2.1. Collagen Gel Product Obtainment
After preparing the different solutions using collagen and acetic acid (AA), lactic acid (LA), lime (LL), and trypsin (OR), the effectiveness of each product was studied. See
Figure 1.
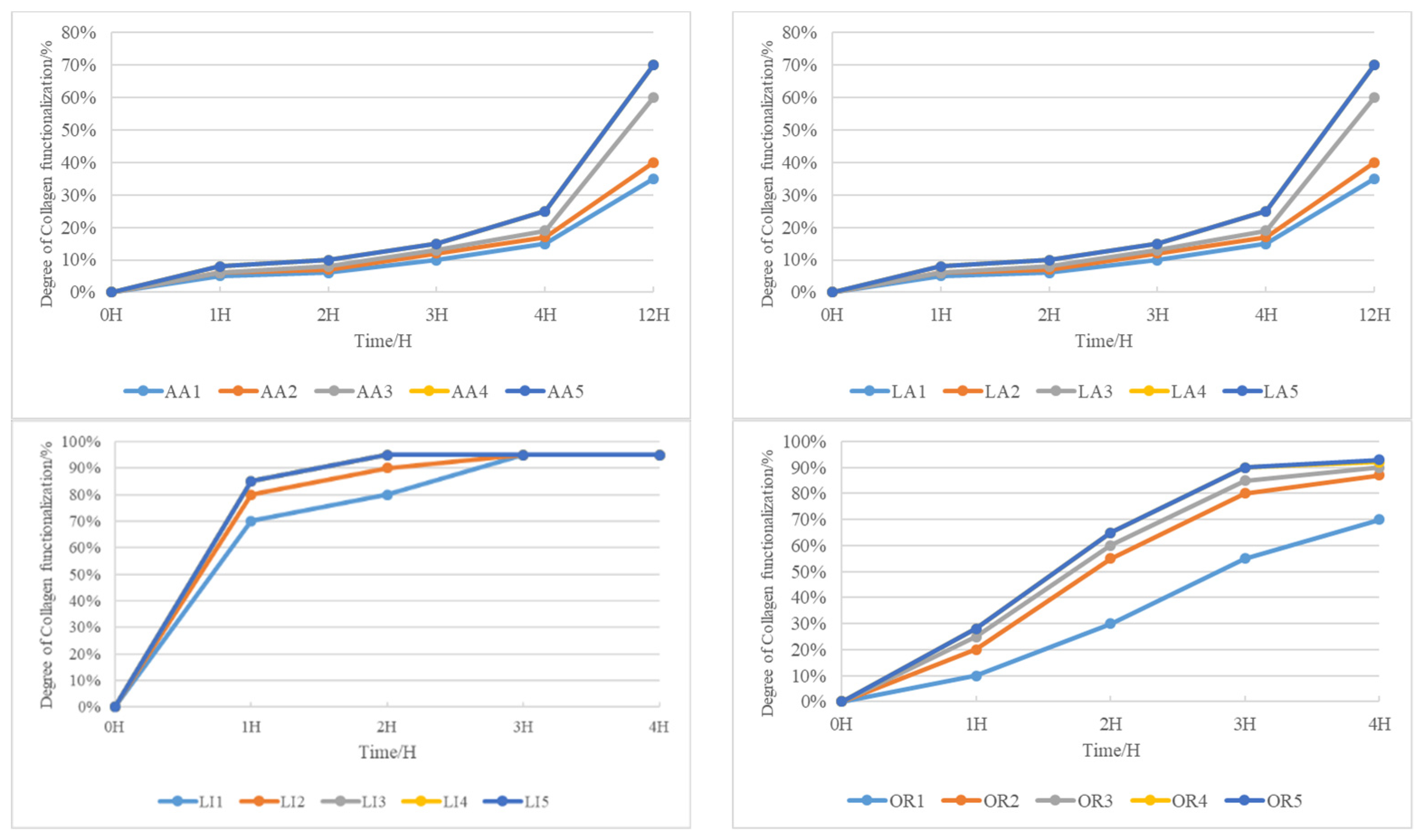
The efficacy of collagen modification with acetic acid exhibits a gradual improvement, becoming more pronounced only after an overnight period. Interestingly, the impact of an increased acetic acid quantity is not immediately apparent within a short timeframe. It takes a prolonged exposure of 12 h for the concentration of acetic acid to significantly enhance collagen modification, reaching its peak at 70%. This underscores the necessity for an extended duration to achieve optimal results when employing acetic acid for collagen modification.
Similar observations apply to the use of lactic acid in collagen modification. The effectiveness is not immediately evident with a short exposure or increased lactic acid concentration. However, after an overnight period, a more conspicuous promotion effect becomes apparent. It takes 12 h for the concentration of lactic acid to significantly enhance collagen modification, reaching a maximum of 70%. Again, this emphasizes the requirement for an extended duration for optimal collagen modification when utilizing lactic acid.
Contrastingly, the modification of collagen with lime occurs rapidly and is completed in just two hours. However, the resulting collagen-based product exhibits a two-phase phenomenon, indicating severe peptide bond disruption due to the intense alkalinity of lime.
In the realm of enzyme-mediated modification, both enzyme quantity and time significantly influence collagen modification. Increasing enzyme quantity enhances modification effectiveness, reaching a saturation point at 2.0 g. Simultaneously, the extension of time greatly improves collagen modification, almost reaching saturation at 4 h. The deepening color of the solution with increased enzyme dosage is noted, and after 5 days, no two-phase phenomenon appears. This rapid and gentle functionalization of collagen using enzymes underscores its efficiency as a modification agent.
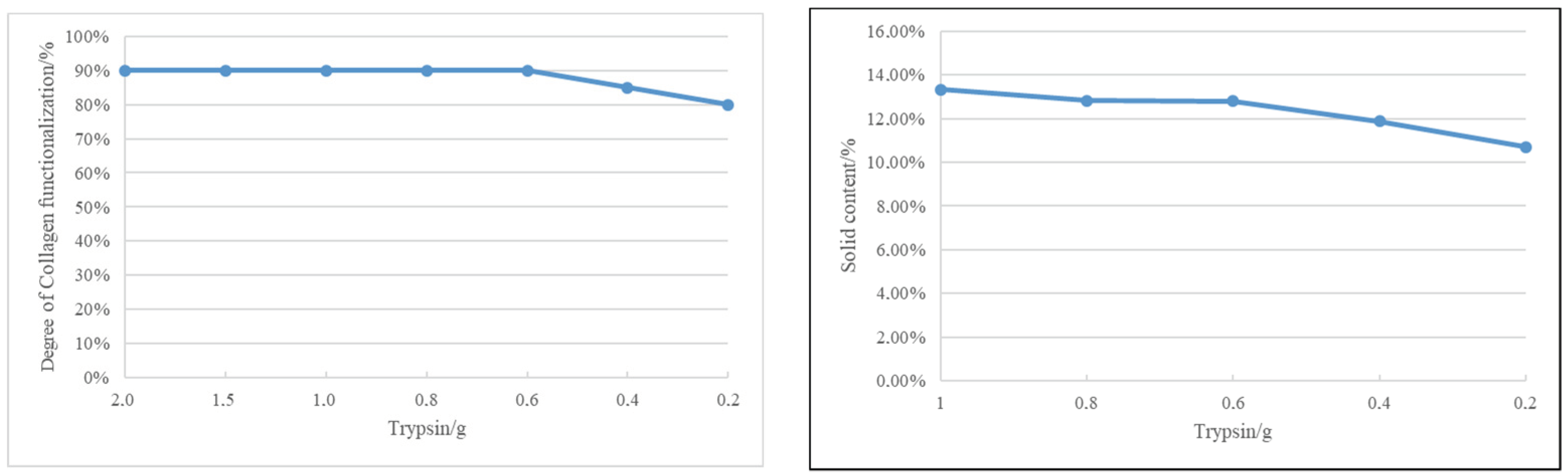
After determining trypsin to be the most suitable modification agent, different parameters were studied (
Figure 2): amount of collagen (CO), temperature, amount of water, amount of pH, and amount of trypsin.
Increasing the amount of collagen inversely affects its functionalization. At collagen amounts ranging from 5.0 g to 7.0 g, there is minimal difference in solubility after 4 h of functionalization. Opting for 7.0 g of collagen is deemed most suitable, taking into account the desired solid content of the final collagen-based product.
Elevated temperatures expedite collagen modification. The accelerated process is particularly noticeable with enzyme- and water-driven modifications. At 3 h, modification efficiency reaches 90%. Among the temperatures tested (40 °C, 45 °C, 50 °C, and 55 °C), 50 °C attains saturation, leading to the decision to set the modification temperature at 50 °C.
At 50 °C and 3 h, varying water amounts show a minimal impact on collagen functionalization, highlighting the predominant role of enzyme quantity. Setting the water amount at 40 g aligns with the desired solid content of the collagen-based coating product.
Two hours into the ultrasonic treatment, collagen functionalization is nearly complete. However, at pH levels 8 and 9, solution stratification occurs. The experiment halts, and subsequent analysis suggests that, despite swift functionalization, certain pH conditions result in undesired outcomes.
Collagen functionalization speed increases with rising pH levels, reaching completion in 2 h. Optimal functionalization is achieved at a pH of 6, with no further improvement. Beyond a pH of 8, a two-phase appearance suggests overfunctionalization. Considering future use in anionic and cationic finishing materials, a neutral pH of 7 is deemed optimal.
In order to obtain the optimum collage gel product, under the same conditions, T = 50 °C, t = 2 h, and pH = 7.0, the amount of trypsin and time were studied. See
Figure 3.
From the depicted graph, it is evident that, for consistent collagen, temperature, time, and pH conditions, the trend in collagen functionalization leans toward a decrease as the enzyme amount diminishes. Though the differences are not substantial, we pragmatically opted for the enzyme quantities of 0.4 g and 0.2 g to delve into the nuanced interplay among collagen functionalization, time, and cost considerations associated with enzyme usage.
Examining
Figure 3, it becomes apparent that at 0.4 g of trypsin, the collagen solution undergoes a two-phase transformation at the 3 h mark. This signifies that an elevated enzyme quantity, coupled with extended time, leads to an excess functionalization of collagen, resulting in the observed two-phase phenomenon.
Contrastingly, when employing a trypsin quantity of 0.2 g, our collagen-based finishing products exhibit no two-phase phenomenon. This suggests that the enzyme dosage of 0.2 g aligns more suitably with the desired outcomes.
At the 0.2 g trypsin level, collagen functionalization sees an increase with time, almost reaching saturation by the 3 h mark. Consequently, we propose that the optimal enzyme quantity for our purposes is 0.2 g, and the most effective time for collagen modification is 3 h.
The culmination of these findings is encapsulated in
Table 1, presenting the final formulation.
The final collagen gel product was characterized as shown in
Table 2.
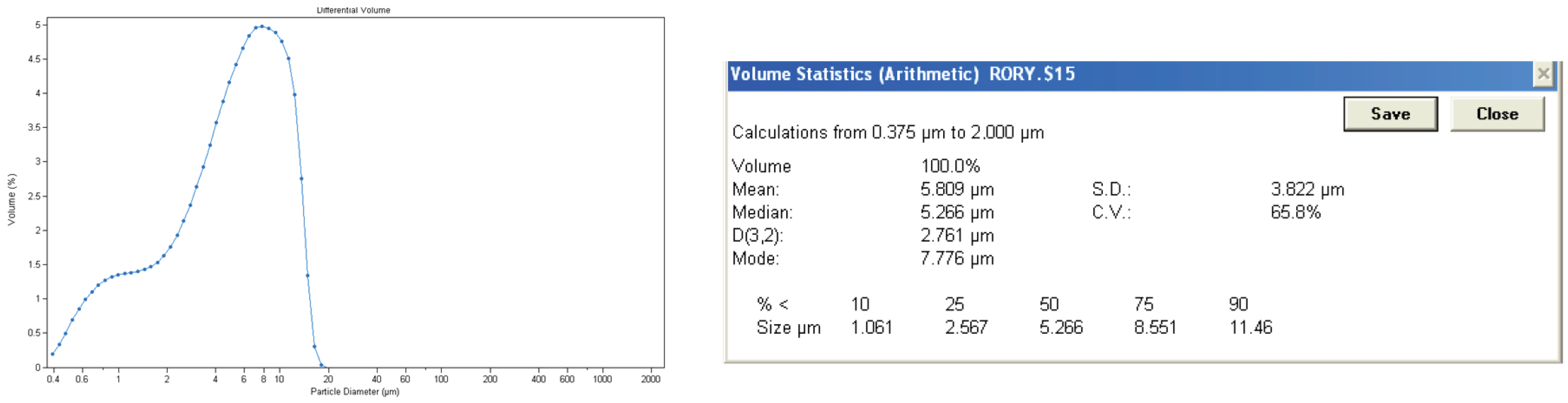
The particle size was measured by Coulter counter as can be seen in
Figure 4.
The particle size of the collagen gel product is not homogeneous, and it has a wide range from 0.3 µm to 20 µm. As it comes from bovine waste, which is a natural material, it is difficult to obtain a homogeneous solution. Its average particle size is 5.809 µm, and 75% of the particles in the collagen gel product have a particle size below 8.551 µm, which indicates that they tend to stay on the surface rather than penetrate deeply into the material.
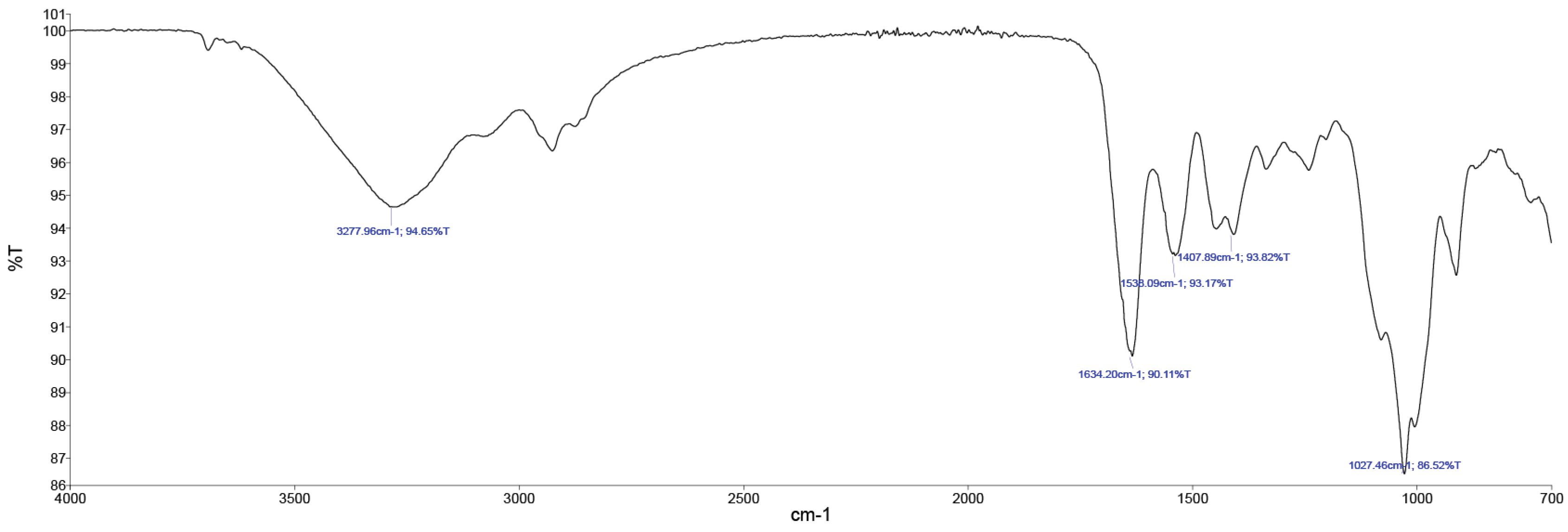
In
Figure 5, the FTIR spectroscopy of the collagen gel product is evident.
The main FTIR peaks include amide A (3277 cm
−1), amide B (2919 cm
−1), amide I (1634 cm
−1), amide II (1538 cm
−1), and amide III (1234 cm
−1) as shown in
Figure 5. The peaks were matched well with those of cow hide. Overall, the FTIR confirmed that the trypsin hydrolysis did not affect the triple-helical structure of collagen.
2.2. Leather Property Evaluation
Once optimized, the evaluations of the collagen gel products with different base coat formulations were carried out according to
Table 3,
Table 4,
Table 5,
Table 6 and
Table 7. BC control is a conventional formulation, and BC 1, BC 2, and BC 3 are the collagen gel product substitutes of some of the conventional polymers used in the base coat. The physical properties obtained by using different formulations can be seen in
Table 3.
According to the findings in
Table 3, the collagen gel product exhibits a noteworthy enhancement in the water vapor permeability of the coating. This improvement contributes to an enhanced level of comfort of the final leather good.
In terms of color fastness to artificial light, the collagen gel product demonstrates remarkable stability, as there is no observed change in color fastness to artificial light when applied to the coating. This underscores the product’s high resistance to fading under artificial light conditions.
All samples showcase commendable color fastness to dry rubbing. However, when evaluating color fastness to wet rubbing and color fastness to water spotting, an interesting trend emerges. As the percentage of collagen gel product in the samples increases, there is a reduction in color fastness to wet rubbing. This suggests that while the product exhibits excellent color fastness to dry rubbing, there is room for improvement in its performance under wet rubbing conditions. This phenomenon aligns with the inherent characteristics of protein binders.
In a parallel way, different top coat formulations were tested on the same base coat formulation (BC control). The physical properties obtained by using different formulations can be seen in
Table 4.
Upon application of the collagen gel product to the top coat, all test results yielded remarkably high values. This implies that its performance is comparable to that of conventional polymers when utilized in the top coat. However, a nuanced observation arises when considering the tactile experience. A subtle increase in stickiness is discernible on the top coat treated with the collagen gel product. This heightened stickiness indicates a pronounced water absorption capacity, resulting in a tendency to absorb moisture on the top coat and consequently imparting a slightly sticky texture upon touch.
Finally, different proportions of collagen gel products were tested to obtain a full biobased coating. The physical properties obtained are shown in
Table 5.
The table reveals that Sample 8 outperforms the conventional coating. Moreover, the complete biobased coating (Sample 9) exhibits excellent performance across various parameters, with the exception of wet resistance. Notably, it excels in both touch and appearance. This confirmation underscores the applicability of the new collagen gel product for finishing bovine hides, meeting all the stringent requirements of the leather market.