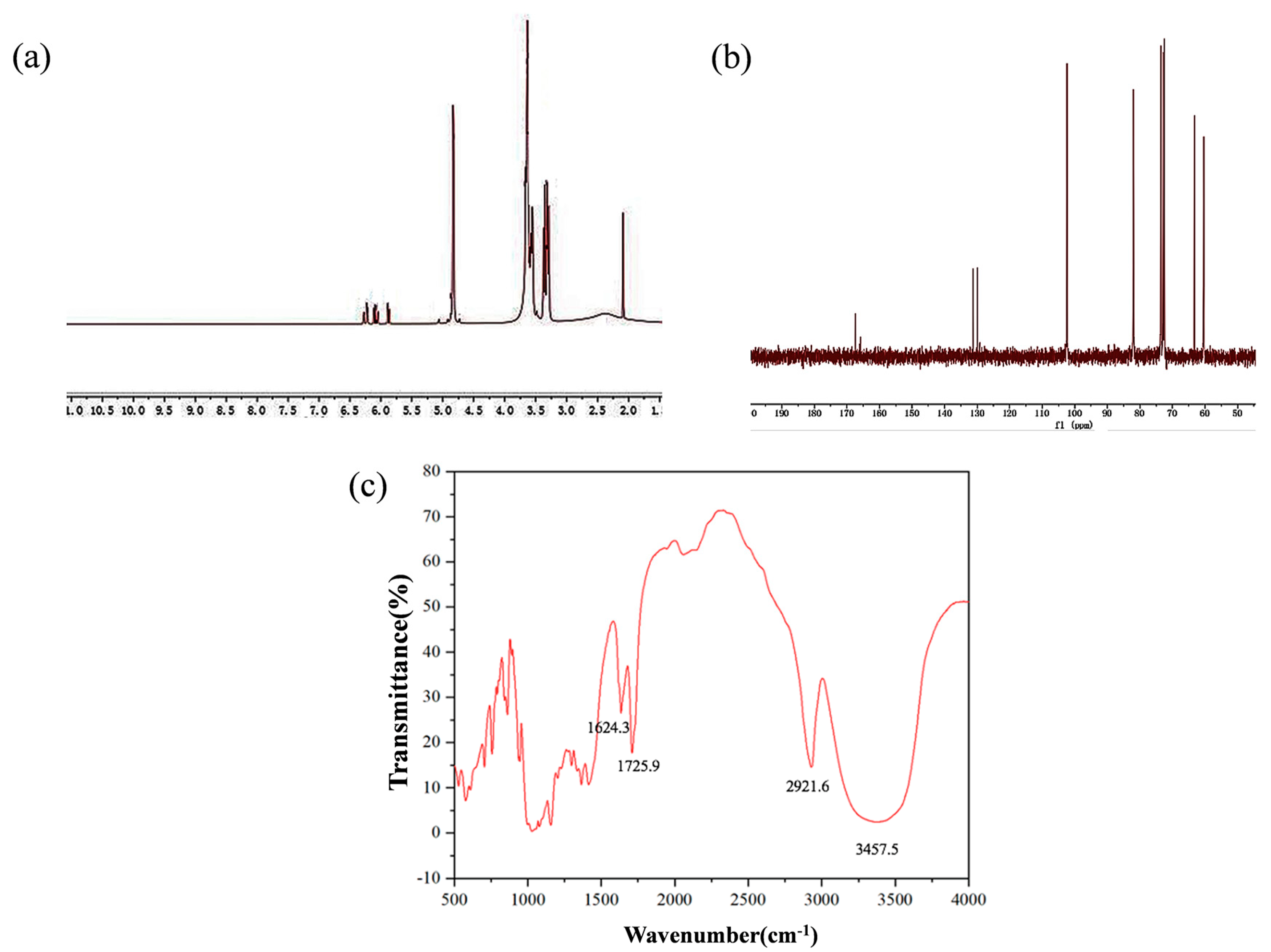
2.1. Characterization of A-β-CD
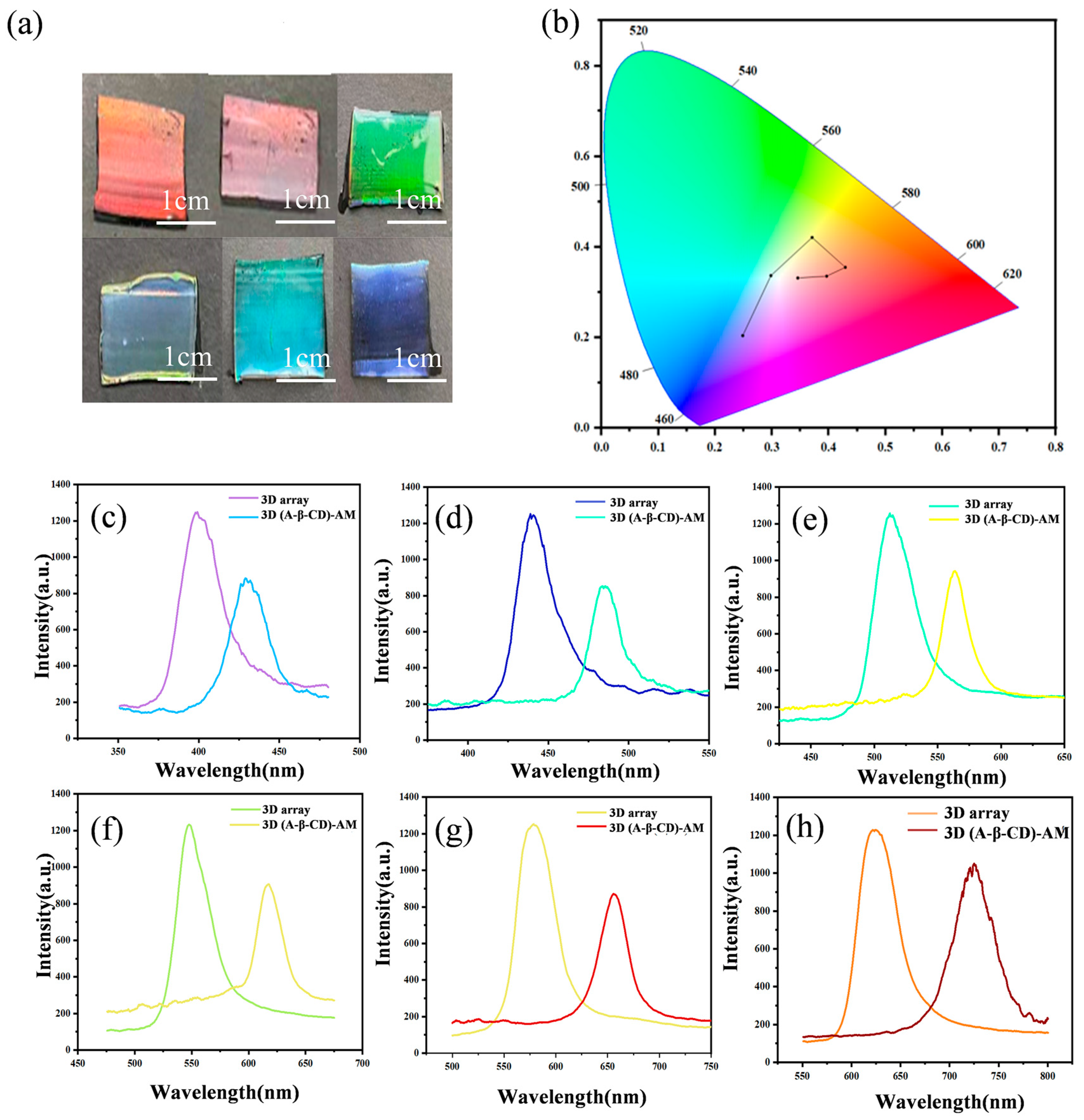
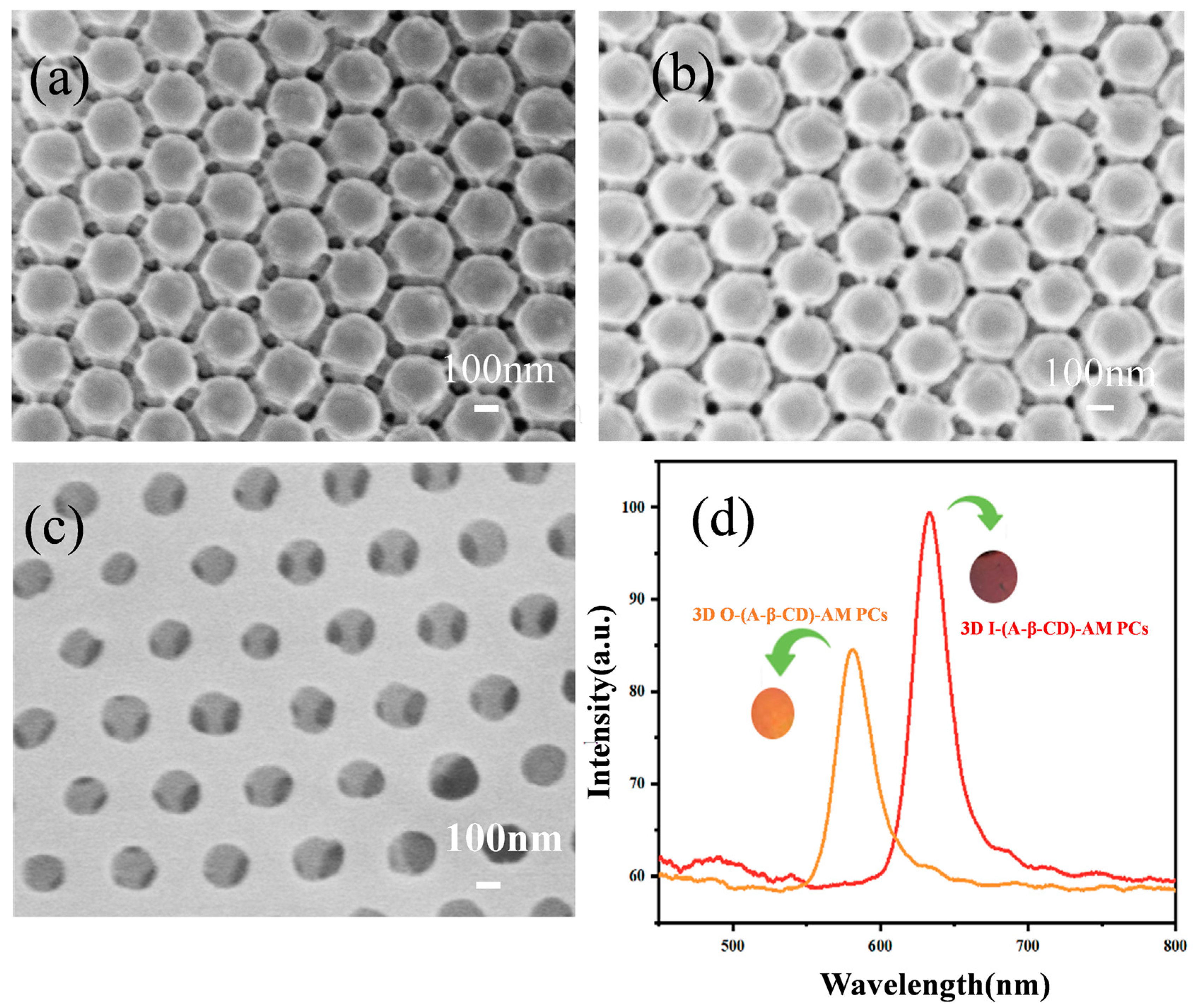
2.2. Characterization of 3D O-(A-β-CD)-AM PC and 3D I-(A-β-CD)-AM PC
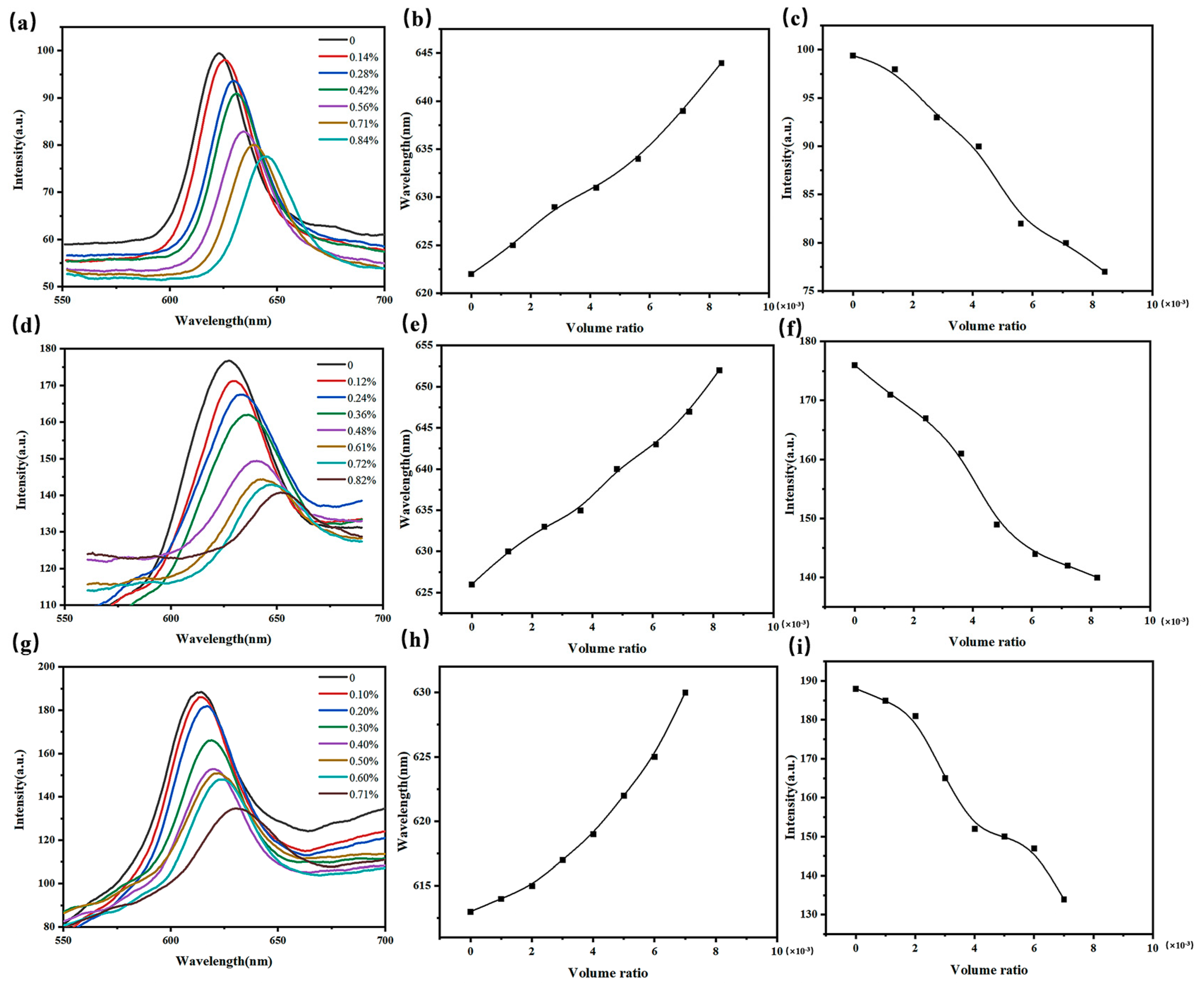
2.3. Response of 3D O-(A-β-CD)-AM PCs to VOCs
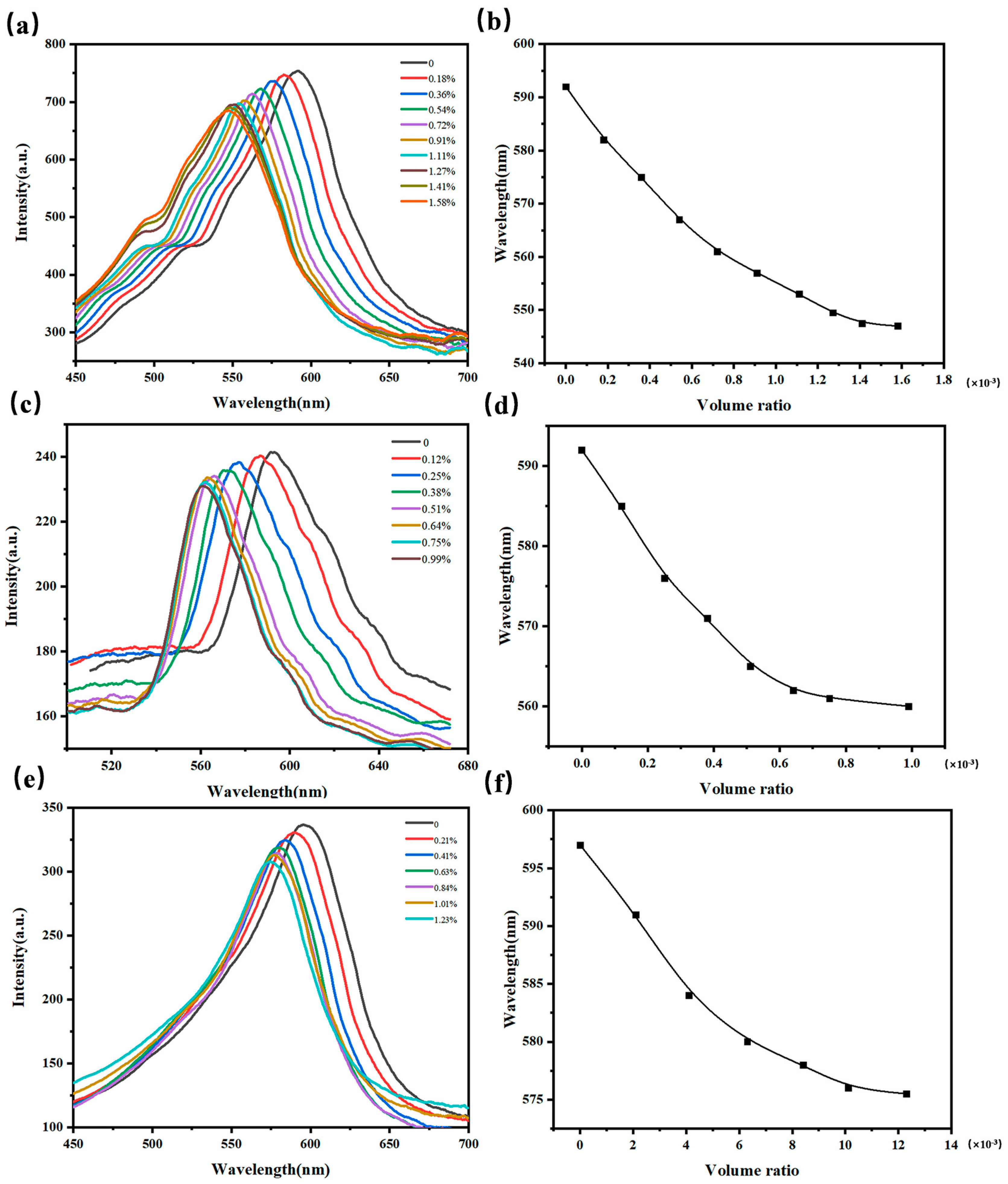
2.4. Response of 3D I-(A-β-CD)-AM to VOCs
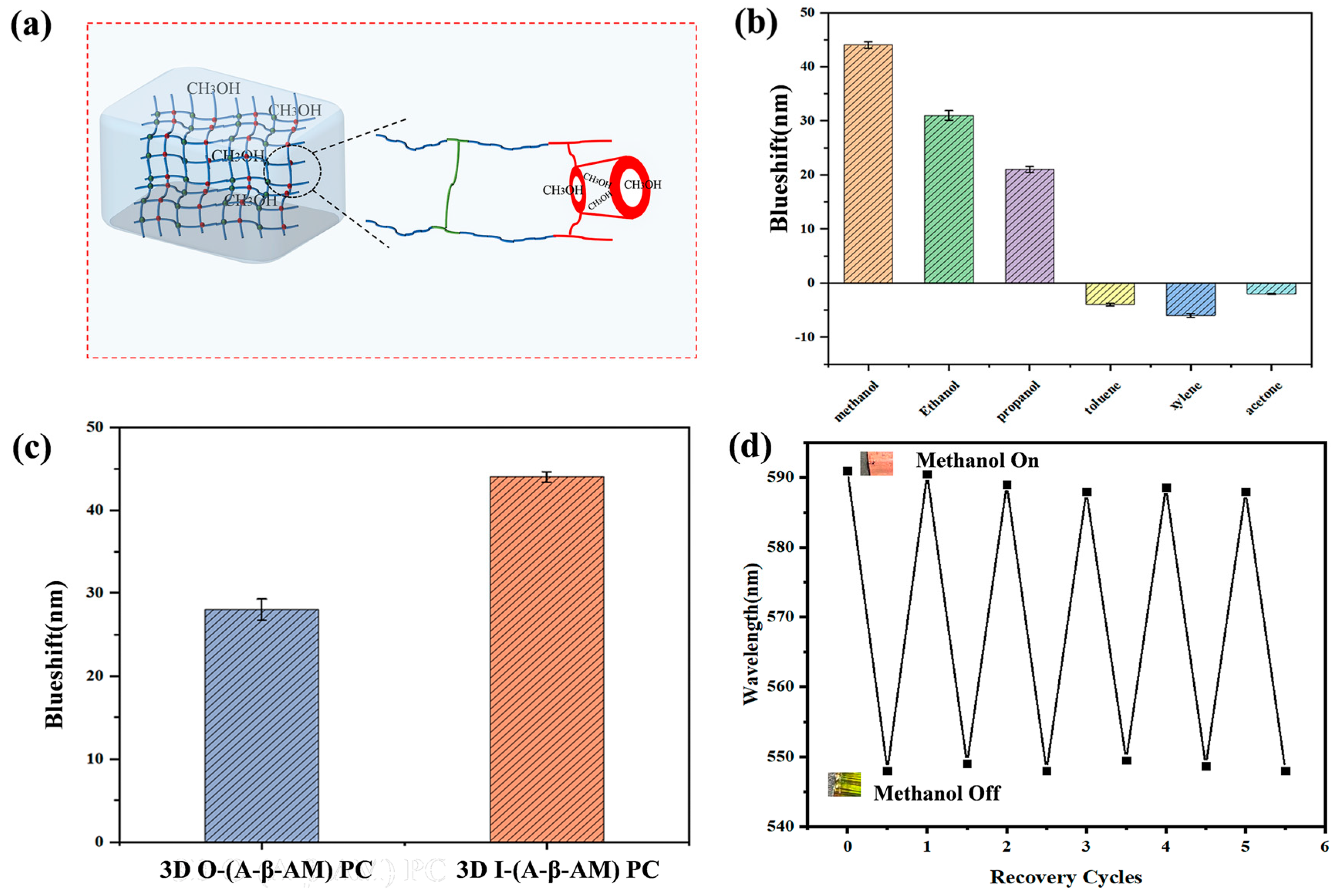
2.5. 3D (A-β-CD)-AM Detection Mechanism
2.6. Response of 3D O-(A-β-CD)-AM PC to Stretching Ability
3. Conclusions
4. Materials and Methods
4.1. Chemical Reagents
4.2. Synthesis of A-β-CD
4.3. Preparation of the PMMA Colloidal Particles and Arrays
4.4. Fabrication of 3D O-(A-β-CD)-AM PhCs and 3D I-(A-β-CD)-AM PhCs
4.5. Materials Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.-L.; Deng, Z.-P.; Dong, X.; Bai, L.; Wang, X.-L.; Wang, Y.-Z.; Song, F. A Surface Diffusion Barrier Strategy toward Water-Resistant Photonic Materials for Accurate Detection of Ethanol. ACS Appl. Mater. Interfaces 2022, 14, 30352–30361. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, B.; Huang, L.; Li, W.; Lu, Q.; Wu, H.; Liang, X.; Liu, T.; Liu, F.; Liu, F.; et al. Highly Selective Mixed Potential Methanol Gas Sensor Based on a Ce0.8Gd0.2O1.95 Solid Electrolyte and Au Sensing Electrode. ACS Sens. 2022, 7, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Santos-Carballal, D.; Magariu, N.; Mishra, A.K.; Ababii, N.; Krüger, H.; Wolff, N.; Vahl, A.; Bodduluri, M.T.; Kohlmann, N.; et al. Al2O3/ZnO Heterostructure-Based Sensors for Volatile Organic Compounds in Safety Applications. ACS Appl. Mater. Interfaces 2022, 14, 29331–29344. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Mohajir, A.E.L.; Berger, F.; Raschetti, M.; Sanchez, J.-B. Dealuminated Zeolite Y/SnO2 Nanoparticle Hybrid Sensors for Detecting Trace Levels of Propanol as a Lung Cancer Biomarker. ACS Appl. Nano Mater. 2022, 5, 9170–9178. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, Y.; Jin, R.; Wang, T.; Liu, F.; Wang, C.; Yan, X.; Sun, P.; Lu, G. Gas sensor based on cobalt-doped 3D inverse opal SnO2 for air quality monitoring. Sensor. Actuat. B-Chem. 2022, 350, 130807. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Z.; Han, D.; Gu, F. Au-modified three-dimensionally ordered macroporous ZnO:In for high-performance ethanol sensors. J. Mater. Chem. C 2020, 8, 2812–2819. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, B.-Y.; Jo, Y.K.; Dai, Z.; Lee, J.-H. Chemiresistive trimethylamine sensor using monolayer SnO2 inverse opals decorated with Cr2O3 nanoclusters. Sens. Actuat. B-Chem. 2020, 309, 127805. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, B.; Yu, Q.; Kou, X.; Sun, P.; Liu, F.; Lu, H.; Yan, X.; Lu, G. Realizing the Control of Electronic Energy Level Structure and Gas-Sensing Selectivity over Heteroatom-Doped In2O3 Spheres with an Inverse Opal Microstructure. ACS Appl. Mater. Interfaces 2019, 11, 9600–9611. [Google Scholar] [CrossRef]
- Chesler, P.; Hornoiu, C.; Anastasescu, M.; Calderon-Moreno, J.M.; Gheorghe, M.; Gartner, M. Cobalt- and Copper-Based Chemiresistors for Low Concentration Methane Detection, a Comparison Study. Gels 2022, 8, 721. [Google Scholar] [CrossRef]
- Guo, X.-X.; Hou, S.-C.; Li, H.-J.; Chen, J.; Haleem, A.; He, W.D. Simultaneous Cryogenic Radical and Oxidative Coupling Polymerizations to Polyaniline/Polyacrylamide Conductive Cryogels for Gas Sensing. Gels 2022, 8, 556. [Google Scholar] [CrossRef]
- Lee, D.; Yun, M.J.; Kim, K.H.; Kim, S.; Kim, H.D. Advanced Recovery and High-Sensitive Properties of Memristor-Based Gas Sensor Devices Operated at Room Temperature. ACS Sens. 2021, 6, 4217–4224. [Google Scholar] [CrossRef]
- Gao, W.; Zhi, Z.; Fan, S.; Hua, Z.; Li, H.; Pan, X.; Sun, W.; Gao, H. Amperometric Hydrogen Sensor Based on Solid Polymer Electrolyte and Titanium Foam Electrode. ACS Omega 2022, 7, 24895–24902. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Qin, M.; Wang, P.; Yang, L.; Zhang, L.; Yan, C.; Zhang, C.; Wang, W. Interface and Sensitive Characteristics of the Viscoelastic Film Used in a Surface Acoustic Wave Gas Sensor. ACS Sens. 2022, 7, 612–621. [Google Scholar] [CrossRef]
- Ng, D.K.T.; Xu, L.; Chen, W.; Wang, H.; Gu, Z.; Chia, X.X.; Fu, Y.H.; Jaafar, N.; Ho, C.P.; Zhang, T.; et al. Miniaturized CO2 Gas Sensor Using 20% ScAlN-Based Pyroelectric Detector. ACS Sens. 2022, 7, 2345–2357. [Google Scholar] [CrossRef]
- Kim, K.J.; Lu, P.; Culp, J.T.; Ohodnicki, P.R. Metal-Organic Framework Thin Film Coated Optical Fiber Sensors: A Novel Waveguide-Based Chemical Sensing Platform. ACS Sens. 2018, 3, 386–394. [Google Scholar] [CrossRef]
- Yan, D.; Qiu, L.; Shea, K.J.; Meng, Z.; Xue, M. Dyeing and Functionalization of Wearable Silk Fibroin/Cellulose Composite by Nanocolloidal Array. ACS Appl. Mater. Interfaces 2019, 11, 39163–39170. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, M.; Fan, J.; Qiu, L.; Zheng, W.; Liu, Y.; Meng, Z. Flory-Huggins VOC Photonics Sensor Made of Cellulose Derivatives. ACS Appl. Mater. Interfaces 2022, 14, 10701–10711. [Google Scholar] [CrossRef]
- John, S. Strong Localization of Photons in Certain Disordered Dielectric Superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef]
- Yablonovitch, E. Inhibited Spontaneous Emission in Solid-State Physics and Electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Shi, Z.; Tan, D.; Yang, X.; Xiong, L.; Li, G.; Lei, Y.; Xue, L. Fast Self-Assembly of Photonic Crystal Hydrogel for Wearable Strain and Temperature Sensor. Small Methods 2022, 6, 2200461. [Google Scholar] [CrossRef]
- Jing, F.A.; Xc, A.; Hao, C.A.; Lei, W.A.; Xiao, D.A.; Wz, A.; Yu, Q.B.; Zm, A.; Lq, A. A Smart Large-Scale Explosive-Responsive Amorphous Photonic Crystal Sensor Based on Color Analysis Method. Chem. Eng. J. 2022, 446, 136450. [Google Scholar] [CrossRef]
- Zheng, W.; Cai, X.; Yan, D.; Murtaza, G.; Meng, Z.; Qiu, L. Dual-Responsive Photonic Crystal Sensors Based on Physical Crossing-Linking SF-PNIPAM Dual-Crosslinked Hydrogel. Gels 2022, 8, 339. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Kozhevnikov, V.; Dai, X.; Turnbull, G.; Chen, X.; Kong, J.; Tang, B.Z.; Li, Y.; Xu, B.B. A flexible topo-optical sensing technology with ultra-high contrast. Nat. Commun. 2020, 11, 1448. [Google Scholar] [CrossRef]
- Wei, Q.; Lv, P.; Zhang, Y.; Zhang, J.; Qin, Z.; de Haan, L.T.; Chen, J.; Wang, D.; Xu, B.B.; Broer, D.J.; et al. Facile Stratification-Enabled Emergent Hyper-Reflectivity in Cholesteric Liquid Crystals. ACS Appl. Mater. Interfaces 2022, 14, 57235–57243. [Google Scholar] [CrossRef]
- Wu, J.; Yang, X.; Wang, Z.; Wu, B.; Wu, X. Tunable multichannel terahertz perfect graphene absorber with Fibonacci quasiperiodic photonic crystal. Adv. Compos. Hybrid Mater. 2022, 5, 2399–2405. [Google Scholar] [CrossRef]
- Dan, Y.Q.; Qiu, L.; Meng, Z.H.; Shen, Y.; Xue, Y.; Xue, M.; Xu, M.; Xu, Z.B.; Liu, Z.B.; Liu, W.F.; et al. Full-Color Natural Rubber Latex with A Photonic Nanostructure Composite. Chem. Commun. 2020, 56, 9604–9607. [Google Scholar]
- Zhang, W.; Xue, M.; Shea, K.J.; Qiu, L.; Xu, Z.; Fan, J.; Yan, D.; Meng, Z. A Biomass Based Photonic Crystal Made of “Konjac Tofu”. Chin. Chem. Lett. 2021, 32, 587–590. [Google Scholar] [CrossRef]
- Völlmecke, K.; Afroz, R.; Bierbach, S.; Brenker, L.J.; Frücht, S.; Glass, A.; Giebelhaus, R.; Hoppe, A.; Kanemaru, K.; Lazarek, M.; et al. Hydrogel-Based Biosensors. Gels 2022, 8, 768. [Google Scholar] [CrossRef]
- Frampton, M.J.; Anderson, H.L. Insulated molecular wires. Angew. Chem. Int. Edit. 2007, 46, 1028–1064. [Google Scholar] [CrossRef]
- Ji, Q.T.; Mu, X.F.; Hu, D.K.; Fan, L.J.; Xiang, S.Z.; Ye, H.J.; Gao, X.H.; Wang, P.Y. Fabrication of Host-Guest Complexes between Adamantane-Functionalized 1,3,4-Oxadiazoles and beta-Cyclodextrin with Improved Control Efficiency against Intractable Plant Bacterial Diseases. ACS Appl. Mater. Interfaces 2022, 14, 2564–2577. [Google Scholar] [CrossRef]
- Roy, A.; Guha Ray, P.; Bose, A.; Dhara, S.; Pal, S. pH-Responsive Copolymeric Network Gel Using Methacrylated beta-Cyclodextrin for Controlled Codelivery of Hydrophilic and Hydrophobic Drugs. ACS Appl. Bio Mater. 2022, 5, 3530–3543. [Google Scholar] [CrossRef]
- Alam, A.U.; Qin, Y.; Catalano, M.; Wang, L.; Kim, M.J.; Howlader, M.M.R.; Hu, N.-X.; Deen, M.J. Tailoring MWCNTs and beta-Cyclodextrin for Sensitive Detection of Acetaminophen and Estrogen. ACS Appl. Mater. Interfaces 2018, 10, 21411–21427. [Google Scholar] [CrossRef]
- Wang, W.; Shao, H.; Zhou, S.; Zhu, D.; Jiang, X.; Yu, G.; Deng, S. Rapid Removal of Perfluoroalkanesulfonates from Water by beta-Cyclodextrin Covalent Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 48700–48708. [Google Scholar] [CrossRef]
- Liu, C.; Wang, P.; Liu, X.; Yi, X.; Zhou, Z.; Liu, D. Multifunctional beta-Cyclodextrin MOF-Derived Porous Carbon as Efficient Herbicides Adsorbent and Potassium Fertilizer. ACS Sustain. Chem. Eng. 2019, 7, 14479–14489. [Google Scholar] [CrossRef]
- Zhu, S.C.; Yu, Y.L.; Shi, M.Z.; Chen, Y.; Cao, J. Ionic Liquid-beta-Cyclodextrin Vesicle-Based Mechanochemical-Assisted Extraction for the Weak Acid Compounds from Mori Fructus. ACS Sustain. Chem. Eng. 2022, 10, 3735–3748. [Google Scholar] [CrossRef]
- Rajha, H.N.; Chacar, S.; Afif, C.; Vorobiev, E.; Louka, N.; Maroun, R.G. beta-Cyclodextrin-Assisted Extraction of Polyphenols from Vine Shoot Cultivars. J. Agric. Food Chem. 2015, 63, 3387–3393. [Google Scholar] [CrossRef]
- Xu, L.; Xing, C.Y.; Ke, D.; Chen, L.; Qiu, Z.J.; Zeng, S.L.; Li, B.J.; Zhang, S. Amino-Functionalized beta-Cyclodextrin to Construct Green Metal-Organic Framework Materials for CO2 Capture. ACS Appl. Mater. Interfaces 2019, 12, 3032–3041. [Google Scholar] [CrossRef]
- Pereva, S.; Himitliiska, T.; Spassov, T.; Stoyanov, S.D.; Arnaudov, L.N.; Dudev, T. Cyclodextrin-Based Solid-Gas Clathrates. J. Agric. Food Chem. 2015, 63, 6603–6613. [Google Scholar] [CrossRef]
- Nishikawa, S.; Ugawa, T.; Fukahori, T. Molecular recognition kinetics of beta-cyclodextrin for several alcohols by ultrasonic relaxation method. J. Phys. Chem. B 2001, 105, 7594–7597. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Ben-Amotz, D. Cavity Hydration and Competitive Binding in Methylated beta-Cyclodextrin. J Phys. Chem. Lett. 2019, 10, 2802–2805. [Google Scholar] [CrossRef]
- Goubet, I.; Dahout, C.; Sémon, E.; Guichard, E.; Le Quéré, J.L.; Voilley, A. Competitive binding of aroma compounds by beta-cyclodextrin. J. Agric. Food Chem. 2001, 49, 5916–5922. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Ugawa, T. Dynamic interaction between cyclodextrin and nonelectrolytes in aqueous solutions by ultrasonic relaxation method. J. Phys. Chem. A 2000, 104, 2914–2918. [Google Scholar] [CrossRef]
- Chen, X.; Parker, S.G.; Zou, G.; Su, W.; Zhang, Q. β-Cyclodextrin-Functionalized Silver Nanoparticles for the Naked Eye Detection of Aromatic Isomers. ACS Nano 2010, 4, 6387–6394. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Liu, Z.; Li, W.; Liu, Y.; Li, J.; Wei, H.; Han, H. Fabrication of a water-retaining, slow-release fertilizer based on nanocomposite double-network hydrogels via ion-crosslinking and free radical polymerization. J. Ind. Eng. Chem. 2021, 93, 375–382. [Google Scholar] [CrossRef]
- Ding, L.; Li, Y.; Jia, D.; Deng, J.; Yang, W. β-Cyclodextrin-based oil-absorbents: Preparation, high oil absorbency and reusability. Carbohydr. Polym. 2011, 83, 1990–1996. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhou, L.J.; Yang, J. Photonic crystal cellulose membrane detection of sulfur dioxide gas. Chin. J. Anal. Chem. 2018, 46, 7. (In Chinese) [Google Scholar]
- Kong, D.; El-Bahy, Z.M.; Algadi, H.; Li, T.; El-Bahy, S.M.; Nassan, M.A.; Li, J.; Faheim, A.A.; Li, A.; Xu, C.; et al. Highly sensitive strain sensors with wide operation range from strong MXene-composited polyvinyl alcohol/sodium carboxymethylcellulose double network hydrogel. Adv. Compos. Hybrid Mater. 2022, 5, 1976–1987. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Z.; Zhang, H.; Huang, Y.; Wang, Z.; Zhou, Y.; Xu, B.B.; Halila, S.; Chen, J. Ultrastretchable, Highly Transparent, Self-Adhesive, and 3D-Printable Ionic Hydrogels for Multimode Tactical Sensing. Chem. Mater. 2021, 33, 6731–6742. [Google Scholar] [CrossRef]
- Chang, X.; Chen, L.; Chen, J.; Zhu, Y.; Guo, Z. Advances in transparent and stretchable strain sensors. Adv. Compos. Hybrid Mater. 2021, 4, 435–450. [Google Scholar] [CrossRef]

| Number | MMA (mL) | KPS (g) | Diameter (nm) |
|---|---|---|---|
| 1 | 9 | 0.9 | 165 |
| 2 | 10 | 0.6 | 180 |
| 3 | 11 | 0.5 | 210 |
| 4 | 12 | 0.4 | 225 |
| 5 | 18 | 0.4 | 240 |
| 6 | 20 | 0.4 | 255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Zhang, X.; Fan, J.; Zheng, W.; Zhang, T.; Qiu, L.; Meng, Z. Specific Alcohol-Responsive Photonic Crystal Sensors Based on Host-Guest Recognition. Gels 2023, 9, 83. https://doi.org/10.3390/gels9020083
Cai X, Zhang X, Fan J, Zheng W, Zhang T, Qiu L, Meng Z. Specific Alcohol-Responsive Photonic Crystal Sensors Based on Host-Guest Recognition. Gels. 2023; 9(2):83. https://doi.org/10.3390/gels9020083
Chicago/Turabian StyleCai, Xiaolu, Xiaojing Zhang, Jing Fan, Wenxiang Zheng, Tianyi Zhang, Lili Qiu, and Zihui Meng. 2023. "Specific Alcohol-Responsive Photonic Crystal Sensors Based on Host-Guest Recognition" Gels 9, no. 2: 83. https://doi.org/10.3390/gels9020083
APA StyleCai, X., Zhang, X., Fan, J., Zheng, W., Zhang, T., Qiu, L., & Meng, Z. (2023). Specific Alcohol-Responsive Photonic Crystal Sensors Based on Host-Guest Recognition. Gels, 9(2), 83. https://doi.org/10.3390/gels9020083









