Three-Dimensional Membranes of Natural Polymer Complex Nanoparticle for Potential Medical Applications
Abstract
:1. Introduction
2. Results and Discussion
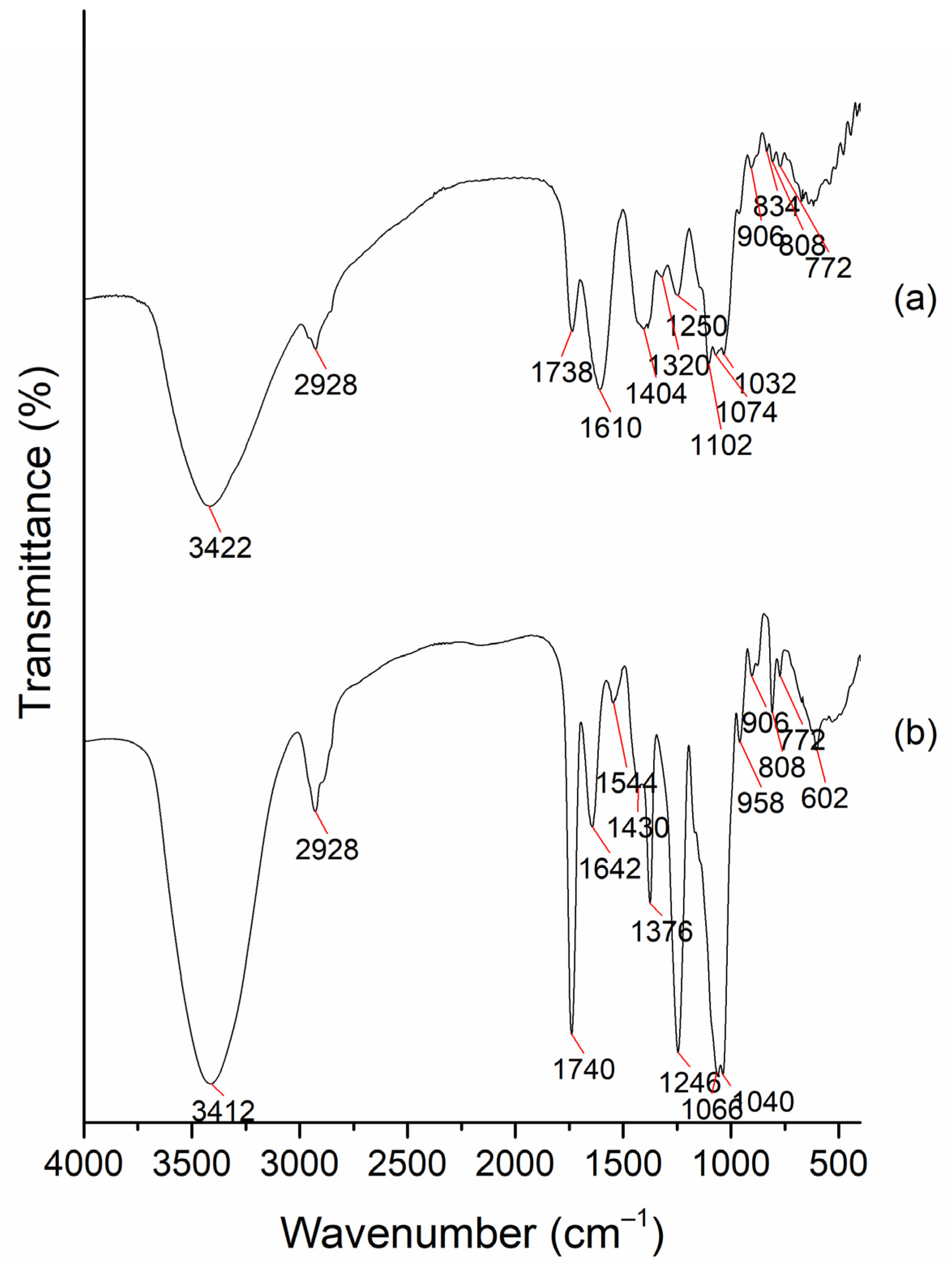
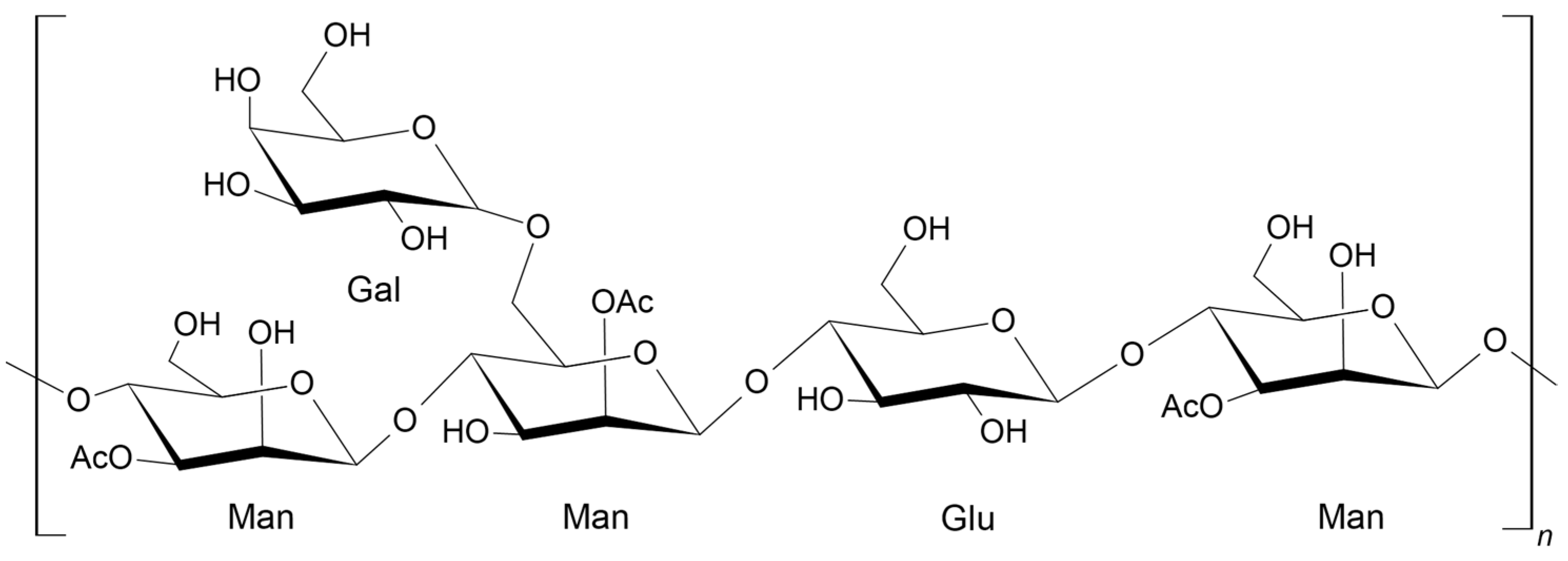
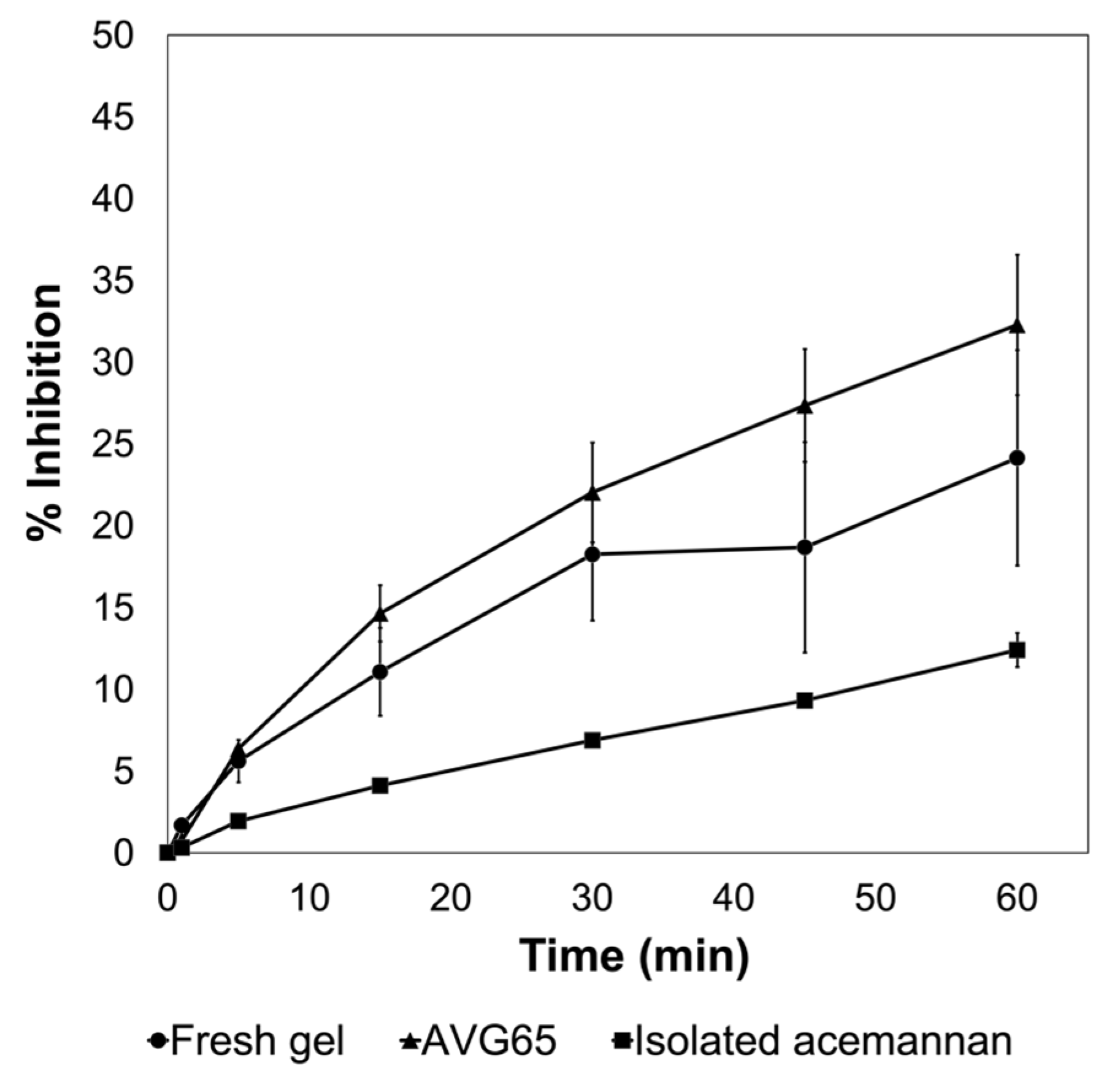
2.1. Characterization of the AVG and Acemannan Isolated from AVG
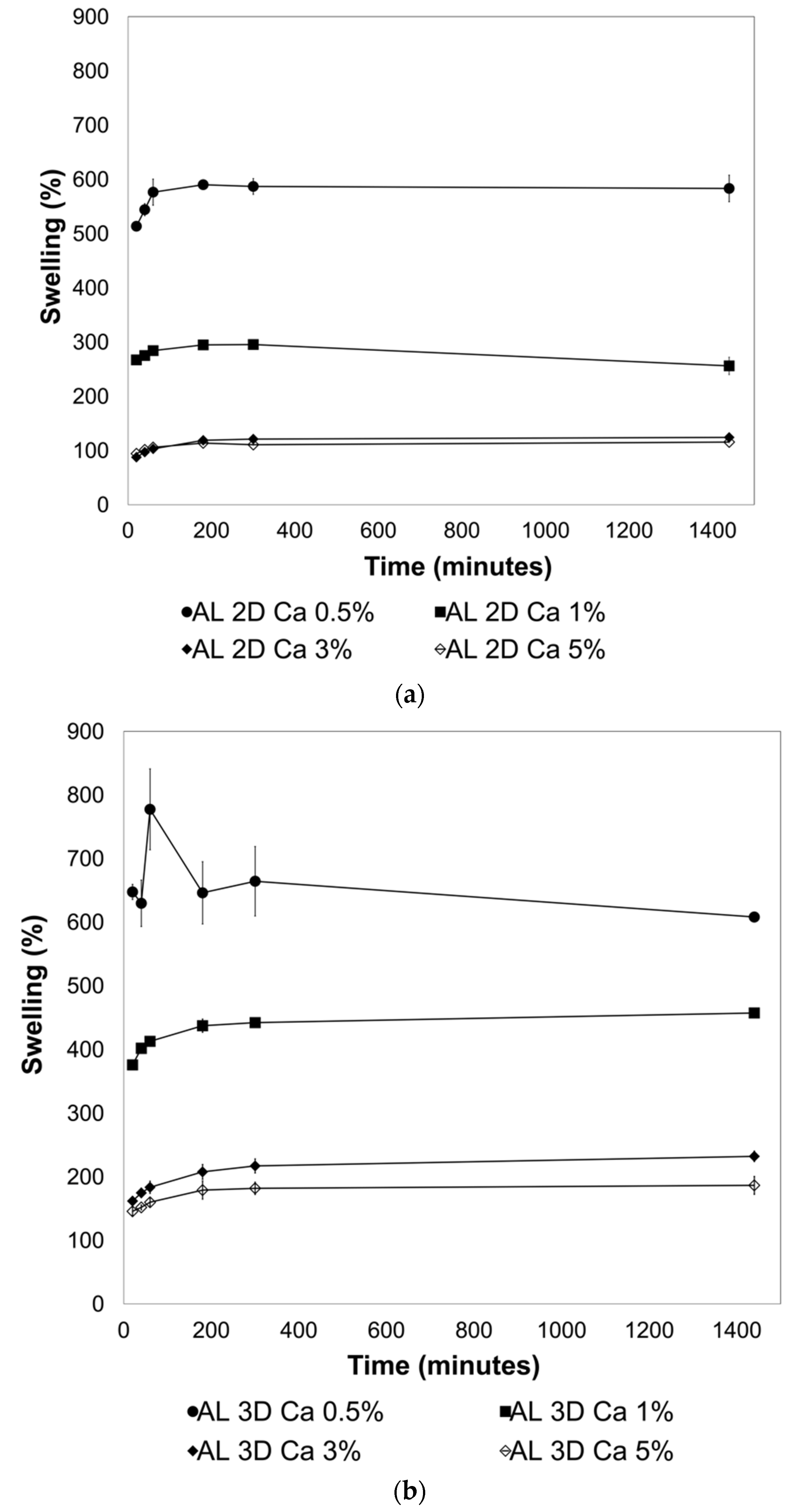
2.2. Effect of Crosslinking on the Swelling Behavior of the 2D and 3D AL Films
2.3. Thickness and Transparency of AL-AVG Membranes
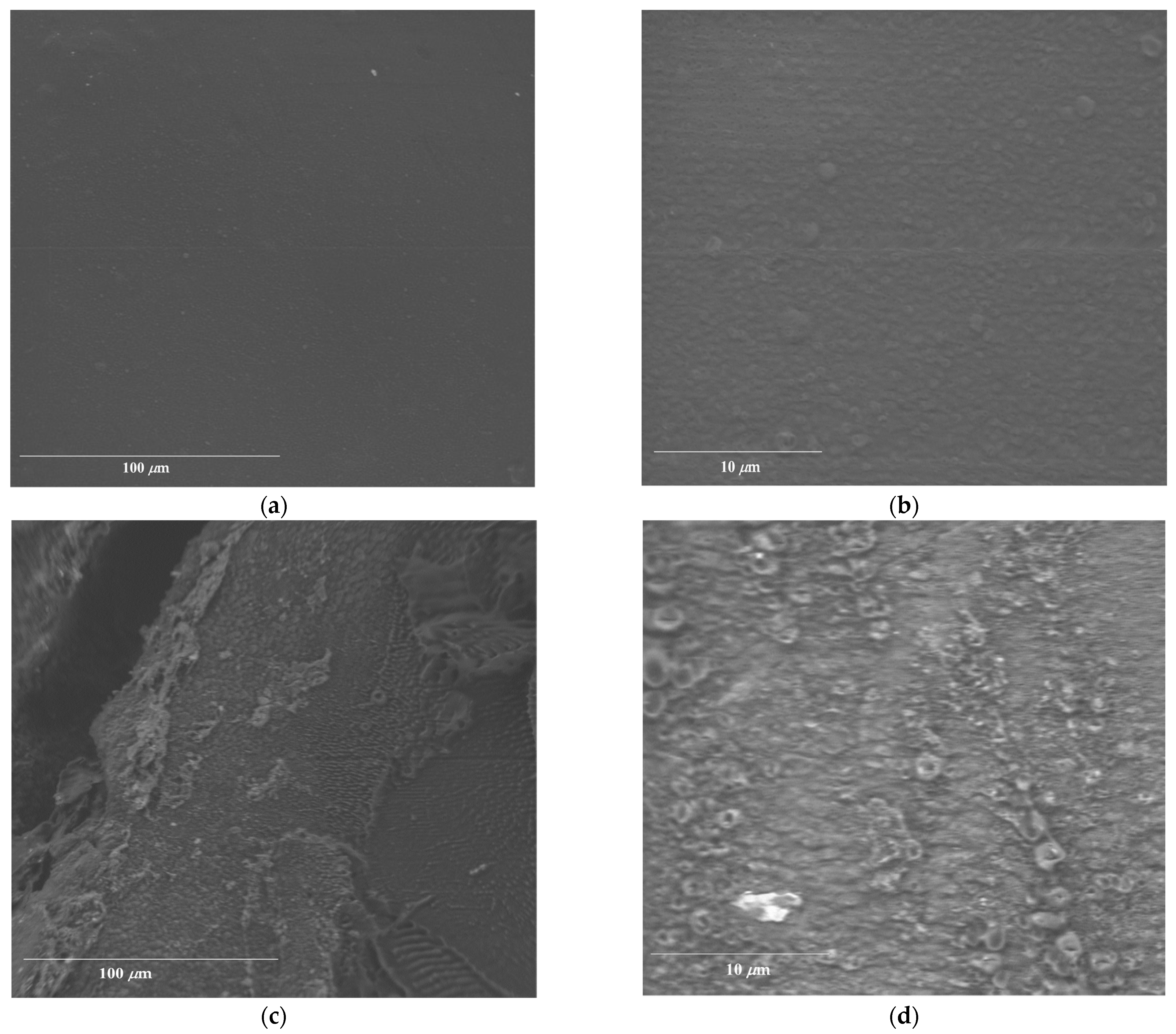
2.4. Morphology of AL-AVG Membranes
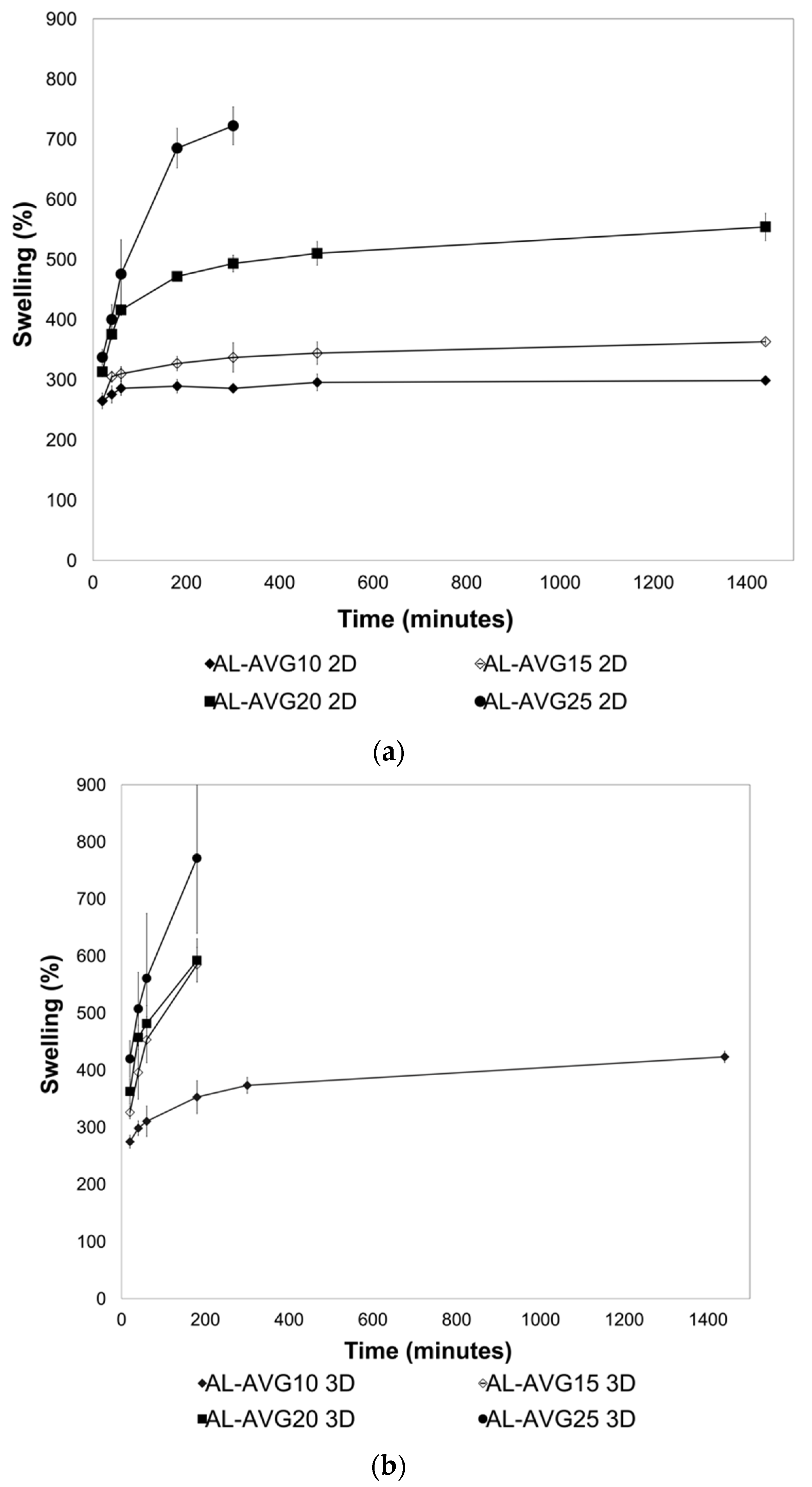
2.5. Swelling of AL-AVG Membranes
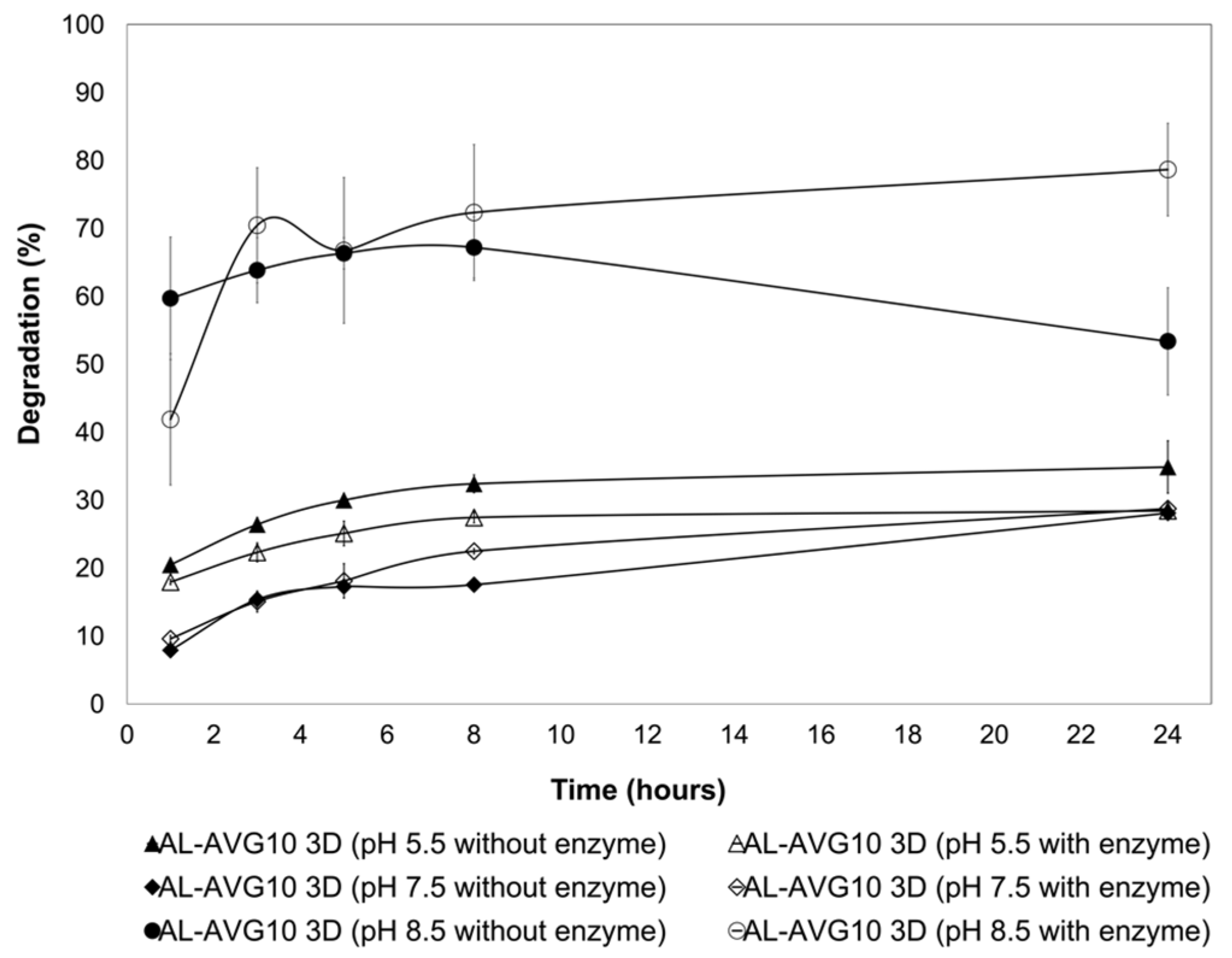
2.6. Degradation of AL-AVG Membranes
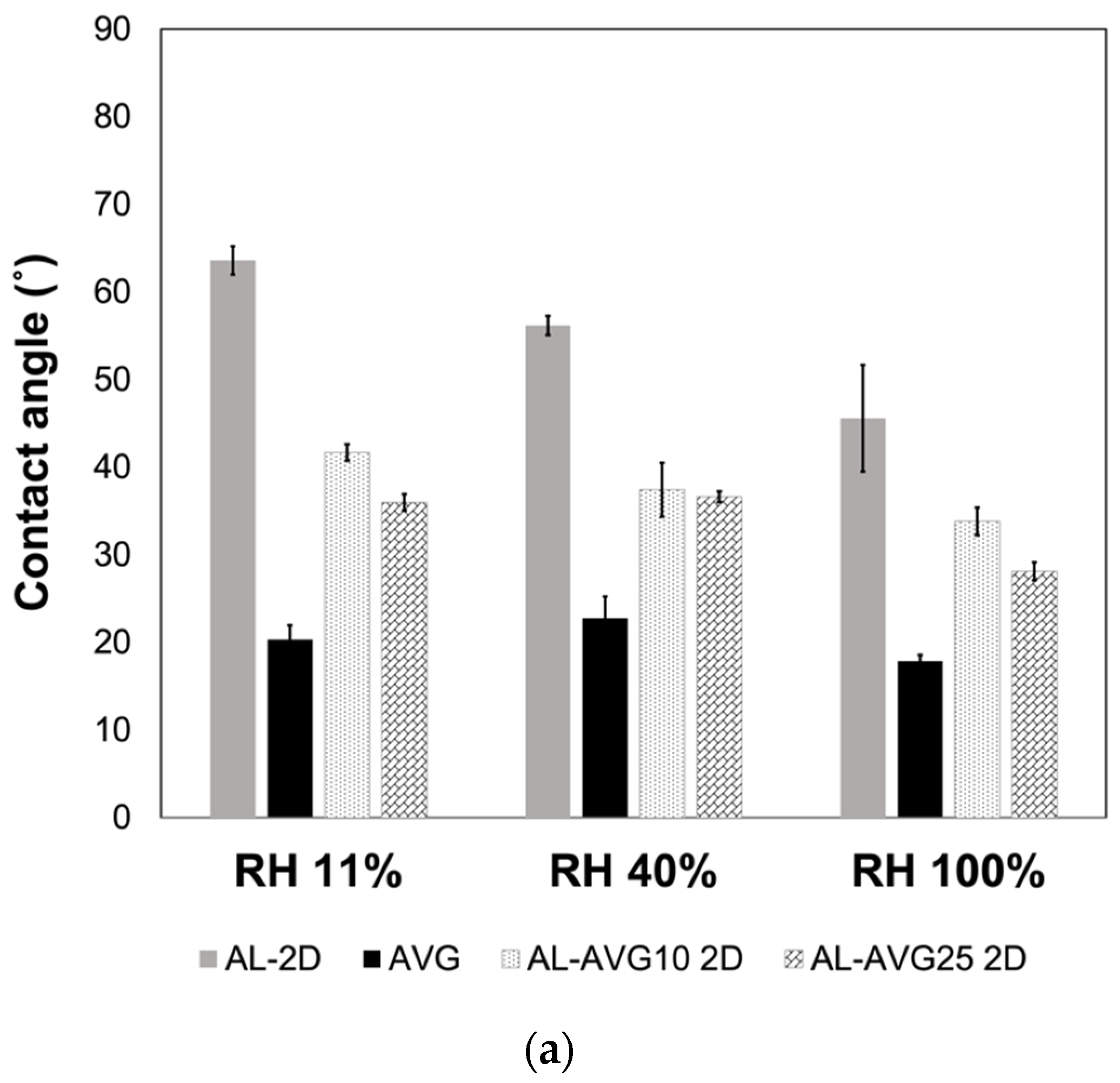
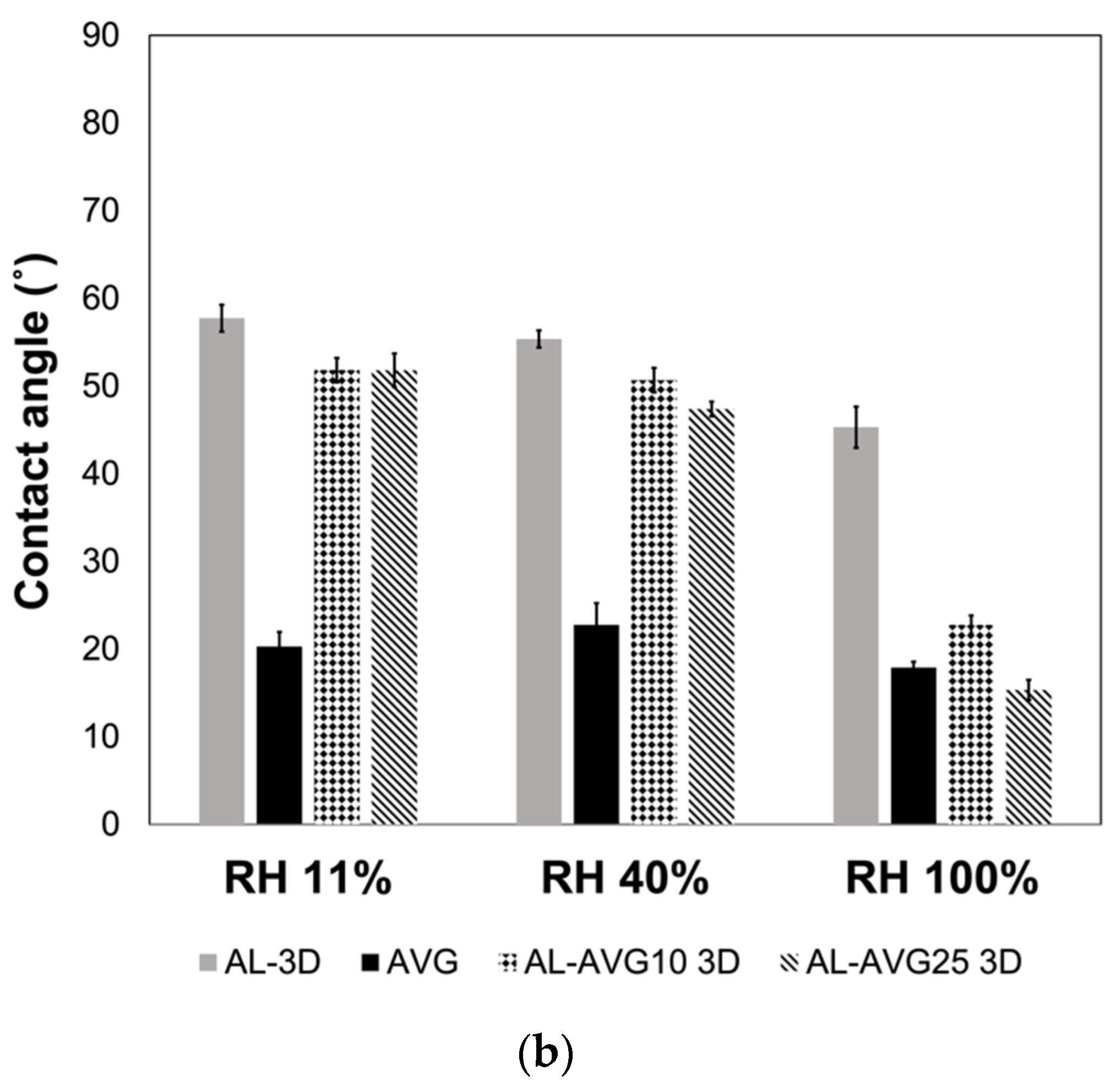
2.7. Surface Wettability of AL-AVG Membranes
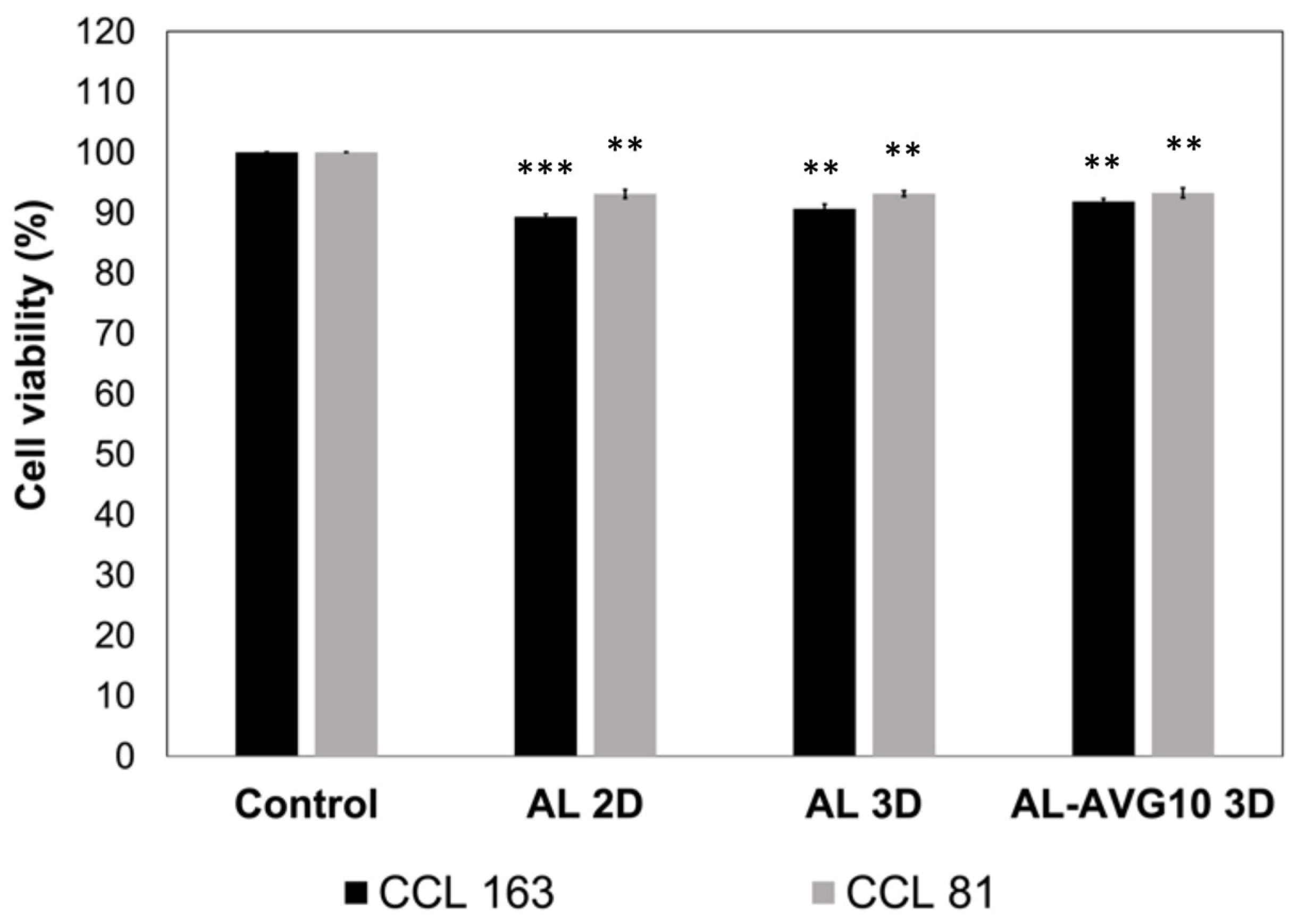
2.8. Cytotoxicity of AL-AVG Membranes
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Aloe Vera Gel (AVG) Preparation and Characterization
4.2.1. AVG Extraction
4.2.2. Isolation of Bioactive Polysaccharides of AVG
4.2.3. Evaluation of Total Water Content of AVG
4.2.4. Fourier-Transformed Infrared (FT-IR) Analysis
4.2.5. Gel Permeation Chromatography (GPC)
4.2.6. Antioxidant Activity Evaluated by ABTS Decoloration Assay
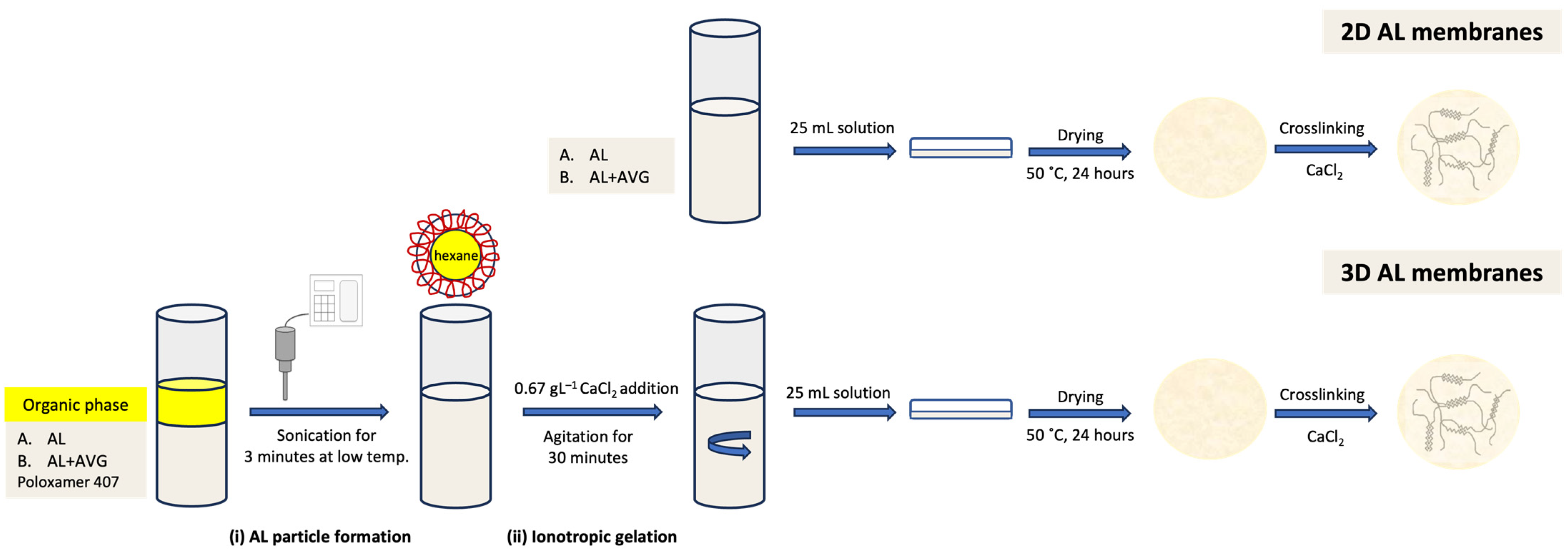
4.3. AL-AVG Membrane Preparation
4.4. Characterization of the AL-AVG Membranes
4.4.1. Thickness and Optical Transparency of AL-AVG Membranes
4.4.2. Surface Morphology
4.4.3. Swelling and Degradation of AL-AVG Membranes
4.4.4. Wettability of AL-AVG Membranes
4.4.5. Cytotoxicity
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheokand, B.; Vats, M.; Kumar, A.; Mohan, C.; Bahadur, I.; Pathak, S. Natural polymers used in the dressing materials for wound healing: Past, present and future. J. Polym. Sci. 2023, 61, 1389–1414. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/hyaluronic acid/alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing-know-how. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef]
- Diller, R.; Tabor, A. The role of the extracellular matrix (ECM) in wound healing: A review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Morton, L.; Phillips, T. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Rippon, M.; Davies, P.; White, R. Taking the trauma out of wound care: The importance of undisturbed healing. J. Wound Care 2012, 21, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Lr, B.; Miller, C.; Summers, R.H.; Minnis, A.; Sussman, G.; McGuiness, G. The pH of wounds during healing and infection: A descriptive literature review. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2017, 25, 63–69. [Google Scholar]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in research on the bioactivity of alginate oligosaccharides. Mar. Drugs. 2020, 18, 144. [Google Scholar] [CrossRef]
- Kim, S.; Nakamatsu, J.; Maurtua, D.; Oliveira, F. Formation, antimicrobial activity, and controlled release from cotton fibers with deposited functional polymers. J. Appl. Polym. Sci. 2016, 133, 43054. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Kim, S. Recent trends and notable advances of alginate based nano-particles for effective biomedical materials: Wound healing and drug delivery. Key Eng. Mater. 2023, 945, 27–32. [Google Scholar] [CrossRef]
- Kekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The effect of Aloe vera clinical trials on prevention and healing of skin wound: A systematic review. Iran. J. Med. Sci. 2019, 44, 1–9. [Google Scholar]
- Kumar, R.; Singh, A.; Gupta, A.; Bishayee, A.; Pandey, A. Therapeutic potential of Aloe vera—A miracle gift of nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Baruah, A.; Bordoloi, M.; Deka, H. Aloe vera: A multipurpose industrial crop. Ind. Crops Prod. 2016, 94, 951–963. [Google Scholar] [CrossRef]
- Rowe, T.; Parks, L. A phytochemical study of Aloe vera leaf. J. Am. Pharm. Assoc. 1941, 30, 262–266. [Google Scholar]
- Sumi, F.A.; Sikder, B.; Rahman, M.M.; Lubna, S.; Ulla, A.; Hossain, M.H.; Jahan, I.A.; Alam, M.A.; Subhan, N. Phenolic Content Analysis of Aloe vera Gel and Evaluation of the Effect of Aloe Gel Supplementation on Oxidative Stress and Fibrosis in Isoprenaline-Administered Cardiac Damage in Rats. Prev. Nutr. Food Sci. 2019, 24, 254–264. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ćirić, A.; Silva, S.; Coelho, E.; Mocan, A.; Calhelha, R.; Soković, M.; Coimbra, M.; et al. Compositional Features and Bioactive Properties of Aloe vera Leaf (Fillet, Mucilage, and Rind) and Flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Teplicki, E.; Ma, Q.; Castillo, D.; Zarei, M.; Hustad, A.; Chen, J.; Li, J. The effects of Aloe vera on wound healing in cell proliferation, migration, and viability. Wounds 2018, 30, 263–268. [Google Scholar] [PubMed]
- Massoud, D.; Alrashdi, B.; Fouda, M.; El-kott, A.; Soliman, S.; Abd-Elhafeez, H. Aloe vera and wound healing: A brief review. Braz. J. Pharm. Sci. 2022, 58, e20837. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, A.; Gil, M.H.; Mendes, A.; Bártolo, P. Influence of Aloe vera on water absorption and enzymatic in vitro degradation of alginate hydrogel films. Carbohydr. Polym. 2013, 98, 311–320. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, Purification, Structural Characteristics, Biological Activities and Pharmacological Applications of Acemannan, a Polysaccharide from Aloe vera: A Review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.; Mendes, A.; Bártolo, P. Preparation and characterization of films based on alginate and Aloe vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Chokboribal, J.; Tachaboonyakiat, W.; Sangvanich, P.; Ruangpornvisuti, V.; Jettanacheawchankit, S.; Thunyakitpisal, P. Deacetylation affects the physical properties and bioactivity of acemannan, an extracted polysaccharide from Aloe vera. Carbohydr. Polym. 2015, 133, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Bajer, D.; Janczak, K.; Bajer, K. Novel Starch/Chitosan/Aloe vera Composites as Promising Biopackaging Materials. J. Polym. Environ. 2020, 28, 1021–1039. [Google Scholar] [CrossRef]
- Miramon-Ortíz, D.; Argüelles-Monal, W.; Carvajal-Millan, E.; López-Franco, Y.; Goycoolea, F.; Lizardi-Mendoza, J. Acemannan Gels and Aerogels. Polymers 2019, 11, 330. [Google Scholar] [CrossRef]
- He, K.; Mergens, B.; Yatcilla, M.; Zheng, Q.; Bao, Z.; Zhang, Y.; Li, X.; Xie, Z. Molecular weight determination of Aloe polysaccharides using size exclusion chromatography coupled with multi-angle lase light scattering and refractive index detectors. J. AOAC Int. 2018, 101, 1729–1740. [Google Scholar] [CrossRef]
- Basha, S.; Muzammil, M.; Dhandayuthabani, R.; Kumari, V. Development of nanoemulsion of Alginate/Aloe vera for oral delivery of insulin. Mater. Today Proc. 2021, 36, 357–363. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Rodríguez-González, V.M.; González-Laredo, R.F.; Eim, V.; González-Centeno, M.R.; Fermenia, A. Effect of different drying procedures on the bioactive polysaccharide acemannan from Aloe vera (Aloe barbadensis Miller). Carbohydr. Polym. 2017, 168, 327–336. [Google Scholar] [CrossRef]
- Riyanto, R.; Wariyah, C. The antioxidative-activity stability of Aloe vera (Aloe vera var. chinensis) instant during storage. Food Res. 2021, 5, 288–293. [Google Scholar] [CrossRef]
- Rodríguez-González, V.M.; Femenia, A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Candelas-Cadillo, M.G.; Ramírez-Baca, P.; Simal, S.; Rosselló, C. Effects of pasteurization on bioactive polysaccharide acemannan and cell wall polymers from Aloe barbadensis Miller. Carbohydr. Polym. 2011, 86, 1675–1683. [Google Scholar] [CrossRef]
- Elgegren, M.; Kim, S.; Cordova, D.; Silva, C.; Noro, J.; Cavaco-Paulo, A.; Nakamatsu, J. Ultrasound-assisted encapsulation of Sacha Inchi (Plukenetia volubilis Linneo.) oil in alginate-chitosan nanoparticles. Polymers 2019, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Barbu, A.; Neamtu, M.; Zăhan, M.; Miresan, V. Trends in alginate-based films and membranes for wound healing. Rom. Biotechnol. Lett. 2020, 25, 1683–1689. [Google Scholar]
- Dodero, A.; Pianella, L.; Vicini, S.; Alloisio, M.; Ottonelli, M.; Castellano, M. Alginate-based hydrogels prepared via ionic gelation: An experimental design approach to predict the crosslinking degree. Eur. Polym. J. 2019, 118, 586–594. [Google Scholar] [CrossRef]
- Ayarza, J.; Coello, Y.; Nakamatsu, J. SEM–EDS study of ionically cross-linked alginate and alginic acid bead formation. Int. J. Polym. Anal. 2017, 22, 1–10. [Google Scholar] [CrossRef]
- Ursini, O.; Angelini, R.; Franco, S.; Cortese, B. Understanding the metal free alginate gelation process. RSC Adv. 2021, 11, 34449–34455. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; He, J.; Huang, Y. A new insight to the effect of calcium concentration on gelation process and physical properties of alginate films. J. Mater. Sci. 2016, 51, 5791–5801. [Google Scholar] [CrossRef]
- Raju, R.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.; Achar, R. Multifunctional and smart wound dressings–A review on recent research advancements in skin regenerative medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef]
- Borbolla-Jiménez, F.; Peña-Corona, S.; Farah, S.; Jiménez-Valdés, M.; Pineda-Pérez, E.; Romero-Montero, A.; Del Prado-Audelo, M.; Berna-Chávez, S.; Magaña, J.; Leyva-Gómez, G. Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics 2023, 15, 1914. [Google Scholar] [CrossRef]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe vera hydrogel films for biomedical applications. Procedia CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- Barbu, A.; Neamtu, B.; Zăhan, M.; Iancu, G.; Bacila, C.; Miresan, V. Current trends in advanced alginate-based wound dressings for chronic wounds. J. Pers. Med. 2021, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Alves-Lima, D.; Lin, H.; Douglas, T.; Kuciel, S.; Zagórska, A.; Tyliszczak, B. Investigations on the impact of the introduction of the Aloe vera into the hydrogel matrix on cytotoxic and hydrophilic properties of these systems considered as potential wound dressings. Mater. Sci. Eng. C 2021, 123, 111977. [Google Scholar] [CrossRef] [PubMed]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Tegl, G.; Rollett, A.; Dopplinger, J.; Gamerith, C.; Guebitz, G. Chitosan based substrates for wound infection detection based on increased lysozyme activity. Carbohydr. Polym. 2020, 151, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Khémiri, I.; Essghaier, B.; Sadfi-Zouaoui, N.; Bitri, L. Antioxidant and antimicrobial potentials of seed oil from Carthamus tinctorius L. in the management of skin injuries. Oxid. Med. Cell Longev. 2020, 2020, 4103418. [Google Scholar] [CrossRef]
- Ercan, D.; Demirci, A. Recent advances for the production and recovery methods of lysozyme. Crit. Rev. Biotechnol. 2016, 36, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Garcez, S.; Cavalcanti, R.; Albuquerque, L.; de Albuquerque, D.; de Souza, A.; Paranhos, L.; Afonso, S.; Gonzaga, M. Analysis of Aloe vera cytotoxicity and genotoxicity associated with endodontic medication and laser photobiomodulation. J. Photochem. Photobiol. B Biol. 2018, 178, 348–354. [Google Scholar] [CrossRef]
- Silva, S.; Caridade, S.; Mano, J.; Reis, R. Effect of crosslinking in chitosan/Aloe vera-based membranes for biomedical applications. Carbohydr. Polym. 2013, 98, 581–588. [Google Scholar] [CrossRef]
- Salah, F.; Ghoul, Y.E.; Mahdhi, A.; Majdoub, H.; Jarroux, N.; Sakli, F. Effect of the deacetylation degree on the antibacterial and antibiofilm activity of acemannan from Aloe vera. Ind. Crops Prod. 2017, 103, 13–18. [Google Scholar] [CrossRef]
- Turner, C.; Williamson, D.; Stroud, P.; Talley, D. Evaluation and comparison of commercially available Aloe vera L. products using size exclusion chromatography with refractive index and multi-angle laser light scattering detection. Int. Immunopharmacol. 2004, 4, 1727–1737. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Liling, G.; Di, Z.; Jiachao, X.; Xin, G.; Xiaoting, F.; Qing, Z. Effects of ionic crosslinking on physical and mechanical properties of alginate mulching films. Carbohydr. Polym. 2016, 136, 259–265. [Google Scholar] [CrossRef]
- Ching, S.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Costa, M.; Marques, A.; Pastrana, L.; Teixeira, J.; Sillankorva, S.; Cerqueira, M. Physicochemical properties of alginate-based films: Effects of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef]
- Norajit, K.; Kim, K.; Ryu, G. Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Amin, M.; Ng, S. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]
- ISO 10993–5:2009; Standard Method: Biological Evaluation of Medical Devices—Part 5: Test for In Vitro Cytotoxicity. Third edition; International Organization for Standardization (ISO): Geneva, Switzerland. Available online: https://www.iso.org/standard/36406.html (accessed on 1 June 2023).
| AVG | Acemannan | |
|---|---|---|
| Mn (kDa) | 34.7 | 3.3 |
| Mw (kDa) | 45.3 | 22.2 |
| Polydispersity (Mw/Mn) | 1.31 | 6.83 |
| Before | After | |||
|---|---|---|---|---|
| Film | Thickness (µm) | Transparency | Thickness (µm) | Transparency |
| AL-AVG10 | 28.00 ± 1.01 | 1.78 ± 0.03 | 27.83 ± 0.71 | 2.24 ± 0.27 |
| AL-AVG15 | 25.42 ± 1.06 | 1.98 ± 0.09 | 25.17 ± 0.94 | 3.03 ± 0.22 |
| AL-AVG20 | 25.92 ± 1.06 | 1.95 ± 0.11 | 24.23 ± 0.82 | 2.57 ± 0.48 |
| AL-AVG25 | 25.11 ± 0.42 | 2.02 ± 0.03 | 23.22 ± 0.92 | 2.76 ± 0.63 |
| Before | After | |||
|---|---|---|---|---|
| Film | Thickness (µm) | Transparency | Thickness (µm) | Transparency |
| AL-AVG10 | 30.11 ± 0.84 | 4.30 ± 0.25 | 21.39 ± 0.25 | 5.71 ± 0.62 |
| AL-AVG15 | 27.92 ± 0.12 | 3.97 ± 0.33 | 25.25 ± 2.24 | 3.60 ± 0.22 |
| AL-AVG20 | 28.58 ± 1.06 | 4.53 ± 0.02 | 20.08 ± 0.59 | 4.56 ± 0.25 |
| AL-AVG25 | 21.83 ± 0.47 | 4.34 ± 0.03 | 18.42 ± 0.35 | 4.17 ± 0.03 |
| Sample | AL 1% (% v/v) | AVG (% v/v) |
|---|---|---|
| AL | 100 | - |
| AL-AVG10 | 90 | 10 |
| AL-AVG15 | 85 | 15 |
| AL-AVG20 | 80 | 20 |
| AL-AVG25 | 75 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgegren, M.; Nakamatsu, J.; Galarreta, B.; Kim, S. Three-Dimensional Membranes of Natural Polymer Complex Nanoparticle for Potential Medical Applications. Gels 2023, 9, 847. https://doi.org/10.3390/gels9110847
Elgegren M, Nakamatsu J, Galarreta B, Kim S. Three-Dimensional Membranes of Natural Polymer Complex Nanoparticle for Potential Medical Applications. Gels. 2023; 9(11):847. https://doi.org/10.3390/gels9110847
Chicago/Turabian StyleElgegren, Mariela, Javier Nakamatsu, Betty Galarreta, and Suyeon Kim. 2023. "Three-Dimensional Membranes of Natural Polymer Complex Nanoparticle for Potential Medical Applications" Gels 9, no. 11: 847. https://doi.org/10.3390/gels9110847
APA StyleElgegren, M., Nakamatsu, J., Galarreta, B., & Kim, S. (2023). Three-Dimensional Membranes of Natural Polymer Complex Nanoparticle for Potential Medical Applications. Gels, 9(11), 847. https://doi.org/10.3390/gels9110847







