Transparent Organogels as a Medium for the Light-Induced Conversion from Spiropyran to Merocyanine
Abstract
:1. Introduction
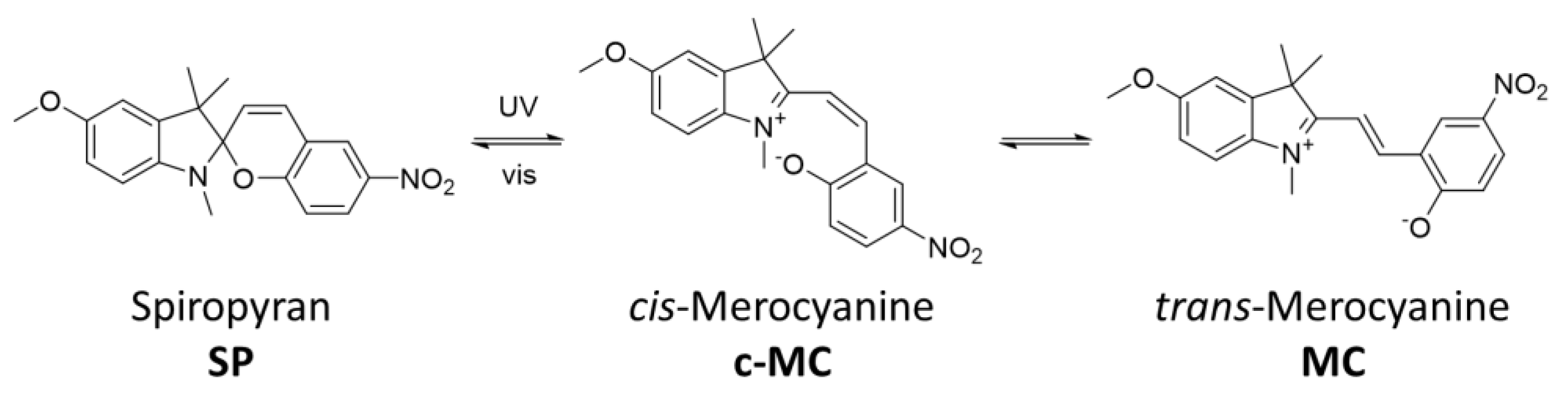
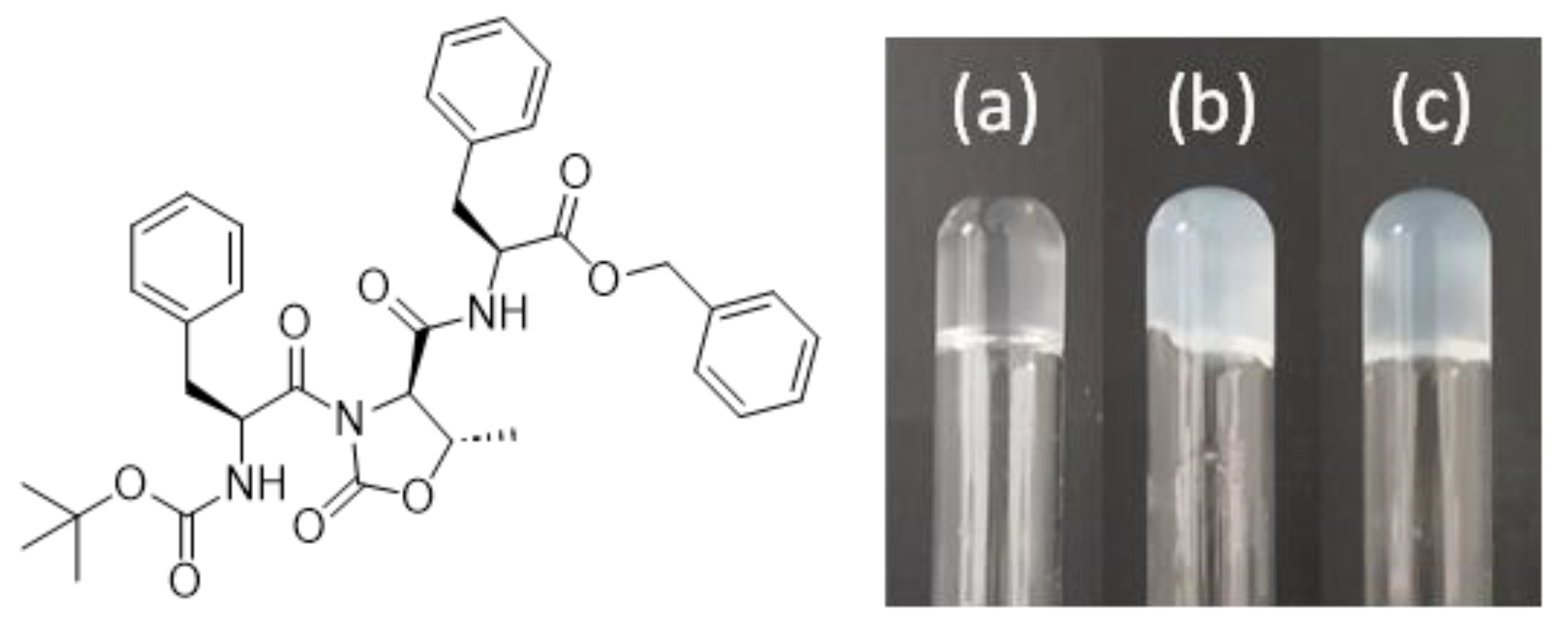
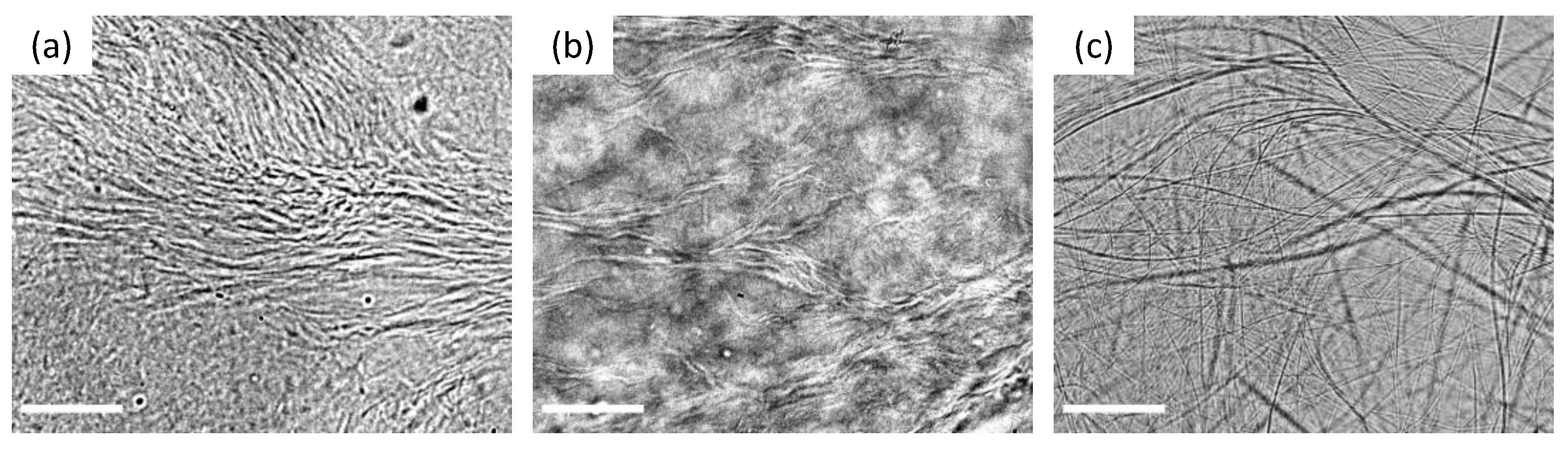
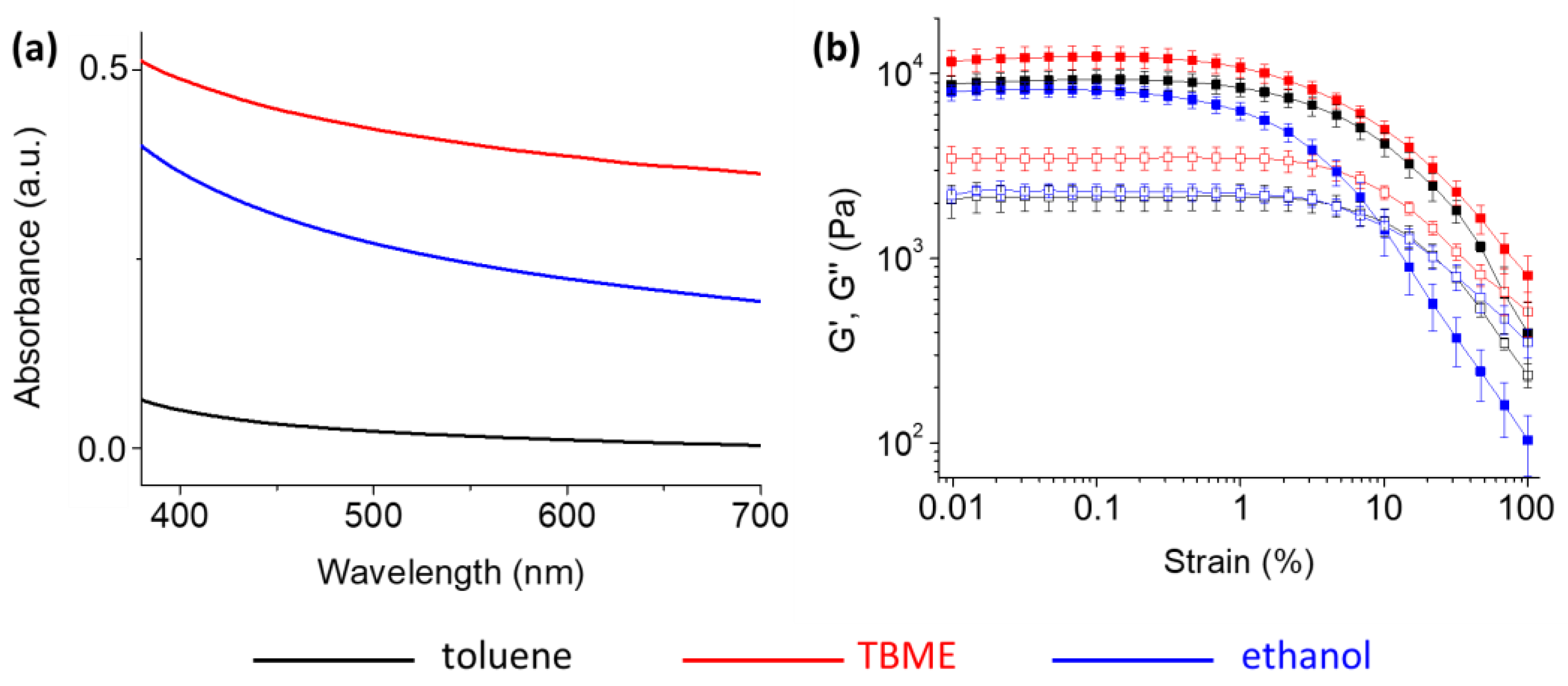
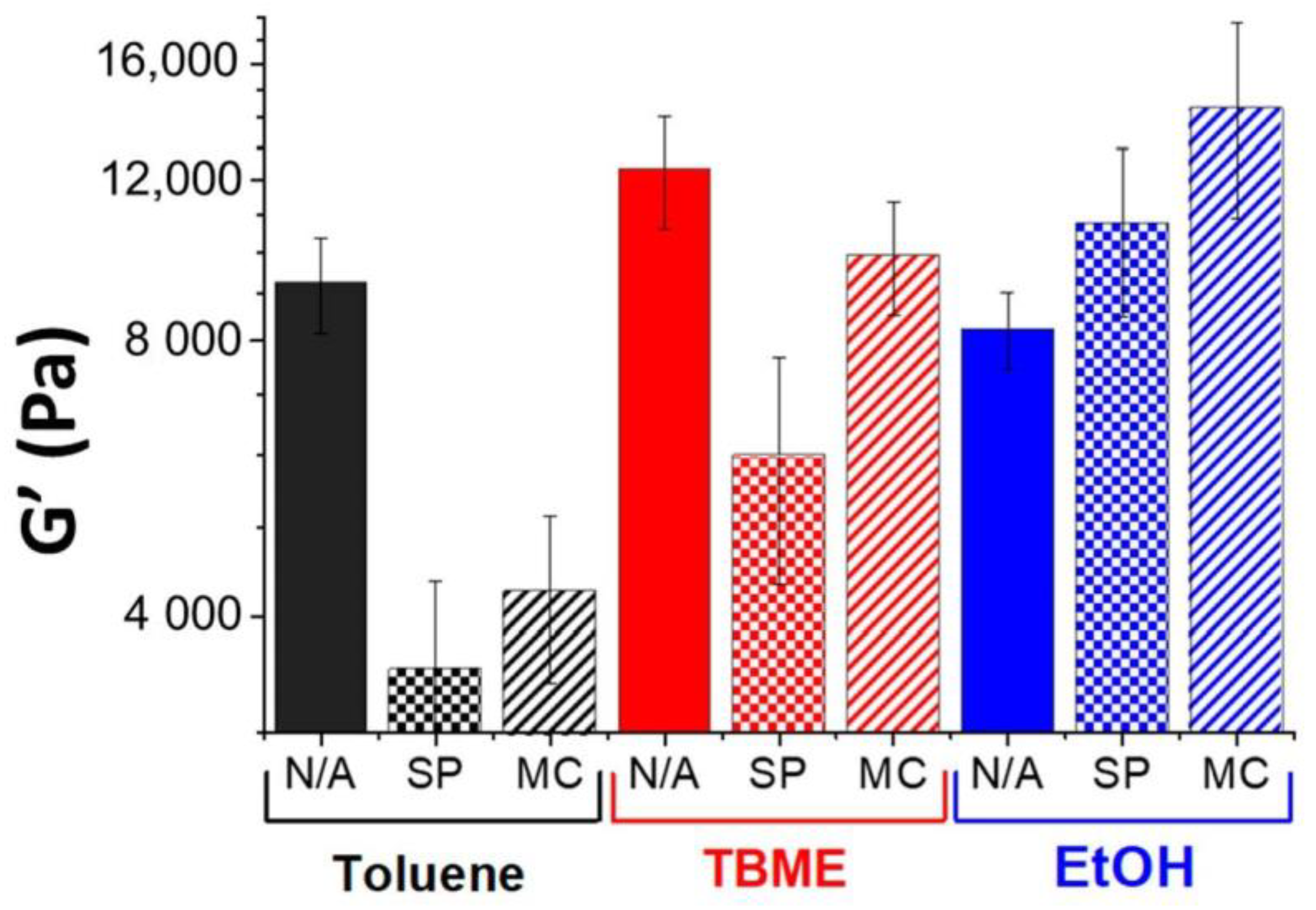
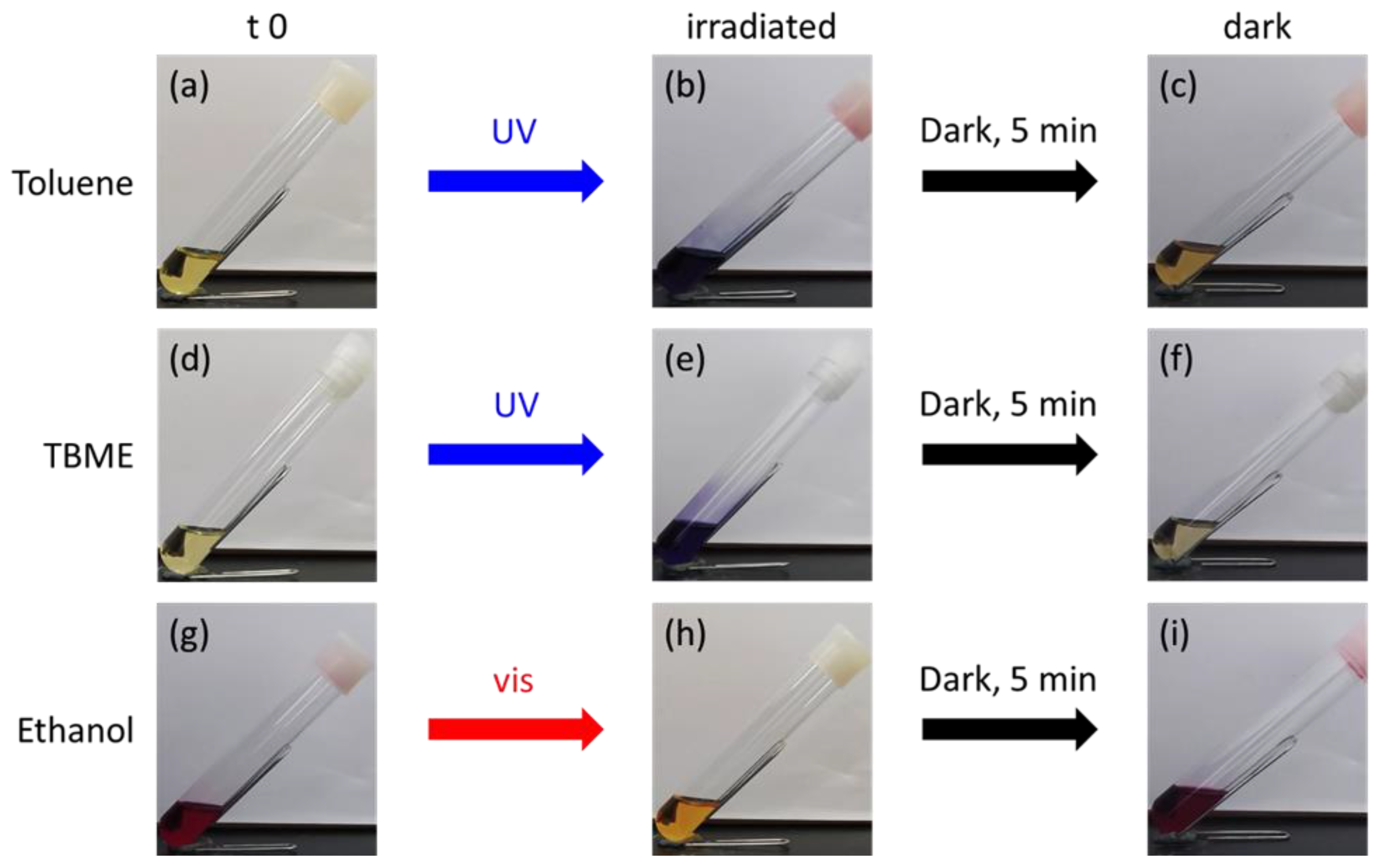
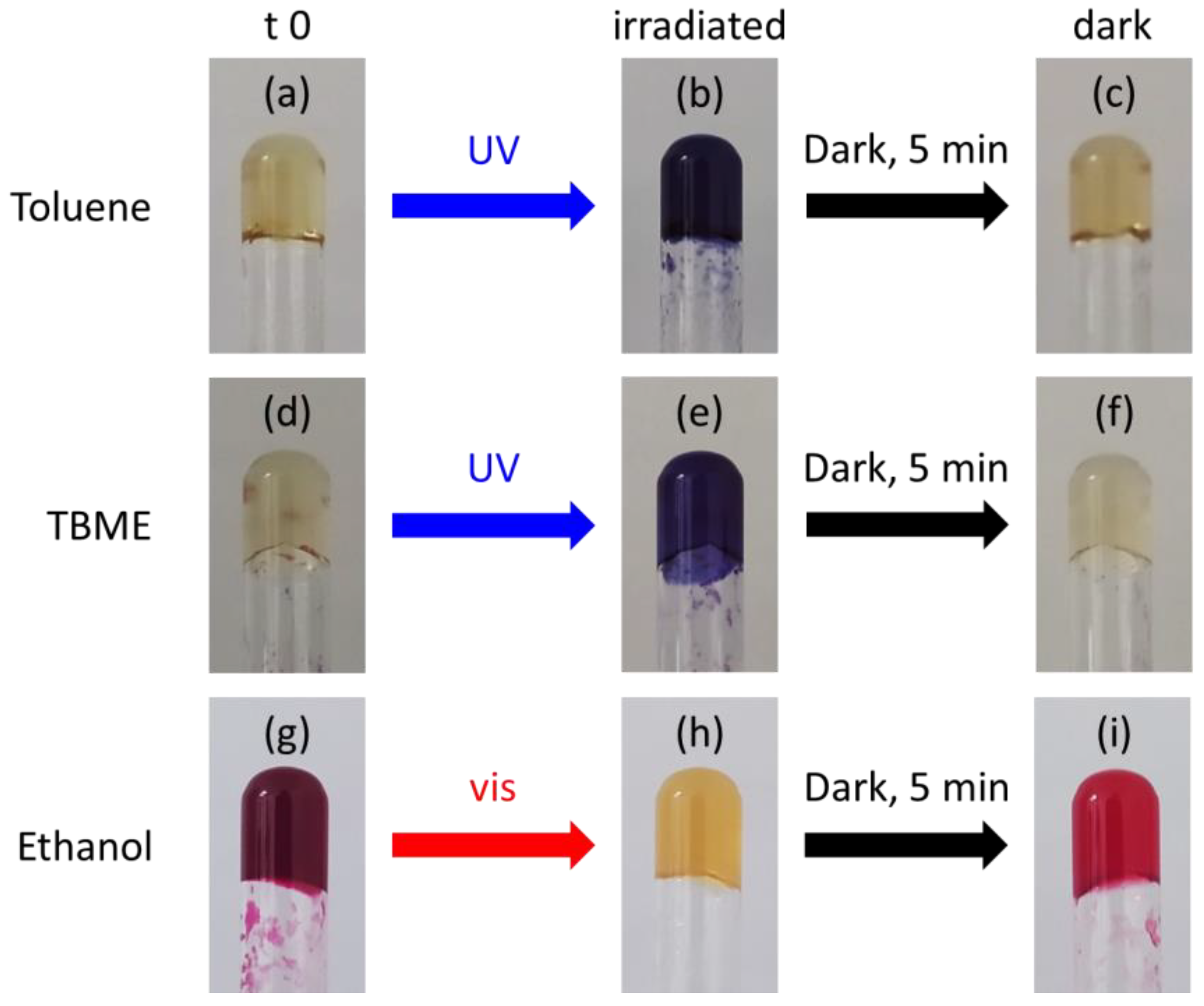
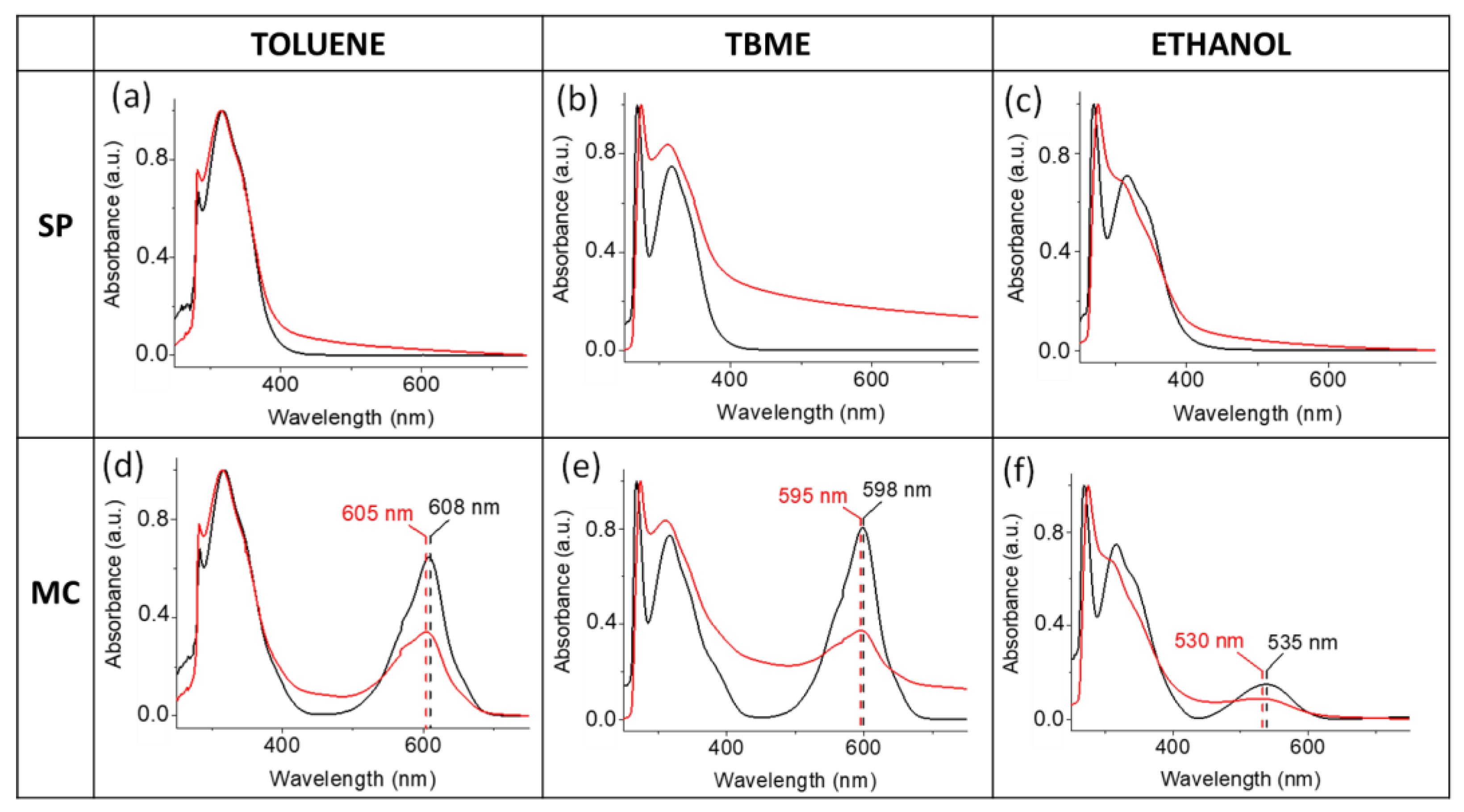
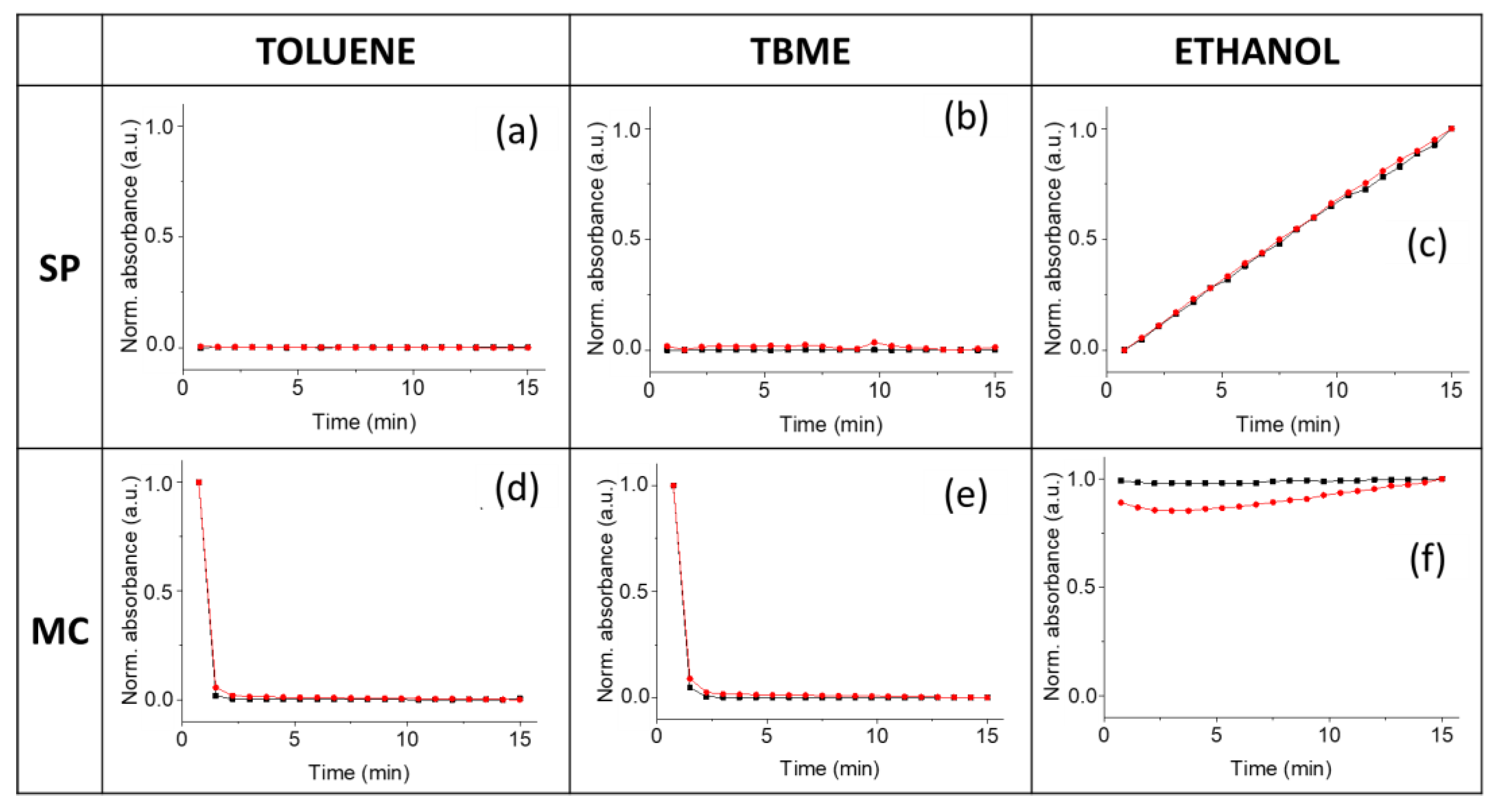
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menger, F.M.; Caran, K.L. Anatomy of a Gel. Amino Acid Derivatives That Rigidify Water at Submillimolar Concentrations. J. Am. Chem. Soc. 2000, 122, 11679–11691. [Google Scholar] [CrossRef]
- De Loos, M.; Feringa, B.L.; Van Esch, J.H. Design and Application of Self-Assembled Low Molecular Weight Hydrogels. European J. Org. Chem. 2005, 2005, 3615–3631. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, J.; Wang, W.; Zhang, X.; Lan, H.; Xiao, S. Pyran-Based Derivative: Non-Conventional Organogel and Tri-Colored High-Contrast Mechanochromism. Tetrahedron Lett. 2022, 100, 153888. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, H.; Liu, Z.-X.; He, Y.-M.; Fan, Q.-H. A Pronounced Halogen Effect on the Organogelation Properties of Peripherally Halogen Functionalized Poly(Benzyl Ether) Dendrons. Chem.—A Eur. J. 2016, 22, 4980–4990. [Google Scholar] [CrossRef]
- Ravarino, P.; Giuri, D.; Faccio, D.; Tomasini, C. Designing a Transparent and Fluorine Containing Hydrogel. Gels 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Ravarino, P.; Di Domenico, N.; Barbalinardo, M.; Faccio, D.; Falini, G.; Giuri, D.; Tomasini, C. Fluorine Effect in the Gelation Ability of Low Molecular. Gels 2022, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A.; Lascialfari, L.; Demitri, N.; Bertolani, A.; Maiolo, D.; Carretti, E.; Metrangolo, P. Halogen Bonding Modulates Hydrogel Formation from Fmoc Amino Acids. CrystEngComm 2017, 19, 1870–1874. [Google Scholar] [CrossRef]
- Awhida, S.; Draper, E.R.; McDonald, T.O.; Adams, D.J. Probing Gelation Ability for a Library of Dipeptide Gelators. J. Colloid. Interface Sci. 2015, 455, 24–31. [Google Scholar] [CrossRef]
- Morris, K.L.; Chen, L.; Rodger, A.; Adams, D.J.; Serpell, L.C. Structural Determinants in a Library of Low Molecular Weight Gelators. Soft Matter 2015, 11, 1174–1181. [Google Scholar] [CrossRef]
- Patrick Gizzi, L.; Pasc, A.; Nicolas Dupuy, L.; Parant, S.; Henry, B.; Gérardin, C. Molecular Tailored Histidine-Based Complexing Surfactants: From Micelles to Hydrogels. European J. Org. Chem. 2009, 2009, 3953–3963. [Google Scholar] [CrossRef]
- Maity, S.; Kumar, P.; Haldar, D. Sonication-Induced Instant Amyloid-like Fibril Formation and Organogelation by a Tripeptide. Soft Matter 2011, 7, 5239–5245. [Google Scholar] [CrossRef]
- Restu, W.K.; Nishida, Y.; Yamamoto, S.; Ishii, J.; Maruyama, T. Short Oligopeptides for Biocompatible and Biodegradable Supramolecular Hydrogels. Langmuir 2018, 34, 8065–8074. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.F.; Giuri, D.; Marchiori, G.; Maglio, M.; Pagani, S.; Fini, M.; Tomasini, C.; Panzavolta, S. Self-Assembling of Fibers inside an Injectable Calcium Phosphate Bone Cement: A Feasibility Study. Mater. Today Chem. 2022, 24, 100991. [Google Scholar] [CrossRef]
- Motulsky, A.; Lafleur, M.; Couffin-Hoarau, A.C.; Hoarau, D.; Boury, F.; Benoit, J.P.; Leroux, J.C. Characterization and Biocompatibility of Organogels Based on L-Alanine for Parenteral Drug Delivery Implants. Biomaterials 2005, 26, 6242–6253. [Google Scholar] [CrossRef] [PubMed]
- Falcone, N.; Kraatz, H.B. Supramolecular Assembly of Peptide and Metallopeptide Gelators and Their Stimuli-Responsive Properties in Biomedical Applications. Chem.—A Eur. J. 2018, 24, 14316–14328. [Google Scholar] [CrossRef]
- Colquhoun, C.; Draper, E.R.; Schweins, R.; Marcello, M.; Vadukul, D.; Serpell, L.C.; Adams, D.J. Controlling the Network Type in Self-Assembled Dipeptide Hydrogels. Soft Matter 2017, 13, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Zanna, N.; Iaculli, D.; Tomasini, C. The Effect of L-DOPA Hydroxyl Groups on the Formation of Supramolecular Hydrogels. Org. Biomol. Chem. 2017, 15, 5797–5804. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Z.; Adams, D.J. Controlling Peptidebased Hydrogelation. Mater. Today 2012, 15, 500–507. [Google Scholar] [CrossRef]
- Buzzaccaro, S.; Ruzzi, V.; Gelain, F.; Piazza, R. A Light Scattering Investigation of Enzymatic Gelation in Self-Assembling Peptides. Gels 2023, 9, 347. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, F.; Chen, Y.; Raymond, J.E.; Zhang, S.; Fan, J.; Zhu, J.; Li, A.; Seetho, K.; He, X.; et al. Responsive Organogels Formed by Supramolecular Self Assembly of PEG-Block-Allyl-Functionalized Racemic Polypeptides into β-Sheet-Driven Polymeric Ribbons. Soft Matter 2013, 9, 5951–5958. [Google Scholar] [CrossRef] [PubMed]
- Nanda, J.; Biswas, A.; Banerjee, A. Single Amino Acid Based Thixotropic Hydrogel Formation and PH-Dependent Morphological Change of Gel Nanofibers. Soft Matter 2013, 9, 4198–4208. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, F.; Gong, R.; Guo, Z.; Chen, C.-F.; Wan, X. A Multi-Stimuli Responsive Organogel Based on a Tetrapeptide–Dithienylcyclopentene Conjugate. Soft Matter 2013, 9, 7538–7544. [Google Scholar] [CrossRef]
- Srivastava, B.K.; Muraleedharan, K.M. Gel-Based Supramolecular ON–OFF Switch from Aryl-Triazolyl Peptides with Excellent Chiro-Optical, Thixotropic, and Self-Healing Characteristics. Soft Matter 2018, 14, 1631–1636. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Zhang, M.; Yi, T. Low-Molecular-Mass Gels Responding to Ultrasound and Mechanical Stress: Towards Self-Healing Materials. Chem. Soc. Rev. 2014, 43, 5346–5371. [Google Scholar] [CrossRef] [PubMed]
- Giuri, D.; D’Agostino, S.; Ravarino, P.; Faccio, D.; Falini, G.; Tomasini, C. Water Remediation from Pollutant Agents by the Use of an Environmentally Friendly Supramolecular Hydrogel. ChemNanoMat 2022, 8, e202200093. [Google Scholar] [CrossRef]
- Giuri, D.; Zanna, N.; Tomasini, C. Low Molecular Weight Gelators Based on Functionalized L-Dopa Promote Organogels Formation. Gels 2019, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.; Giuri, D.; Zanna, N.; Calvaresi, M.; Montalti, M.; Tomasini, C. Biocompatible and Light-Penetrating Hydrogels for Water Decontamination. ACS Omega 2018, 3, 8122–8128. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying Low-Molecular Weight Supramolecular Gelators in an Environmental Setting-Self-Assembled Gels as Smart Materials for Pollutant Removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef]
- Fortunato, A.; Mba, M. A Peptide-Based Hydrogel for Adsorption of Dyes and Pharmaceuticals in Water Remediation. Gels 2022, 8, 672. [Google Scholar] [CrossRef]
- Rizzo, C.; Marullo, S.; D’Anna, F. Carbon-Based Ionic Liquid Gels: Alternative Adsorbents for Pharmaceutically Active Compounds in Wastewater. Environ. Sci. Nano 2021, 8, 131–145. [Google Scholar] [CrossRef]
- Bisker, G.; Iverson, N.M.; Ahn, J.; Strano, M.S. A Pharmacokinetic Model of a Tissue Implantable Insulin Sensor. Adv. Healthc. Mater. 2015, 4, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Wulf, V.; Bisker, G. Single-Walled Carbon Nanotubes as Fluorescent Probes for Monitoring the Self-Assembly and Morphology of Peptide/Polymer Hybrid Hydrogels. Nano Lett. 2022, 22, 9205–9214. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-González, O.; Campos-Escamilla, C.; Flores-Ibarra, A.; Esturau-Escofet, N.; Arreguin-Espinosa, R.; Stojanoff, V.; Cuéllar-Cruz, M.; Moreno, A. Crystal Growth in Gels from the Mechanisms of Crystal Growth to Control of Polymorphism: New Trends on Theoretical and Experimental Aspects. Crystals 2019, 9, 443. [Google Scholar] [CrossRef]
- Giuri, D.; Jurković, L.; Fermani, S.; Kralj, D.; Falini, G.; Tomasini, C. Supramolecular Hydrogels with Properties Tunable by Calcium Ions: A Bio-Inspired Chemical System. ACS Appl. Bio Mater. 2019, 2, 5819–5828. [Google Scholar] [CrossRef]
- Bonifazi, E.L.; Mac Cormack, A.S.; Busch, V.M.; Japas, M.L.; Di Bari, L.; Di Chenna, P.H. Chiral Self-Assembly and Water Effect on a Supramolecular Organogel Stable towards Aqueous Interfaces. J. Solgel Sci. Technol. 2022, 102, 30–40. [Google Scholar] [CrossRef]
- Okesola, B.O.; Wu, Y.; Derkus, B.; Gani, S.; Wu, D.; Knani, D.; Smith, D.K.; Adams, D.J.; Mata, A. Supramolecular Self-Assembly To Control Structural and Biological Properties of Multicomponent Hydrogels. Chem. Mater. 2019, 31, 7883–7897. [Google Scholar] [CrossRef]
- Gačanin, J.; Hedrich, J.; Sieste, S.; Glaßer, G.; Lieberwirth, I.; Schilling, C.; Fischer, S.; Barth, H.; Knöll, B.; Synatschke, C.V.; et al. Autonomous Ultrafast Self-Healing Hydrogels by PH-Responsive Functional Nanofiber Gelators as Cell Matrices. Adv. Mater. 2019, 31, 1805044. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, G.; Black, L.M.; Ravarino, P.; D’Agostino, S.; Faccio, D.; Tomasini, C.; Giuri, D. Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. Int. J. Mol. Sci. 2022, 23, 3105. [Google Scholar] [CrossRef]
- Cenciarelli, F.; Falini, G.; Giuri, D.; Tomasini, C. Controlled Lactonization of O-Coumaric Esters Mediated by Supramolecular Gels. Gels 2023, 9, 350. [Google Scholar] [CrossRef]
- Shariati Pour, S.R.; Oddis, S.; Barbalinardo, M.; Ravarino, P.; Cavallini, M.; Fiori, J.; Giuri, D.; Tomasini, C. Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules 2023, 28, 2528. [Google Scholar] [CrossRef]
- Valls, A.; Castillo, A.; Porcar, R.; Hietala, S.; Altava, B.; Garcĺa-Verdugo, E.; Luis, S.V. Urea-Based Low-Molecular-Weight Pseudopeptidic Organogelators for the Encapsulation and Slow Release of (R)-Limonene. J. Agric. Food Chem. 2020, 68, 7051–7061. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Bartolomei, M.; Bollati, C.; Boschin, G.; Arnoldi, A.; Lammi, C. Gel-Forming of Self-Assembling Peptides Functionalized with Food Bioactive Motifs Modulate DPP-IV and ACE Inhibitory Activity in Human Intestinal Caco-2 Cells. Biomedicines 2022, 10, 330. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Molecular Organogels. Soft Matter Comprised of Low-Molecular-Mass Organic Gelators and Organic Liquids. Acc. Chem. Res. 2006, 39, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Ishi-i, T.; Shinkai, S. Dye-Based Organogels: Stimuli-Responsive Soft Materials Based on One-DimensionalSelf-Assembling Aromatic Dyes. In Supermolecular Dye Chemistry; Würthner, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 119–160. ISBN 978-3-540-31458-5. [Google Scholar]
- Estroff, L.A.; Hamilton, A.D. Water Gelation by Small Organic Molecules. Chem. Rev. 2004, 104, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; D’agostino, S.; D’amen, E.; Grepioni, F. Polymorphs from Supramolecular Gels: Four Crystal Forms of the Same Silver(I) Supergelator Crystallized Directly from Its Gels. Chem. Commun. 2011, 47, 5154–5156. [Google Scholar] [CrossRef] [PubMed]
- Podder, D.; Chowdhury, S.R.; Nandi, S.K.; Haldar, D. Tripeptide Based Super-Organogelators: Structure and Function. New J. Chem. 2019, 43, 3743–3749. [Google Scholar] [CrossRef]
- Mazzier, D.; Carraro, F.; Crisma, M.; Rancan, M.; Toniolo, C.; Moretto, A. A Terminally Protected Dipeptide: From Crystal Structure and Self-Assembly, through Co-Assembly with Carbon-Based Materials, to a Ternary Catalyst for Reduction Chemistry in Water. Soft Matter 2016, 12, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Das, A.K.; Banerjee, A. Smart Oligopeptide Gels: In Situ Formation and Stabilization of Gold and Silver Nanoparticles within Supramolecular Organogel Networks. Chem. Commun. 2006, 26, 2816–2818. [Google Scholar] [CrossRef]
- Bouas-Laurent, H.; Dürr, H. Organic Photochromism. Pure Appl. Chem. 2001, 73, 639–665. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, Q.; Tian, H. Photochromic Materials: More than Meets the Eye. Adv. Mater. 2013, 25, 378–399. [Google Scholar] [CrossRef]
- Matsuda, K.; Irie, M. Photoswitching of Intramolecular Magnetic Interaction Using Photochromic Compounds. In Chemistry of Nanomolecular Systems: Towards the Realization of Molecular Devices; Nakamura, T., Matsumoto, T., Tada, H., Sugiura, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 25–40. ISBN 978-3-662-05250-1. [Google Scholar]
- Tian, H.; Yang, S. Recent Progresses on Diarylethene Based Photochromic Switches. Chem. Soc. Rev. 2004, 33, 85–97. [Google Scholar] [CrossRef]
- Mortimer, R.J. Organic Electrochromic Materials. Electrochim. Acta 1999, 44, 2971–2981. [Google Scholar] [CrossRef]
- Park, I.S.; Heo, E.J.; Kim, J.M. A Photochromic Phenoxyquinone Based Cyanide Ion Sensor. Tetrahedron Lett. 2011, 52, 2454–2457. [Google Scholar] [CrossRef]
- Ercole, F.; Davis, T.P.; Evans, R.A. Photo-Responsive Systems and Biomaterials: Photochromic Polymers, Light-Triggered Self-Assembly, Surface Modification, Fluorescence Modulation and Beyond. Polym. Chem. 2010, 1, 37–54. [Google Scholar] [CrossRef]
- Cui, B.; Guo, C.; Fu, G.; Zhang, Z. Photochromic Performance of Hydrogel Based on Deep Eutectic Solvent Induced Water Soluble Cu-Doped WO3 Hybrids with Antibacterial Property. J. Photochem. Photobiol. A Chem. 2023, 435, 114320. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, J.; Lin, G.; Wang, S.; Zhou, Y.; Yin, J.; Lee, P.S.; Long, Y. Smart Windows: Electro-, Thermo-, Mechano-, Photochromics, and Beyond. Adv. Energy Mater. 2019, 9, 1902066. [Google Scholar] [CrossRef]
- Vasantha, V.A.; Chen, J.; Zhao, W.; Parthiban, A. Cast, Dip, Spray, and Print─Covalently Embedded Photochromic Materials from a Versatile Spiropyran Conjugate. ACS Appl. Polym. Mater. 2023, 5, 1696–1706. [Google Scholar] [CrossRef]
- Qiu, Z.; Yu, H.; Li, J.; Wang, Y.; Zhang, Y. Spiropyran-Linked Dipeptide Forms Supramolecular Hydrogel with Dual Responses to Light and to Ligand-Receptor Interaction. Chem. Commun. 2009, 23, 3342–3344. [Google Scholar] [CrossRef]
- Wang, W.; Hu, J.; Zheng, M.; Zheng, L.; Wang, H.; Zhang, Y. Multi-Responsive Supramolecular Hydrogels Based on Merocyanine-Peptide Conjugates. Org. Biomol. Chem. 2015, 13, 11492–11498. [Google Scholar] [CrossRef]
- Chang, D.; Yan, W.; Yang, Y.; Wang, Q.; Zou, L. Reversible Light-Controllable Intelligent Gel Based on Simple Spiropyran-Doped with Biocompatible Lecithin. Dye. Pigment. 2016, 134, 186–189. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Jin, Q.; Zhang, L.; Liu, M. A Chiroptical Logic Circuit Based on Self-Assembled Soft Materials Containing Amphiphilic Spiropyran. Adv. Mater. 2016, 28, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Jiao, T. Reversible Chiral Optical Switching Based on Co-Assembled Spiropyran Gels. Langmuir 2022, 38, 13668–13673. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gordievskaya, Y.D.; Lomadze, N.; Bekir, M.; Jung, S.H.; Pich, A.; Santer, S. Making Microgels Photo-Responsive by Complexation with a Spiropyran Surfactant. Soft Matter 2023, 19, 4088–4098. [Google Scholar] [CrossRef] [PubMed]
- Nekoonam, N.; Vera, G.; Goralczyk, A.; Mayoussi, F.; Zhu, P.; Böcherer, D.; Shakeel, A.; Helmer, D. Controllable Wetting Transitions on Photoswitchable Physical Gels. ACS Appl. Mater. Interfaces 2023, 15, 27234–27242. [Google Scholar] [CrossRef] [PubMed]
- Minkin, V.I. Photo-, Thermo-, Solvato-, and Electrochromic Spiroheterocyclic Compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Xiong, C.; Ma, X.; Ma, W.; Sun, R. A Multiresponsive Hydrophobic Associating Hydrogel Based on Azobenzene and Spiropyran. Chin. J. Chem. 2019, 37, 793–798. [Google Scholar] [CrossRef]
- Keyvan Rad, J.; Balzade, Z.; Mahdavian, A.R. Spiropyran-Based Advanced Photoswitchable Materials: A Fascinating Pathway to the Future Stimuli-Responsive Devices. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100487. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, Y.; Zhang, D.; Zhang, G.; Fan, Q.; Sun, S.; Zhu, D. Light-Triggered Self-Assembly of a Spiropyran-Functionalized Dendron into Nano-/Micrometer-Sized Particles and Photoresponsive Organogel with Switchable Fluorescence. Adv. Funct. Mater. 2010, 20, 36–42. [Google Scholar] [CrossRef]
- Miao, W.; Wang, S.; Liu, M. Reversible Quadruple Switching with Optical, Chiroptical, Helicity, and Macropattern in Self-Assembled Spiropyran Gels. Adv. Funct. Mater. 2017, 27, 1701368. [Google Scholar] [CrossRef]
- Milli, L.; Castellucci, N.; Tomasini, C. Turning around the L-Phe-D-Oxd Moiety for a Versatile Low-Molecular-Weight Gelator. European J. Org. Chem. 2014, 2014, 5954–5961. [Google Scholar] [CrossRef]
- Marchesan, S.; Vargiu, A.V.; Styan, K.E. The Phe-Phe Motif for Peptide Self-Assembly in Nanomedicine. Molecules 2015, 20, 19775–19788. [Google Scholar] [CrossRef]
- Kralj, S.; Bellotto, O.; Parisi, E.; Garcia, A.M.; Iglesias, D.; Semeraro, S.; Deganutti, C.; D’Andrea, P.; Vargiu, A.V.; Geremia, S.; et al. Heterochirality and Halogenation Control Phe-Phe Hierarchical Assembly. ACS Nano 2020, 14, 16951–16961. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, M.C.; Romano, C.; Parisi, E.; Waddington, L.J.; Melchionna, M.; Semeraro, S.; De Zorzi, R.; Grönholm, M.; Marchesan, S. Bioadhesive Supramolecular Hydrogel from Unprotected, Short d,l-Peptides with Phe-Phe and Leu-Asp-Val Motifs. Chem. Commun. 2020, 56, 3015–3018. [Google Scholar] [CrossRef] [PubMed]
- Kol, N.; Adler-Abramovich, L.; Barlam, D.; Shneck, R.Z.; Gazit, E.; Rousso, I. Self-Assembled Peptide Nanotubes Are Uniquely Rigid Bioinspired Supramolecular Structures. Nano Lett. 2005, 5, 1343–1346. [Google Scholar] [CrossRef]
- Creasey, R.C.G.; Louzao, I.; Arnon, Z.A.; Marco, P.; Adler-Abramovich, L.; Roberts, C.J.; Gazit, E.; Tendler, S.J.B. Disruption of Diphenylalanine Assembly by a Boc-Modified Variant. Soft Matter 2016, 12, 9451–9457. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R.; Mironi-Harpaz, I.; Adler-Abramovich, L.; Mossou, E.; Mitchell, E.P.; Forsyth, V.T.; Gazit, E.; Seliktar, D. The Rheological and Structural Properties of Fmoc-Peptide-Based Hydrogels: The Effect of Aromatic Molecular Architecture on Self-Assembly and Physical Characteristics. Langmuir 2012, 28, 2015–2022. [Google Scholar] [CrossRef]
- Diaferia, C.; Morelli, G.; Accardo, A. Fmoc-Diphenylalanine as a Suitable Building Block for the Preparation of Hybrid Materials and Their Potential Applications. J. Mater. Chem. B 2019, 7, 5142–5155. [Google Scholar] [CrossRef]
- Diaferia, C.; Rosa, E.; Gallo, E.; Smaldone, G.; Stornaiuolo, M.; Morelli, G.; Accardo, A. Self-Supporting Hydrogels Based on Fmoc-Derivatized Cationic Hexapeptides for Potential Biomedical Applications. Biomedicines 2021, 9, 678. [Google Scholar] [CrossRef]
- Balmond, E.I.; Tautges, B.K.; Faulkner, A.L.; Or, V.W.; Hodur, B.M.; Shaw, J.T.; Louie, A.Y. Comparative Evaluation of Substituent Effect on the Photochromic Properties of Spiropyrans and Spirooxazines. J. Org. Chem. 2016, 81, 8744–8758. [Google Scholar] [CrossRef]
- Rosario, R.; Gust, D.; Hayes, M.; Springer, J.; Garcia, A.A. Solvatochromic Study of the Microenvironment of Surface-Bound Spiropyrans. Langmuir 2003, 19, 8801–8806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuri, D.; Ravarino, P.; Tomasini, C. Transparent Organogels as a Medium for the Light-Induced Conversion from Spiropyran to Merocyanine. Gels 2023, 9, 932. https://doi.org/10.3390/gels9120932
Giuri D, Ravarino P, Tomasini C. Transparent Organogels as a Medium for the Light-Induced Conversion from Spiropyran to Merocyanine. Gels. 2023; 9(12):932. https://doi.org/10.3390/gels9120932
Chicago/Turabian StyleGiuri, Demetra, Paolo Ravarino, and Claudia Tomasini. 2023. "Transparent Organogels as a Medium for the Light-Induced Conversion from Spiropyran to Merocyanine" Gels 9, no. 12: 932. https://doi.org/10.3390/gels9120932
APA StyleGiuri, D., Ravarino, P., & Tomasini, C. (2023). Transparent Organogels as a Medium for the Light-Induced Conversion from Spiropyran to Merocyanine. Gels, 9(12), 932. https://doi.org/10.3390/gels9120932








