Abstract
Gel-based membranes, a fusion of polymer networks and liquid components, have emerged as versatile tools in a variety of technological domains thanks to their unique structural and functional attributes. Historically rooted in basic filtration tasks, recent advancements in synthetic strategies have increased the mechanical strength, selectivity, and longevity of these membranes. This review summarizes their evolution, emphasizing breakthroughs that have positioned them at the forefront of cutting-edge applications. They have the potential for desalination and pollutant removal in water treatment processes, delivering efficiency that often surpasses conventional counterparts. The biomedical field has embraced them for drug delivery and tissue engineering, capitalizing on their biocompatibility and tunable properties. Additionally, their pivotal role in energy storage as gel electrolytes in batteries and fuel cells underscores their adaptability. However, despite monumental progress in gel-based membrane research, challenges persist, particularly in scalability and long-term stability. This synthesis provides an overview of the state-of-the-art applications of gel-based membranes and discusses potential strategies to overcome current limitations, laying the foundation for future innovations in this dynamic field.
1. Introduction
The concept of membranes can be traced back to ancient civilizations, where simple forms of filtration using natural materials like sand, gravel, and charcoal were employed to purify water [1]. The 19th century saw the birth of dialysis; in 1861, Thomas Graham described the process of separating dissolved substances using “parchment or some analogous membrane” [2]. In the early 20th century, researchers began to use cellulose-based materials to make membranes. In the 1960s, the development of asymmetric cellulose acetate membranes led to the commercialization of the reverse osmosis process, a significant breakthrough for desalination and water purification. Around the same time, ultrafiltration membranes were developed using new polymer materials, enhancing their ability to separate macromolecules. In the latter half of the 20th century, there was significant interest in gas separation [3]. Membranes became a focus for separating gases such as oxygen and nitrogen from air. With the advancement of nanotechnology, new materials like zeolites, metal-organic frameworks (MOFs), and carbon-based materials (e.g., graphene oxide) started being explored for membrane fabrication. Membranes began to be designed not just for separation but also for additional functions such as catalysis, sensing, and antimicrobial activity. Given the increasing concerns over environmental pollution, there was a rising interest in membranes for wastewater treatment, desalination, and other sustainable applications [4,5].
The exploration of membranes for separation processes began with traditional materials like ceramics and polymers. As technology advanced, researchers sought materials that offered more flexibility, adaptability, and specificity. The development of hydrogels, comprising polymer chains crosslinked in a way to retain significant amounts of water, provided a foundation for a new type of membrane. When these hydrogels were further engineered to have controlled pore sizes and mechanical stability, they emerged as viable membrane materials [6,7,8].
The main advantages of the gel-based membranes are their adaptability to different environments due to their tunable nature, better selectivity due to controlled pore sizes, enhanced flux because of their higher water content, and improved biocompatibility, which makes them ideal for biological and medical applications [9,10]. As the search for efficient, adaptable, and sustainable materials in separation technologies intensifies, gel-based membranes have emerged as a beacon of innovation, bridging the gap between functionality and environmental responsibility. The importance of gel-based membranes can be found in multiple areas, such as biomedical applications, water treatment, environmental remediations and energy, durability and stability, sensing and detection, and economic and environmental impact [11,12].
Figure 1 shows the publication trend (2000–2022) for the applications of the “gel-based membranes” field. There are multiple applications of gel-based membranes, spanning various fields like water treatment, food application, biomedical application, and energy and environment applications. Their high water content and tunable pore sizes make them especially useful for water purification, including desalination and contaminant removal [13]. Many gel-based membranes are constructed from biopolymers or are designed to be biocompatible, making them particularly suitable for biomedical applications like drug delivery, tissue engineering [14], and hemodialysis [10]. Gel-based membranes made from biopolymers are increasingly being recognized for their potential in wound healing applications. Being derived from natural sources, biopolymers are typically biocompatible, meaning they are less likely to cause adverse reactions when in contact with body tissues [15,16,17,18].
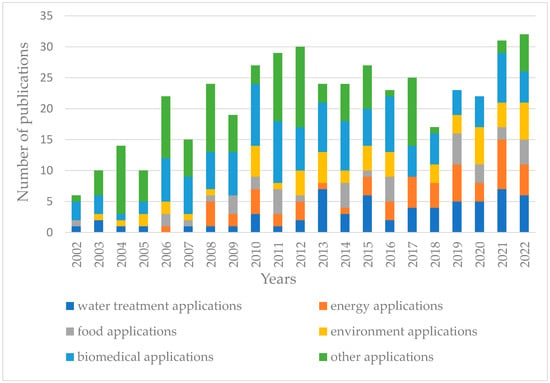
Figure 1.
Publication trend (2000–2022) for the applications of the “gel-based membranes” field. (Source of raw data: Document Search - SciELO Citation Index (webofscience.com), accessed on 15 October; search keywords: “gel-based membranes applications”).
Their ability to house functional groups and change properties in response to specific stimuli also made them suitable for sensing applications [19,20]. In the energy sector, gel-based electrolytes became pivotal in batteries and fuel cells. Their advantages regarding adaptability and ionic conductivity compared to solid electrolytes were significant [21,22]. Their selectivity and adaptability made them good candidates for air purification, carbon capture, and hazardous waste containment [23,24]. In addition to having the flexibility of gels, these membranes were enhanced to maintain their mechanical stability, making them reliable for their long-term use [25]. Due to their efficient separation and low energy requirements, gel-based membranes have the potential to offer sustainable solutions with reduced environmental footprints [26].
This review paper presents a state-of-the-art analysis of advances and current applications of gel-based membranes by highlighting the latest developments and bridging the gaps in existing research by introducing a novel perspective and providing a comprehensive multidisciplinary approach. It not only synthesizes the current body of knowledge but also paves the way for future innovations in different fields (e.g., medical, environmental, and industrial).
2. Fundamental Properties of Gel-Based Membranes
Understanding the composition and structure of gel-based membranes is crucial as these factors directly influence their performance in various applications. The versatility in design and adaptability of these membranes comes from the ability to tweak these fundamental properties, which can lead to their widespread utilization in diverse technological fields [9]. Most gel-based membranes are made from a polymeric backbone. This could be natural polymers like agarose, chitosan, and alginate, or synthetic polymers like polyvinyl alcohol (PVA), polyacrylamide (PAM), and polyethylene glycol (PEG). To provide stability and determine the mechanical properties of the gel, crosslinking agents are added. Examples include glutaraldehyde for PVA or N,N’-methylenebisacrylamide for PAM. Depending on the intended use, specific functional monomers can be added to the gel composition, providing specialized properties like increased hydrophilicity, ionic conductivity, or reactive sites for further modifications [27,28,29]. To tailor specific properties or enhance functionality, various additives can be integrated, such as nanoparticles, salts, or organic solvents. At the microscopic level, gel-based membranes exhibit a porous network structure [30,31]. The size and distribution of these pores are crucial determinants of the membrane’s selectivity and permeability. Due to the high water content, these membranes usually exist in a swollen state, where water molecules are trapped within the polymeric matrix. This makes them highly hydrophilic, which in turn affects their permeability and rejection characteristics. The density and nature of the cross-linking play pivotal roles in determining the mechanical strength and flexibility of the membrane [32,33]. A higher cross-linking density usually leads to a stiffer but less swellable membrane. Advanced gel-based membranes might have multiple layers or a composite structure, each layer offering a specific function. For instance, a composite membrane might have a highly selective skin layer and a supportive and mechanically robust sublayer. Gel-based membranes have distinct characteristics that set them apart from other conventional membrane types [34,35]. Due to their gelatinous nature, these membranes exhibit a high degree of swelling, allowing them to retain significant amounts of water. This feature not only enhances their hydrophilicity but also improves their permeation characteristics, especially in aqueous environments [36]. The properties of gel-based membranes, such as pore size, mechanical strength, and chemical functionality, can be easily tailored during the synthesis process by varying the type of monomer, cross-linking agent, and polymerization conditions [37,38]. Some gel-based membranes have the unique ability to heal themselves after being damaged, owing to their dynamic cross-linked network [39,40]. Also, gel-based membranes can be engineered to respond to external stimuli such as pH, temperature, or ionic strength. This responsive behavior can be leveraged for controlled substance release or selective separation applications [41,42]. Unlike rigid ceramic membranes or certain polymeric membranes, gel-based membranes are inherently soft and flexible, which can reduce the risk of fracturing under mechanical stress [43,44]. Despite their soft nature, many gel-based membranes exhibit remarkable stability under various thermal and chemical conditions, especially when designed with specific cross-linkers or additives [45,46]. Also, the high water content and hydrophilic nature of gel-based membranes can reduce the affinity of foulants to the membrane surface, leading to decreased fouling tendencies in applications like water purification [47,48]. Some gel-based membranes, especially those integrated with specific nanoparticles or dyes, can showcase tunable optical properties, which are useful in sensing and detection applications [49]. These distinct properties make gel-based membranes versatile and adaptable to a plethora of applications. Their ability to combine the strengths of both polymeric and ceramic membranes, along with their unique features, underpins their growing significance in the field of advanced separation and filtration technologies.
3. Advances in Gel-Based Membrane Technology
The modern synthesis techniques are not only more efficient but also allow for greater tunability and specificity of the membrane properties [50,51,52]. Some key advances in the synthesis methods for gel-based membranes are presented in Figure 2.
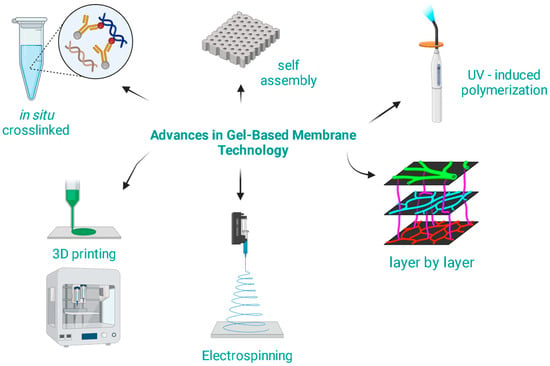
Figure 2.
Advances in gel-based membrane technology.
- (a)
- Interfacial polymerization involves the reaction of two immiscible solutions at their interface, leading to the formation of a thin, dense polymeric layer [47]. By adjusting the monomers and conditions, one can tailor the properties of the resulting gel-based membrane. Yuan et al. [48] introduced a novel method for creating thin film composite nanofiltration membranes to tackle global water shortages exacerbated by population growth and water pollution. This technique utilized hydrogel-assisted interfacial polymerization, which incorporated piperazine monomers within a hydrogel that acted as the aqueous phase. This approach facilitated a more uniform polymerization process at the interface, slowed the diffusion of piperazine monomers, and enhanced the mechanical robustness of the resulting polyamide selective layer. Furthermore, the underlying principles contributing to the high permeance were investigated through theoretical simulations of the hydrogel-assisted interfacial polymerization. Significantly, the production method for these hydrogel-thin film composite membranes was straightforward, suggesting potential for cost-effective and scalable manufacturing, which is crucial for widespread application in addressing water scarcity challenges [53,54].
- (b)
- Electrospinning allows for the creation of membranes with high porosity and adjustable fiber diameters. By applying a high voltage to a polymer solution, fibers can be drawn and deposited on a substrate, resulting in nanofibrous gel-based membranes [55,56]. Al-Baadani et al. [55] explored polycaprolactone/gelatin (PCL/Gel) composite membranes, assessing their biocompatibility and controlled drug release capabilities for the first time. Gelatin enhanced osteoblast adhesion and differentiation, while polycaprolactone improved mechanical durability. The study found that adjusting the amount and size of PCL fibers can control the degradation of gelatin and the release patterns of hydrophilic drugs or proteins. Lower PCL content led to a quick dissolution of gelatin fibers and a rapid drug release within one week. Conversely, higher PCL levels slowed the release rate, extending the duration to over two weeks, which could be beneficial for bone regeneration applications. The findings indicate that PCL/Gel composite membranes fabricated via co-electrospinning could be an effective method for creating customizable drug delivery systems that support bone healing.
- (c)
- Layer-by-Layer (LbL) assembly uses the alternate deposition of positively and negatively charged polymers, building up multi-layered structures. The method allows for the precise control of membrane thickness and functionality. LbL techniques can help in crafting multilayered membranes, where each layer can cater to a specific separation requirement, offering improved overall selectivity. In a review paper, Liu et al. [57] focused on multi-layered hydrogels, which are preferred materials for biomedical applications due to their organized functional layers. It compiled recent advancements in multi-layered hydrogels, categorizing them based on their fabrication techniques such as layer-by-layer self-assembly, stepwise, photo-polymerization, and sequential electrospinning. The review also examined the morphology of these hydrogels and their various biomedical uses. It concluded by addressing the current challenges faced in the development of multi-layered hydrogels and suggested that 3D printing technology may offer innovative solutions for designing these materials, potentially broadening their biomedical applications.
- (d)
- During in situ cross-linking, the membrane is formed by introducing cross-linking agents directly into the polymer solution or gel, triggering cross-linking in place. This can provide enhanced mechanical stability and tailor the swelling behavior of the gel-based membrane [58,59]. Leone et al. [53] studied the production of such hydrogels designed for wound healing, particularly in treating complex blast injuries. In situ-forming hydrogels are emerging as versatile biomaterials for patient-specific biomedical applications like cell therapy and drug delivery. Researchers developed bionanocomposite hydrogels by integrating oxidized polysaccharides (which have aldehyde groups), chitosan (rich in amine groups), and nanostructured zinc oxide. They thoroughly examined the physicochemical properties of these components, their cytotoxicity towards HaCat skin cells, and the release profile of zinc ions on synthetic skin models. The hydrogels formed rapidly in situ, exhibited no toxicity at functional levels for HaCat cells, and successfully released Zn2+ ions, indicating the potential to promote wound healing.
- (e)
- UV-induced polymerization uses ultraviolet light to initiate polymerization and cross-linking within the gel precursor. It offers rapid synthesis and the potential for spatial control over membrane properties [60,61]. Siccardi et al. [55] introduced a novel approach using UV-induced, solvent-free radical copolymerization to create a solid polymer electrolyte with enhanced properties. Solid polymer electrolytes offer a safer alternative for lithium metal batteries, but their practical use is hindered by low ionic conductivity and poor cyclability at room temperature. When activated with a small amount of liquid electrolyte, these polymers demonstrated high thermal resistance, good lithium-ion conductivity, and a wide electrochemical window. The polymers exhibited excellent interfacial stability, enabling stable lithium metal plating and stripping at room temperature. This research led to a significant advancement toward developing safer, room-temperature operable, self-healing quasi-solid-state lithium metal batteries.
- (f)
- With the self-assembly method, some gel-based systems can spontaneously organize into structured networks through non-covalent interactions like hydrogen bonding, electrostatic forces, or π-π stacking. This bottom-up approach can lead to membranes with highly ordered nanostructures [62,63]. Braun et al. [57] investigated the self-assembly of the hydrogel-forming peptide, revealing the microscopic steps of its formation. By applying theoretical models of linear polymerization to kinetic data, it was discovered that peptide fibril formation is predominantly driven by fibril-catalyzed secondary nucleation. Furthermore, the peptide’s self-assembly processes exhibited enzyme-like saturation, indicating that they are not simply chemical reactions but are regulated in a manner like biological systems. The study quantified the rates of these processes at various concentrations and the evolution of these rates throughout the assembly. This novel mechanistic approach, distinct from traditional material science methods, could lead to more a sophisticated design and application of self-assembling hydrogels in medicine.
- (g)
- Microfluidic fabrication allows for the controlled synthesis of gel particles or fibers with uniform sizes and shapes. When these are used to fabricate membranes, they can offer highly reproducible transport properties [64,65]. Correa et al. [58] explored the biofabrication of stable, aligned collagen hydrogels within microfluidic devices, aiming to improve tissue and organ models for extended culture times. Collagen-alginate microgels were created by cross-linking with calcium ions within a microfluidic channel, using a chitosan membrane to allow ion diffusion without convection. These gels formed rapidly into isolated structures and their growth was self-regulating. By adjusting the calcium concentration and the flow rate of the collagen-alginate solution, gel thickness could be precisely controlled between 30 and 200 μm. Less calcium and higher flow rates resulted in more compressed gels, especially further from the pores. The study also showed that the gels allowed size-dependent diffusion of molecules, making them suitable for on-chip models that require the invasion of a dense extracellular matrix, cancer growth, and targeted drug delivery. This demonstrates the potential of controlled physicochemical parameters for collagen gel formation in microfluidic applications.
- (h)
- Incorporation of nanomaterials: Modern synthesis methods have adapted to integrate various nanomaterials like metal nanoparticles, carbon nanotubes, or graphene oxide into the gel matrix. This enhances the functional properties of the membrane, from improved mechanical strength to specialized separation characteristics [66,67].
- (i)
- 3D Printing techniques are being explored to create gel-based membranes with intricate structures, customizable geometries, and multi-functional regions [68,69]. Tayebi et al. [63] highlighted the creation of scaffolds designed for the cultivation of full-thickness oral mucosa, representing a type of heterogeneous tissue. By exploring these dimensions, the paper provided insights into the nuanced production of biologically relevant models using 3D printing technology, which holds potential for advancing tissue engineering and regenerative medicine. Biological membranes, while seemingly two-dimensional, possess intricate structures extending into the third dimension. Three-dimensional printing, particularly through layer-by-layer assembly, emerged as a sophisticated technique for crafting models that embody this complexity. Nonetheless, printing certain hydrogels like gelatin can be challenging due to their unique rheological properties. The authors tackled these challenges by analyzing the complexities of 3D printing gelatin, proposing a reproducible method to surmount the associated experimental hurdles, and detailing the design specifications and fabrication process for 3D printed gelatin membranes.
These modern synthesis methods underscore the versatility and adaptability of gel-based membrane technology. By leveraging these techniques, researchers can design membranes for specific applications with unprecedented precision, paving the way for innovations across diverse industrial domains.
The advancements in gel-based membrane technology have ushered in significant enhancements in mechanical strength, selectivity, and longevity. The density and nature of cross-linking have been optimized to provide higher mechanical robustness [70]. For instance, dual cross-linking, involving both physical and chemical bonds, can significantly boost strength [71]. The introduction of nanofillers like silica nanoparticles, carbon nanotubes, or graphene oxide can reinforce the gel matrix, leading to improved tensile strength and toughness [72]. Creating hybrid structures by incorporating both organic and inorganic components can marry the flexibility of polymers with the rigidity of inorganic materials, leading to enhanced mechanical properties [73]. Advanced synthesis methods allow for precise control over pore sizes, enabling high selectivity based on the size of molecules or ions. The introduction of specific functional groups or ligands can enhance selectivity based on chemical interactions, such as hydrogen bonding, electrostatic attractions, or even affinity-based separations [74]. Incorporation of hydrophilic groups or zwitterionic components can provide antifouling properties, reducing membrane fouling and thereby extending its lifespan for applications. Chemical modifications or the inclusion of stabilizing agents can render the membranes more resistant to harsh conditions like extreme pH, high temperatures, or aggressive solvents [75]. Some modern gel-based membranes are imbued with self-healing capabilities, where minor damage can be auto-repaired, thus prolonging their operational lifespan [76,77]. The addition of protective coatings or layers can shield the membrane from mechanical abrasions, aggressive chemicals, or microbial attacks, thereby extending its durability [78]. The development of efficient cleaning-in-place (CIP) and maintenance protocols has further bolstered the longevity of gel-based membranes in industrial applications [79].
These enhancements in mechanical strength, selectivity, and longevity underline the commitment of researchers and industries to optimize gel-based membrane technology. Such improvements ensure that these membranes can meet the rigorous demands of contemporary applications while maintaining operational efficiency over extended periods. Incorporating nanoparticles or functional groups into membranes has provided an avenue to tailor membrane properties to specific needs [80]. This customizable approach ensures that the resultant membranes are not only more efficient but also versatile in handling a wide range of applications. For example, by incorporating metal and metal oxide nanoparticles like silver (Ag), gold (Au), titanium dioxide (TiO2), and zinc oxide (ZnO), they can impart antimicrobial properties, improve thermal stability, and enhance mechanical strength [81,82,83]. Also, incorporating magnetic nanoparticles is useful in applications where remote actuation or controlled movement is required, like in drug delivery or targeted separations. Nanoparticles can add size-exclusion properties or even specific affinity interactions that can enhance the selectivity of membranes [84]. Certain nanoparticles can improve the porosity and hydrophilicity of the membranes, leading to improved permeation rates [85]. Incorporating groups like -OH, -COOH, or -NH2 can also enhance the hydrophilicity of the membrane, reducing fouling and improving water flux. Zwitterionic Groups have both positive and negative charges, which can greatly reduce protein or microbial fouling due to their unique surface properties [86].
Functional groups like epoxy, carboxyl, or amine can allow for further modifications, tethering of other molecules, or even specific interactions with target substances [87].
The introduction of charged moieties (like sulfonic or quaternary ammonium groups) can enhance the ionic selectivity of the membrane, useful in processes like desalination or ion exchange [88]. Functional groups that have a specific affinity for certain contaminants (e.g., chelating agents for heavy metal capture) can be introduced into membranes to provide selectivity based on chemical interactions [89].
The integration of nanoparticles and functional groups into water treatment membranes has set a new benchmark for efficiency, selectivity, and sustainability. These advanced membranes not only ensure cleaner water but also promise more energy-efficient and eco-friendly water treatment solutions. Gel-based membranes, with their unique properties and adaptability, hold great potential in revolutionizing water treatment processes, providing both efficiency and sustainability [90].
4. Applications of Gel-Based Membranes
One of the defining characteristics of gel-based membranes is their unique combination of elasticity and mechanical strength. This combination allows for potential self-healing properties and resistance to physical damage [91]. The hydrophilic and smooth nature of gel-based membranes can lead to reduced biofouling and organic fouling, enhancing the membrane’s lifespan [11]. The porous structure of gel-based membranes can be finely tuned to achieve the desired permeability and selectivity [92]. Gel-based membranes, especially those derived from natural polymers, are often biocompatible, making them suitable for applications where avoiding toxic by-products is essential [93]. Figure 3 shows some current applications of gel-based membranes.
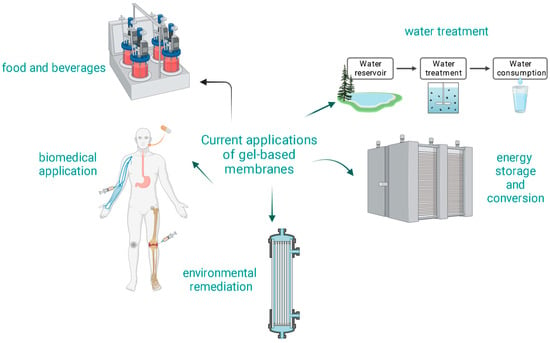
Figure 3.
Current applications of gel-based membranes.
4.1. Applications in Water Treatment
By embedding nanoparticles or other agents, gel-based membranes can be endowed with multiple functionalities, enabling them to perform several tasks simultaneously, like separation and catalysis [94].
Gel-based membranes for water treatment combine the advantages of hydrophilicity, selectivity, and anti-fouling properties, making them highly effective for various water purification needs [11,95]. Gel-based membranes are typically synthesized from hydrophilic polymers. Common choices include polyvinyl alcohol (PVA), polyethylene glycol (PEG), and various polysaccharides. To form a stable gel structure, these polymers are often cross-linked. Chemical cross-linkers like glutaraldehyde or physical methods such as UV irradiation can be used. Their structure allows for the selective permeation of water molecules while retaining contaminants like heavy metals, organic pollutants, or pathogens. The hydrophilic nature helps resist fouling, a common challenge in membrane-based water treatment. These membranes must be chemically stable in various water conditions, including different pH levels and the presence of various contaminants. While gel membranes are flexible, they need sufficient mechanical strength to withstand operational pressures in water treatment systems. Their design and synthesis are tailored to the specific requirements of the water treatment application, such as desalination (removing salts to produce potable water from seawater or brackish water), pollutant removal (effective in trapping heavy metals like lead, arsenic, and mercury from industrial wastewater), removal of organic compounds (e.g., endocrine-disrupting chemicals, pharmaceuticals, and personal care products), pathogen filtration (with the possibility to be tailored for the removal of bacteria, viruses, and other pathogens), and reduction of hardness-causing ions like calcium and magnesium [96,97,98,99,100,101].
Table 1 presents some applications of gel-based membranes in water treatment.

Table 1.
Applications of Gel-Based Membranes in Water Treatment.
4.2. Biomedical Applications of Gel-Based Membranes
The adaptability and intrinsic properties of gel-based membranes make them immensely suitable for a range of biomedical applications. Gel-based membranes made from biopolymers are increasingly being recognized for their potential in wound healing applications. Examples of biopolymers used in these membranes include collagen, chitosan, alginate, and hyaluronic acid, among others [102]. Each of these has unique properties, making them suitable for different wound healing applications, from minor cuts and abrasions to more severe burns and surgical wounds. These membranes offer several advantages like biocompatibility, moisture maintenance, permeability, natural degradation, drug delivery, and structural and mechanical properties [103,104]. Biopolymers are often biodegradable, meaning they can break down naturally in the body, eliminating the need for removal and reducing the risk of chronic inflammation [105]. Gel-based biopolymer membranes can maintain a moist environment, which is beneficial for wound healing [106]. A moist environment can facilitate cell migration and proliferation, essential for tissue regeneration. These membranes are often semi-permeable, allowing for gas exchange while keeping out pathogens. This characteristic is crucial for protecting the wound from infections while allowing it to “breathe” [107]. Some biopolymer membranes can be engineered to deliver therapeutic agents directly to the wound site, such as antibiotics, pain relievers, or growth factors to promote healing. Gel-based biopolymer membranes can be tailored to have specific structural and mechanical properties, such as elasticity and strength, to match the needs of different types of wounds [108,109,110].
Hemodialysis membranes are typically synthesized from biocompatible hydrogels, such as cellulose or synthetic polymers like polyether sulfone. Cross-linking methods, like radiation or chemical cross-linking, are employed to enhance membrane stability. These membranes have a porous structure with a controlled pore size, allowing the selective diffusion of waste products from the blood while retaining essential proteins and blood cells. Hemodialysis membranes exhibit high biocompatibility, hemocompatibility, and good mechanical strength. They have a high surface area for efficient dialysis, and their properties are optimized to prevent clotting and inflammation during treatment.
Transdermal drug delivery membranes are synthesized from biocompatible and permeable hydrogels. The synthesis process may involve incorporating drugs or active compounds into the gel matrix. These membranes are typically thin and flexible, designed to adhere to the skin’s surface. They control drug release through diffusion or other controlled mechanisms. Transdermal drug delivery membranes provide controlled release of drugs over time, offering a convenient and non-invasive method for drug administration [111,112,113,114,115,116,117,118,119].
Their potential to be engineered precisely as per the application’s demands ensures that they will remain at the forefront of biomedical research and innovations (Table 2).

Table 2.
Applications of some Gel-Based Membranes in Biomedical Applications.
4.3. Applications of Gel-Based Membranes in Energy Storage and Conversion
In the arena of energy storage and conversion (Table 3), gel-based membranes offer a suite of advantages that can potentially lead to more efficient, safer, and more durable devices. As research in this domain continues, it is highly plausible that these membranes will play a significant role in the next generation of energy technologies. The role of gel-based membranes in energy storage and conversion is multifaceted, encompassing electrolytes in batteries and fuel cells, separators in supercapacitors, and functional components in solar cells and electrochromic devices. Their design and synthesis are heavily tailored to meet the specific electrical, ionic, and mechanical demands of each application [120,121].

Table 3.
Applications of some Gel-Based Membranes in Energy Storage and Conversion.
Proton-conductive gel-based membranes for fuel cells are synthesized from sulfonated or acid-functionalized hydrogels. The synthesis process often includes sulfonation or functionalization of the polymer chains. These membranes have a well-defined structure with proton-conductive groups distributed throughout the polymer matrix. The structure ensures efficient proton transport. Fuel cell membranes exhibit high proton conductivity, chemical stability, and mechanical strength. They enable the efficient conversion of hydrogen and oxygen into electricity. The synthesis often involves conductive polymers like polyaniline, polypyrrole, or polythiophene to ensure electrical conductivity. To enhance their electrochemical properties, electroactive materials such as metal oxides or conductive nanoparticles may be incorporated. For applications like batteries and fuel cells, the ionic conductivity of the membrane is crucial. This is often achieved by incorporating ionic liquids or salts into the gel matrix. Creating composite membranes with materials like graphene, carbon nanotubes, or metal-organic frameworks can enhance electrical and ionic conductivity, as well as mechanical strength.
Gel-based membranes are used in lithium-ion batteries, sodium-ion batteries, and other types of rechargeable batteries, serving as electrolytes that facilitate ion transport [122,123,124,125]. They are used as proton exchange membranes (PEMs) or electrolytes in fuel cells, including PEM fuel cells, facilitating the transport of ions while preventing electron flow [126,127,128,129]. In supercapacitors, they act as separators and electrolytes, contributing to the device’s overall capacitance and energy density [130]. Some gel-based membranes are used in photovoltaic cells, particularly in organic solar cells, as they can be engineered to improve light absorption and charge transport [131,132]. Also, they are used in smart windows and displays, where their ability to conduct ions plays a crucial role in the color-changing mechanism [133].
4.4. Applications of Gel-Based Membranes in Food and Beverages
Gel-based membranes have also been used in the food and beverage industry (Table 4). They can act as selective barriers to oxygen, carbon dioxide, and ethylene, which is crucial in food packaging for shelf life extension. In the context of the food and beverage industry, gel-based membranes offer solutions that can enhance product quality, safety, and sensory attributes. Their potential to be customized for specific applications ensures that they will continue to be of interest in food science and technology research. In the food and beverage industry, gel-based membranes offer innovative solutions for packaging, processing, and ensuring product quality and safety. Membranes for food applications are typically made from food-grade, non-toxic materials like alginate, or pectin. Natural extracts or antimicrobial agents can be incorporated to extend shelf life and improve food safety. The synthesis often focuses on achieving specific permeability properties to target certain molecules, such as gases, flavors, or nutrients. Stability under various temperature conditions is important for processing and storage. Used in packaging to extend shelf life, improve safety, or maintain quality. For example, oxygen-scavenging membranes help prevent oxidation in packaged foods. They can be applied as thin, edible coatings on fruits and vegetables to extend shelf life and reduce spoilage [134]. In beverage processing, these membranes can be used for selective infusion or removal of flavors, colors, or nutrients They can also be used in the dairy industry for processes like lactose reduction and protein concentration [135,136,137,138,139,140].

Table 4.
Applications of Gel-Based Membranes in Food and Beverages.
4.5. Applications of Some Gel-Based Membranes in Environmental Remediation
Gel-based membranes, due to their versatility and adaptability, have been explored for various environmental remediation applications (Table 5). Due to their multifunctionality and adaptability, they hold great promise in addressing the current pressing environmental challenges. Their ability to be tailored for specific contaminants ensures targeted and effective remediation. As research progresses, these membranes will likely play a more prominent role in sustainable environmental solutions.
In environmental remediation, gel-based membranes offer the advantage of tailored selectivity for various pollutants, high efficiency, and the potential for regeneration and reuse. Their application spans from water treatment, targeting a variety of pollutants, to air purification and greenhouse gas capture, highlighting their versatility and importance in addressing environmental challenges [141,142].

Table 5.
Applications of some Gel-Based Membranes in Environmental Remediation.
Table 5.
Applications of some Gel-Based Membranes in Environmental Remediation.
| Applications | Characteristics of Gel-Based Membranes | References |
|---|---|---|
| Air Purification | ||
| Volatile Organic Compound (VOC) Removal | Gel-based membranes can be engineered to selectively capture and remove VOCs from indoor and industrial air streams. Their porous structure allows for high surface area interaction with contaminants. | [143] |
| Particulate Matter Capture | Certain gel membranes can be designed to effectively trap particulate matter, including fine and ultrafine particles, which are detrimental to human health. | [144] |
| Bioaerosol Capture | With functionalized surfaces, gel-based membranes can capture and potentially neutralize bioaerosols, including bacteria, viruses, and fungal spores, contributing to cleaner air in healthcare and public spaces. | [145] |
| Carbon Capture | ||
| Enhanced Gas Selectivity | Gel-based membranes, especially when embedded with functional groups or materials that have an affinity for CO2, can effectively separate carbon dioxide from gas mixtures, such as flue gases from power plants. | [146] |
| Reduced Energy Penalty | Traditional carbon capture methods can be energy-intensive. Gel-based membranes, with their potential for high permeability and selectivity, can reduce the energy penalty associated with the separation process. | [147] |
| Durability | Properly designed gel-based membranes can offer stability and resistance to fouling, ensuring long-term performance in CO2 capture applications. | [148] |
| Hazardous Waste Containment | ||
| Heavy Metal Removal | Gel-based membranes, when functionalized with chelating agents or other selective groups, can effectively bind and remove heavy metals from wastewater. | [98] |
| Organic Pollutant Capture | Membranes can be tailored to selectively adsorb organic contaminants, including oil, pesticides, and certain industrial chemicals, from water sources. | [51] |
| Radioactive Waste Containment | Some gel-based membranes are being explored for their potential in containing and isolating radioactive compounds, especially in liquid radioactive waste treatment. | [149] |
| Encapsulation and Immobilization | Hazardous compounds can be effectively encapsulated within gel matrices, preventing their migration and leaching into the environment. This is particularly useful for the long-term containment of certain pollutants. | [150] |
The polymers chosen for synthesis, such as polyacrylamide, chitosan, or alginate, often have specific affinities for certain pollutants. The introduction of functional groups or active sites that can selectively bind to or react with specific contaminants is common. This can include groups that target heavy metals, organic pollutants, or radioactive materials. Materials like activated carbon, zeolites, or metal-organic frameworks can be incorporated into the membrane matrix to enhance adsorption properties. The development of composite membranes, which might include inorganic materials or nanoparticles, can improve mechanical strength, chemical resistance, and pollutant removal efficiency. The structure is typically porous, allowing for efficient flow-through of water while trapping contaminants. These membranes often exhibit swelling behavior, which can be tuned for specific applications to enhance pollutant capture. The morphology, including pore size and distribution, is often optimized for specific types of pollutants [143,144,145].
Gel-based membranes are effective in capturing heavy metals from industrial wastewater, such as lead, mercury, and cadmium, and are used to remove organic contaminants, including pesticides, pharmaceuticals, and dyes from water. They can be specialized for the separation of oil from water, which is crucial in treating oil spills and industrial emulsions. Also, some gel-based membranes are designed to capture radioactive isotopes, aiding in the cleanup of nuclear waste. They can also be applied in gas separation processes, removing pollutants like sulfur dioxide or nitrogen oxides from industrial emissions. Specialized gel membranes can be used for carbon capture, helping to reduce greenhouse gas emissions [51,98,146,147,148,149,150].
Table 6 shows important information about the production of the gel-based membrane.

Table 6.
Some applications of Gel-Based Membranes.
5. Challenges and Future Perspectives of Gel-Based Membranes
While gel-based membranes have shown remarkable potential, addressing the current challenges is crucial for their widespread adoption. The ongoing research and technological innovations in this domain are promising and suggest a bright future for these versatile materials. One of the key challenges with gel-based membranes is their mechanical fragility compared to other membrane types. They may not withstand high pressures or aggressive operating conditions over long durations. Over time, gel-based membranes can face fouling, especially when used in wastewater treatment or other applications with high organic loadings. This reduces their performance and lifespan. Some gel membranes tend to swell excessively in the presence of certain solvents or conditions, leading to a loss in their selective properties. Manufacturing large-scale, uniform, and defect-free gel-based membranes, especially for industrial applications, can be challenging and costly. Introducing cross-links in the polymer network can enhance the mechanical strength and stability of the membranes. Incorporating nanoparticles or nano-fillers into the gel matrix can improve properties such as mechanical strength, selectivity, and resistance to fouling. The use of state-of-the-art manufacturing techniques, like 3D printing, could enable the production of customized and defect-free membranes at larger scales. Adding specific functional groups or coatings can enhance selectivity, reduce fouling, and improve overall performance. Developing efficient cleaning techniques that do not compromise membrane integrity can extend the life and efficiency of these membranes. The integration of sensing elements or responsive components can lead to ‘smart’ membranes that adapt or respond to changes in their environment, improving efficiency and selectivity. With an increasing emphasis on sustainability, there will be a rise in the research and development of biodegradable or compostable gel-based membranes. Combining gel-based membranes with other technologies, such as advanced oxidation processes or biofiltration, can lead to integrated systems with enhanced efficiency. In the biomedical domain, gel-based membranes might be customized for individual needs, particularly in areas like drug delivery or tissue engineering. As the technology matures and overcomes its current limitations, there will likely be an uptick in the commercial applications of gel-based membranes in various industries, from environmental to biomedical fields.
6. Conclusions
Gel-based membranes, with their unique blend of structural, chemical, and functional properties, have undeniably carved a niche in modern technological applications. From purifying water to enhancing energy storage, safeguarding the environment, and pushing the boundaries in biomedical applications, they have demonstrated unparalleled versatility. Their tailoring ability allows for bespoke solutions to challenges across various industries, emphasizing their centrality in addressing contemporary issues. However, as with any evolving technology, they come with their set of challenges. Future research must not only aim to harness their potential but also innovatively address their limitations. Advanced research, cross-disciplinary collaborations, and industrial partnerships are pivotal in this regard.
Looking forward, the horizon for gel-based membranes seems promising. As we tread into an era marked by sustainability, efficiency, and precision, these membranes are poised to play a pivotal role. Their continued evolution will not only augment existing technologies but also pave the way for novel applications yet to be envisioned. The future holds a multitude of opportunities for gel-based membrane applications due to their multifaceted potential.
Author Contributions
Conceptualization, C.U., R.Z., and G.T.; methodology, C.U.; software, S.R.; validation, C.B., C.U., and R.Z.; resources, C.U. and S.R.; data curation, C.B.; writing—original draft preparation, C.U. and S.R.; writing—review and editing, C.U., R.Z., and G.T.; visualization, C.B.; supervision, C.U.; project administration, C.U. and S.R.; funding acquisition, C.U. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
C.U., R.Z., and G.T. gratefully acknowledge the support of a grant from the Ministry of Research, Innovation, and Digitization, CCCDI—UEFISCDI, project number PN-III-P2-2.1-PED-2021-0042, within PNCDI III; S.R. gratefully acknowledges the support from a grant from the National Program for Research of the National Association of Technical Universities—GNAC ARUT 2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| MOFs | metal-organic frameworks |
| PVA | polyvinyl alcohol |
| PAM | polyacrylamide |
| PEG | polyethylene glycol |
| LbL | Layer-by-Layer |
| CIP | cleaning-in-place |
| Ag | silver |
| Au | gold |
| TiO2 | titanium dioxide |
| ZnO | Zinc oxide |
| RO | Reverse Osmosis |
| FO | Forward Osmosis |
| ED | Electrodialysis |
| LIBs | Lithium-Ion Batteries |
| PEMFCs | Proton Exchange Membrane Fuel Cells |
| DMFCs | Direct Methanol Fuel Cells |
| VOCs | Volatile Organic Compounds |
| PCL/Gel | polycaprolactone/gelatin |
| EDC/NHS | 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide/N-hydroxysuccinimide |
| PVA | polyvinyl alcohol |
| CS | Chitosan |
| XG/GG/HA | xanthan gum/gellan gum/hyaluronan hydrogel |
| PAM | polyacrylamide |
| DN | Double network |
| DEA | N,N-diethylacrylamide |
| DMA | N,N-dimethylacrylamide |
| PVDF-HFP | poly(vinylidene fluoride-hexafluoro propylene |
| PBMA | poly (butyl methacrylate) |
References
- Malik, S.; Khyalia, P.; Singh Laura, J. Chapter 7—Conventional methods and materials used for water treatment in rural areas. In Water Resources Management for Rural Development; Madhav, S., Srivastav, A.L., Chibueze Izah, S., Hullebusch, E.v., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 79–90. [Google Scholar]
- Ling, G.N. Chapter 6—Colloid, the Brain Child of a Chemist. In Life at the Cell and Below-Cell Level. The Hidden History of a Fundamental Revolution in Biology; Pacific Press: New York, NY, USA, 2001; pp. 29–34. [Google Scholar]
- Linder, C.; Kedem, O. History of Nanofiltration Membranes from 1960 to 1990. In Nanofiltration; Wiley: Hoboken, NJ, USA, 2021; pp. 1–34. [Google Scholar]
- Kujawa, J.; Al-Gharabli, S.; Muzioł, T.M.; Knozowska, K.; Li, G.; Dumée, L.F.; Kujawski, W. Crystalline porous frameworks as nano-enhancers for membrane liquid separation—Recent developments. Coord. Chem. Rev. 2021, 440, 213969. [Google Scholar] [CrossRef]
- Căprărescu, S.; Zgârian, R.G.; Tihan, G.T.; Purcar, V.; Eftimie Totu, E.; Modrogan, C.; Chiriac, A.-L.; Nicolae, C.A. Biopolymeric Membrane Enriched with Chitosan and Silver for Metallic Ions Removal. Polymers 2020, 12, 1792. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Shanmugam, D.K.; Dasan, A.; Mosas, K.K.A. Hydrogels—A Promising Materials for 3D Printing Technology. Gels 2023, 9, 260. [Google Scholar] [CrossRef]
- Atia, G.A.N.; Shalaby, H.K.; Ali, N.G.; Morsy, S.M.; Ghobashy, M.M.; Attia, H.A.N.; Barai, P.; Nady, N.; Kodous, A.S.; Barai, H.R. New Challenges and Prospective Applications of Three-Dimensional Bioactive Polymeric Hydrogels in Oral and Craniofacial Tissue Engineering: A Narrative Review. Pharmaceuticals 2023, 16, 702. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Radu, E.R.; Voicu, S.I.; Thakur, V.K. Polymeric Membranes for Biomedical Applications. Polymers 2023, 15, 619. [Google Scholar] [CrossRef]
- Rando, G.; Sfameni, S.; Plutino, M.R. Development of Functional Hybrid Polymers and Gel Materials for Sustainable Membrane-Based Water Treatment Technology: How to Combine Greener and Cleaner Approaches. Gels 2023, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Burratti, L.; Prosposito, P.; Venditti, I. Functionalized Gels for Environmental Applications. Gels 2023, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazeq, H.; Khraisheh, M.; Ashraf, H.M.; Ebrahimi, P.; Kunju, A. Sustainable Innovation in Membrane Technologies for Produced Water Treatment: Challenges and Limitations. Sustainability 2021, 13, 6759. [Google Scholar] [CrossRef]
- Tihan, G.T.; Zgarian, R.G.; Berteanu, E.; Ionita, D.; Totea, G.; Iordachel, C.; Tatia, R.; Prodana, M.; Demetrescu, I. Alkaline Phosphatase Immobilization on New Chitosan Membranes with Mg2+ for Biomedical Applications. Mar. Drugs 2018, 16, 287. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Ehtesabi, H.; Ebrahimi, S. Incorporation of Saqez essential oil into polyvinyl alcohol/chitosan bilayer hydrogel as a potent wound dressing material. Int. J. Biol. Macromol. 2023, 226, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, C.; Ionita, D.; Berteanu, E.; Tcacenco, L.; Zuav, A.; Demetrescu, I. Improving Natural Biopolymeric Membranes Based on Chitosan and Collagen for Biomedical Applications Introducing Silver. J. Braz. Chem. Soc. 2015, 26, 458–465. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. 3D-Printed Hydrogels and Aerogels for Water Treatment and Energy Storage Applications. ChemistrySelect 2023, 8, e202300738. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Y.; Wan, Y.; Carvalho, W.; Hu, L.; Serpe, M.J. Stimuli-Responsive Polymers for Sensing and Reacting to Environmental Conditions. Prog. Polym. Sci. 2021, 116, 101386. [Google Scholar] [CrossRef]
- Chakrapani, G.; Zare, M.; Ramakrishna, S. Intelligent hydrogels and their biomedical applications. Mater. Adv. 2022, 3, 7757–7772. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Santos, D.M.F. Applications of Polymer Electrolytes in Lithium-Ion Batteries: A Review. Polymers 2023, 15, 3907. [Google Scholar] [CrossRef]
- Cho, Y.-G.; Hwang, C.; Cheong, D.S.; Kim, Y.-S.; Song, H.-K. Gel/Solid Polymer Electrolytes Characterized by In Situ Gelation or Polymerization for Electrochemical Energy Systems. Adv. Mater. 2019, 31, 1804909. [Google Scholar] [CrossRef]
- Sodiq, A.; Abdullatif, Y.; Aissa, B.; Ostovar, A.; Nassar, N.; El-Naas, M.; Amhamed, A. A review on progress made in direct air capture of CO2. Environ. Technol. Innov. 2023, 29, 102991. [Google Scholar] [CrossRef]
- Ansaloni, L.; Salas-Gay, J.; Ligi, S.; Baschetti, M.G. Nanocellulose-based membranes for CO2 capture. J. Membr. Sci. 2017, 522, 216–225. [Google Scholar] [CrossRef]
- Nandi, A.K.; Chatterjee, D.P. Hybrid polymer gels for energy applications. J. Mater. Chem. A 2023, 11, 12593–12642. [Google Scholar] [CrossRef]
- Xue, N.; Lu, J.; Gu, D.; Lou, Y.; Yuan, Y.; Li, G.; Kumagai, S.; Saito, Y.; Yoshioka, T.; Zhang, N. Carbon footprint analysis and carbon neutrality potential of desalination by electrodialysis for different applications. Water Res. 2023, 232, 119716. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Maktedar, S.S. Structural, functional and mechanical performance of advanced Graphene-based composite hydrogels. Results Chem. 2023, 6, 101029. [Google Scholar] [CrossRef]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Chapter 2—Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, K.; Ali, M.; Gingrich, K.; Porter, D.L.; Chong, S.; Riley, B.J.; Peak, C.W.; Naleway, S.E.; Zharov, I.; Carlson, K. Sol-gel derived silica: A review of polymer-tailored properties for energy and environmental applications. Microporous Mesoporous Mater. 2022, 336, 111874. [Google Scholar] [CrossRef]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2023, 80, 7247–7312. [Google Scholar] [CrossRef]
- Sahu, A.; Dosi, R.; Kwiatkowski, C.; Schmal, S.; Poler, J.C. Advanced Polymeric Nanocomposite Membranes for Water and Wastewater Treatment: A Comprehensive Review. Polymers 2023, 15, 540. [Google Scholar] [CrossRef]
- Nafti Mateur, M.; Gonzalez Ortiz, D.; Jellouli Ennigrou, D.; Horchani-Naifer, K.; Bechelany, M.; Miele, P.; Pochat-Bohatier, C. Porous Gelatin Membranes Obtained from Pickering Emulsions Stabilized with h-BNNS: Application for Polyelectrolyte-Enhanced Ultrafiltration. Membranes 2020, 10, 144. [Google Scholar] [CrossRef]
- Hou, Y.; Ma, S.; Hao, J.; Lin, C.; Zhao, J.; Sui, X. Construction and Ion Transport-Related Applications of the Hydrogel-Based Membrane with 3D Nanochannels. Polymers 2022, 14, 4037. [Google Scholar] [CrossRef]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels: Present Challenges and Future Opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef]
- Morteza, B.; Naimeh, M.; Mehdi, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Sutapa Biswas, M., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 2. [Google Scholar]
- Devi, V.K.A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Quan, L.; Xin, Y.; Wu, X.; Ao, Q. Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polymers 2022, 14, 2184. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Kaner, P.; Bengani-Lutz, P.; Sadeghi, I.; Asatekin, A. Responsive filtration membranes by polymer self-assembly. Technology 2016, 04, 217–228. [Google Scholar] [CrossRef]
- Hamoudi, L.; Akretche, D.E.; Hadadi, A.; Amrane, A.; Mouni, L. Comparative Study of Ceramic Membranes Developed on Different Algerian Natural Clays for Industrial-Effluent Filtration. Minerals 2023, 13, 273. [Google Scholar] [CrossRef]
- National Research Council. Polymer Science and Engineering: The Shifting Research Frontiers. In 3. Manufacturing: Materials and Processing; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Mashabela, L.T.; Maboa, M.M.; Miya, N.F.; Ajayi, T.O.; Chasara, R.S.; Milne, M.; Mokhele, S.; Demana, P.H.; Witika, B.A.; Siwe-Noundou, X.; et al. A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders. Gels 2022, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with Surface-Enhanced Antifouling Properties for Water Purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Amin, S.; Mohamed, A.A. Fouling in reverse osmosis membranes: Monitoring, characterization, mitigation strategies and future directions. Heliyon 2023, 9, e14908. [Google Scholar] [CrossRef]
- Quazi, M.Z.; Hwang, J.; Song, Y.; Park, N. Hydrogel-Based Biosensors for Effective Therapeutics. Gels 2023, 9, 545. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Rabiee, N.; Sharma, R.; Foorginezhad, S.; Jouyandeh, M.; Asadnia, M.; Rabiee, M.; Akhavan, O.; Lima, E.C.; Formela, K.; Ashrafizadeh, M.; et al. Green and Sustainable Membranes: A review. Environ. Res. 2023, 231, 116133. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Xue, Y.-R.; Xu, Z.-K. Alginate Hydrogel Assisted Controllable Interfacial Polymerization for High-Performance Nanofiltration Membranes. Membranes 2021, 11, 435. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, G.; Zhu, J.; Mamrol, N.; Liu, S.; Mai, Z.; Van Puyvelde, P.; Van der Bruggen, B. Hydrogel assisted interfacial polymerization for advanced nanofiltration membranes. J. Mater. Chem. A 2020, 8, 3238–3245. [Google Scholar] [CrossRef]
- Al-Baadani, M.A.; Hii Ru Yie, K.; Al-Bishari, A.M.; Alshobi, B.A.; Zhou, Z.; Fang, K.; Dai, B.; Shen, Y.; Ma, J.; Liu, J.; et al. Co-electrospinning polycaprolactone/gelatin membrane as a tunable drug delivery system for bone tissue regeneration. Mater. Des. 2021, 209, 109962. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.Y.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.-Y.L.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres—Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ding, Z.; Yuan, Q.; Xie, H.; Gu, Z. Multi-Layered Hydrogels for Biomedical Applications. Front. Chem. 2018, 6, 439. [Google Scholar] [CrossRef] [PubMed]
- Xiangdong, B.; Aiye, L. In Situ-Forming Cross-linking Hydrogel Systems: Chemistry and Biomedical Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Sutapa Biswas, M., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 6. [Google Scholar]
- Leone, F.; Firlak, M.; Challen, K.; Bonnefin, W.; Onida, B.; Wright, K.L.; Hardy, J.G. In Situ Crosslinking Bionanocomposite Hydrogels with Potential for Wound Healing Applications. J. Funct. Biomater. 2019, 10, 50. [Google Scholar] [CrossRef]
- Nair, J.R.; Chiappone, A.; Destro, M.; Jabbour, L.; Meligrana, G.; Gerbaldi, C. UV-Induced Radical Photo-Polymerization: A Smart Tool for Preparing Polymer Electrolyte Membranes for Energy Storage Devices. Membranes 2012, 2, 687–704. [Google Scholar] [CrossRef]
- Siccardi, S.; Amici, J.; Colombi, S.; Carvalho, J.T.; Versaci, D.; Quartarone, E.; Pereira, L.; Bella, F.; Francia, C.; Bodoardo, S. UV-cured self-healing gel polymer electrolyte toward safer room temperature lithium metal batteries. Electrochim. Acta 2022, 433, 141265. [Google Scholar] [CrossRef]
- Zhi, K.; Wang, J.; Zhao, H.; Yang, X. Self-assembled small molecule natural product gel for drug delivery: A breakthrough in new application of small molecule natural products. Acta Pharm. Sin. B 2020, 10, 913–927. [Google Scholar] [CrossRef]
- Braun, G.A.; Ary, B.E.; Dear, A.J.; Rohn, M.C.H.; Payson, A.M.; Lee, D.S.M.; Parry, R.C.; Friedman, C.; Knowles, T.P.J.; Linse, S.; et al. On the Mechanism of Self-Assembly by a Hydrogel-Forming Peptide. Biomacromolecules 2020, 21, 4781–4794. [Google Scholar] [CrossRef]
- Correa, S.O.; Luo, X.; Raub, C.B. Microfluidic fabrication of stable collagen microgels with aligned microstructure using flow-driven co-deposition and ionic gelation. J. Micromech. Microeng. 2020, 30, 085002. [Google Scholar] [CrossRef]
- De Geest, B.G.; Urbanski, J.P.; Thorsen, T.; Demeester, J.; De Smedt, S.C. Synthesis of Monodisperse Biodegradable Microgels in Microfluidic Devices. Langmuir 2005, 21, 10275–10279. [Google Scholar] [CrossRef]
- Herrera-Ruiz, A.; Tovar, B.B.; García, R.G.; Tamez, M.F.L.; Mamidi, N. Nanomaterials-Incorporated Chemically Modified Gelatin Methacryloyl-Based Biomedical Composites: A Novel Approach for Bone Tissue Engineering. Pharmaceutics 2022, 14, 2645. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent advances in 3D printing of tough hydrogels: A review. Compos. Part B 2022, 238, 109895. [Google Scholar] [CrossRef]
- Tayebi, L.; Rasoulianboroujeni, M.; Cui, Z.; Ye, H. 3D-printed thick structured gelatin membrane for engineering of heterogeneous tissues. Mater. Lett. 2018, 217, 39–43. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Carrico, J.D.; Tyler, T.; Leang, K.K. A comprehensive review of select smart polymeric and gel actuators for soft mechatronics and robotics applications: Fundamentals, freeform fabrication, and motion control. Int. J. Smart Nano Mater. 2017, 8, 144–213. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Bose, M.; Das, T.K.; Mondal, S.; Das, A.K.; Das, N.C. Sonochemical green reduction to prepare Ag nanoparticles decorated graphene sheets for catalytic performance and antibacterial application. Ultrason. Sonochem. 2017, 39, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Sachot, N.; Engel, E.; Castano, O. Hybrid Organic-Inorganic Scaffolding Biomaterials for Regenerative Therapies. Curr. Org. Chem. 2014, 18, 2299–2314. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, J.; Pan, L.; Shi, Y.; Yu, G. Energy gels: A bio-inspired material platform for advanced energy applications. Nano Today 2016, 11, 738–762. [Google Scholar] [CrossRef]
- Amira, A.; Huu, D.; Ali, L. Applications of Biomimetic and Bioinspired Membranes. In Biomimetic and Bioinspired Membranes for New Frontiers in Sustainable Water Treatment Technology; Amira, A., Huu, D., Ali, L., Eds.; IntechOpen: Rijeka, Croatia, 2017; Chapter 8. [Google Scholar]
- Wei, Z.; Yang, J.H.; Zhou, J.; Xu, F.; Zrínyi, M.; Dussault, P.H.; Osada, Y.; Chen, Y.M. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem. Soc. Rev. 2014, 43, 8114–8131. [Google Scholar] [CrossRef]
- Xu, C.; Zhan, W.; Tang, X.; Mo, F.; Fu, L.; Lin, B. Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polym. Test. 2018, 66, 155–163. [Google Scholar] [CrossRef]
- Ray, P.; Chakraborty, R.; Banik, O.; Banoth, E.; Kumar, P. Surface Engineering of a Bioartificial Membrane for Its Application in Bioengineering Devices. ACS Omega 2023, 8, 3606–3629. [Google Scholar] [CrossRef]
- Chung, M.M.S.; Arbour, A.J.; Huang, J.-Y. Microbubble-Assisted Cleaning-in-Place Process for Ultrafiltration System and Its Environmental Performance. Membranes 2023, 13, 424. [Google Scholar] [CrossRef]
- Liu, S.; Low, Z.-X.; Hegab, H.M.; Xie, Z.; Ou, R.; Yang, G.; Simon, G.P.; Zhang, X.; Zhang, L.; Wang, H. Enhancement of desalination performance of thin-film nanocomposite membrane by cellulose nanofibers. J. Membr. Sci. 2019, 592, 117363. [Google Scholar] [CrossRef]
- Khdary, N.H.; Almuarqab, B.T.; El Enany, G. Nanoparticle-Embedded Polymers and Their Applications: A Review. Membranes 2023, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Salim, S.H.; Al-Anbari, R.H.; Haider, A.J. Polymeric Membrane with Nanomaterial’s for Water Purification: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012103. [Google Scholar] [CrossRef]
- Sadeghi, A.; PourEskandar, S.; Askari, E.; Akbari, M. Polymeric Nanoparticles and Nanogels: How Do They Interact with Proteins? Gels 2023, 9, 632. [Google Scholar] [CrossRef]
- Khraisheh, M.; Elhenawy, S.; AlMomani, F.; Al-Ghouti, M.; Hassan, M.K.; Hameed, B.H. Recent Progress on Nanomaterial-Based Membranes for Water Treatment. Membranes 2021, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhuang, B.; Yu, J. Functional Zwitterionic Polymers on Surface: Structures and Applications. Chem. Asian J. 2020, 15, 2060–2075. [Google Scholar] [CrossRef]
- Basinska, T.; Gadzinowski, M.; Mickiewicz, D.; Slomkowski, S. Functionalized Particles Designed for Targeted Delivery. Polymers 2021, 13, 2022. [Google Scholar] [CrossRef]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of Transport Processes in Ion-Exchange Membranes: Relationship with the Structure and Methods for Its Improvement. Int. J. Mol. Sci. 2020, 21, 5517. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Kolya, H.; Kang, C.-W. Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration. Polymers 2023, 15, 3421. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hong, X.; Zhao, M.; Liu, N.; Liu, H.; Zhao, J.; Shao, L.; Xue, W.; Zhang, H.; Zhu, P.; et al. Nanocomposite hydrogels for biomedical applications. Bioeng. Transl. Med. 2022, 7, e10315. [Google Scholar] [CrossRef]
- Nagarajan, S.; Abessolo Ondo, D.; Gassara, S.; Bechelany, M.; Balme, S.; Miele, P.; Kalkura, N.; Pochat-Bohatier, C. Porous Gelatin Membrane Obtained from Pickering Emulsions Stabilized by Graphene Oxide. Langmuir 2018, 34, 1542–1549. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, X.; Shi, X.; Wang, S.; Wang, S.; Wu, Q.; Ma, Y.; Wang, J.; Wan, D.; Pan, J. Simultaneously enhancing purification, catalysis and in situ separation in a continuous cross-flow catalytic degradation process of multi-component organic pollutants by a double-layer PVDF composite membrane. J. Environ. Chem. Eng. 2022, 10, 107160. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Wang, Y.; Tang, C.Y.; Huo, F. Mesoporous Silica Gel–Based Mixed Matrix Membranes for Improving Mass Transfer in Forward Osmosis: Effect of Pore Size of Filler. Sci. Rep. 2015, 5, 16808. [Google Scholar] [CrossRef]
- Bakly, S.; Ibrar, I.; Saleem, H.; Yadav, S.; Al-Juboori, R.; Naji, O.; Altaee, A.; Zaidi, S.J. Chapter 13—Polymer-based nano-enhanced forward osmosis membranes. In Advancement in Polymer-Based Membranes for Water Remediation; Nayak, S.K., Dutta, K., Gohil, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 471–501. [Google Scholar]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants— Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar]
- Available online: https://www.sciencedirect.com/bookseries/journal-of-chromatography-library/vol/61/suppl/C (accessed on 30 October 2023).
- Kusworo, T.D.; Kumoro, A.C.; Aryanti, N.; Kurniawan, T.A.; Dalanta, F.; Alias, N.H. Photocatalytic polysulfone membrane incorporated by ZnO-MnO2@SiO2 composite under UV light irradiation for the reliable treatment of natural rubber-laden wastewater. Chem. Eng. J. 2023, 451, 138593. [Google Scholar] [CrossRef]
- Ramnath, L. Application of Magnetic Nanoparticles and Reactive Filter Materials for Wastewater Treatment. Doctoral Thesis, Royal Institute of Technology, School of Biotechnology Stockholm, Stockholm, Sweden, 2013. [Google Scholar]
- Gardikiotis, I.; Cojocaru, F.-D.; Mihai, C.-T.; Balan, V.; Dodi, G. Borrowing the Features of Biopolymers for Emerging Wound Healing Dressings: A Review. Int. J. Mol. Sci. 2022, 23, 8778. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Prete, S.; Dattilo, M.; Patitucci, F.; Pezzi, G.; Parisi, O.I.; Puoci, F. Natural and Synthetic Polymeric Biomaterials for Application in Wound Management. J. Funct. Biomater. 2023, 14, 455. [Google Scholar] [CrossRef]
- Berteanu, E.; Ionita, D.; Simoiu, M.; Paraschiv, M.; Tatia, R.; Apatean, A.; Sidoroff, M.; Tcacenco, L. Evaluation of biodegradation and biocompatibility of collagen/chitosan/alkaline phosphatase biopolymeric membranes. Bull. Mater. Sci. 2016, 39, 377–383. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Souza, P. alpha-N-heterocyclic thiosemicarbazone derivatives as potential antitumor agents: A structure-activity relationships approach. Mini Rev. Med. Chem. 2009, 9, 1389–1396. [Google Scholar] [CrossRef]
- Güiza-Argüello, V.R.; Solarte-David, V.A.; Pinzón-Mora, A.V.; Ávila-Quiroga, J.E.; Becerra-Bayona, S.M. Current Advances in the Development of Hydrogel-Based Wound Dressings for Diabetic Foot Ulcer Treatment. Polymers 2022, 14, 2764. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Yasin, S.N.N.; Said, Z.; Halib, N.; Rahman, Z.A.; Mokhzani, N.I. Polymer-Based Hydrogel Loaded with Honey in Drug Delivery System for Wound Healing Applications. Polymers 2023, 15, 3085. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers. Polymers 2022, 14, 3806. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Karimi, M.; Eslami, M.; Sahandi-Zangabad, P.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 696–716. [Google Scholar] [CrossRef]
- Wei, Z.; Volkova, E.; Blatchley, M.R.; Gerecht, S. Hydrogel vehicles for sequential delivery of protein drugs to promote vascular regeneration. Adv. Drug Deliv. Rev. 2019, 149–150, 95–106. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Nazari, S.; Abdelrasoul, A. Impact of Membrane Modification and Surface Immobilization Techniques on the Hemocompatibility of Hemodialysis Membranes: A Critical Review. Membranes 2022, 12, 1063. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Abdelrasoul, A.; Shoker, A. A critical review of recent advances in hemodialysis membranes hemocompatibility and guidelines for future development. Mater. Chem. Phys. 2020, 248, 122911. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T.; Mizuno, T.; Kubota, K. Antithrombogenic Properties of Amphiphilic Block Copolymer Coatings: Evaluation of Hemocompatibility Using Whole Blood. ACS Biomater. Sci. Eng. 2015, 1, 352–362. [Google Scholar] [CrossRef]
- Li, C.; Zhang, K.; Cheng, X.; Li, J.; Jiang, Y.; Li, P.; Wang, B.; Peng, H. Polymers for flexible energy storage devices. Prog. Polym. Sci. 2023, 143, 101714. [Google Scholar] [CrossRef]
- Gerbaldi, C.; Nair, J.R.; Meligrana, G.; Bongiovanni, R.; Bodoardo, S.; Penazzi, N. Highly ionic conducting methacrylic-based gel-polymer electrolytes by UV-curing technique. J. Appl. Electrochem. 2009, 39, 2199–2207. [Google Scholar] [CrossRef]
- Sun, J.; He, C.; Li, Y.; Zhang, Q.; Hou, C.; De Volder, M.; Li, K.; Wang, H. Solid-state nanocomposite ionogel electrolyte with in-situ formed ionic channels for uniform ion-flux and suppressing dendrite formation in lithium metal batteries. Energy Stor. Mater. 2023, 54, 40–50. [Google Scholar] [CrossRef]
- Tombolesi, S.; Zanieri, N.; Bargnesi, L.; Mernini, M.; Lacarbonara, G.; Arbizzani, C. A Sustainable Gel Polymer Electrolyte for Solid-State Electrochemical Devices. Polymers 2023, 15, 3087. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ding, C.; Fu, X.; Huang, Y. Advanced gel polymer electrolytes for safe and durable lithium metal batteries: Challenges, strategies, and perspectives. Energy Storage Mater. 2021, 34, 515–535. [Google Scholar] [CrossRef]
- Manuel Stephan, A. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Prakash, S.; Mustain, W.E.; Kohl, P.A. Chapter 1—Electrolytes for Long-Life, Ultra Low-Power Direct Methanol Fuel Cells. In Micro Fuel Cells; Zhao, T.S., Ed.; Academic Press: Boston, MA, USA, 2009; pp. 1–50. [Google Scholar]
- Sahu, A.K.; Selvarani, G.; Pitchumani, S.; Sridhar, P.; Shukla, A.K. A Sol-Gel Modified Alternative Nafion-Silica Composite Membrane for Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2007, 154, B123. [Google Scholar] [CrossRef]
- Lai, W.-C.; Fan, R.-W. A simple low-cost method to prepare gel electrolytes incorporating graphene oxide with increased ionic conductivity and electrochemical stability. J. Electroanal. Chem. 2022, 907, 115889. [Google Scholar] [CrossRef]
- Sharma, S.; Chand, P. Supercapacitor and electrochemical techniques: A brief review. Results Chem. 2023, 5, 100885. [Google Scholar] [CrossRef]
- Available online: https://www.chemistryworld.com/news/hydrogel-boosts-power-of-self-cooling-solar-panels/4011770.article?utm_campaign=cw_shared&utm_medium=post&utm_source=navigator (accessed on 8 December 2023).
- Lv, T.; Sun, L.; Yang, Y.; Huang, J. Bio-inspired hydrogel with all-weather adhesion, cooling and reusability functions for photovoltaic panels. Sol. Energy 2021, 216, 358–364. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, X.; Mi, Y.; Fan, F.; Xu, Q.; Zhao, H.; Wang, S.; Long, Y. Hydrogel smart windows. J. Mater. Chem. A 2020, 8, 10007–10025. [Google Scholar] [CrossRef]
- Ungureanu, C.; Tihan, G.; Zgârian, R.; Pandelea, G. Bio-Coatings for Preservation of Fresh Fruits and Vegetables. Coatings 2023, 13, 1420. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Encapsulation of Flavours and Fragrances into Polymeric Capsules and Cyclodextrins Inclusion Complexes: An Update. Molecules 2020, 25, 5878. [Google Scholar] [CrossRef] [PubMed]
- Kłosowska, A.; Wawrzyńczak, A.; Feliczak-Guzik, A. Microencapsulation as a Route for Obtaining Encapsulated Flavors and Fragrances. Cosmetics 2023, 10, 26. [Google Scholar] [CrossRef]
- Noore, S.; Rastogi, N.K.; O’Donnell, C.; Tiwari, B. Novel Bioactive Extraction and Nano-Encapsulation. Encyclopedia 2021, 1, 632–664. [Google Scholar] [CrossRef]
- Gernat, D.C.; Brouwer, E.; Ottens, M. Aldehydes as Wort Off-Flavours in Alcohol-Free Beers—Origin and Control. Food Bioprocess Technol. 2020, 13, 195–216. [Google Scholar] [CrossRef]
- Patel, V.B.; Chatterjee, S.; Dhoble, A.S. A review on pectinase properties, application in juice clarification, and membranes as immobilization support. J. Food Sci. 2022, 87, 3338–3354. [Google Scholar] [CrossRef]
- Sarbatly, R.; Sariau, J.; Krishnaiah, D. Recent Developments of Membrane Technology in the Clarification and Concentration of Fruit Juices. Food Eng. Rev. 2023, 15, 420–437. [Google Scholar] [CrossRef]
- Soffian, M.S.; Abdul Halim, F.Z.; Aziz, F.; Rahman, M.A.; Mohamed Amin, M.A.; Awang Chee, D.N. Carbon-based material derived from biomass waste for wastewater treatment. Environ. Adv. 2022, 9, 100259. [Google Scholar] [CrossRef]
- Zubair, N.; Abouzari-Lotf, E.; Nasef, M.; Abdullah, E. Aerogel-based materials for adsorbent applications in material domains. E3S Web Conf. 2019, 90, 01003. [Google Scholar] [CrossRef]
- Franco, P.; Cardea, S.; Tabernero, A.; De Marco, I. Porous Aerogels and Adsorption of Pollutants from Water and Air: A Review. Molecules 2021, 26, 4440. [Google Scholar] [CrossRef]
- Saleh, W.M.; Ahmad, M.I.; Yahya, E.B.; HPS, A.K. Nanostructured Bioaerogels as a Potential Solution for Particulate Matter Pollution. Gels 2023, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality: A Review of Cleaning Technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Y.; Geng, C.; Zhang, Z.; Qiao, Z.; Zhong, C. Improved CO2/N2 separation performance by relatively continuous and defect-free distribution of IL-encapsulated ZIF-67 in ion gel membranes. J. Membr. Sci. 2023, 683, 121818. [Google Scholar] [CrossRef]
- Fam, W.; Mansouri, J.; Li, H.; Chen, V. Improving CO2 separation performance of thin film composite hollow fiber with Pebax®1657/ionic liquid gel membranes. J. Membr. Sci. 2017, 537, 54–68. [Google Scholar] [CrossRef]
- Pasichnyk, M.; Stanovsky, P.; Polezhaev, P.; Zach, B.; Šyc, M.; Bobák, M.; Jansen, J.C.; Přibyl, M.; Bara, J.E.; Friess, K.; et al. Membrane technology for challenging separations: Removal of CO2, SO2 and NOx from flue and waste gases. Sep. Purif. Technol. 2023, 323, 124436. [Google Scholar] [CrossRef]
- Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/TRS431_web.pdf (accessed on 30 October 2023).
- Li, Y.; Zhu, X.; Qi, X.; Shu, B.; Zhang, X.; Li, K.; Wei, Y.; Wang, H. Removal and immobilization of arsenic from copper smelting wastewater using copper slag by in situ encapsulation with silica gel. Chem. Eng. J. 2020, 394, 124833. [Google Scholar] [CrossRef]
- Refat, M.S.; Elsabawy, K.M.; Alhadhrami, A.; Almalki, A.S.A.; El-Sayed, M.Y.; Hassan, R.F. Development of medical drugs: Synthesis and in vitro bio-evaluations of nanomedicinal zinc–penicillins polymeric hydrogel membranes for wound skin dressing by new chemical technology. J. Mol. Liq. 2018, 255, 462–470. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS PharmSciTech 2007, 8, 21. [Google Scholar] [CrossRef]
- Hou, Y.; Brower, M.; Pollard, D.; Kanani, D.; Jacquemart, R.; Kachuik, B.; Stout, J. Advective hydrogel membrane chromatography for monoclonal antibody purification in bioprocessing. Biotechnol. Prog. 2015, 31, 974–982. [Google Scholar] [CrossRef]
- Kuo, S.M.; Chang, S.J.; Wang, H.-Y.; Tang, S.C.; Yang, S.-W. Evaluation of the ability of xanthan gum/gellan gum/hyaluronan hydrogel membranes to prevent the adhesion of postrepaired tendons. Carbohydr. Polym. 2014, 114, 230–237. [Google Scholar] [CrossRef]
- Miao, H.; Chen, B.; Li, S.; Wu, X.; Wang, Q.; Zhang, C.; Sun, Z.; Li, H. All-solid-state flexible zinc-air battery with polyacrylamide alkaline gel electrolyte. J. Power Sources 2020, 450, 227653. [Google Scholar] [CrossRef]
- Ye, Y.N.; Frauenlob, M.; Wang, L.; Tsuda, M.; Sun, T.L.; Cui, K.; Takahashi, R.; Zhang, H.J.; Nakajima, T.; Nonoyama, T.; et al. Tough and Self-Recoverable Thin Hydrogel Membranes for Biological Applications. Adv. Funct. Mater. 2018, 28, 1801489. [Google Scholar] [CrossRef]
- Getachew, B.A.; Kim, S.-R.; Kim, J.-H. Self-Healing Hydrogel Pore-Filled Water Filtration Membranes. Environ. Sci. Technol. 2017, 51, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, P.; Gao, G.; Qin, L.; Yang, H.; Zhang, X. A smart microhydrogel membrane sensor realized by pipette tip. Biosens. Bioelectron. 2022, 211, 114341. [Google Scholar] [CrossRef]
- Saroja, A.P.V.K.; Kumar, A.; Moharana, B.C.; Kamaraj, M.; Ramaprabhu, S. Design of porous calcium phosphate based gel polymer electrolyte for Quasi-solid state sodium ion battery. J. Electroanal. Chem. 2020, 859, 113864. [Google Scholar] [CrossRef]
- Akkaya, R.; Ulusoy, U. Adsorptive features of chitosan entrapped in polyacrylamide hydrogel for Pb2+, UO22+, and Th4+. J. Hazard. Mater. 2008, 151, 380–388. [Google Scholar] [CrossRef]
- Ur Rehman, H.; Nawaz, M.A.; Pervez, S.; Jamal, M.; Attaullah, M.; Aman, A.; Ul Qader, S.A. Encapsulation of pectinase within polyacrylamide gel: Characterization of its catalytic properties for continuous industrial uses. Heliyon 2020, 6, e04578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).