Strength Assessment of Water–Glass Sand Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Specimens
2.2. Mechanical Tests
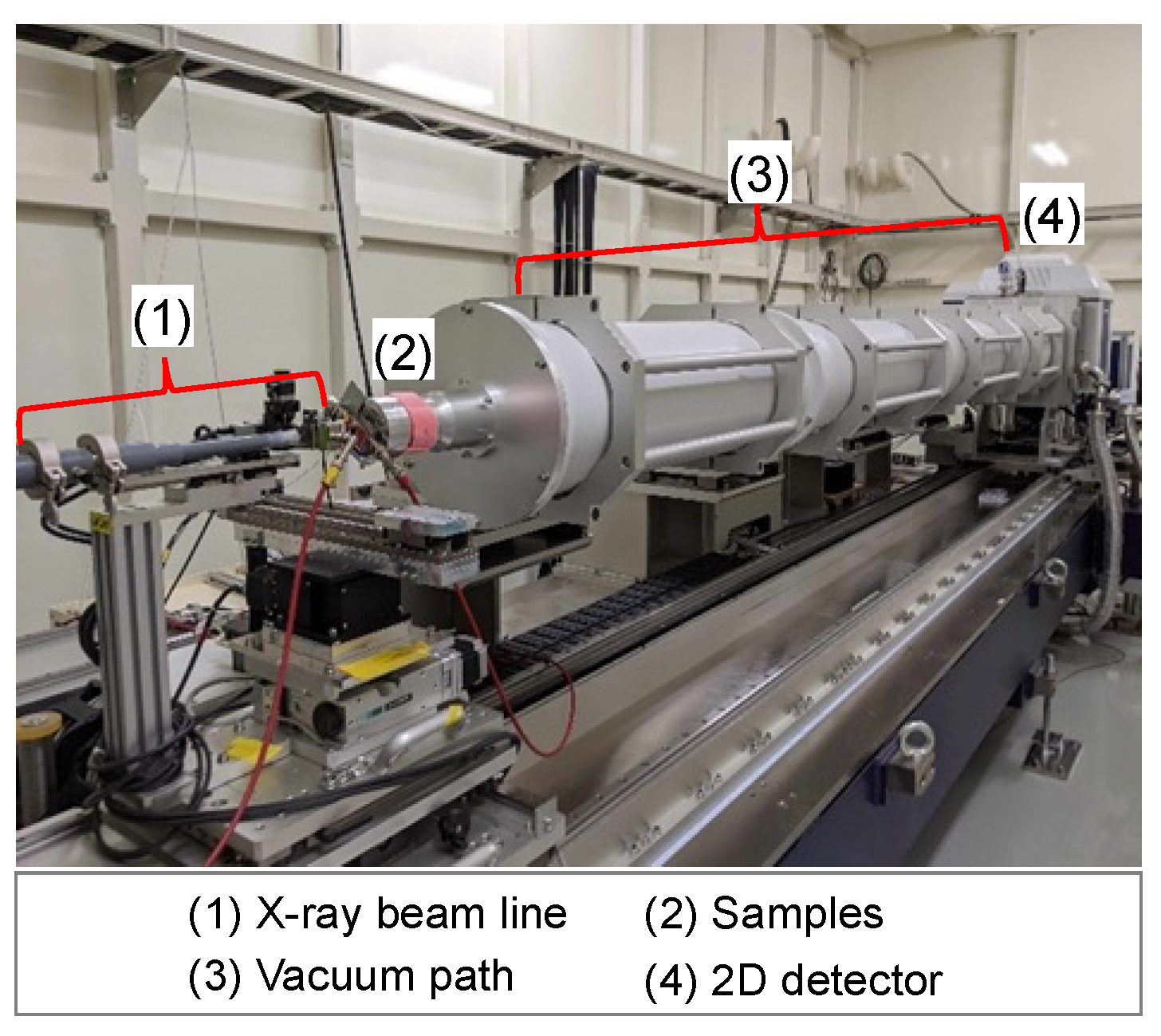
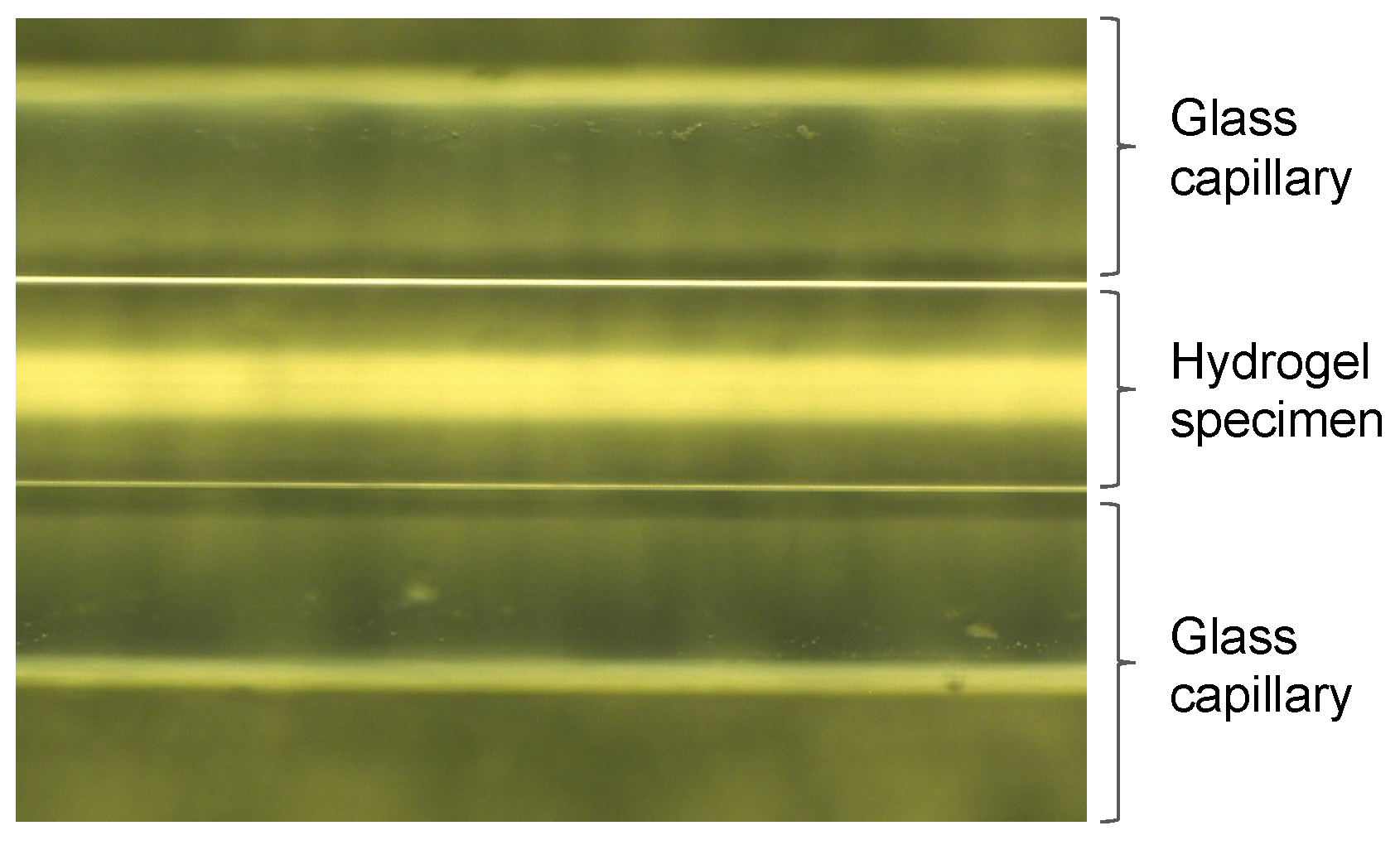
2.3. Small-Angle X-ray Scattering Tests (SAXS Tests)
- (1)
- A quartz glass tube of ϕ2 mm for X-ray diffraction is filled with the chemical solution and sealed at the end.
- (2)
- After gelation of the chemical solution, the tubes are cured at different curing temperatures of 10, 20, 45, and 60 °C.
- (3)
- The SAXS tests are conducted on the hydrogel specimens at each material age, namely, 1, 7, 14, 21, and 28 days. The SAXS test apparatus used for the tests was the beamline BL8S3 (see Figure 2) operated at the Aichi Synchrotron Radiation Center, Japan.
- (4)
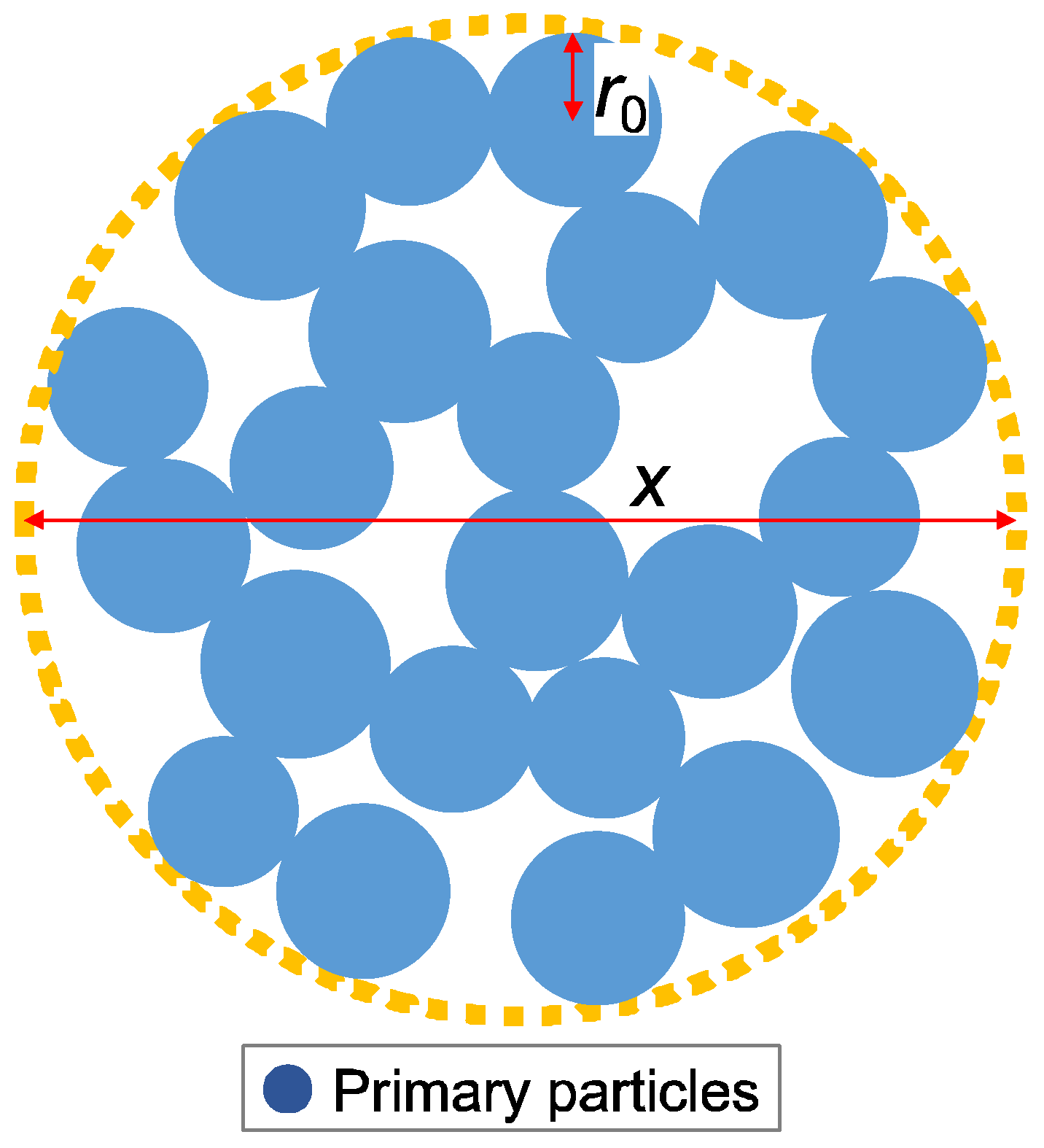
- The measurement data obtained through the SAXS tests are fitted using the aggregate analysis model shown in Figure 3, and lower limit censoring length (assumed to be distributed in the analysis) r0, mass fractal dimension D, and censored length (correlation length) ξ are calculated.
- (5)
3. Results and Discussion
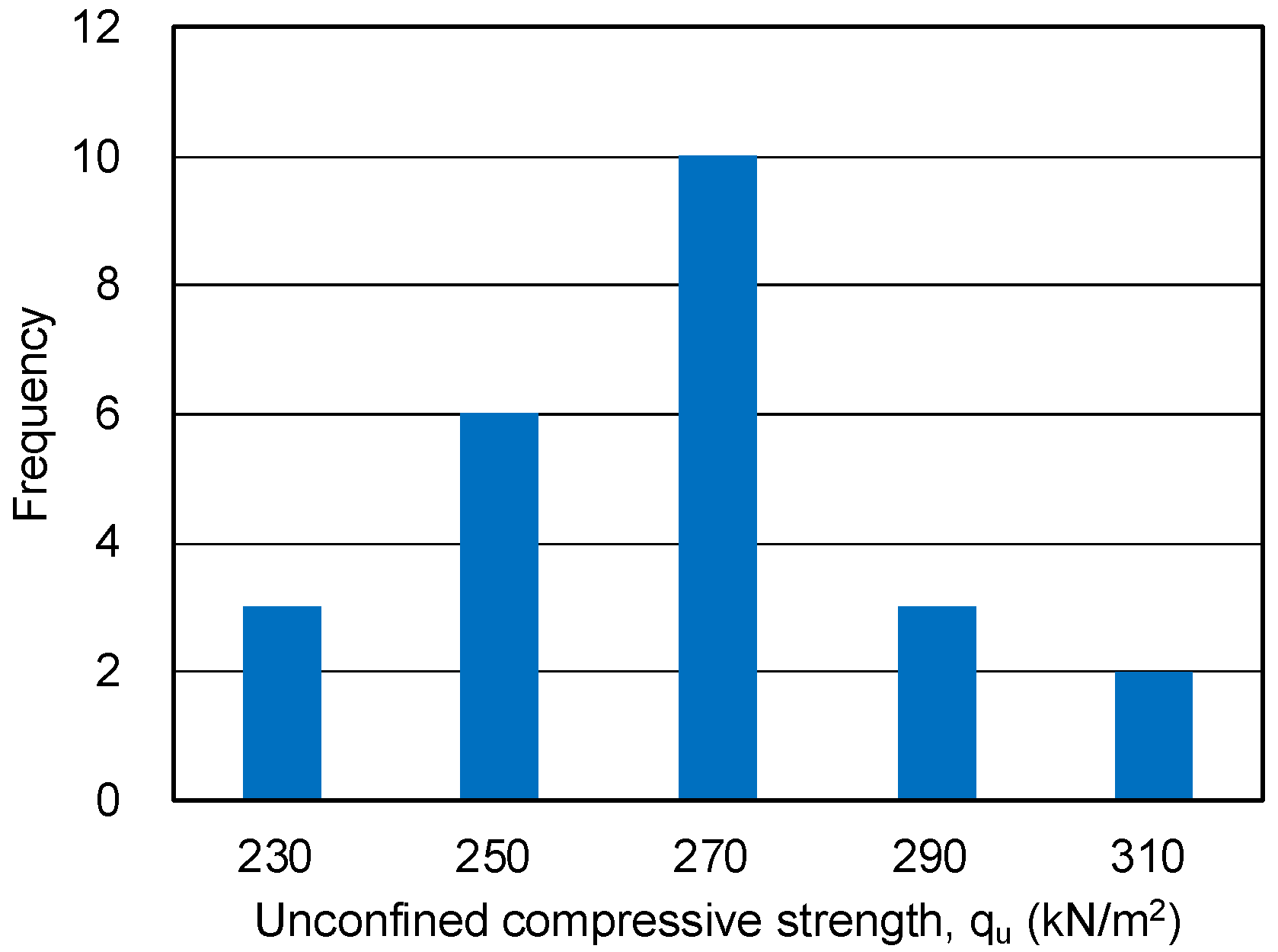
3.1. UC Tests for Sand-Gel Specimens
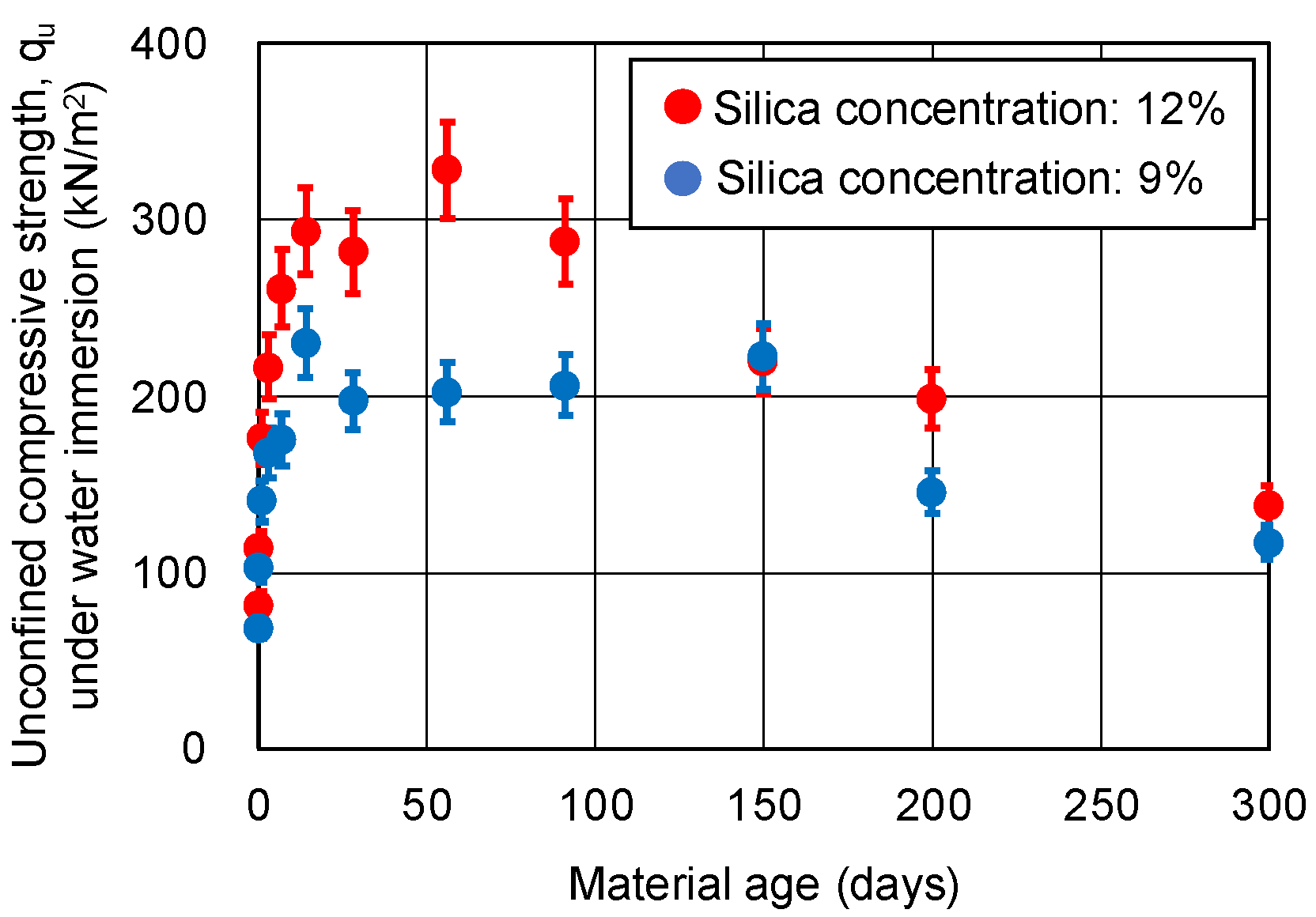
3.2. UC Tests under Water Immersion for Sand-Gel Specimens
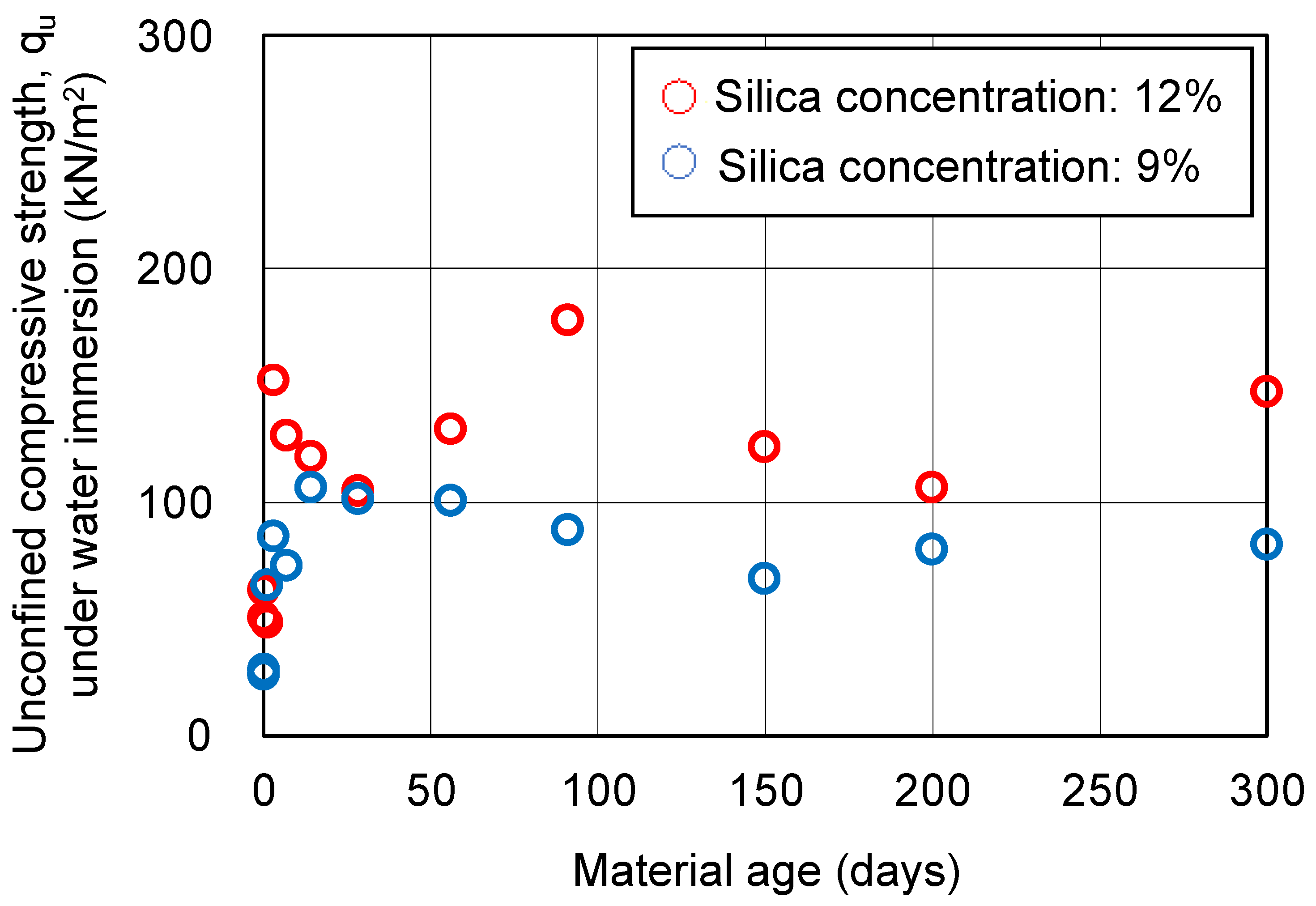
3.3. UC Tests for Hydrogel Specimens
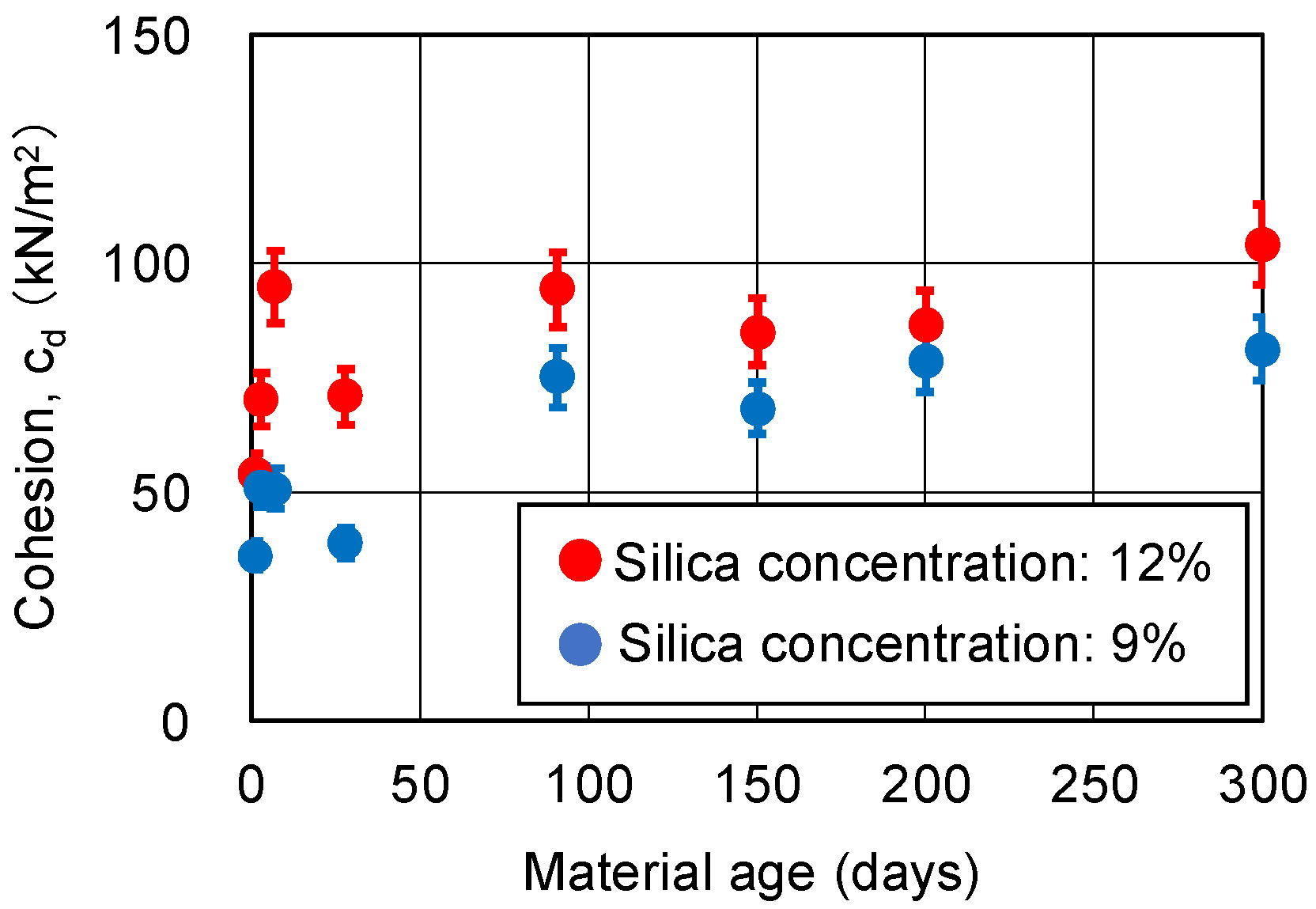
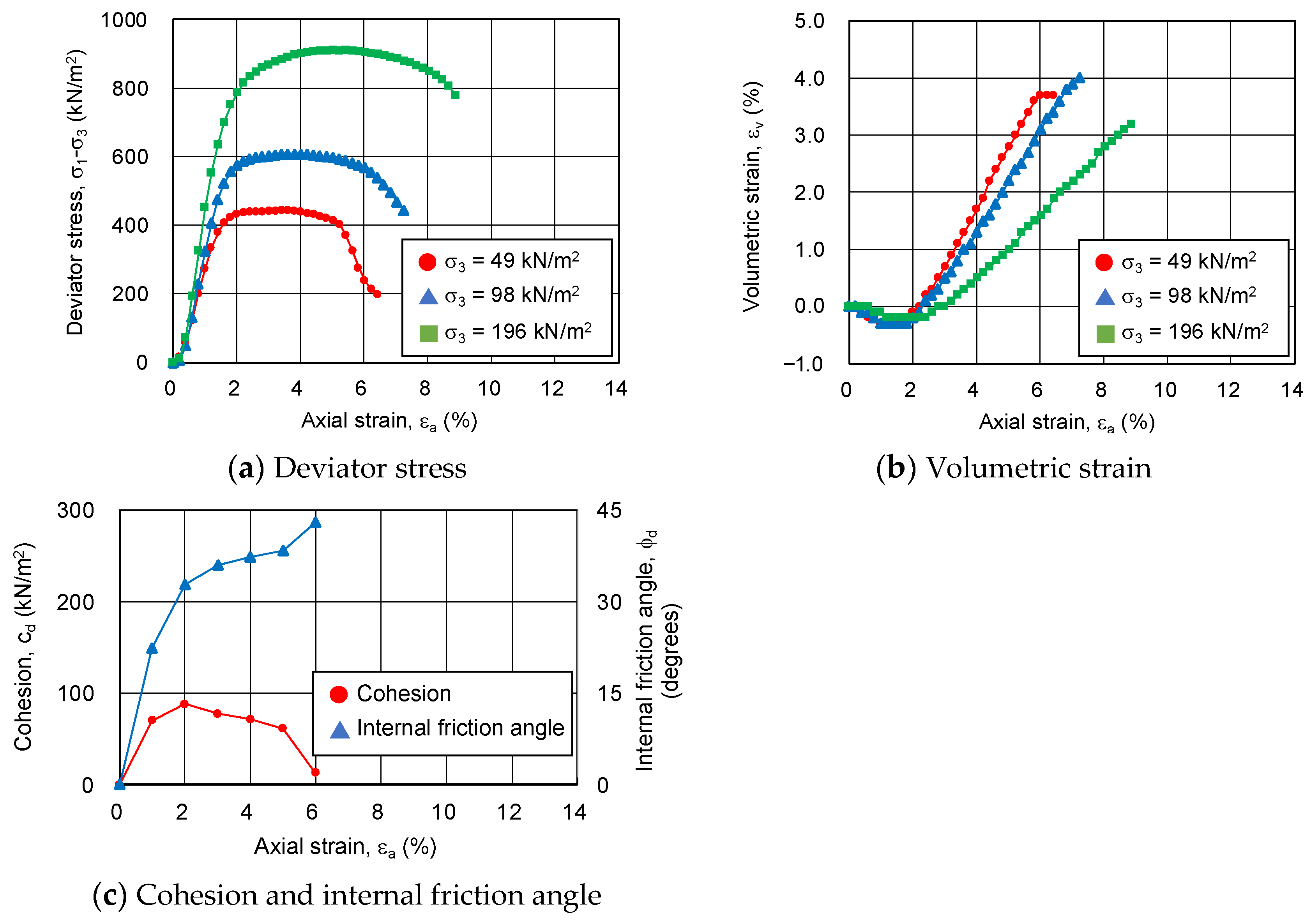
3.4. CD Tests for Sand-Gel Specimens
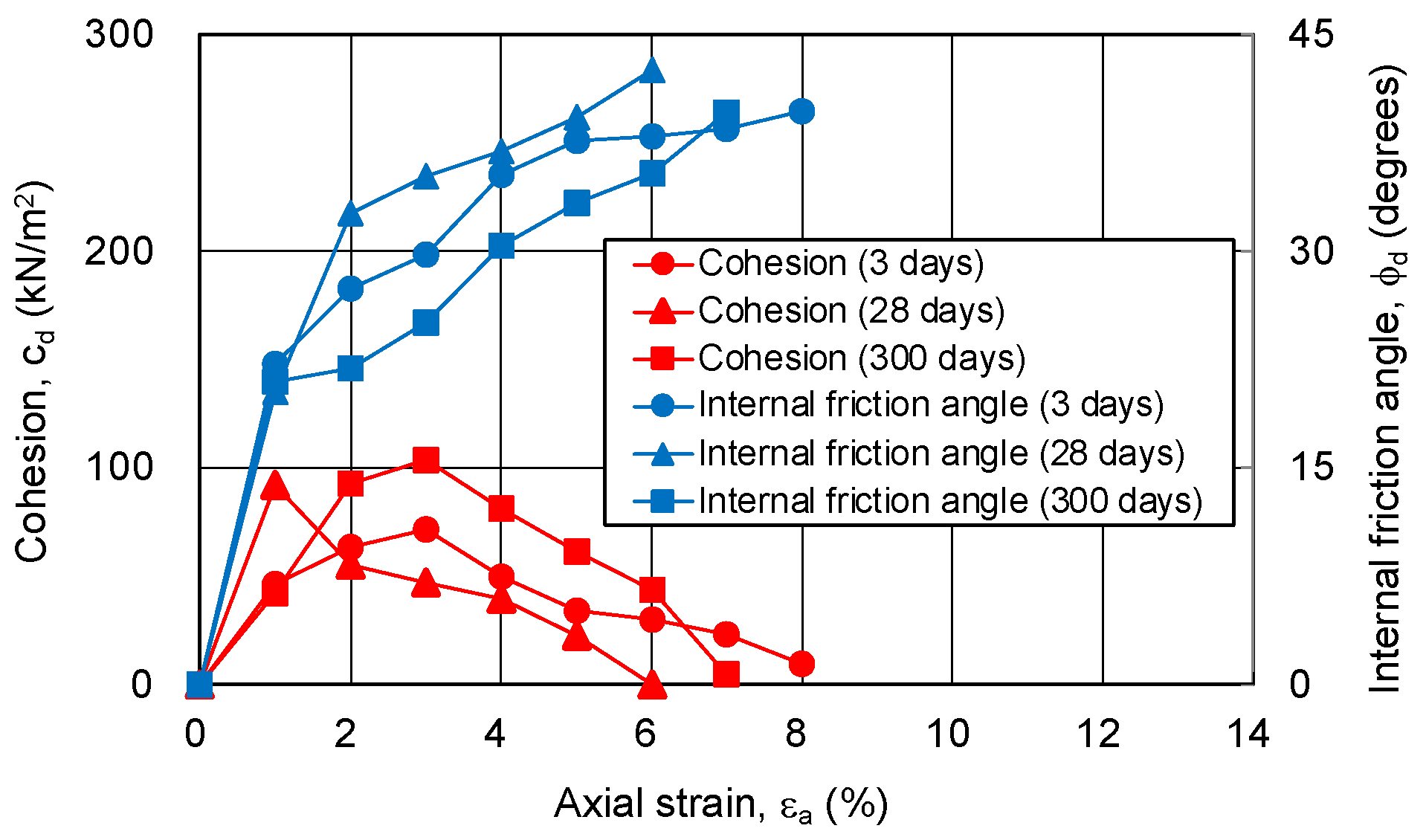
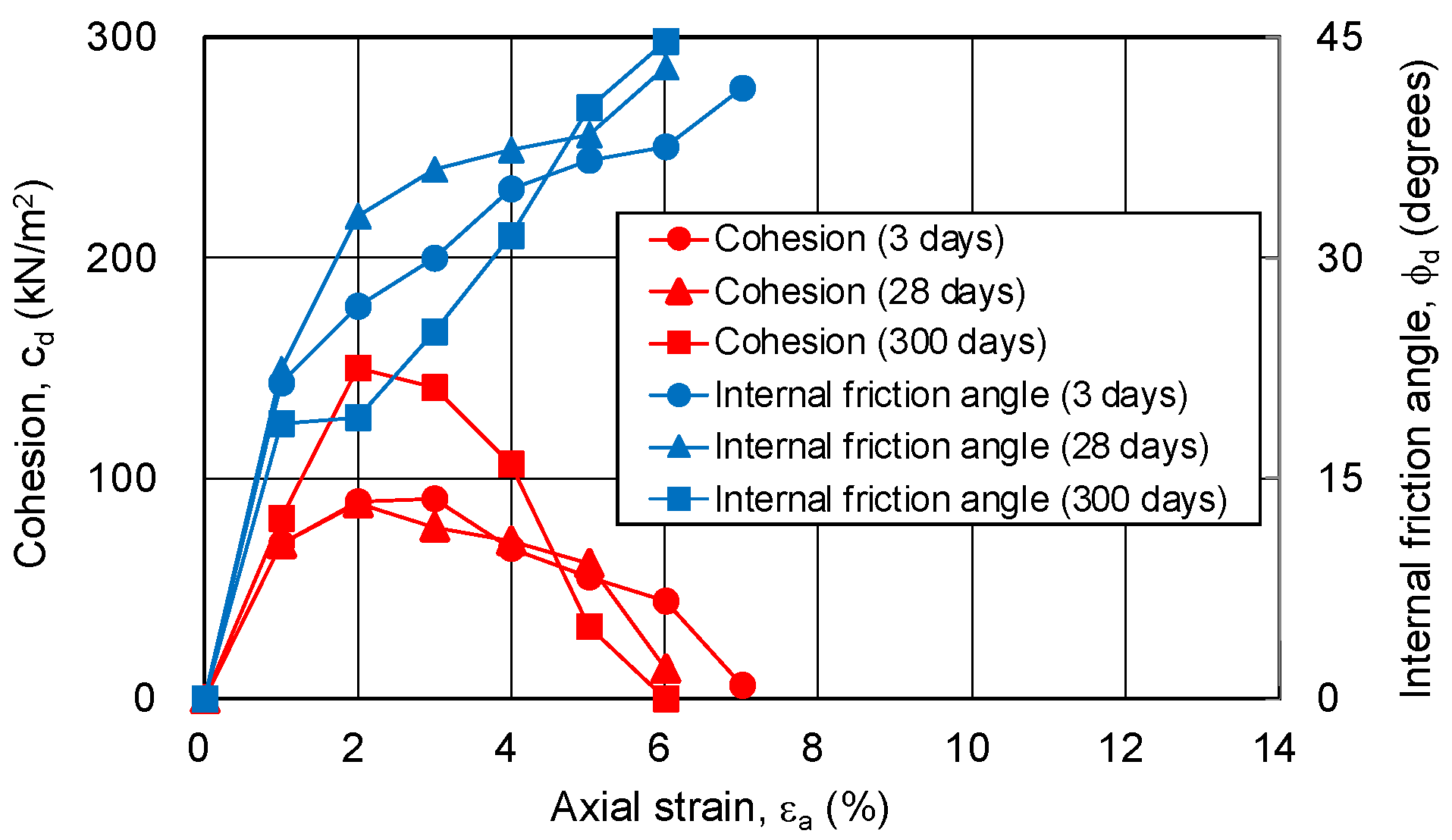
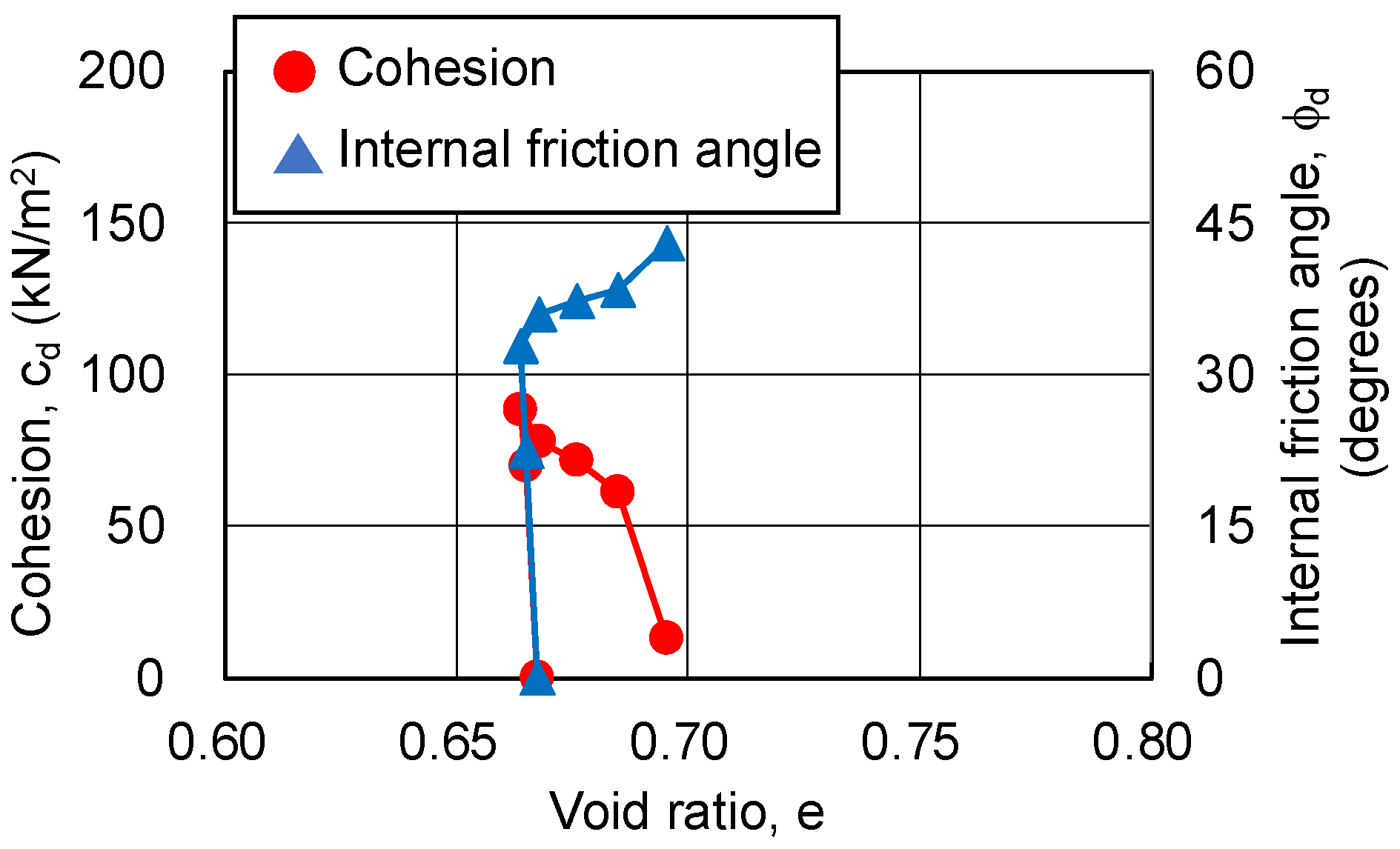
3.4.1. Relationship between Strength Components and Axial Strain
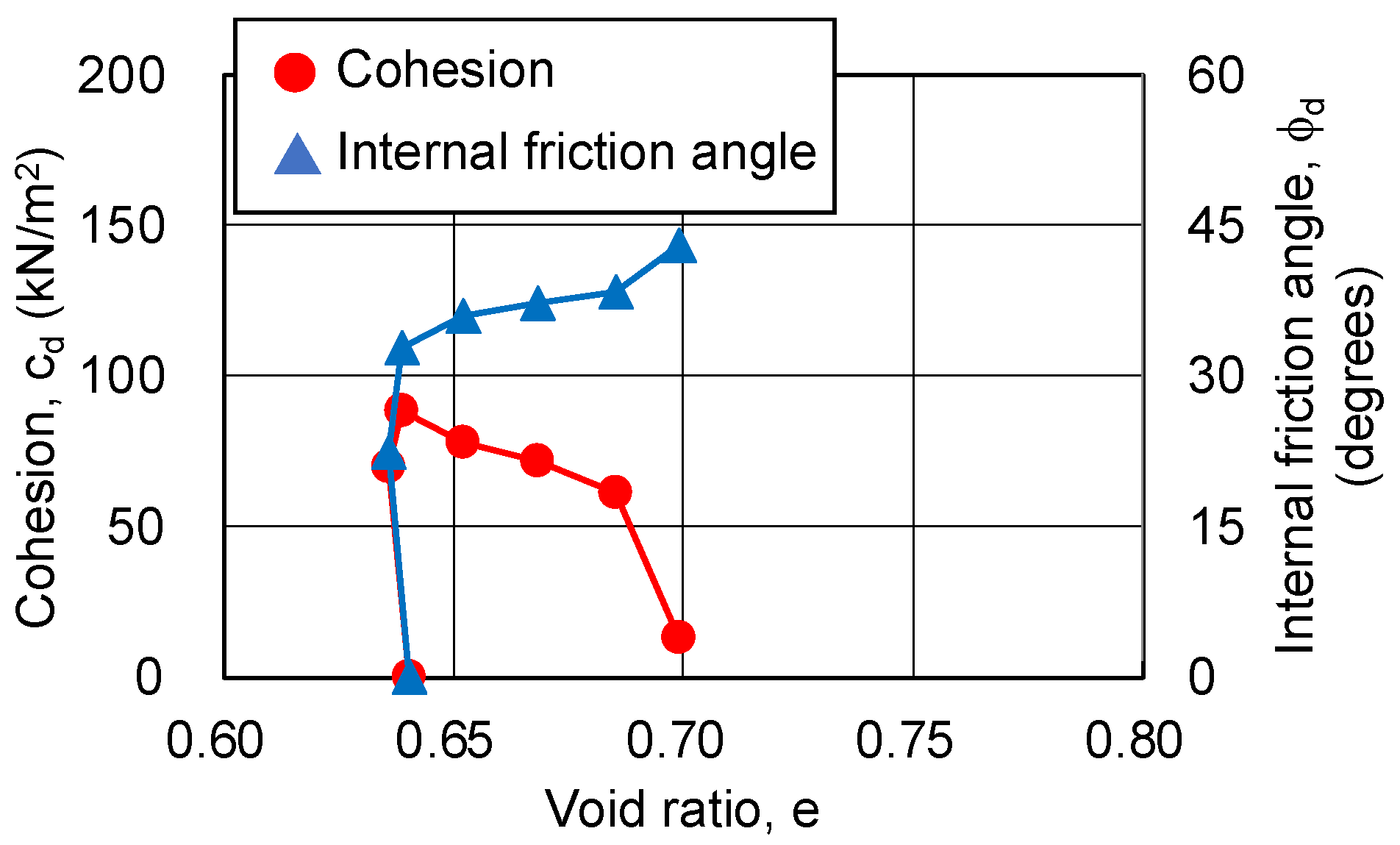
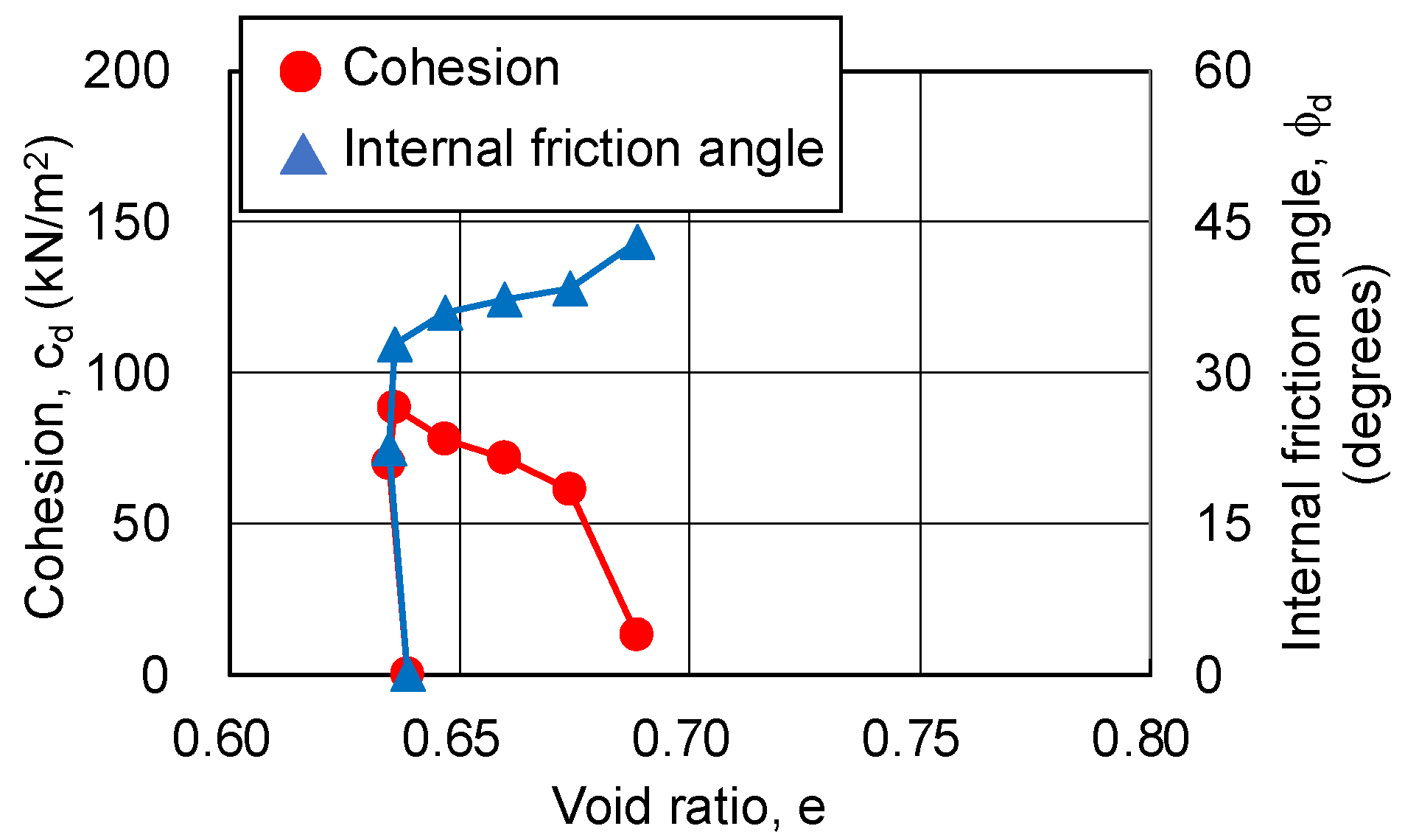
3.4.2. Relationship between Strength Components and Hvorslev Failure Criteria
- (1)
- The wet density at each axial strain is calculated from the volume change (amount of water absorbed or drained) at each axial strain.
- (2)
- The pore ratio at each axial strain is calculated from the sandy soil particle density, saturation, and wet density.
- (3)
- The pore ratio is related to the change in cohesion.
- (1)
- Consolidation and shearing cause changes in volume, and the amount of drainage or water supply at these times correspond to changes in the volume and mass of the sand-gel specimens (density of 1.0 g/cm3 as water).
- (2)
- The saturation of the specimens does not change from before shearing up to the end of the CD test.
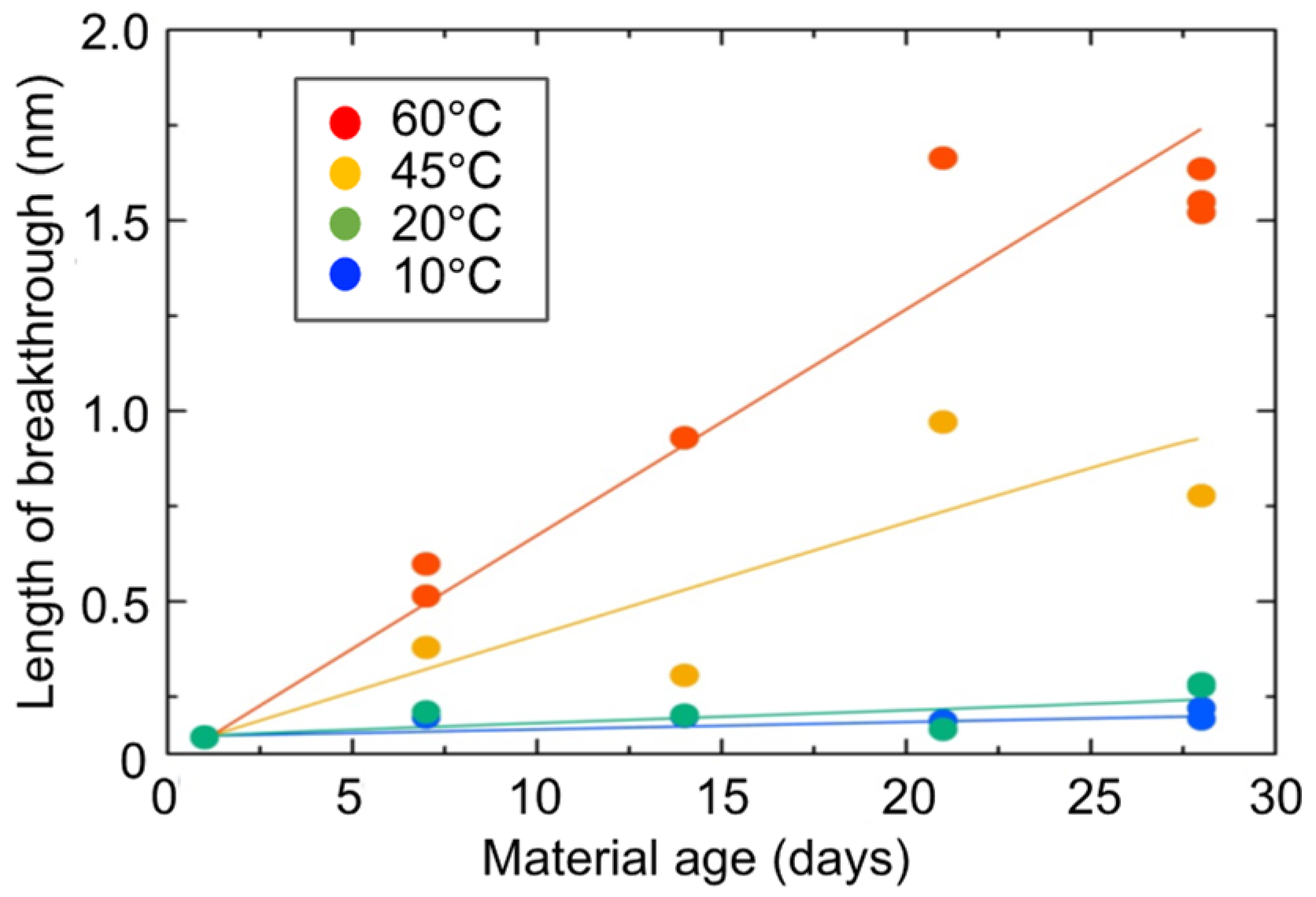
3.5. Volumetric Shrinkage Tests for Hydrogel Specimens
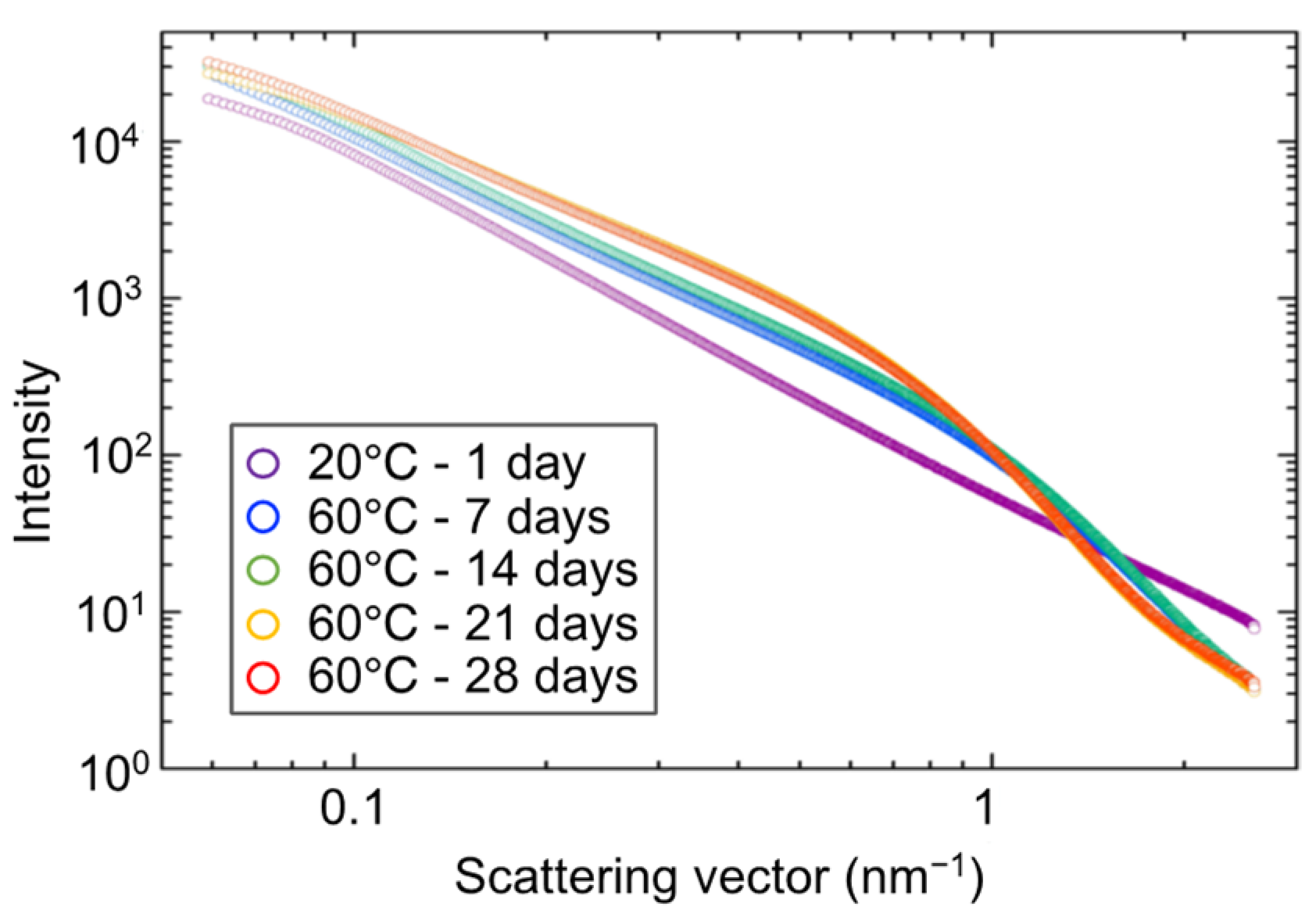
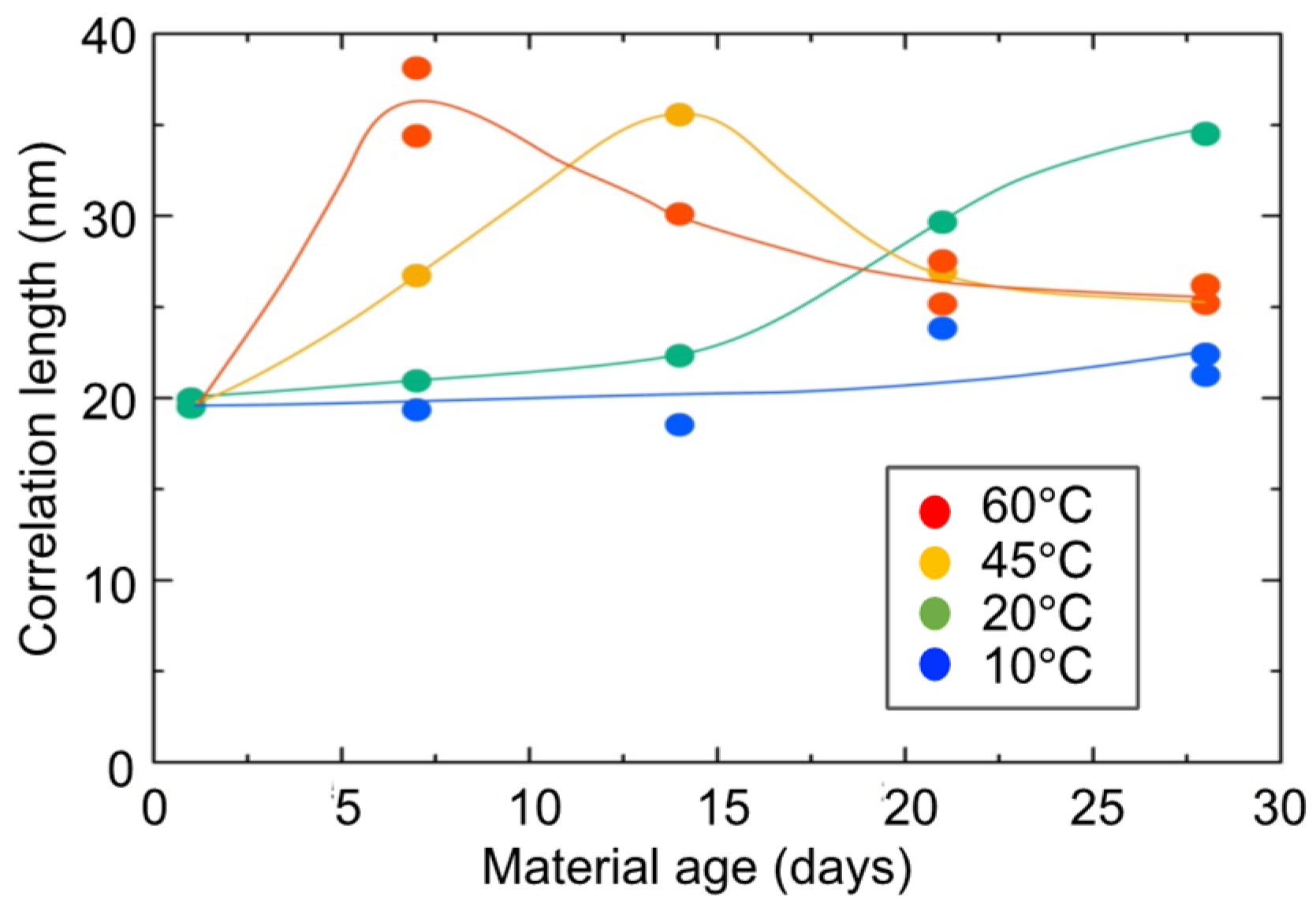
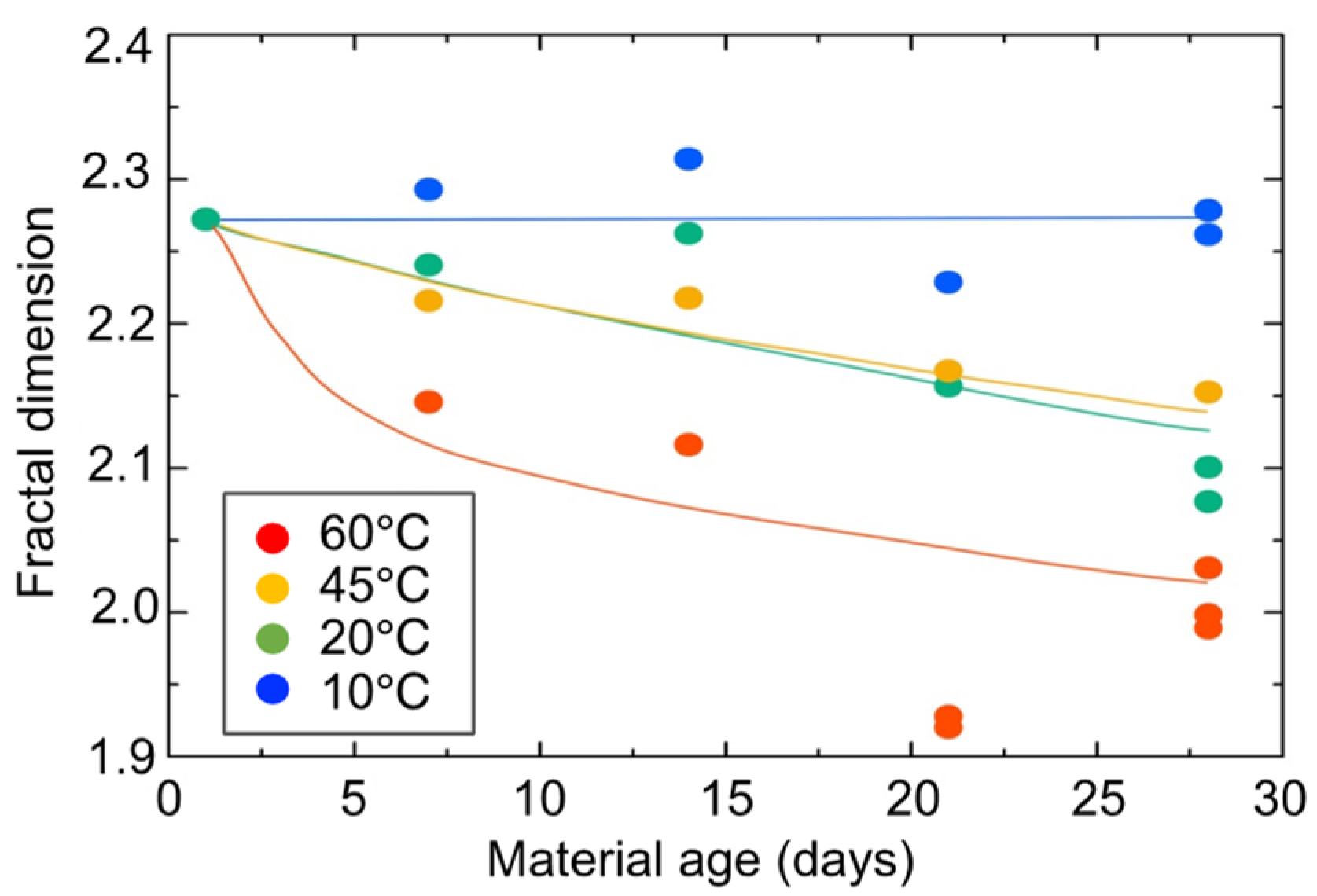
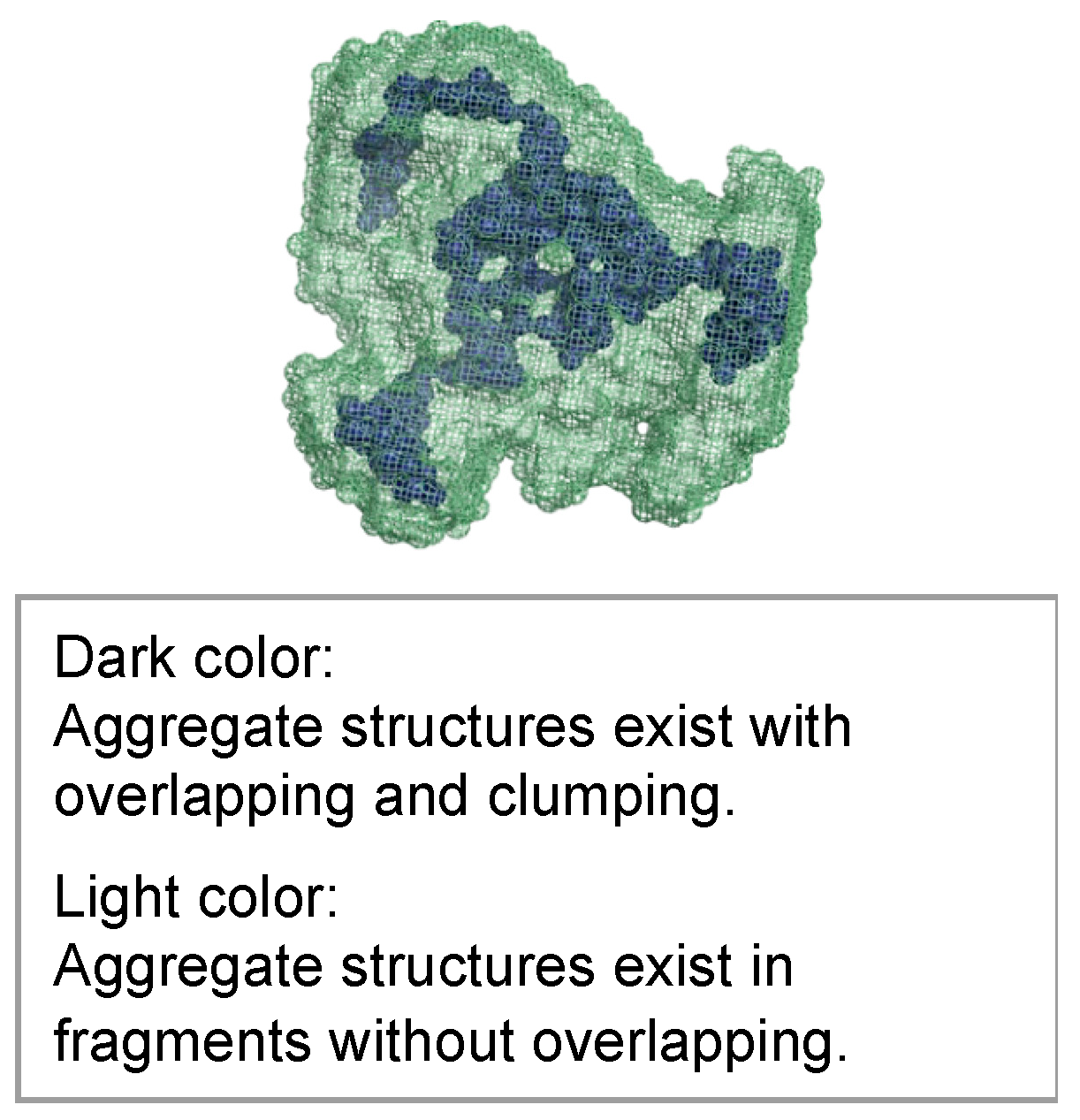
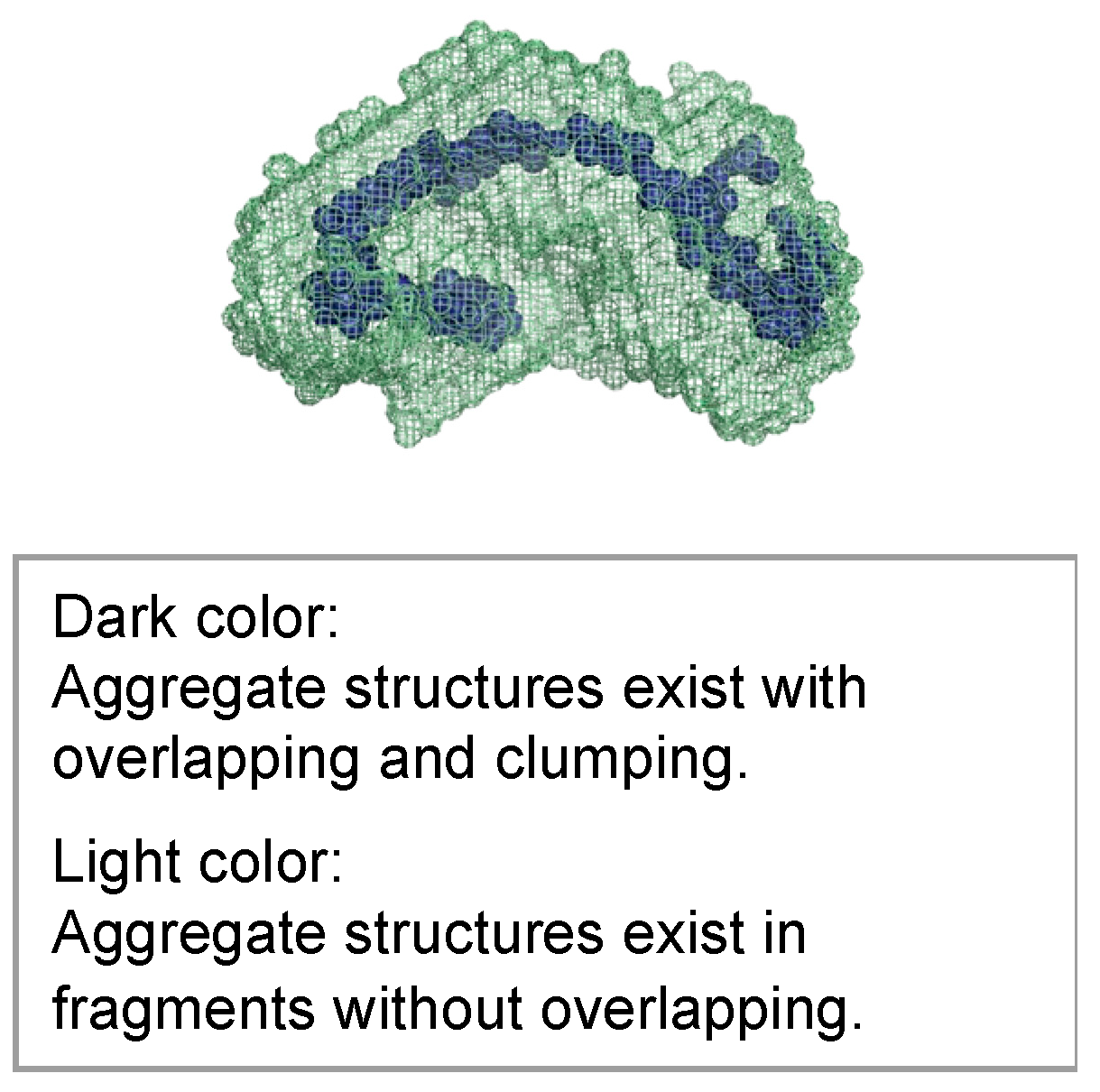
3.6. SAXS Tests for Hydrogel Specimens
3.7. Strength Assessment
4. Conclusions
- (1)
- The strength development of the sandy soil improved via chemical injection can be explained by the increases in cohesion and the internal friction angle of the sandy soil particles fixed by the chemical solution.
- (2)
- Under triaxial conditions with confining pressure, such as in CD tests, the strength component of the sandy soil improved via chemical injection shows no long-term loss of strength.
- (3)
- No physically visible volumetric shrinkage of the hydrogel occurs in the pore spaces of the soil particles.
- (4)
- The failure of the sandy soil improved via chemical injection is the failure of the aggregate structure of the hydrogel itself.
- (5)
- The aggregate structure inside the hydrogel changes to a sparse state due to the shrinkage of the microscopic molecular structure of the hydrogel over time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karol, R.H. Chemical Grouting and Soil Stabilization, 3rd ed.; Revised and expanded; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Varol, A.; Dalgıç, S. Grouting applications in the Istanbul metro, Turkey. Tunn. Undergr. Space Technol. 2006, 21, 602–612. [Google Scholar] [CrossRef]
- Kazemian, S.; Huat, B.B.; Arun, P.; Barghchi, M. A review of stabilization of soft soils by injection of chemical grouting. Aust. J. Basic Appl. Sci. 2010, 4, 5862–5868. [Google Scholar]
- Anagnostopoulos, C.A.; Papaliangas, T.; Manolopoulou, S.; Dimopoulos, T. Physical and mechanical properties of chemically grouted sand. Tunn. Undergr. Space Technol. 2011, 26, 718–724. [Google Scholar] [CrossRef]
- Liang, Y.; Sui, W.; Qi, J. Experimental investigation on chemical grouting of inclined fracture to control sand and water flow. Tunn. Undergr. Space Technol. 2019, 83, 82–90. [Google Scholar] [CrossRef]
- Spagnoli, G. A review of soil improvement with non-conventional grouts. Int. J. Geotech. Eng. 2021, 15, 273–287. [Google Scholar] [CrossRef]
- Mori, A.; Tamura, M. Cohesion and dilatancy of sands stabilised by chemical grout. Jpn. J. JSCE 1986, 370/III-5, 123–132. [Google Scholar] [CrossRef]
- Mori, A.; Tamura, M. Effect of dilatancy on permeability in sands stabilized by chemical grout. Soils Found. 1986, 26, 96–104. [Google Scholar] [CrossRef]
- Mori, A.; Tamura, M.; Komine, H. Mechanism for the improvement of cohesive soils by chemical grouting and the governing condition. Jpn. J. JSCE 1988, 400/III-10, 55–64. [Google Scholar] [CrossRef][Green Version]
- Mori, A.; Tamura, M.; Fukui, Y. Fracturing pressure of soil ground by viscous materials. Soils Found. 1990, 30, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Tamura, M.; Komine, H.; Ogawa, Y. A method of determine the critical injection rate considering permeated shapes in chemical grouting. Soils Found. 1993, 33, 159–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sasaki, T.; Oyama, T.; Shimada, S.; Suemasa, N. Influence of gelling time on permeability and strength of ground improved by chemical grouting method. In Proceedings of the 21nd International Offshore and Polar Engineering Conference, Rhodes, Greece, 17–22 June 2012. ISOPE-1-12-480. [Google Scholar]
- Uemura, K.; Sasaki, T.; Shimada, S.; Suemasa, N.; Nagao, K. A study on infusion of silica microparticles into soil particles. In Proceedings of the 25th International Offshore and Polar Engineering Conference, Big Island, HI, USA, 21–26 June 2015. ISOPE-I-15-421. [Google Scholar]
- Kaga, M.; Mori, A. Basic study on chemical stability and strength durability of silicate grout sand. Jpn. J. JSCE 1994, 496/V-24, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Adachi, K.; Yonekura, R.; Tokoro, T. Estimation method on the mechanical stability and water cut-off capacity of sand solidified by chemical grouting. Jpn. J. JSCE 2001, 693/VI-53, 25–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, T.; Senesi, A.J.; Lee, B. Small angle X-ray scattering for nanoparticle research. Chem. Rev. 2016, 116, 11128–11180. [Google Scholar] [CrossRef]
- Manalastas-Cantos, K.; Konarev, P.V.; Hajizadeh, N.R.; Kikhney, A.G.; Petoukhov, M.V.; Molodenskiy, D.S.; Panjkovich, A.; Mertens, H.D.T.; Gruzinov, A.; Borges, C.; et al. ATSAS 3.0: Expanded functionality and new tools for small-angle scattering data analysis. J. Appl. Crystallogr. 2021, 54, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Elkins, J.G.; Davison, B.H.; Kelley, E.G.; Nickels, J. Implementation of a self-consistent slab model of bilayer structure in the SasView suite. J. Appl. Crystallogr. 2021, 54, 363–370. [Google Scholar] [CrossRef]
- Schmertmann, J.H. An Experimental Study of the Development of Cohesion and Friction with Axial Strain in Saturated Cohesive Soils. Ph.D. thesis, Northwestern University, Evanston, IL, USA, 1962. [Google Scholar]
- Schmertmann, J.H. Static cone to compute static settlement over sand. J. Soil Mech. Found. Div. 1970, 96, 1011–1043. [Google Scholar] [CrossRef]
- Hvorslev, M.J. Physical Properties of Remolded Cohesive Soils; US Army Engineer Waterways Experiment Station: Vicksburg, MI, USA, 1969. [Google Scholar]
- Yao, Y.; Gao, Z.; Zhao, J.; Wan, Z. Modified UH model: Constitutive modeling of overconsolidated clays based on a parabolic Hvorslev envelope. J. Geotech. Geoenviron. 2012, 138, 860–868. [Google Scholar] [CrossRef]
- Tsiampousi, A.; Zdravković, L.; Potts, D.M. A new Hvorslev surface for critical state type unsaturated and saturated constitutive models. Comput. Geotech. 2013, 48, 156–166. [Google Scholar] [CrossRef]
- Uno, T.; Sugii, T.; Kamiya, K. Considerations of permeability related to physical properties of particles based on the measurement of specific surface area. Jpn. J. JSCE 1993, 469/III-23, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lambe, T.W.; Whitman, R.V. Soil Mechanics; John Wiley and Sons: Hoboken, NJ, USA, 1969. [Google Scholar]
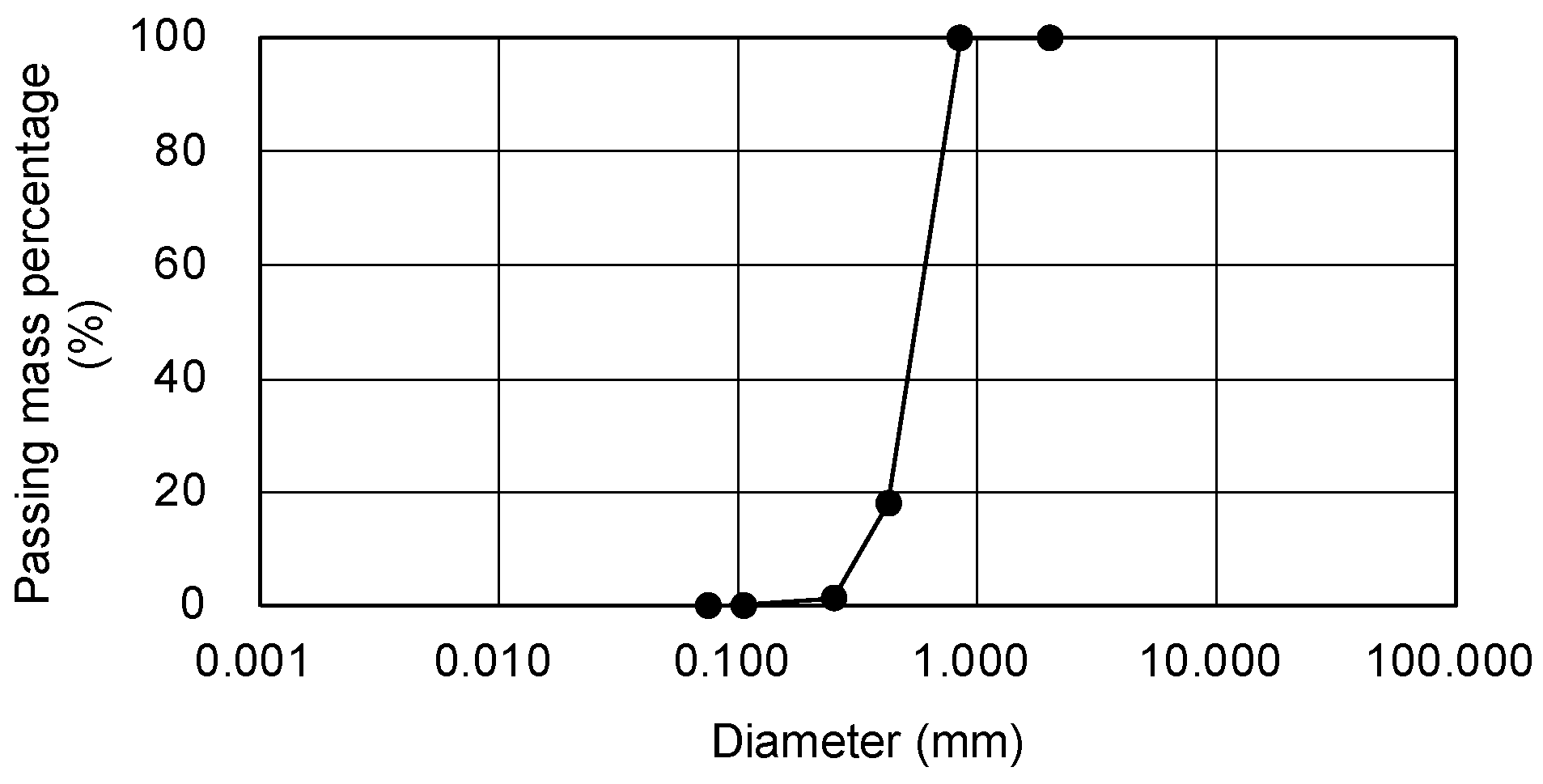



| Test | Specimen | SiO2 |
|---|---|---|
| Unconfined compression test (UC test) | Sand-gel | 12% |
| Hydrogel | 12%, 9% | |
| Unconfined compression test under water immersion (UC test under water immersion) | Sand-gel | 12%, 9% |
| Consolidation drainage triaxial compression tests (CD test) | Sand-gel | 12%, 9% |
| Volumetric shrinkage test (VS test) | Hydrogel | 12%, 9%, 7% |
| Small-angle X-ray scattering test (SAXS test) | Hydrogel | 7% |
| Basic Physical Properties Index | |||
|---|---|---|---|
| Wet density | ρt | 1.995 | g/cm3 |
| Dry density | ρd | 1.591 | g/cm3 |
| Natural water content | wn | 25.4 | % |
| Void ratio | e | 0.659 | |
| Degree of saturation | Sr | 101.7 | % |
| Relative density | Dr | 58.0 | % |
| Materials | Average Diameter, (cm) | (cm2/cm3) |
|---|---|---|
| Standard sand | 0.0191 | 314.1 |
| Glass capillary | 0.0150 | 400.0 |
| Plastic mold | 5.0000 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motohashi, T.; Sasahara, S.; Inazumi, S. Strength Assessment of Water–Glass Sand Mixtures. Gels 2023, 9, 850. https://doi.org/10.3390/gels9110850
Motohashi T, Sasahara S, Inazumi S. Strength Assessment of Water–Glass Sand Mixtures. Gels. 2023; 9(11):850. https://doi.org/10.3390/gels9110850
Chicago/Turabian StyleMotohashi, Toshiyuki, Shigeo Sasahara, and Shinya Inazumi. 2023. "Strength Assessment of Water–Glass Sand Mixtures" Gels 9, no. 11: 850. https://doi.org/10.3390/gels9110850
APA StyleMotohashi, T., Sasahara, S., & Inazumi, S. (2023). Strength Assessment of Water–Glass Sand Mixtures. Gels, 9(11), 850. https://doi.org/10.3390/gels9110850






