Monolayer/Bilayer Equilibrium of Phospholipids in Gel or Liquid States: Interfacial Adsorption via Monomer or Liposome Diffusion?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Temperature Dependence of DPPC and DSPC and Cholesterol
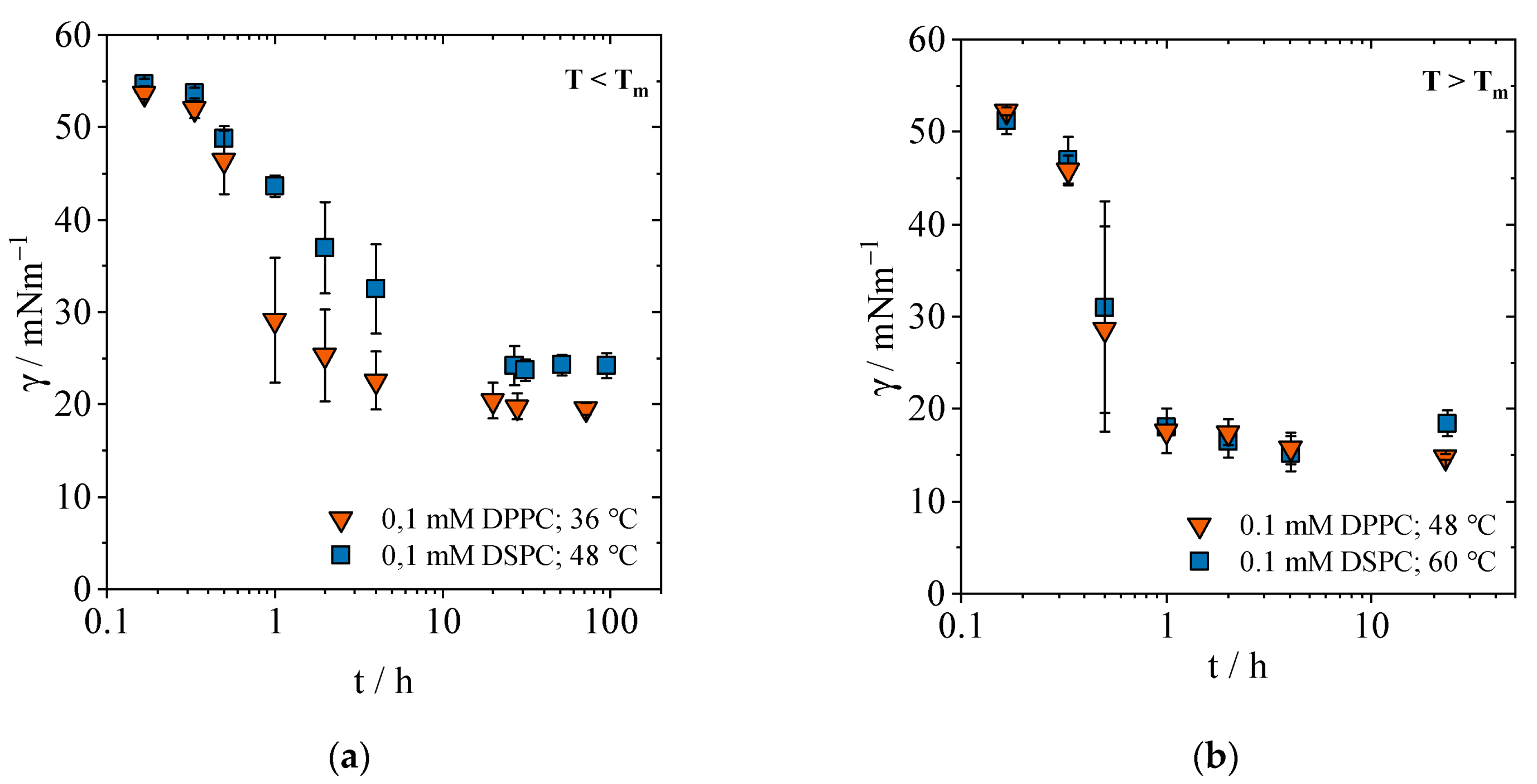
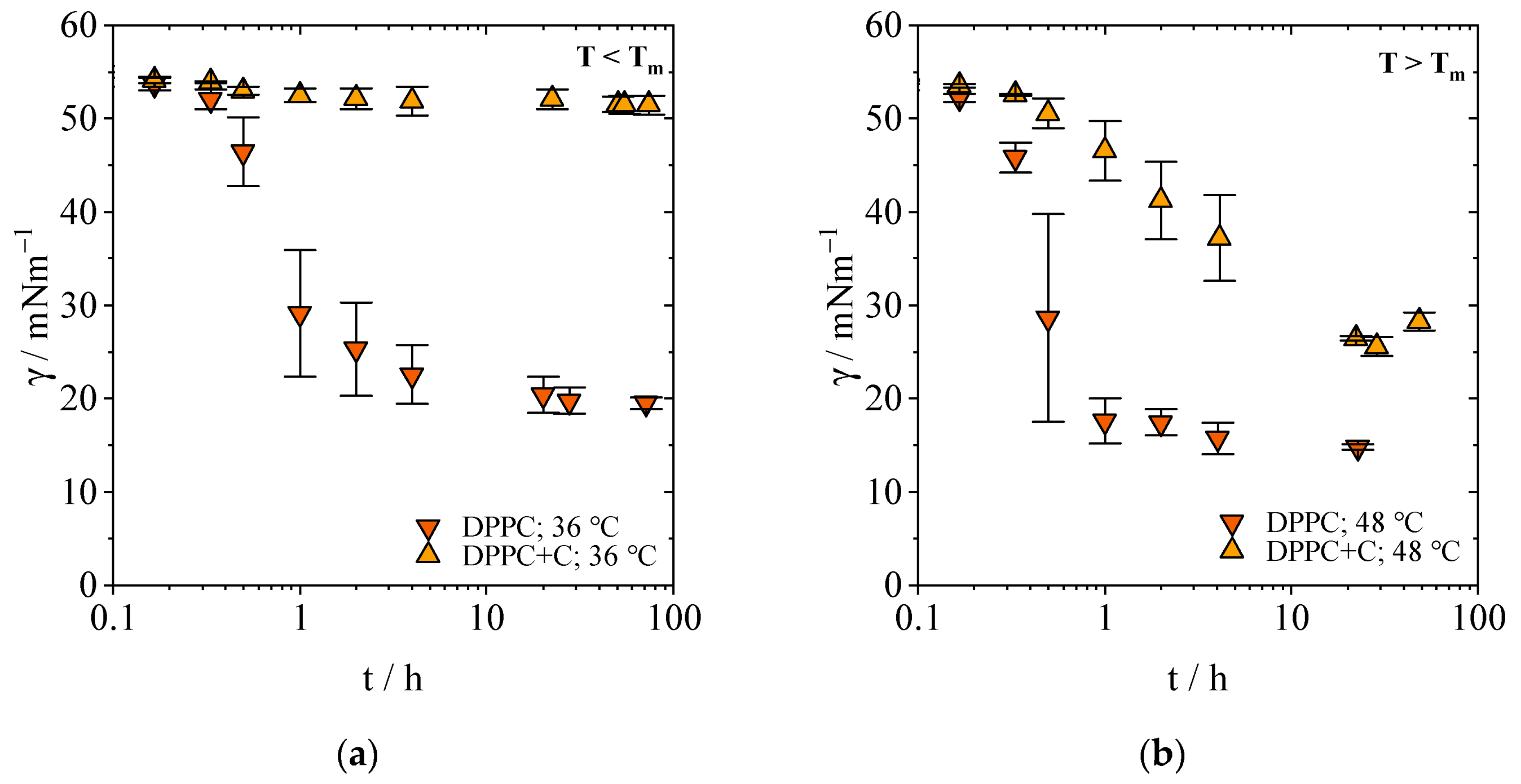
2.1.1. Equilibrium Interfacial Tensions
2.1.2. Concentration-Dependent Measurements
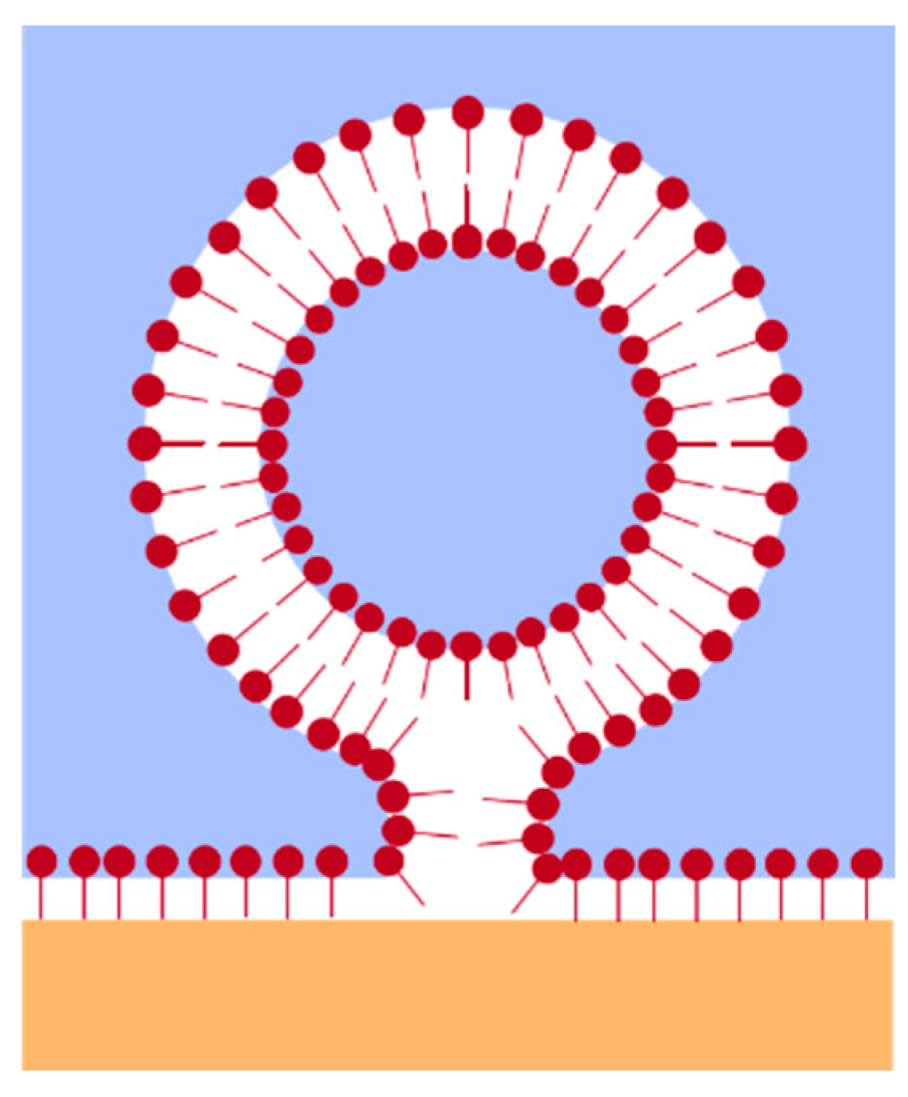
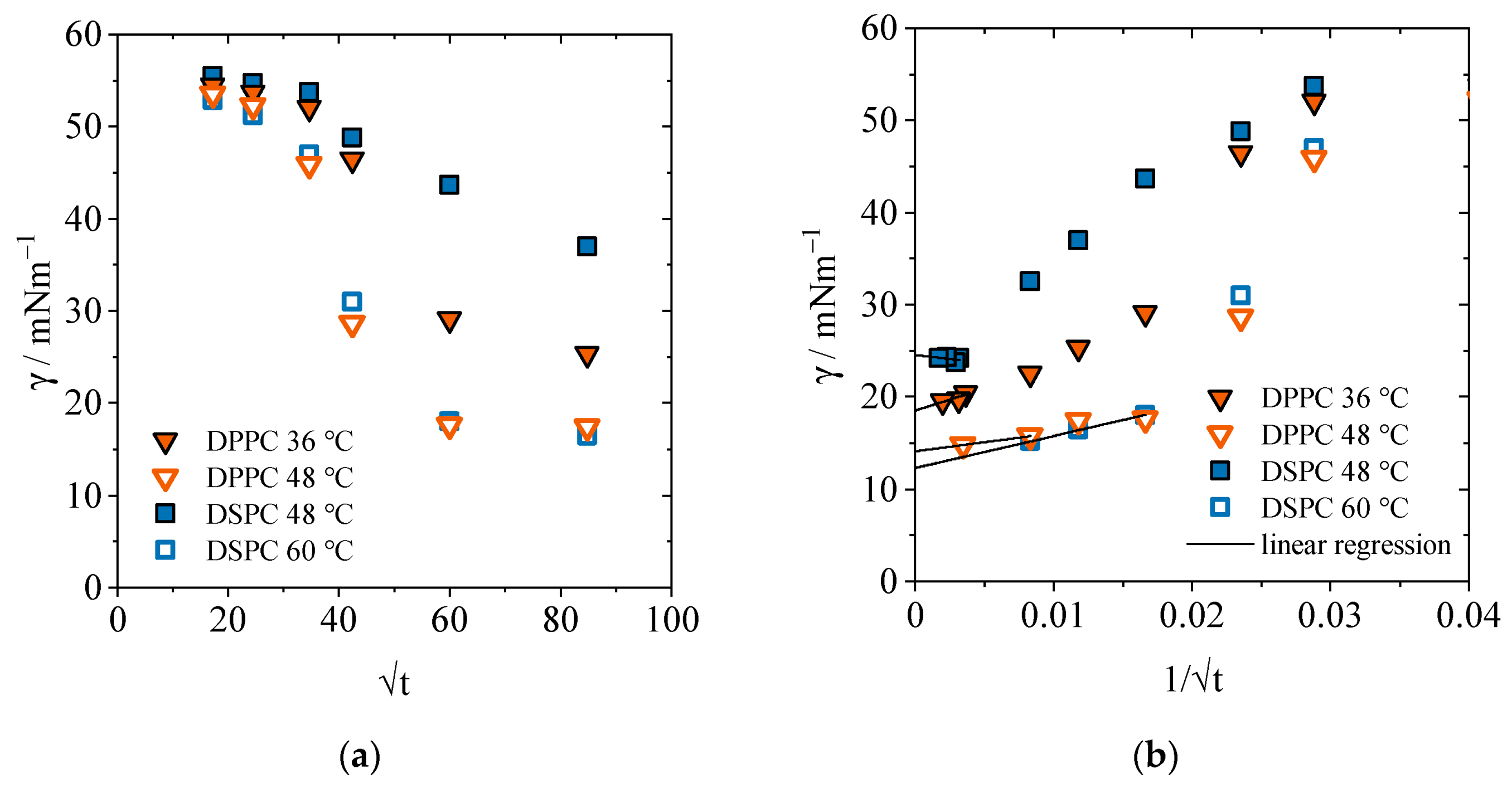
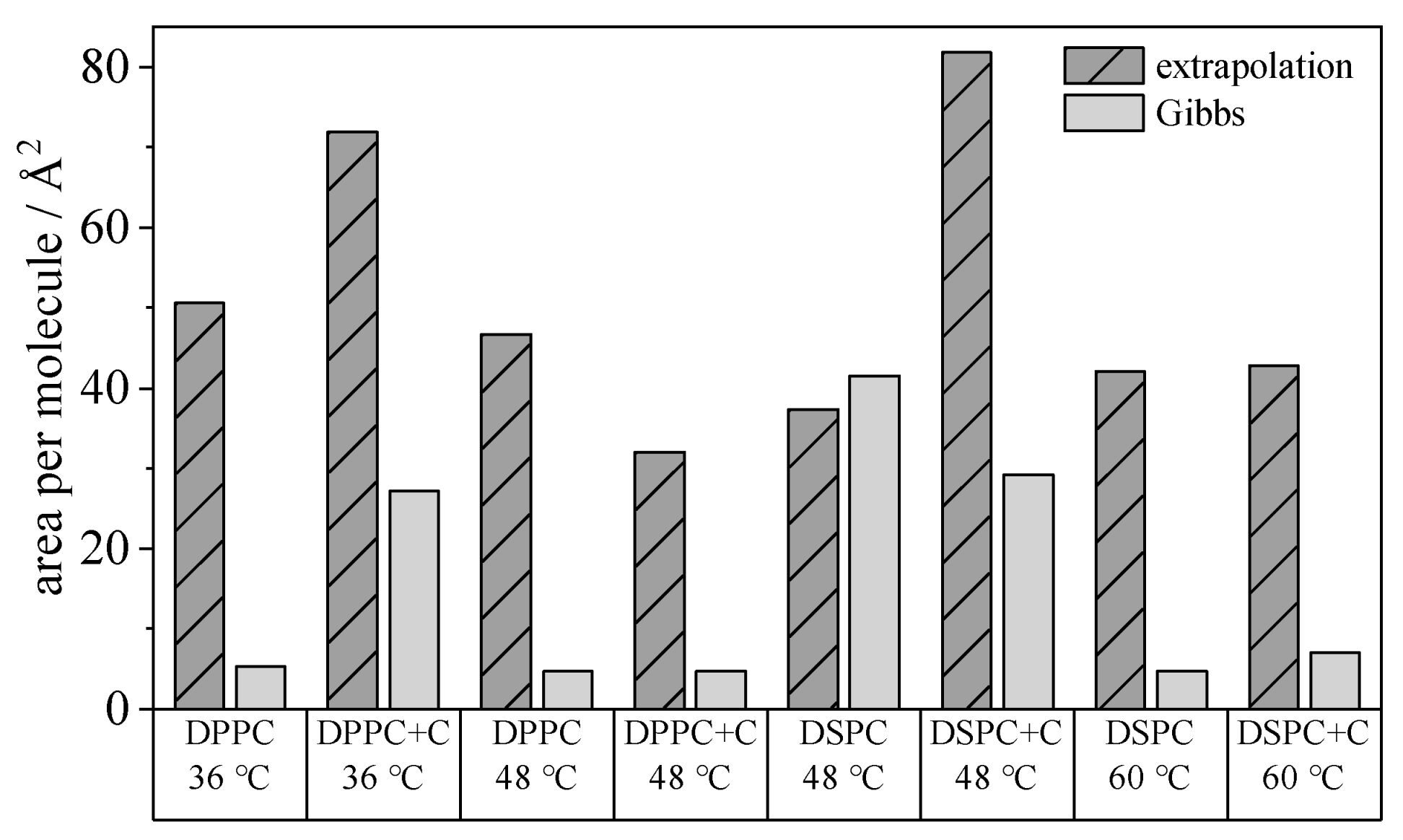
2.2. Concentration and Time-Dependent Calculation of the Area per Molecule
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Lipid Suspensions
4.3. Tensiometry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Hoogevest, P. Review—An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 175–193. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Lasic, D.D.; Frederik, P.M.; Stuart, M.C.A.; Barenholz, Y.; McIntosh, T.J. Gelation of liposome interior A novel method for drug encapsulation. FEBS Lett. 1992, 312, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.J. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature 1978, 274, 923–924. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Scie. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Klimuk, S.K.; Harasym, T.O.; Dos Santos, N.; Ansell, S.M.; Wong, K.F.; Maurer, N.; Stark, H.; Cullis, P.R.; Hope, M.J.; et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: Formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta. Biomembr. 2001, 1510, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Dass, C.R. Lipoplex-mediated delivery of nucleic acids: Factors affecting in vivo transfection. J. Mol. Med. 2004, 82, 579–591. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Q.; Wei, T.; Yu, X.; Johnson, L.T.; Farbiak, L.; Siegwart, D.J. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 2021, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neurotransmitter Release: The Last Millisecond in the Life of a Synaptic Vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Chorlay, A.; Forêt, L.; Thiam, A.R. Origin of gradients in lipid density and surface tension between connected lipid droplet and bilayer. Biophys. J. 2021, 120, 5491–5503. [Google Scholar] [CrossRef]
- Ullmann, K.; Leneweit, G.; Nirschl, H. How to Achieve High Encapsulation Efficiencies for Macromolecular and Sensitive APIs in Liposomes. Pharmaceutics 2021, 13, 691. [Google Scholar] [CrossRef]
- Ullmann, K.; Meier, M.; Benner, C.; Leneweit, G.; Nirschl, H. Water-in-Fluorocarbon Nanoemulsions Stabilized by Phospholipids and Characterized for Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8, 2001376. [Google Scholar] [CrossRef]
- Lauth, G.J.; Kowalczyk, J. Einführung in Die Physik und Chemie der Grenzflächen und Kolloide; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-47017-6. [Google Scholar]
- Marsh, D. Analysis of the chainlength dependence of lipid phase transition temperatures: Main and pretransitions of phosphatidylcholines; main and non-lamellar transitions of phosphatidylethanolamines. Biochim. Biophys. Acta. Biomembr. 1991, 1062, 1–6. [Google Scholar] [CrossRef]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug. Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Kučerka, N.; Nieh, M.-P.; Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta 2011, 1808, 2761–2771. [Google Scholar] [CrossRef]
- Leonenko, Z.V.; Finot, E.; Ma, H.; Dahms, T.E.S.; Cramb, D.T. Investigation of temperature-induced phase transitions in DOPC and DPPC phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys. J. 2004, 86, 3783–3793. [Google Scholar] [CrossRef]
- Purdon, A.D.; Tinker, D.O.; Neumann, A.W. The Temperature Dependence of Surface Tension and Critical Micelle Concentration of Egg Lysolecithin. Colloid Polym. Sci. 1980, 1980, 1062–1069. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Lu, G.; Miller, R.; Möhwald, H.; Li, J. Dynamic adsorption and characterization of phospholipid and mixed phospholipid/protein layers at liquid/liquid interfaces. Adv. Colloid Interface Sci. 2008, 140, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kabalnov, A.; Weers, J.; Arlauskas, R.; Tarara, T. Phospholipids as Emulsion Stabilizers. 1. Interfacial Tensions. Langmuir 1995, 11, 2966–2974. [Google Scholar] [CrossRef]
- Anastasiadis, S.H.; Gancarz, I.; Koberstein, J.T. Interfacial tension of immiscible polymer blends: Temperature and molecular weight dependence. Macromolecules 1988, 21, 2980–2987. [Google Scholar] [CrossRef]
- Banks, R.E.; Smart, B.E.; Tatlow, J.C. Organofluorine Chemistry: Principles and Commercial Applications; Plenum Press: New York, NY, USA; p. 1994. ISBN 1489912029.
- Krafft, M.P.; Riess, J.G. Highly fluorinated amphiphiles and colloidal systems, and their applications in the biomedical field. A contribution. Biochimie 1998, 80, 489–514. [Google Scholar] [CrossRef]
- Sadtler, V.M.; Krafft, M.P.; Riess, J.G. Achieving Stable, Reverse Water-in-Fluorocarbon Emulsions. Angew. Chem. Int. Ed. Engl. 1996, 35, 1976–1978. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Ling, J.; Yan, Y.; Hu, J.; Cheng, Y. The fluorination effect of fluoroamphiphiles in cytosolic protein delivery. Nat. Commun. 2018, 9, 1377. [Google Scholar] [CrossRef]
- Riess, J.G. Highly fluorinated systems for oxygen transport, diagnosis and drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 1994, 84, 33–48. [Google Scholar] [CrossRef]
- Ullmann, K.; Poggemann, L.; Nirschl, H.; Leneweit, G. Adsorption process for phospholipids of different chain lengths at a fluorocarbon/water interface studied by Du Noüy ring and spinning drop. Colloid Polym. Sci. 2020, 298, 407–417. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.H.; Needham, D. Equilibrium and Dynamic Interfacial Tension Measurements at Microscopic Interfaces Using a Micropipet Technique. 2. Dynamics of Phospholipid Monolayer Formation and Equilibrium Tensions at the Water−Air Interface. Langmuir 2001, 17, 5544–5550. [Google Scholar] [CrossRef]
- Pan, J.; Tristram-Nagle, S.; Nagle, J.F. Effect of cholesterol on structural and mechanical properties of membranes depends on lipid chain saturation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2009, 80, 21931. [Google Scholar] [CrossRef] [PubMed]
- Tamai, N.; Uemura, M.; Takeichi, T.; Goto, M.; Matsuki, H.; Kaneshina, S. A new interpretation of eutectic behavior for distearoylphosphatidylcholine-cholesterol binary bilayer membrane. Biophys. Chem. 2008, 135, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Vist, M.R.; Davis, J.H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: Deuterium nuclear magnetic resonance and differential scanning calorimetry. Biochemistry 1990, 29, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Lönnfors, M.; Peter Slotte, J.; Kato, S. A detailed analysis of partial molecular volumes in DPPC/cholesterol binary bilayers. Biochim. Biophys. Acta. Biomembr. 2014, 1838, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Wilfling, F.; Thiam, A.R.; Olarte, M.-J.; Wang, J.; Beck, R.; Gould, T.J.; Allgeyer, E.S.; Pincet, F.; Bewersdorf, J.; Farese Robert, V., Jr.; et al. Arf1/COPI machinery acts directly on lipid droplets and enables their connection to the ER for protein targeting. eLife 2014, 3, e01607. [Google Scholar] [CrossRef]
- Anglin, T.C.; Conboy, J.C. Lateral pressure dependence of the phospholipid transmembrane diffusion rate in planar-supported lipid bilayers. Biophys. J. 2008, 95, 186–193. [Google Scholar] [CrossRef]
- Mansour, H.M.; Zografi, G. Relationships between Equilibrium Spreading Pressure and Phase Equilibria of Phospholipid Bilayers and Monolayers at the Air−Water Interface. Langmuir 2007, 23, 3809–3819. [Google Scholar] [CrossRef]
- Marsh, D. Lateral pressure in membranes. Biochim. Et Biophys. Acta (BBA) Rev. Biomembr. 1996, 1286, 183–223. [Google Scholar] [CrossRef]
- Li, J.; Miller, R.; Möhwald, H. Characterisation of phospholipid layers at liquid interfaces 2. Comparison of isotherms of insoluble and soluble films of phospholipids at different fluid/water interfaces. Colloids Surf. A Physicochem. Eng. Asp. 1996, 114, 123–130. [Google Scholar] [CrossRef]
- Hildebrandt, E.; Dessy, A.; Sommerling, J.-H.; Guthausen, G.; Nirschl, H.; Leneweit, G. Interactions between Phospholipids and Organic Phases: Insights into Lipoproteins and Nanoemulsions. Langmuir 2016, 32, 5821–5829. [Google Scholar] [CrossRef]
- Schlossman, M.L.; Tikhonov, A.M. Molecular Ordering and Phase Behavior of Surfactants at Water-Oil Interfaces as Probed by X-Ray Surface Scattering. Annu. Rev. Phys. Chem. 2008, 59, 153–177. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Kolpakov, A.F. Phospholipids at the oil/water interface: Adsorption and interfacial phenomena in an electric field. Adv. Colloid Interface Sci. 1991, 35, 31–138. [Google Scholar] [CrossRef]
- Ward, A.F.H.; Tordai, L. Time-Dependence of Boundary Tensions of Solutions I. Role Diffus. Time-Effects. J. Chem. Phys. 1946, 14, 453–461. [Google Scholar] [CrossRef]
- van Hunsel, J.; Joos, P. Adsorption kinetics at the oil/water interface. Colloids Surf. 1987, 24, 139–158. [Google Scholar] [CrossRef]
- Leekumjorn, S.; Sum, A.K. Molecular Simulation Study of Structural and Dynamic Properties of Mixed DPPC/DPPE Bilayers. Biophys. J. 2006, 90, 3951–3965. [Google Scholar] [CrossRef] [PubMed]
- Demel, R.A.; van Deenen, L.L.M.; Pethica, B.A. Monolayer Interactions of Phospholipids and Cholesterol. Biochim. Biophys. Acta 1967, 135, 11–19. [Google Scholar] [CrossRef]
- Hildebrandt, E.; Sommerling, J.-H.; Guthausen, G.; Zick, K.; Stürmer, J.; Nirschl, H.; Leneweit, G. Phospholipid adsorption at oil in water versus water in oil interfaces: Implications for interfacial densities and bulk solubilities. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 56–63. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The Colorimetric Determination of Phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]

| Temperature/°C | tε,DPPC/h | tε,DPPC+C/h | tε,DSPC/h | tε,DSPC+C/h |
|---|---|---|---|---|
| 20 | <48 1 | <48 | <168 1 | <168 |
| 36 | <48 | <48 | - | - |
| 48 | <24 | <48 | <72 | <168 |
| 60 | - | - | <24 | <144 |
| Hypothesis H0 | df | Critical t-Value | Calculated t-Value | p-Value |
|---|---|---|---|---|
| (DPPC) = (DSPC) | 6 | 2.36 | −2.07 | 0.079 |
| (DPPC+C) = (DSPC+C) | 7 | 2.44 | 0.14 | 0.89 |
| Phospholipid (Temperature) | MBE/mM | Amin/Å2 [Equation (3)] | /mN m−1 | ∏M/mN/m |
|---|---|---|---|---|
| DPPC (36 °C) | 0.100 | 5.3 | 19.31 ± 0.93 | 35.69 |
| DPPC (48 °C) | 0.101 | 4.7 | 13.67 ± 0.45 | 41.33 |
| DPPC+C (36 °C) | 0.530 | 27.1 | 26.35 ± 1.81 | 28.65 |
| DPPC+C (48 °C) | 0.101 | 4.8 | 24.28 ± 3.73 | 30.72 |
| DSPC (48 °C) | 0.101 | 41.6 | 24.16 ± 1.47 | 30.84 |
| DSPC (60 °C) | 0.103 | 4.7 | 14.34 ± 1.34 | 40.66 |
| DSPC+C (48 °C) | 0.934 | 29.2 | 25.33 ± 1.62 | 29.67 |
| DSPC+C (60 °C) | 0.110 | 7.0 | 23.28 ± 4.34 | 31.72 |
| Phospholipid (Temperature) | D/ m2 s−1 | Amin, E /Å2 | Amin,G /Å2 | /mN m−1 | /mN m−1 | /mN m−1 |
|---|---|---|---|---|---|---|
| DPPC (36 °C) | 2.43 × 10−13 | 50.6 | 5.3 | 18.50 ± 0.72 | 19.05 ± 0.50 | 19.31 ± 0.93 |
| DPPC (48 °C) | 2.30 × 10−12 | 46.7 | 4.7 | 14.11 ± 0.00 | 14.81 ± 0.33 | 13.67 ± 0.45 |
| DPPC+C (36 °C) | 7.51× 10−14 | 71.9 | 27.1 | 51.44 ± 0.13 | 51.44 ± 1.00 | 26.35 ± 1.81 |
| DPPC+C (48 °C) | 7.70× 10−14 | 32.1 | 4.8 | 21.74 ± 1.69 | 28.28 ± 0.95 | 24.28 ± 3.73 |
| DSPC (48 °C) | 1.14 × 10−13 | 37.4 | 41.6 | 24.53 ± 0.60 | 24.19 ± 1.08 | 24.16 ± 1.47 |
| DSPC (60 °C) | 1.23 × 10−12 | 42.1 | 4.7 | 12.31 ± 0.10 | 18.40 ± 1.40 | 14.34 ± 1.34 |
| DSPC+C (48 °C) | 8.30 × 10−14 | 81.8 | 29.2 | 51.26± 0.17 | 52.16 ± 0.64 | 25.33 ± 1.62 |
| DSPC+C (60 °C) | 1.57 × 10−15 | 42.9 | 7.0 | 16.27 ± 0.82 | 28.72 ± 0.86 | 23.28 ± 4.34 |
| Phospholipid | Tm/°C | T1 < Tm/°C | T2 > Tm/°C |
|---|---|---|---|
| DPPC | 41 | 36 | 48 |
| DSPC | 55 | 48 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullmann, K.; Fachet, L.; Nirschl, H.; Leneweit, G. Monolayer/Bilayer Equilibrium of Phospholipids in Gel or Liquid States: Interfacial Adsorption via Monomer or Liposome Diffusion? Gels 2023, 9, 803. https://doi.org/10.3390/gels9100803
Ullmann K, Fachet L, Nirschl H, Leneweit G. Monolayer/Bilayer Equilibrium of Phospholipids in Gel or Liquid States: Interfacial Adsorption via Monomer or Liposome Diffusion? Gels. 2023; 9(10):803. https://doi.org/10.3390/gels9100803
Chicago/Turabian StyleUllmann, Kirsten, Lea Fachet, Hermann Nirschl, and Gero Leneweit. 2023. "Monolayer/Bilayer Equilibrium of Phospholipids in Gel or Liquid States: Interfacial Adsorption via Monomer or Liposome Diffusion?" Gels 9, no. 10: 803. https://doi.org/10.3390/gels9100803
APA StyleUllmann, K., Fachet, L., Nirschl, H., & Leneweit, G. (2023). Monolayer/Bilayer Equilibrium of Phospholipids in Gel or Liquid States: Interfacial Adsorption via Monomer or Liposome Diffusion? Gels, 9(10), 803. https://doi.org/10.3390/gels9100803






