Amino-Functionalized Cellulose Nanofiber/Lignosulfonate New Aerogel Adsorbent for the Removal of Dyes and Heavy Metals from Wastewater
Abstract
:1. Introduction
2. Results and Discussion
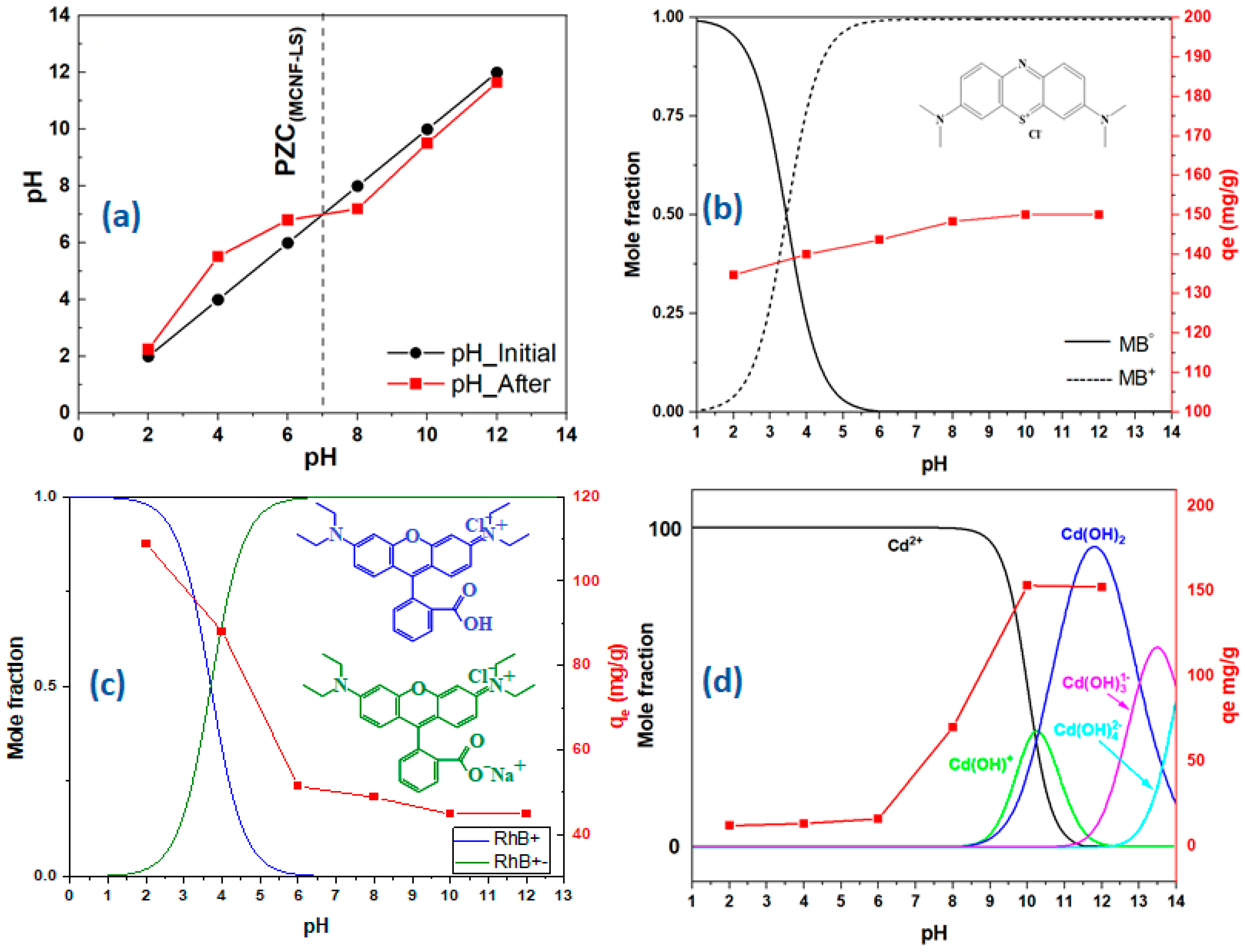
2.1. Point of Zero Charge (PZC)
2.2. Characterization of Amf-CNF/LS Aerogel
2.3. Adsorption Study
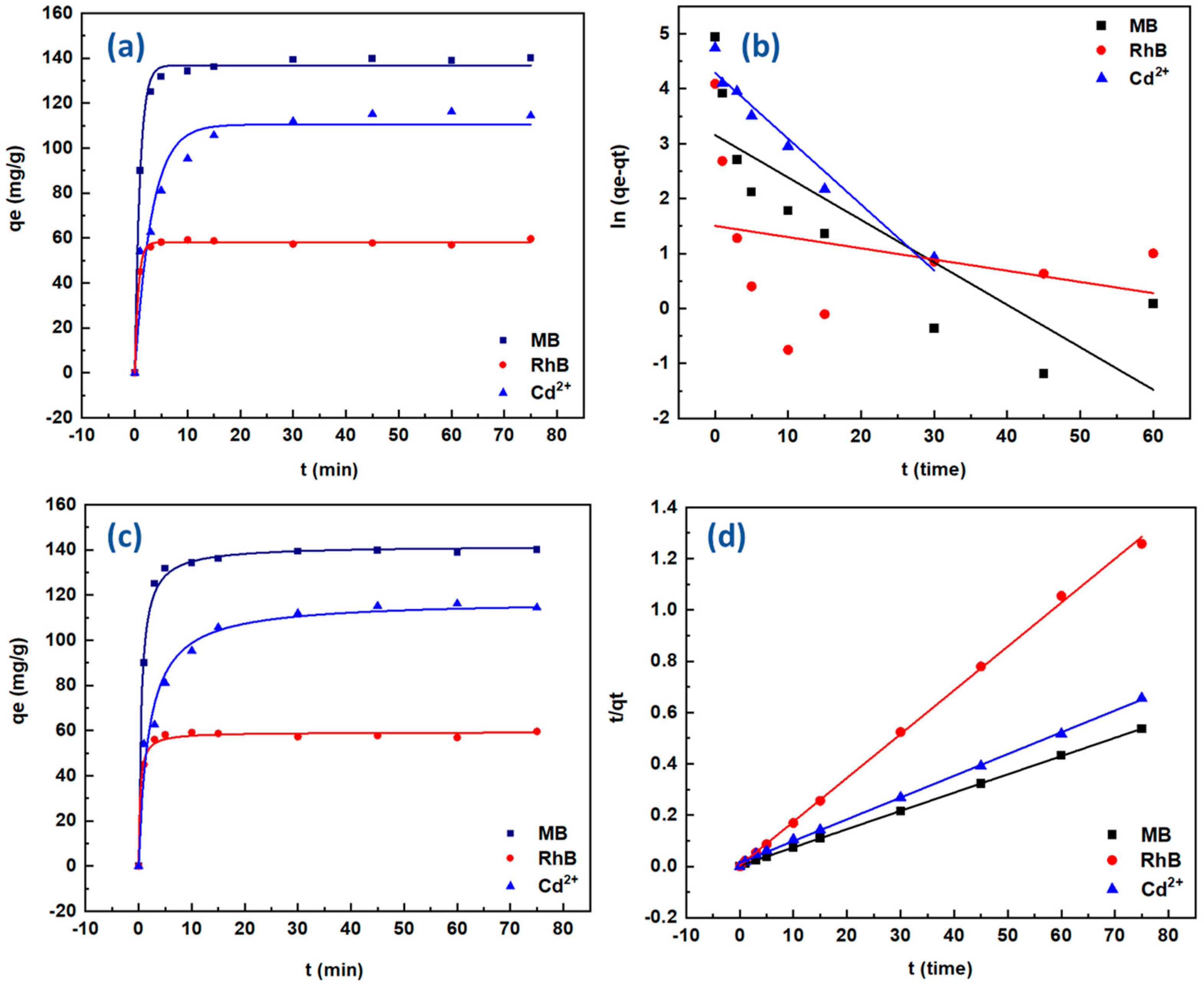
2.3.1. Adsorption Kinetics
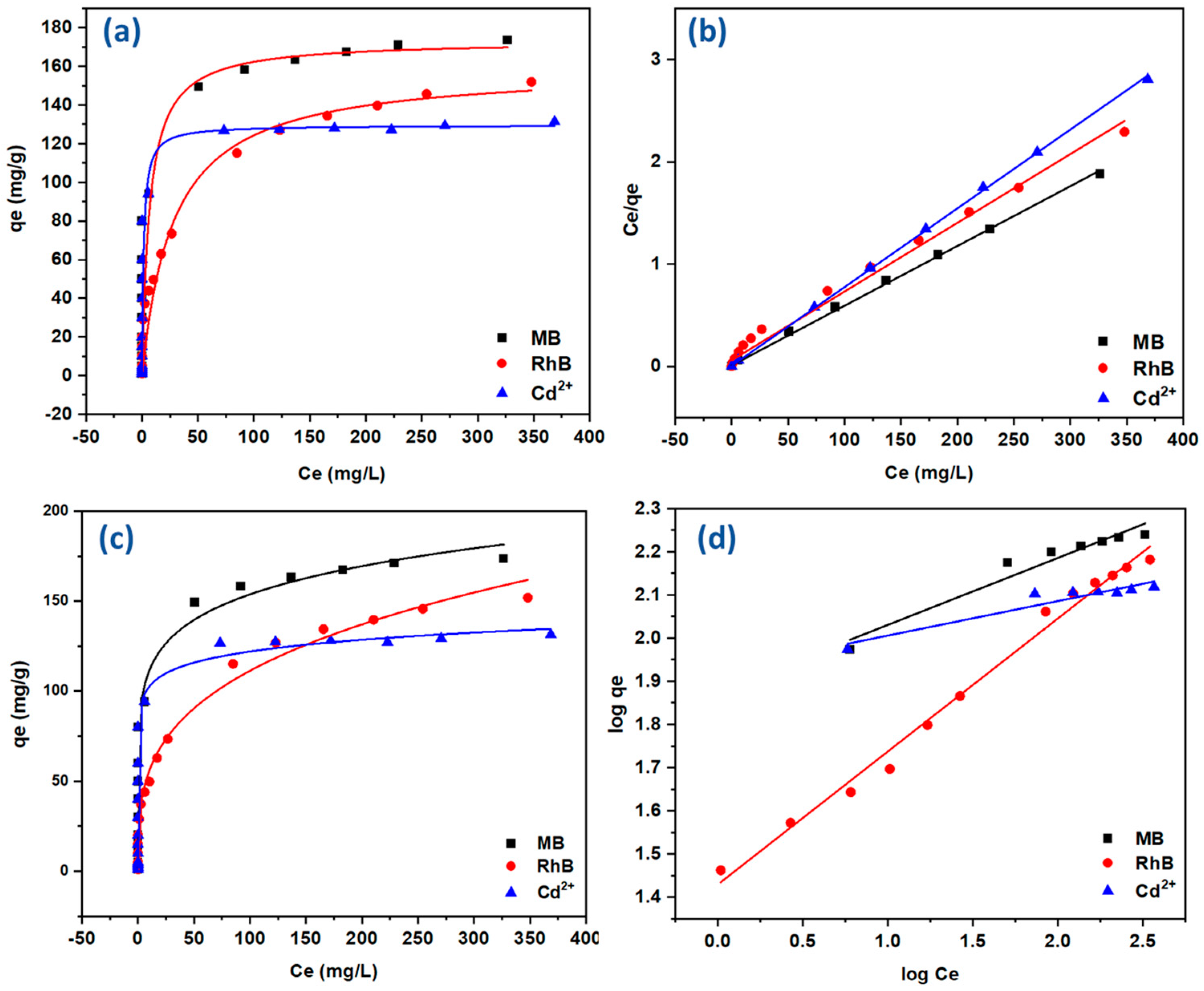
2.3.2. Adsorption Isotherm
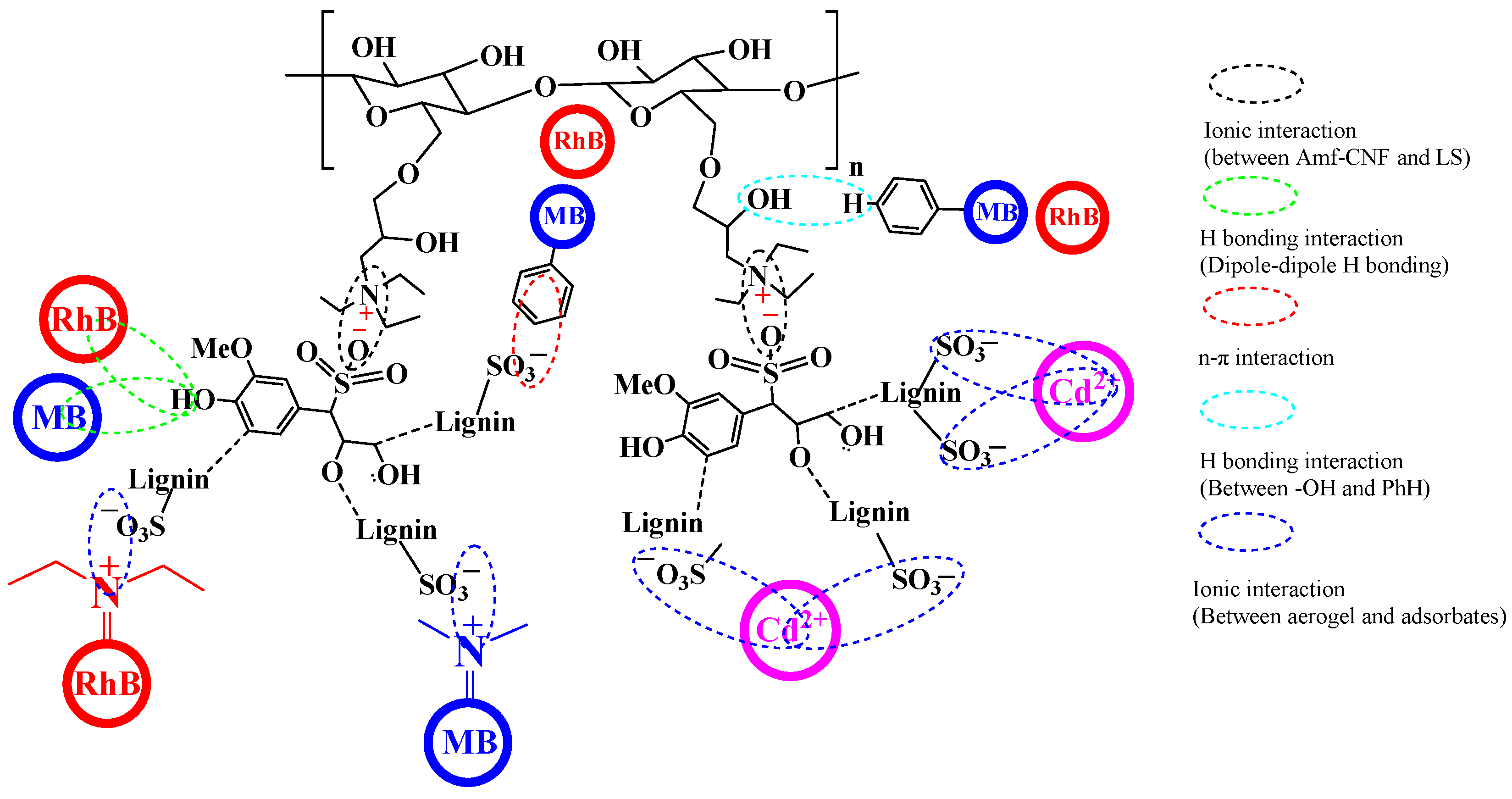
2.3.3. Adsorption Mechanism
2.3.4. Recyclability
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Regenerated Cellulose
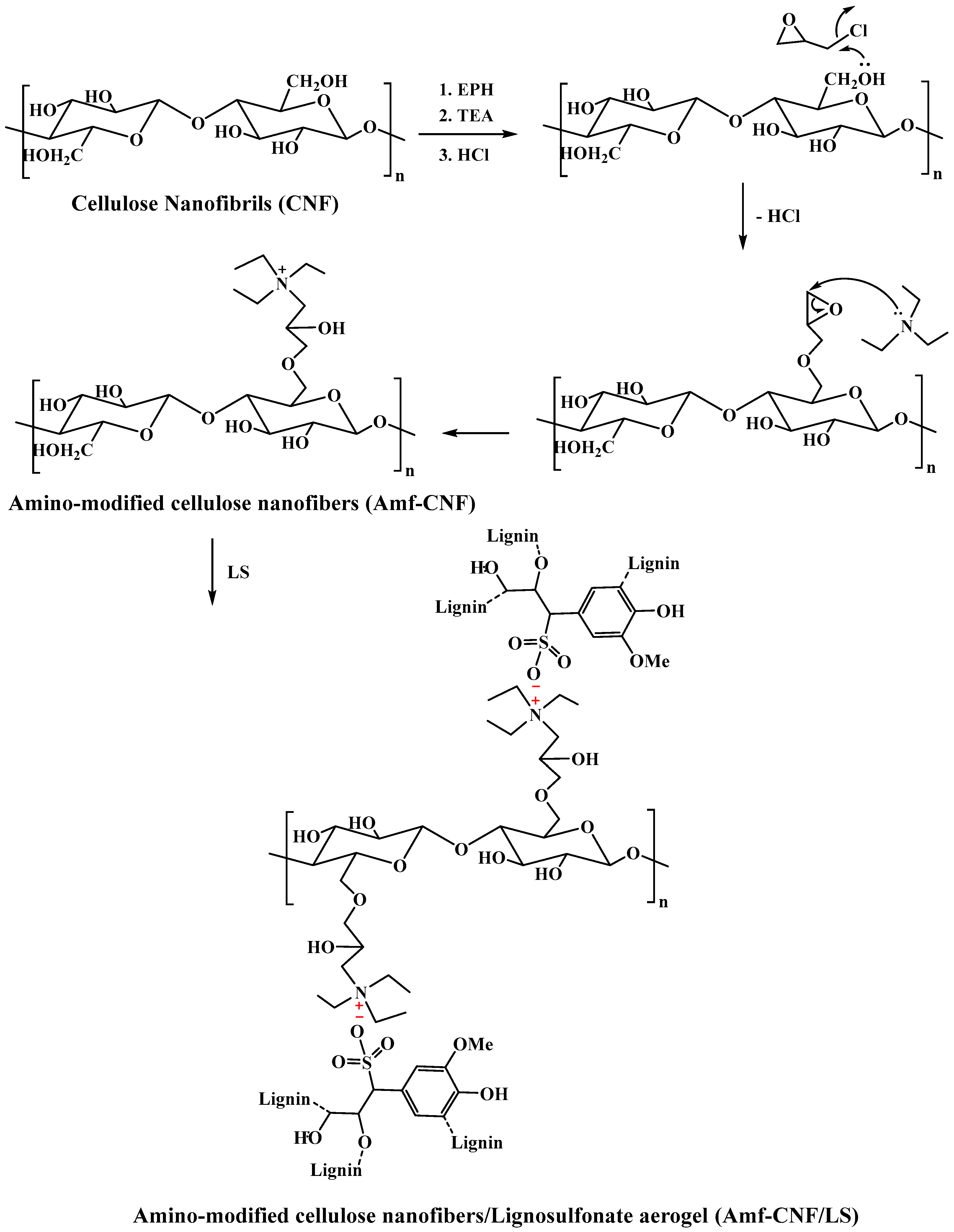
4.3. Preparation of Amino-Modified Cellulose Nanofibers (Amf-CNF)
4.4. Preparation of Amino-Modified Cellulose Nanofibers/Lignosulfonate Aerogel (Amf-CNF/LS)
4.5. Characterization of Amf-CNF/LS Aerogel
4.5.1. PZC Measurements
4.5.2. Effect of pH
4.6. Adsorption Experiments
4.6.1. Adsorption Kinitics
4.6.2. Adsorption Isotherms
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Pal, D.B.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Bioresour. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef] [PubMed]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ. Adv. 2020, 2, 100007. [Google Scholar] [CrossRef]
- Davarcı, D.; Duyar, C.; Zorlu, Y. 3D Ag(I) coordination polymer constructed from a flexible pyridyloxycyclotetraphoshazene linker: Synthesis, crystal structure and dye adsorption properties. Polyhedron 2023, 231, 116250. [Google Scholar] [CrossRef]
- Hassanpour, M.; Safardoust-Hojaghan, H.; Salavati-Niasari, M. Degradation of methylene blue and Rhodamine B as water pollutants via green synthesized Co3O4/ZnO nanocomposite. J. Mol. Liq. 2017, 229, 293–299. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Nunell, G.V.; Bonelli, P.R.; Cukierman, A.L. Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Ind. Crops Prod. 2014, 62, 437–445. [Google Scholar] [CrossRef]
- Tahir, S.S.; Rauf, N. Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 2006, 63, 1842–1848. [Google Scholar] [CrossRef]
- Visa, M.; Enesca, A. Opportunities for Recycling PV Glass and Coal Fly Ash into Zeolite Materials Used for Removal of Heavy Metals (Cd, Cu, Pb) from Wastewater. Materials 2023, 16, 239. [Google Scholar] [CrossRef]
- Saraydin, D.; Karadağ, E.; Güven, O. Use of superswelling acrylamide/maleic acid hydrogels for monovalent cationic dye adsorption. J. Appl. Polym. Sci. 2001, 79, 1809–1815. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Roa, K.; Oyarce, E.; Boulett, A.; Alsamman, M.; Oyarzún, D.; Pizarro, G.D.C.; Sánchez, J. Lignocellulose-based materials and their application in the removal of dyes from water: A review. Sustain. Mater. Technol. 2021, 29, e00320. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Karishma, S. Review on biopolymers and composites—Evolving material as adsorbents in removal of environmental pollutants. Environ. Res. 2022, 212, 113114. [Google Scholar] [CrossRef]
- Zubair, M.; Ullah, A. Chapter 14—Biopolymers in environmental applications: Industrial wastewater treatment. In Biopolymers and Their Industrial Applications; Thomas, S., Gopi, S., Amalraj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 331–349. [Google Scholar]
- Guan, Y.; Rao, J.; Wu, Y.; Gao, H.; Liu, S.; Chen, G.; Peng, F. Hemicelluloses-based magnetic aerogel as an efficient adsorbent for Congo red. Int. J. Biol. Macromol. 2020, 155, 369–375. [Google Scholar] [CrossRef]
- Wang, F.; Huang, K.; Xu, Z.; Cao, F.; Chen, C.; Shi, F.; Chen, N. Preparation of high-strength dynamic polysaccharide nanocomposite hydrogels and their application towards dye adsorption. Ind. Crops Prod. 2022, 189, 115704. [Google Scholar] [CrossRef]
- Song, G.; Zhang, L.; He, C.; Fang, D.-C.; Whitten, P.G.; Wang, H. Facile Fabrication of Tough Hydrogels Physically Cross-Linked by Strong Cooperative Hydrogen Bonding. Macromolecules 2013, 46, 7423–7435. [Google Scholar] [CrossRef]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose–lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Ciolacu, D.; Doroftei, F.; Cazacu, G.; Cazacu, M. Morphological and surface aspects of cellulose-lignin hydrogels. Cellul. Chem. Technol. 2013, 47, 377–386. [Google Scholar]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Li, Z.; Kong, Y.; Ge, Y. Synthesis of porous lignin xanthate resin for Pb2+ removal from aqueous solution. Chem. Eng. J. 2015, 270, 229–234. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Mo, Q.; Yang, X.; Wang, J.; Lin, X.; Shang, D.; Li, Y.; Zhang, Y. Removal of cadmium and tetracycline by lignin hydrogels loaded with nano-FeS: Nanoparticle size control and content calculation. J. Hazard. Mater. 2021, 416, 126262. [Google Scholar] [CrossRef]
- Alekhina, M.; Ershova, O.; Ebert, A.; Heikkinen, S.; Sixta, H. Softwood kraft lignin for value-added applications: Fractionation and structural characterization. Ind. Crops Prod. 2015, 66, 220–228. [Google Scholar] [CrossRef]
- Megiatto, J.D.; Cerrutti, B.M.; Frollini, E. Sodium lignosulfonate as a renewable stabilizing agent for aqueous alumina suspensions. Int. J. Biol. Macromol. 2016, 82, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, A.; Verşan Kök, M.; Özel, R. Performance analysis of drilling fluid liquid lubricants. J. Pet. Sci. Eng. 2013, 108, 64–73. [Google Scholar] [CrossRef]
- Pan, Y.; Zhan, J.; Pan, H.; Wang, W.; Tang, G.; Song, L.; Hu, Y. Effect of Fully Biobased Coatings Constructed via Layer-by-Layer Assembly of Chitosan and Lignosulfonate on the Thermal, Flame Retardant, and Mechanical Properties of Flexible Polyurethane Foam. ACS Sustain. Chem. Eng. 2016, 4, 1431–1438. [Google Scholar] [CrossRef]
- Areskogh, D.; Li, J.; Gellerstedt, G.; Henriksson, G. Investigation of the Molecular Weight Increase of Commercial Lignosulfonates by Laccase Catalysis. Biomacromolecules 2010, 11, 904–910. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Hao, C.; Dai, X.; Zhu, F.; Ge, C. Ultrasonic synthesis and properties of a sodium lignosulfonate–grafted poly(acrylic acid-co-acryl amide) composite super absorbent polymer. New J. Chem. 2014, 38, 6057–6063. [Google Scholar] [CrossRef]
- Barbosa, V.; Ramires, E.C.; Razera, I.A.T.; Frollini, E. Biobased composites from tannin–phenolic polymers reinforced with coir fibers. Ind. Crops Prod. 2010, 32, 305–312. [Google Scholar] [CrossRef]
- Sena Neto, A.R.; Araujo, M.A.M.; Barboza, R.M.P.; Fonseca, A.S.; Tonoli, G.H.D.; Souza, F.V.D.; Mattoso, L.H.C.; Marconcini, J.M. Comparative study of 12 pineapple leaf fiber varieties for use as mechanical reinforcement in polymer composites. Ind. Crops Prod. 2015, 64, 68–78. [Google Scholar] [CrossRef]
- Yu, C.; Wang, F.; Zhang, C.; Fu, S.; Lucia, L.A. The synthesis and absorption dynamics of a lignin-based hydrogel for remediation of cationic dye-contaminated effluent. React. Funct. Polym. 2016, 106, 137–142. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, K.; Nan, J.; Tang, C.; Chen, Y.; Hu, Y. Synthesis and characterization of lignosulfonate-graft-poly (acrylic acid)/hydroxyethyl cellulose semi-interpenetrating hydrogels. React. Funct. Polym. 2017, 115, 28–35. [Google Scholar] [CrossRef]
- Panzarasa, G.; Osypova, A.; Ribera, J.; Schwarze, F.W.M.R.; Quasso, F.; Consolati, G. Hybrid Adsorbent Materials Obtained by the Combination of Poly(ethylene-alt-maleic anhydride) with Lignin and Lignosulfonate. J. Polym. Environ. 2018, 26, 4293–4302. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Q.; Chang, M.; Jang, J.; Sui, W.; Si, C.; Ni, Y. Novel Fe3O4@lignosulfonate/phenolic core-shell microspheres for highly efficient removal of cationic dyes from aqueous solution. Ind. Crops Prod. 2019, 127, 110–118. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Moreira, M.M.; Paíga, P.; Dias, D.; Bernardo, M.; Carvalho, M.; Lapa, N.; Fonseca, I.; Morais, S.; Figueiredo, S.; et al. Evaluation of the adsorption potential of biochars prepared from forest and agri-food wastes for the removal of fluoxetine. Bioresour. Technol. 2019, 292, 121973. [Google Scholar] [CrossRef] [PubMed]
- Sayğılı, H.; Güzel, F.; Önal, Y. Conversion of grape industrial processing waste to activated carbon sorbent and its performance in cationic and anionic dyes adsorption. J. Clean. Prod. 2015, 93, 84–93. [Google Scholar] [CrossRef]
- Zheng, T.; Zheng, D.; Li, X.; Cai, C.; Lou, H.; Liu, W.; Qiu, X. Synthesis of Quaternized Lignin and Its Clay-Tolerance Properties in Montmorillonite-Containing Cement Paste. ACS Sustain. Chem. Eng. 2017, 5, 7743–7750. [Google Scholar] [CrossRef]
- Ji, X.; Guo, M.; Zhu, L.; Du, W.; Wang, H. Synthesis Mechanism of an Environment-Friendly Sodium Lignosulfonate/Chitosan Medium-Density Fiberboard Adhesive and Response of Bonding Performance to Synthesis Mechanism. Materials 2020, 13, 5697. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Guizani, C.; Grosseau, P.; Chaussy, D.; Beneventi, D. Thermal characterization and kinetic analysis of microfibrillated cellulose/lignosulfonate blends. J. Anal. Appl. Pyrolysis 2017, 124, 25–34. [Google Scholar] [CrossRef]
- Zhang, X.; Elsayed, I.; Navarathna, C.; Schueneman, G.T.; Hassan, E.I.B. Biohybrid Hydrogel and Aerogel from Self-Assembled Nanocellulose and Nanochitin as a High-Efficiency Adsorbent for Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 46714–46725. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, Y.-d.; Yang, Z.-k.; Nagarajan, D.; Chang, J.-S.; Ren, N.-q. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour. Technol. 2017, 246, 142–149. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Removal of lead(II) from aqueous solution using date seed-derived biochar: Batch and column studies. Appl. Water Sci. 2018, 8, 181. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Gao, J.; Hu, G.; Zhao, Y.; Tang, X.; Han, X. Efficient Removal of Methylene Blue by Bio-Based Sodium Alginate/Lignin Composite Hydrogel Beads. Polymers 2022, 14, 2917. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Liu, Y.-g.; Hu, X.-j.; Liu, S.-b.; Zeng, G.-m.; Zheng, B.-h.; Jiang, L.-h.; Guo, F.-y.; Ding, Y.; Xu, Y. Decontamination of methylene blue from aqueous solution by magnetic chitosan lignosulfonate grafted with graphene oxide: Effects of environmental conditions and surfactant. RSC Adv. 2016, 6, 19298–19307. [Google Scholar] [CrossRef]
- Mondal, A.K.; Xu, D.; Wu, S.; Zou, Q.; Lin, W.; Huang, F.; Ni, Y. High lignin containing hydrogels with excellent conducting, self-healing, antibacterial, dye adsorbing, sensing, moist-induced power generating and supercapacitance properties. Int. J. Biol. Macromol. 2022, 207, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin—A biosorbent. J. Colloid Interface Sci. 2006, 297, 489–504. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, L.; Han, C.; Lv, X.; Sun, X.; Wang, T. Hybrid amino-functionalized TiO2/sodium lignosulfonate surface molecularly imprinted polymer for effective scavenging of methylene blue from wastewater. J. Clean. Prod. 2022, 337, 130457. [Google Scholar] [CrossRef]
- Abboud, M.; Sahlabji, T.; Haija, M.A.; El-Zahhar, A.A.; Bondock, S.; Ismail, I.; Keshk, S.M.A.S. Synthesis and characterization of lignosulfonate/amino-functionalized SBA-15 nanocomposites for the adsorption of methylene blue from wastewater. New J. Chem. 2020, 44, 2291–2302. [Google Scholar] [CrossRef]
- Geng, J.; Gu, F.; Chang, J. Fabrication of magnetic lignosulfonate using ultrasonic-assisted in situ synthesis for efficient removal of Cr(Ⅵ) and Rhodamine B from wastewater. J. Hazard. Mater. 2019, 375, 174–181. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Wang, B.; Wu, Y.; Ma, M.; Zhang, X. Synthesis of Magnetic Lignin-Based Hollow Microspheres: A Highly Adsorptive and Reusable Adsorbent Derived from Renewable Resources. ACS Sustain. Chem. Eng. 2016, 4, 5523–5532. [Google Scholar] [CrossRef]
- Suteu, D.; Bilba, D.; Zaharia, C.; Popescu, A. Removal of dyes from textile wastewater by sorption onto ligno-cellulosic materials. Sci. Study Res. 2008, 9, 293–302. [Google Scholar]
- Gu, F.; Geng, J.; Li, M.; Chang, J.; Cui, Y. Synthesis of Chitosan–Ignosulfonate Composite as an Adsorbent for Dyes and Metal Ions Removal from Wastewater. ACS Omega 2019, 4, 21421–21430. [Google Scholar] [CrossRef]
- Chen, F.; Shahabadi, S.I.S.; Zhou, D.; Liu, W.; Kong, J.; Xu, J.; Lu, X. Facile preparation of cross-linked lignin for efficient adsorption of dyes and heavy metal ions. React. Funct. Polym. 2019, 143, 104336. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Xin, J.; Liu, G.; Wu, G.; Kong, Z.; Zhang, J. Clickable Synthesis of 1,2,4-Triazole Modified Lignin-Based Adsorbent for the Selective Removal of Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 4086–4093. [Google Scholar] [CrossRef]
- Demirbas, A. Adsorption of lead and cadmium ions in aqueous solutions onto modified lignin from alkali glycerol delignication. J. Hazard. Mater. 2004, 109, 221–226. [Google Scholar] [CrossRef]
- Luo, M.; Lin, H.; Li, B.; Dong, Y.; He, Y.; Wang, L. A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour. Technol. 2018, 259, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Okoronkwo, A.E.; Adebayo, A.O.; Omotunde, O.I. Sorptive removal of cadmium from aqueous solutions by Delonix regia derived lignin: Effect of amination. Desalination Water Treat. 2013, 51, 5026–5034. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, J.; Li, X.; Lin, Q.; Xiao, R.; Zhang, M.; Yin, G.; Zhou, Y. Effect of lignosulfonate on the adsorption performance of hematite for Cd(II). Sci. Total Environ. 2020, 738, 139952. [Google Scholar] [CrossRef]
- Elsayed, I.; Madduri, S.; El-Giar, E.M.; Hassan, E.B. Effective removal of anionic dyes from aqueous solutions by novel polyethylenimine-ozone oxidized hydrochar (PEI-OzHC) adsorbent. Arab. J. Chem. 2022, 15, 103757. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mohammed, I.A.; Abdulhameed, A.S. Tuning of Fly Ash Loading into Chitosan-Ethylene Glycol Diglycidyl Ether Composite for Enhanced Removal of Reactive Red 120 Dye: Optimization Using the Box–Behnken Design. J. Polym. Environ. 2020, 28, 2720–2733. [Google Scholar] [CrossRef]


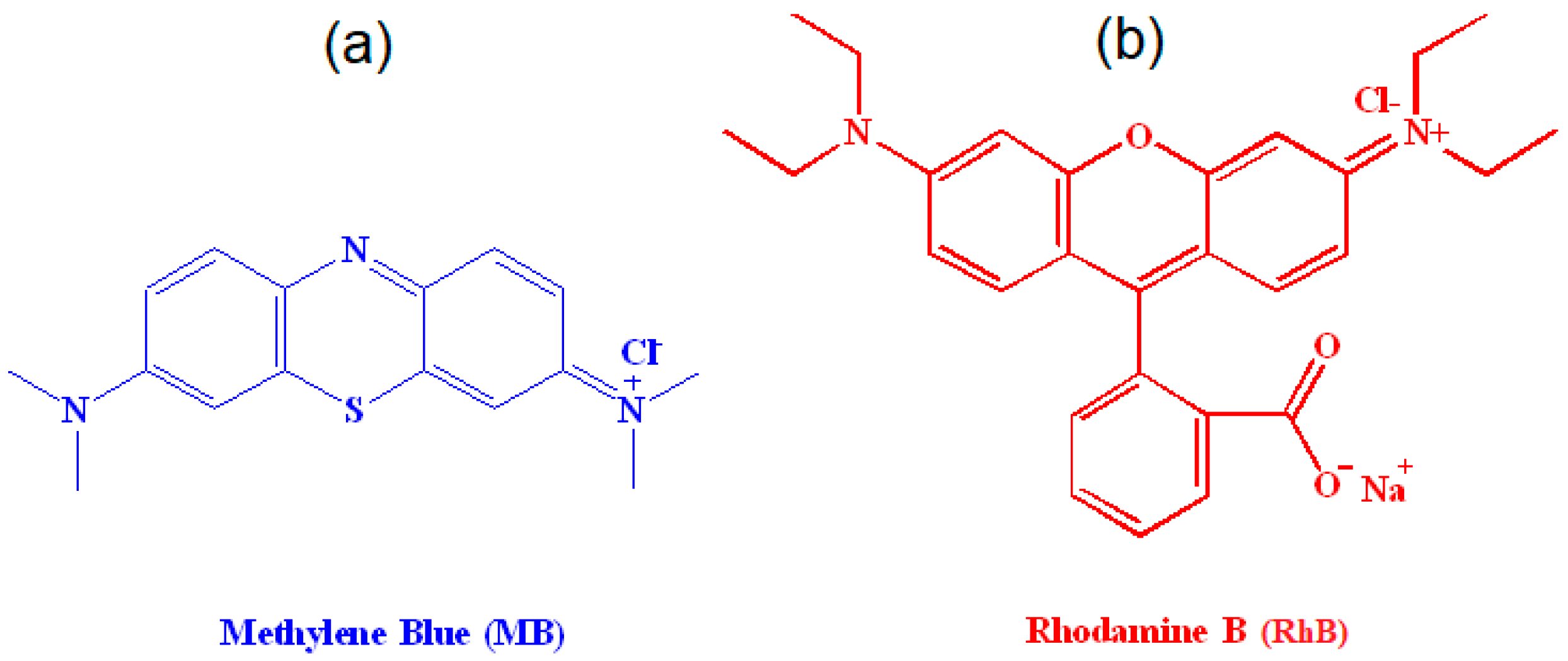
| Sample | Elemental Analysis | Surface Area (m2/g) | |||
|---|---|---|---|---|---|
| %C | %H | %N | %O | ||
| CNF | 42.87 | 6.27 | 0.06 | 50.82 | 2.82 |
| Amf-CNF | 45.15 | 7.17 | 2.09 | 45.59 | 9.18 |
| Amf-CNF/LS | 48.23 | 6.66 | 1.58 | 43.55 | 12.13 |
| Cont. Type | Pseudo-First-Order | |||||
| qe(exp) (mg/g) | qe(calc) (mg/g) | K1 (min−1) | R2 | Reduced Chi-Sqr (χ2) | Root-MSE (SD) | |
| MB | 140.01 | 23.48 | 0.078 | 0.694 | 1.397 | 1.182 |
| RhB | 59.61 | 4.49 | 0.020 | 0.090 | 2.23 | 1.492 |
| Cd2+ | 116.08 | 72.85 | 0.120 | 0.959 | 0.083 | 0.289 |
| Pseudo-Second-Order | ||||||
| qe (exp) (mg/g) | qe(calc) (mg/g) | K2 (min−1) | R2 | Reduced Chi-Sqr (χ2) | Root-MSE (SD) | |
| MB | 140.01 | 140.45 | 0.02 | 0.999 | 2.09 × 10−6 | 1.44 × 10−3 |
| RhB | 59.61 | 58.51 | 0.061 | 0.999 | 1.97 × 10−4 | 1.40 × 10−2 |
| Cd2+ | 116.08 | 117.93 | 0.004 | 0.998 | 5.50 × 10−5 | 7.42 × 10−3 |
| Cont. Type | Langmuir Model | ||||
| Qmax (mg/g) | KL(L/mg) | R2 | Reduced Chi-Sqr (χ2) | Root-MSE (SD) | |
| MB | 171.23 | 0.76 | 0.999 | 3.43 × 10−4 | 0.019 |
| RhB | 148.81 | 0.12 | 0.989 | 5.37 × 10−3 | 0.073 |
| Cd2+ | 129.87 | 1.92 | 0.999 | 1.84 × 10−4 | 1.36 × 10−2 |
| Freundlich Model | |||||
| Kf | n | R2 | Reduced Chi-Sqr (χ2) | Root-MSE (SD) | |
| MB | 74.98 | 6.44 | 0.945 | 5.76 × 10−4 | 0.024 |
| RhB | 26.88 | 3.25 | 0.990 | 7.29 × 10−4 | 0.027 |
| Cd2+ | 84.31 | 12.51 | 0.686 | 2.83 × 10−4 | 0.017 |
| Adsorbent | Adsorbate | pH | Qmax (mg/g) | Reference |
|---|---|---|---|---|
| Amf-CNF/LS | MB | 8 | 171.23 | This study |
| Amino-functionalizedTiO2/sodium lignosulfonate | 11 | 37 | [56] | |
| Sodium alginate/lignin −20% beads | 12 | 254.3 | [52] | |
| Activated lignin-chitosan (Lig/CS) | 7 | 36.25 | [13] | |
| Lignosulfonate/amino-functionalized SBA-15 | 9 | 62.89 | [57] | |
| Magnetic chitosan lignosulfonate (MGLS) | 10 | 81.4 | [53] | |
| Magnetic chitosan lignosulfonate/graphene oxide | 10 | 253.53 | [53] | |
| Fe3O4@lignosulfonate/phenolic core-shell microspheres | 10 | 283.6 | [42] | |
| Amf-CNF/LS | RhB | 6 | 148.81 | This study |
| Magnetic lignosulfonate | 2 | 22.47 | [58] | |
| Magnetic lignin microspheres from larch lignin | — | 17.62 | [59] | |
| Ligno-cellulosic solid wastes | 5.7 | 7.309 | [60] | |
| Chitosan–lignosulfonate Composite | 7 | 126.58 | [61] | |
| Lignosulfonate/poly acrylic acid/Al hydrogels | 7 | 334.64 | [54] | |
| Cross-linked lignin | — | 156.4 | [62] | |
| Amf-CNF/LS | Cd2+ | 7 | 129.87 | This study |
| 1,2,4-triazole modified lignin-based adsorbent | 6 | 87.4 | [63] | |
| Modified lignin from beech and poplar woods | 4.5 | 6.7–7.5 | [64] | |
| Kraft lignin | 4.5 | 137.14 | [55] | |
| Modified lignin on corncob-based biochar | 7 | 85.65 | [65] | |
| Aminated lignin | 5 | 43.2 | [66] | |
| lignin hydrogels loaded with nano-FeS | 7 | 215 | [30] | |
| Hematite/lignosulfonate composite (HLS) | 5 | 53.65 | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, I.; Schueneman, G.T.; El-Giar, E.M.; Hassan, E.B. Amino-Functionalized Cellulose Nanofiber/Lignosulfonate New Aerogel Adsorbent for the Removal of Dyes and Heavy Metals from Wastewater. Gels 2023, 9, 154. https://doi.org/10.3390/gels9020154
Elsayed I, Schueneman GT, El-Giar EM, Hassan EB. Amino-Functionalized Cellulose Nanofiber/Lignosulfonate New Aerogel Adsorbent for the Removal of Dyes and Heavy Metals from Wastewater. Gels. 2023; 9(2):154. https://doi.org/10.3390/gels9020154
Chicago/Turabian StyleElsayed, Islam, Gregory T. Schueneman, Emad M. El-Giar, and El Barbary Hassan. 2023. "Amino-Functionalized Cellulose Nanofiber/Lignosulfonate New Aerogel Adsorbent for the Removal of Dyes and Heavy Metals from Wastewater" Gels 9, no. 2: 154. https://doi.org/10.3390/gels9020154
APA StyleElsayed, I., Schueneman, G. T., El-Giar, E. M., & Hassan, E. B. (2023). Amino-Functionalized Cellulose Nanofiber/Lignosulfonate New Aerogel Adsorbent for the Removal of Dyes and Heavy Metals from Wastewater. Gels, 9(2), 154. https://doi.org/10.3390/gels9020154





