Enhancing Conductivity and Self-Healing Properties of PVA/GEL/OSA Composite Hydrogels by GO/SWNTs for Electronic Skin
Abstract
1. Introduction
2. Results and Discussion
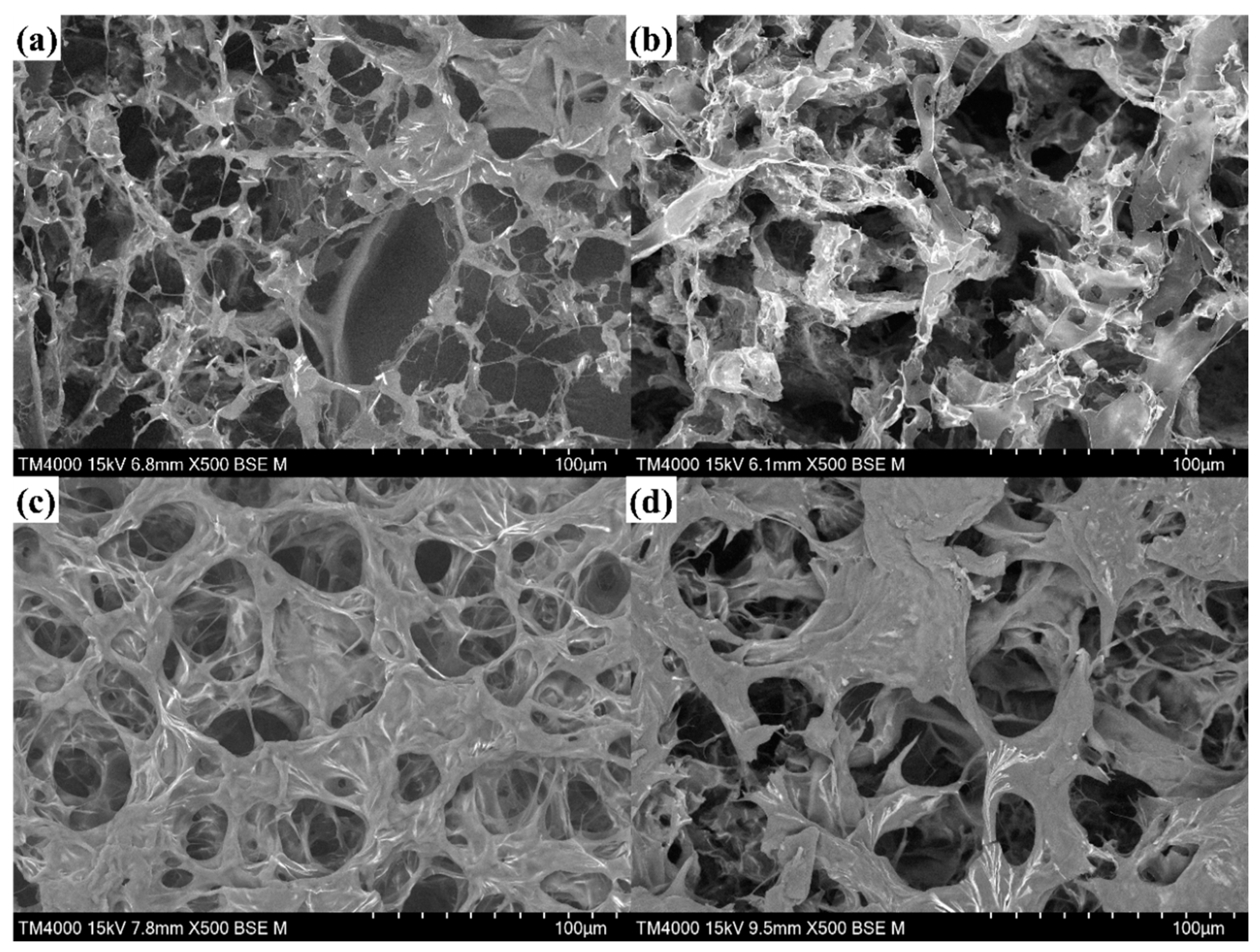
2.1. Microtopography of Hydrogels
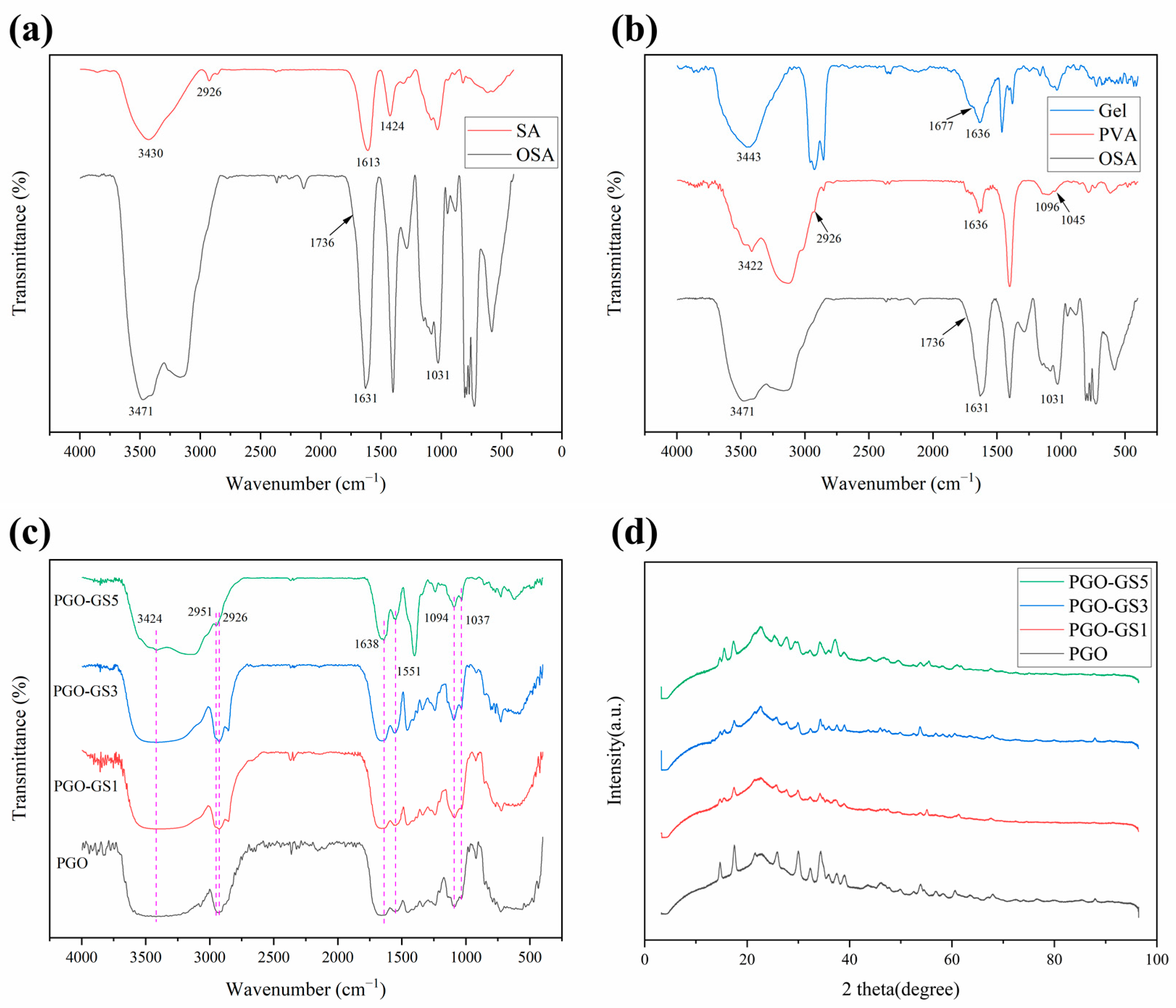
2.2. Chemical Structure of the Hydrogels
2.3. Mechanical Properties of Hydrogels
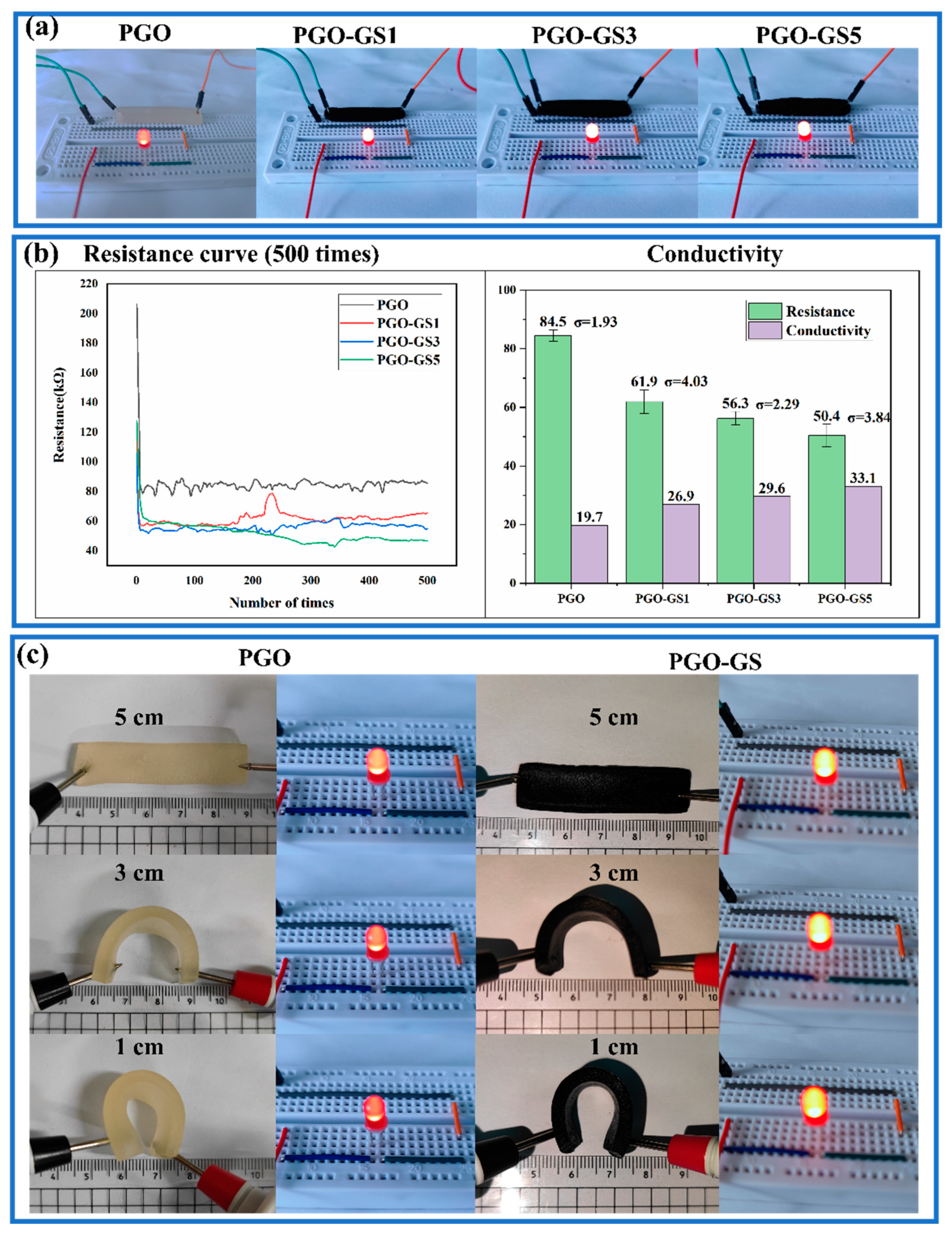
2.4. Conductive Properties of Hydrogels
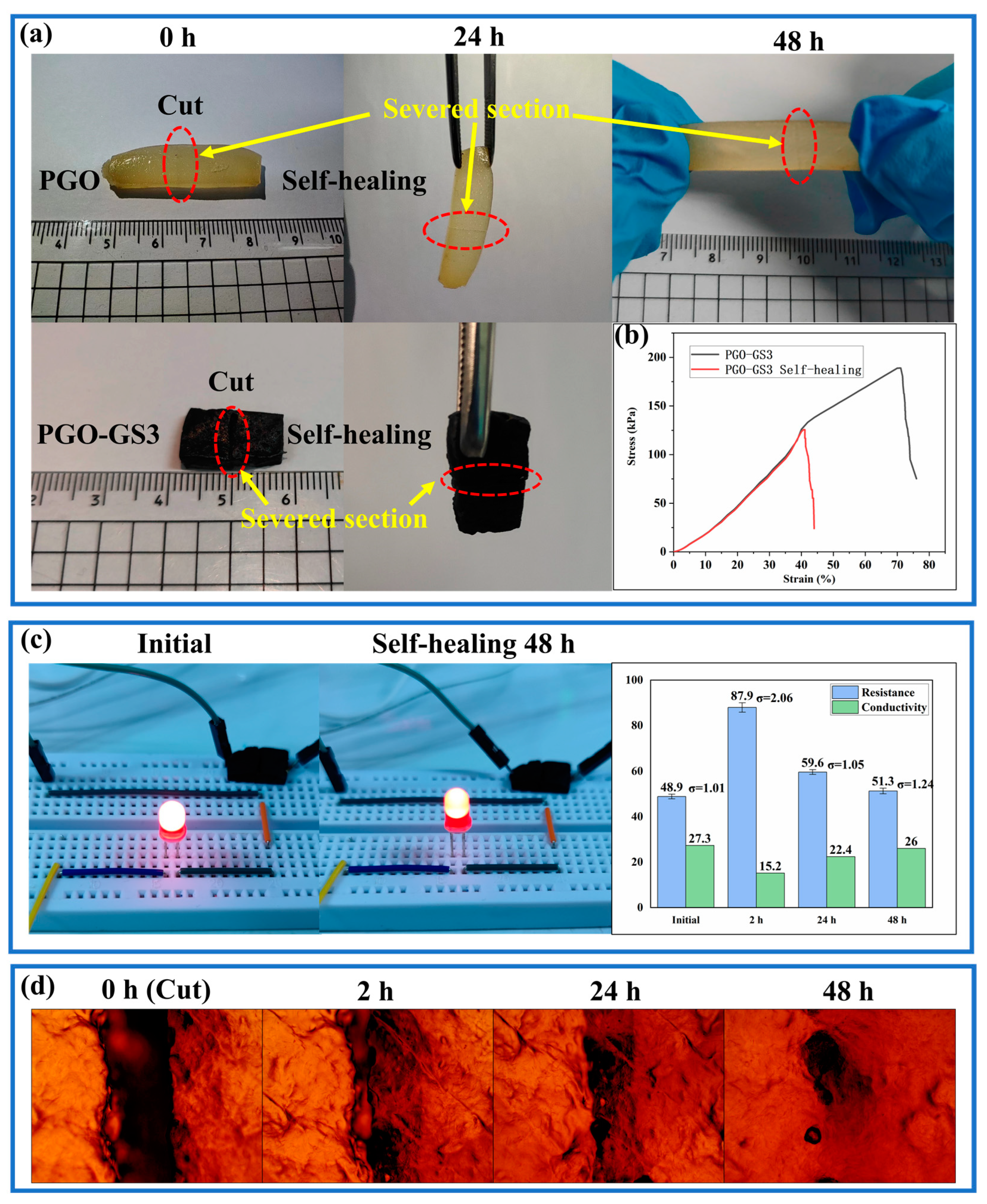
2.5. Self-Healing Property of Hydrogels
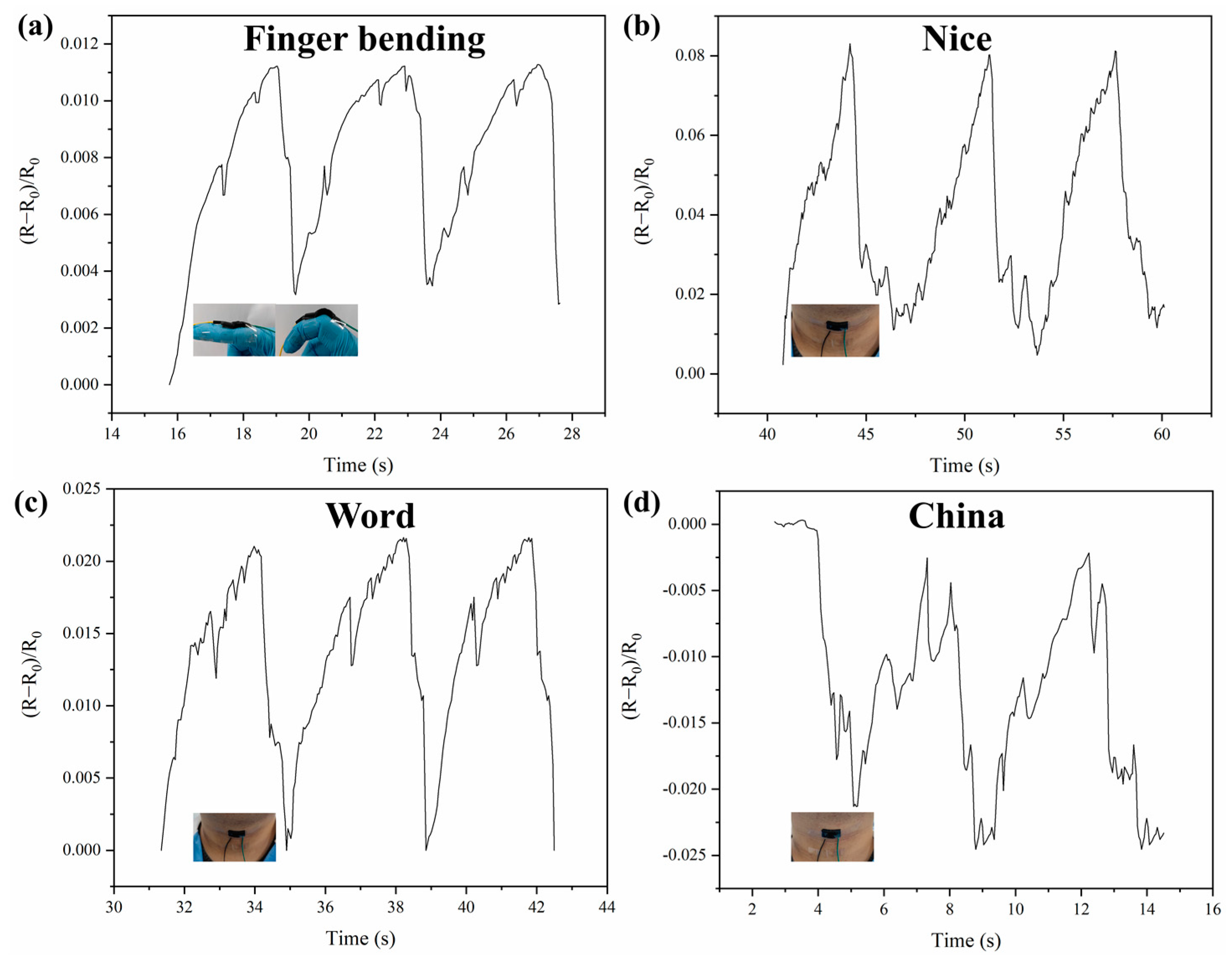
2.6. Electronic Skin
2.7. Cytocompatibility
3. Conclusions
4. Materials and Methods
4.1. Materials
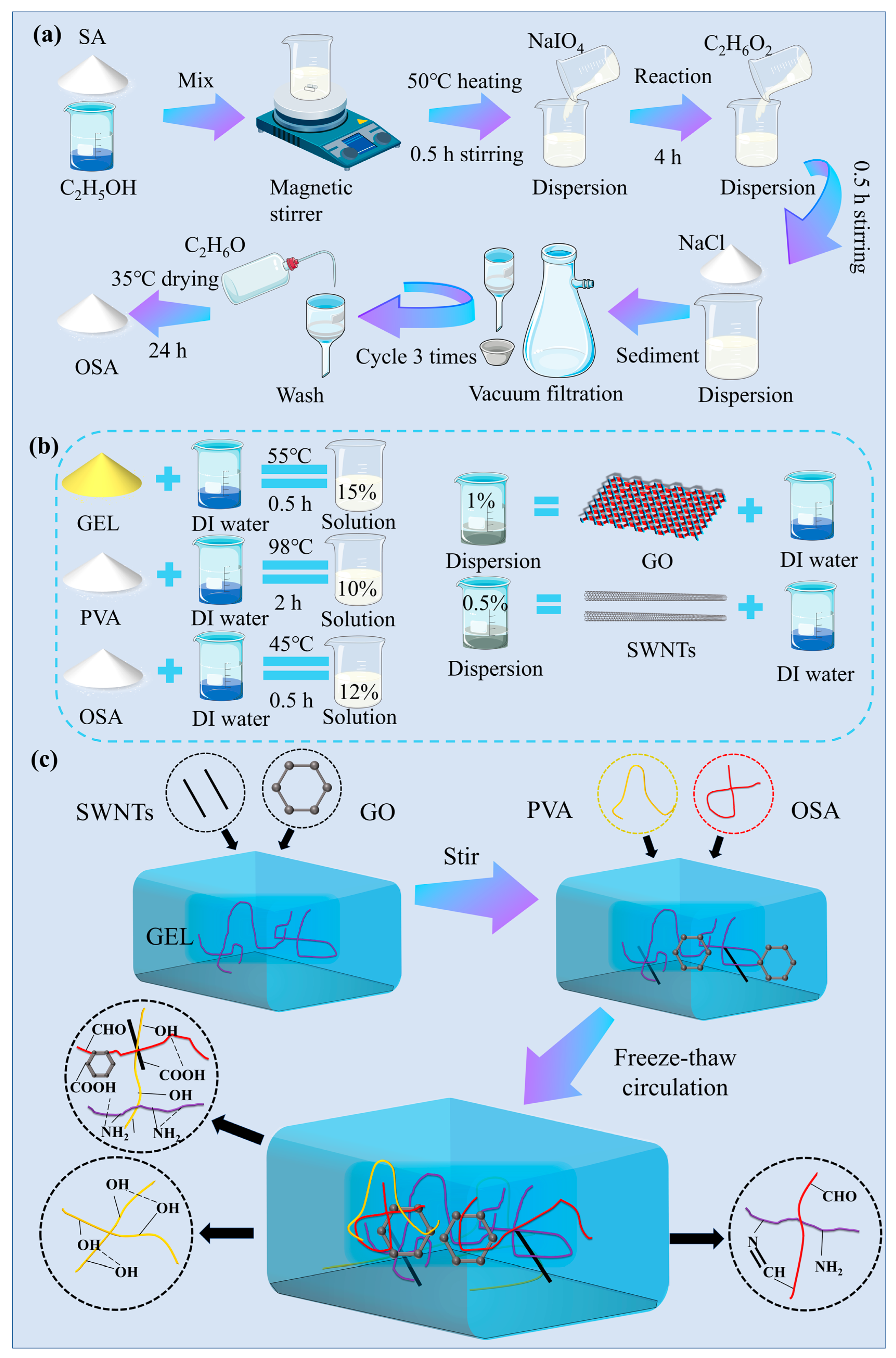
4.2. Preparation of OSA
4.3. Preparation of Hydrogel
4.4. Characterization
4.4.1. Constituent Analysis
4.4.2. Structural Analysis
4.4.3. Mechanical Performance
4.4.4. Conductive Property Measurement
4.4.5. Self-Healing Behaviors
4.4.6. Cytocompatibility Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobin, D.J. Biochemistry of human skin—Our brain on the outside. Chem. Soc. Rev. 2006, 35, 52–67. [Google Scholar] [CrossRef]
- Xu, K.; Lu, Y.; Takei, K. Multifunctional Skin-Inspired Flexible Sensor Systems for Wearable Electronics. Adv. Mater. Technol. 2019, 4, 1800628. [Google Scholar] [CrossRef]
- Yang, T.; Xie, D.; Li, Z.; Zhu, H. Recent advances in wearable tactile sensors: Materials, sensing mechanisms, and device performance. Mater. Sci. Eng. R Rep. 2017, 115, 1–37. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, K.; Lou, Z.; Chen, D.; Shen, G. Recent Developments in Graphene-Based Tactile Sensors and E-Skins. Adv. Mater. Technol. 2018, 3, 1700248. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Cui, K.; Ge, S.; Cheng, X.; Yan, M.; Yu, J.; Liu, H. Flexible Electronics Based on Micro/Nanostructured Paper. Adv. Mater. 2018, 30, 1801588. [Google Scholar] [CrossRef]
- He, Z.; Gao, B.; Li, T.; Liao, J.; Liu, B.; Liu, X.; Wang, C.; Feng, Z.; Gu, Z. Piezoelectric-Driven Self-Powered Patterned Electrochromic Supercapacitor for Human Motion Energy Harvesting. ACS Sustain. Chem. Eng. 2019, 7, 1745–1752. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, C.; Zou, D.; Liu, X.; Cai, L.; Li, X.; Shi, M. A Structured Design for Highly Stretchable Electronic Skin. Adv. Mater. Technol. 2019, 4, 1900492. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Guo, M.-M.; Zhang, Y.; Tang, C.Y.; Jiang, C.; Dong, Y.; Law, W.-C.; Du, F.-P. Flexible, stretchable and conductive PVA/PEDOT:PSS composite hydrogels prepared by SIPN strategy. Polym. Test. 2020, 81, 106213. [Google Scholar] [CrossRef]
- Huang, H.; Han, L.; Fu, X.; Wang, Y.; Yang, Z.; Pan, L.; Xu, M. Multiple Stimuli Responsive and Identifiable Zwitterionic Ionic Conductive Hydrogel for Bionic Electronic Skin. Adv. Electron. Mater. 2020, 6, 2000239. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, M.; Shen, J.; Qiu, Q.; Yu, J.; Ding, B. All-Fiber Structured Electronic Skin with High Elasticity and Breathability. Adv. Funct. Mater. 2020, 30, 1908411. [Google Scholar] [CrossRef]
- Nie, B.; Liu, S.; Qu, Q.; Zhang, Y.; Zhao, M.; Liu, J. Bio-inspired flexible electronics for smart E-skin. Acta Biomater. 2022, 139, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Cheng, W.; Zhang, J.-H.; Zhou, Z.; Sun, X.; Li, L.; Liang, J.-G.; Shi, Y.; Pan, L. Challenges in Materials and Devices of Electronic Skin. ACS Mater. Lett. 2022, 4, 577–599. [Google Scholar] [CrossRef]
- Tran, V.V.; Lee, K.; Nguyen, T.N.; Lee, D. Recent Advances and Progress of Conducting Polymer-Based Hydrogels in Strain Sensor Applications. Gels 2023, 9, 12. [Google Scholar] [CrossRef]
- Tang, L.; Wu, S.; Qu, J.; Gong, L.; Tang, J. A Review of Conductive Hydrogel Used in Flexible Strain Sensor. Materials 2020, 13, 3947. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lin, Z.; Luo, Z.; Jiang, T.; Shang, J.; Yang, Y. Development of conductive hydrogels: from design mechanisms to frontier applications. Bio-Design Manuf. 2022, 5, 729–756. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Z.; Zhang, H.; Huang, Y.; Wang, Z.; Zhou, Y.; Xu, B.B.; Halila, S.; Chen, J. Ultrastretchable, Highly Transparent, Self-Adhesive, and 3D-Printable Ionic Hydrogels for Multimode Tactical Sensing. Chem. Mater. 2021, 33, 6731–6742. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, X.; Gao, S.; Yue, W.; Li, Y.; Shen, G. Recent Advances in Carbon Material-Based Multifunctional Sensors and Their Applications in Electronic Skin Systems. Adv. Funct. Mater. 2021, 31, 2104288. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Kang, Q.; Zhu, L.; Qin, G.; Chen, Q. Freezing-tolerant and robust gelatin-based supramolecular conductive hydrogels with double-network structure for wearable sensors. Polym. Test. 2021, 93, 106879. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, A.; Li, J.; Li, Q.; Han, Q.; Zheng, X.; Cao, H.; Bai, S. Preparation of conductive and transparent dipeptide hydrogels for wearable biosensor. Bio-Design Manuf. 2022, 5, 153–162. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Y.; Liu, M.; Wang, F.; Liu, P.; Yu, Y.; Feng, Q.; Xiao, X. Highly Stretchable, Tough, and Conductive Ag@Cu Nanocomposite Hydrogels for Flexible Wearable Sensors and Bionic Electronic Skins. Macromol. Mater. Eng. 2021, 306, 2100341. [Google Scholar] [CrossRef]
- Hammock, M.L.; Chortos, A.; Tee, B.C.-K.; Tok, J.B.-H.; Bao, Z. 25th Anniversary Article: The Evolution of Electronic Skin (E-Skin): A Brief History, Design Considerations, and Recent Progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef]
- Liu, C.; Huang, N.; Xu, F.; Tong, J.; Chen, Z.; Gui, X.; Fu, Y.; Lao, C. 3D Printing Technologies for Flexible Tactile Sensors toward Wearable Electronics and Electronic Skin. Polymers 2018, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Wang, R.; Xu, X.; Cheng, Y.; Wang, X.; Shi, L.; Sun, J. High-Performance Piezoresistive Electronic Skin with Bionic Hierarchical Microstructure and Microcracks. ACS Appl. Mater. Interfaces 2017, 9, 14911–14919. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, K.; Shen, G. Wearable, Implantable, and Interventional Medical Devices Based on Smart Electronic Skins. Adv. Mater. Technol. 2021, 6, 2100107. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Z.; Zhao, Y.; Hu, J.; Wang, H. Highly stretchable porous composite hydrogels with stable conductivity for strain sensing. Compos. Sci. Technol. 2021, 213, 108968. [Google Scholar] [CrossRef]
- Wen, N.; Jiang, B.; Wang, X.; Shang, Z.; Jiang, D.; Zhang, L.; Sun, C.; Wu, Z.; Yan, H.; Liu, C.; et al. Overview of Polyvinyl Alcohol Nanocomposite Hydrogels for Electro-Skin, Actuator, Supercapacitor and Fuel Cell. Chem. Rec. 2020, 20, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhong, W. Facile Preparation of an Excellent Mechanical Property Electroactive Biopolymer-Based Conductive Composite Film and Self-Enhancing Cellulose Hydrogel to Construct a High-Performance Wearable Supercapacitor. ACS Sustain. Chem. Eng. 2020, 8, 7879–7891. [Google Scholar] [CrossRef]
- Wu, X.; Xie, Y.; Xue, C.; Chen, K.; Yang, X.; Xu, L.; Qi, J.; Zhang, D. Preparation of PVA-GO composite hydrogel and effect of ionic coordination on its properties. Mater. Res. Express 2019, 6, 075306. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Pan, L.; Wang, H.; Bin, Y. MWCNTs reinforced conductive, self-healing polyvinyl alcohol/carboxymethyl chitosan/oxidized sodium alginate hydrogel as the strain sensor. J. Appl. Polym. Sci. 2021, 138, 49800. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Li, P.; Fan, Y. Ternary hydrogels with tunable mechanical and self-healing properties based on the synergistic effects of multiple dynamic bonds. J. Mater. Chem. B 2020, 8, 4660–4671. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wei, Q.; Wang, Y.; Lei, M.; Li, M.; Li, D.; Zhang, L.; Wu, Y. Self-Healing Mechanism and Conductivity of the Hydrogel Flexible Sensors: A Review. Gels 2021, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, H.; Ma, P.X.; Guo, B. Self-healing conductive hydrogels: Preparation, properties and applications. Nanoscale 2020, 12, 1224–1246. [Google Scholar] [CrossRef] [PubMed]
- Strandman, S.; Zhu, X.X. Self-Healing Supramolecular Hydrogels Based on Reversible Physical Interactions. Gels 2016, 2, 16. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wei, Q.; Wang, Y.; Li, M.; Li, D.; Zhang, L. A 3D printable, highly stretchable, self-healing hydrogel-based sensor based on polyvinyl alcohol/sodium tetraborate/sodium alginate for human motion monitoring. Int. J. Biol. Macromol. 2022, 219, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, B.; Chen, Y.; Feng, X. Biocompatible and Ultra-Flexible Inorganic Strain Sensors Attached to Skin for Long-Term Vital Signs Monitoring. IEEE Electron Device Lett. 2016, 37, 496–499. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Sun, Y.; Zhao, S.; Feng, M.; Xu, G.; Zhu, H.; Ji, P.; Mao, H.; He, Y.; et al. A double-network polysaccharide-based composite hydrogel for skin wound healing. Carbohydr. Polym. 2021, 261, 117870. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Cong, H.-P.; Wang, P.; Yu, S.-H. Highly Elastic and Superstretchable Graphene Oxide/Polyacrylamide Hydrogels. Small 2014, 10, 448–453. [Google Scholar] [CrossRef]
- Ionita, M.; Crica, L.E.; Tiainen, H.; Haugen, H.J.; Vasile, E.; Dinescu, S.; Costache, M.; Iovu, H. Gelatin–poly(vinyl alcohol) porous biocomposites reinforced with graphene oxide as biomaterials. J. Mater. Chem. B 2015, 4, 282–291. [Google Scholar] [CrossRef]
- Lin, J.; Yao, X.; Basquiroto de Souza, F.; Sagoe-Crentsil, K.; Duan, W. Mechanisms of dispersion of nanoparticle-decorated graphene oxide nanosheets in aqueous media: Experimental and molecular dynamics simulation study. Carbon 2021, 184, 689–697. [Google Scholar] [CrossRef]
- Atif, R.; Inam, F. Reasons and remedies for the agglomeration of multilayered graphene and carbon nanotubes in polymers. Beilstein J. Nanotechnol. 2016, 7, 1174–1196. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-H.; Pan, X.-R.; Zhou, Y.; Chen, L.-Y.; Xie, W.-G.; Long, Z.-H.; Zheng, H. Preparation and characterization of crosslinked carboxymethyl chitosan–oxidized sodium alginate hydrogels. J. Appl. Polym. Sci. 2011, 122, 2331–2337. [Google Scholar] [CrossRef]
- Wang, L.; Deng, F.; Wang, W.; Li, A.; Lu, C.; Chen, H.; Wu, G.; Nan, K.; Li, L. Construction of Injectable Self-Healing Macroporous Hydrogels via a Template-Free Method for Tissue Engineering and Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 36721–36732. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Nakajima, T.; Takanashi, H.; Ohki, A. Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry. Fuel 2009, 88, 139–144. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Cao, Y.; Guo, Z.; Li, F.; Wang, L. Construction of Chitosan-Based Hydrogel Incorporated with Antimonene Nanosheets for Rapid Capture and Elimination of Bacteria. Adv. Funct. Mater. 2020, 30, 2003196. [Google Scholar] [CrossRef]

| Hydrogel Samples | Density (g/cm3) | Porosity (%) |
|---|---|---|
| PGO | 0.205 ± 0.009 | 40.59 ± 2.71 |
| PGO-GS1 | 0.109 ± 0.003 | 60.77 ± 3.65 |
| PGO-GS3 | 0.101 ± 0.019 | 62.28 ± 7.30 |
| PGO-GS5 | 0.114 ± 0.012 | 58.58 ± 6.82 |
| Group Name | Absorbance | Sur % |
|---|---|---|
| Blank group | 0.35469 | N/A |
| Control group | 1.19736 | 100 |
| PGO-GS3 | 0.99315 | 75.8 |
| PGO | 1.15768 | 95.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, H.; Cui, J.; Wang, Y.; Li, M.; Zhang, J.; Wang, C.; Liu, Z.; Wei, Q. Enhancing Conductivity and Self-Healing Properties of PVA/GEL/OSA Composite Hydrogels by GO/SWNTs for Electronic Skin. Gels 2023, 9, 155. https://doi.org/10.3390/gels9020155
Chen X, Zhang H, Cui J, Wang Y, Li M, Zhang J, Wang C, Liu Z, Wei Q. Enhancing Conductivity and Self-Healing Properties of PVA/GEL/OSA Composite Hydrogels by GO/SWNTs for Electronic Skin. Gels. 2023; 9(2):155. https://doi.org/10.3390/gels9020155
Chicago/Turabian StyleChen, Xiaohu, Haonan Zhang, Jiashu Cui, Yanen Wang, Mingyang Li, Juan Zhang, Changgeng Wang, Zhisheng Liu, and Qinghua Wei. 2023. "Enhancing Conductivity and Self-Healing Properties of PVA/GEL/OSA Composite Hydrogels by GO/SWNTs for Electronic Skin" Gels 9, no. 2: 155. https://doi.org/10.3390/gels9020155
APA StyleChen, X., Zhang, H., Cui, J., Wang, Y., Li, M., Zhang, J., Wang, C., Liu, Z., & Wei, Q. (2023). Enhancing Conductivity and Self-Healing Properties of PVA/GEL/OSA Composite Hydrogels by GO/SWNTs for Electronic Skin. Gels, 9(2), 155. https://doi.org/10.3390/gels9020155






