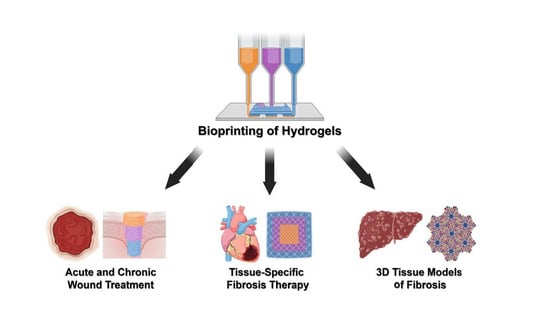
Bioprinted Hydrogels for Fibrosis and Wound Healing: Treatment and Modeling
Abstract
1. Introduction
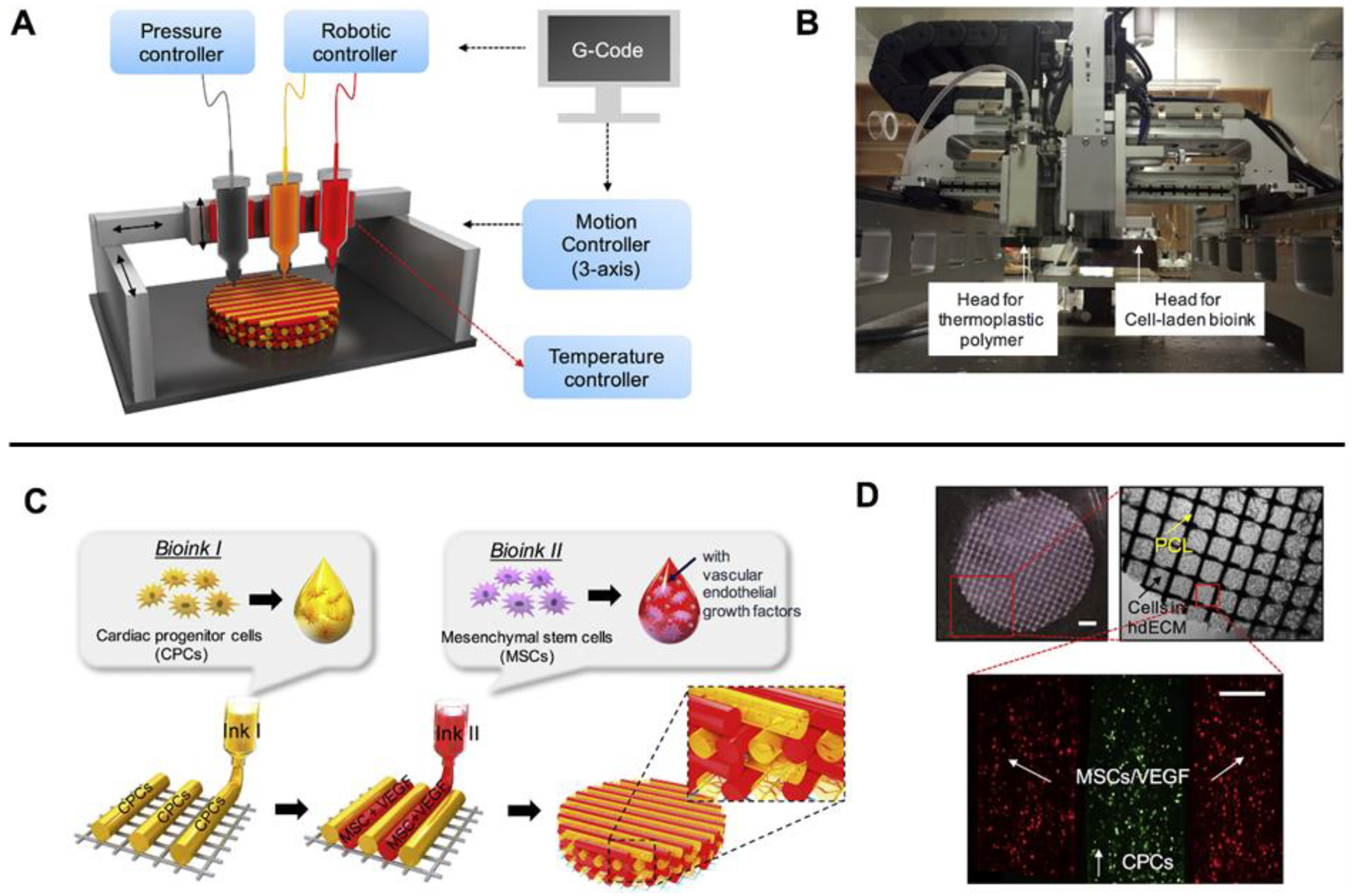
2. Bioprinting Methodologies
3. Treatment of Fibrosis with Bioprinted Hydrogels
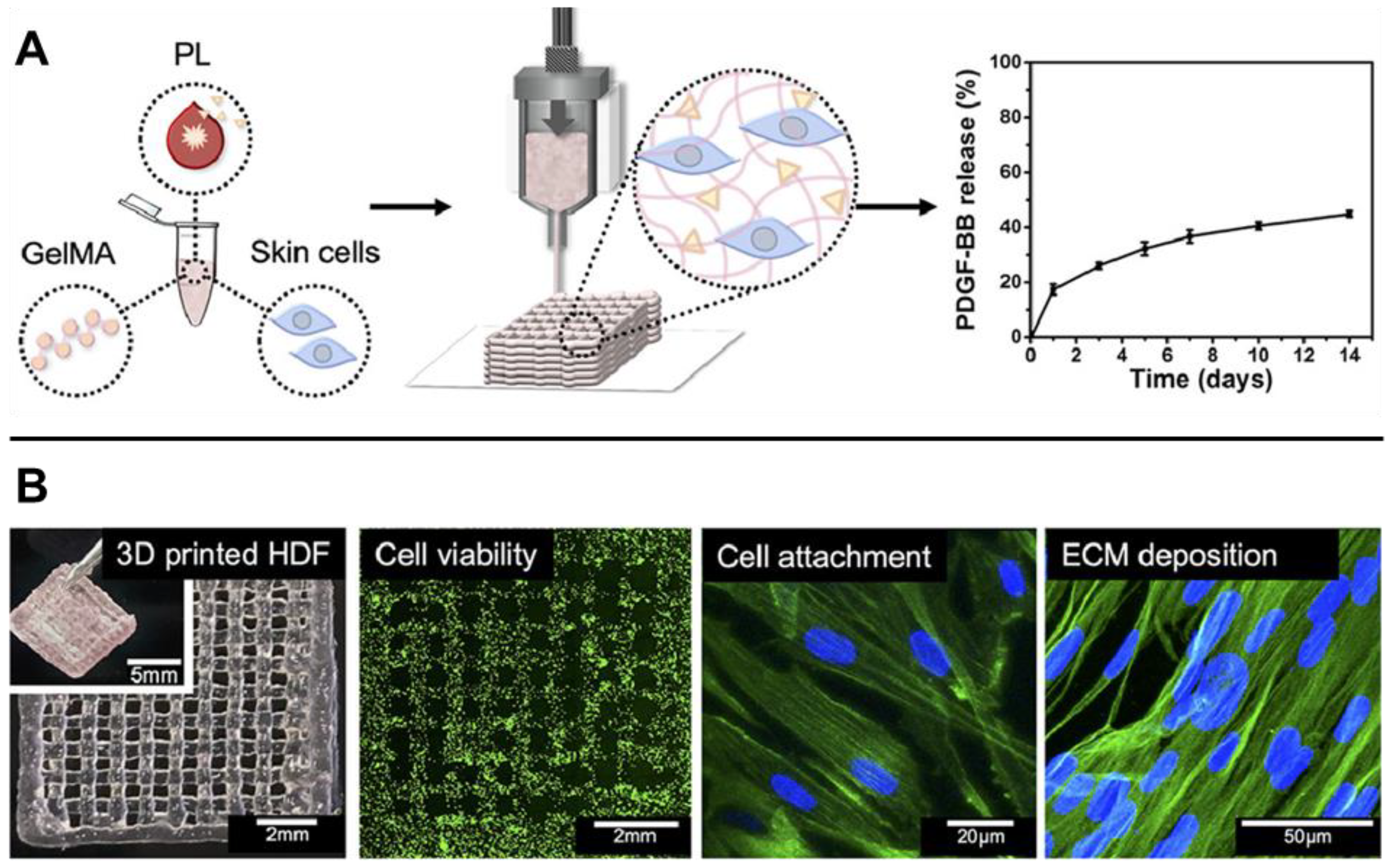
3.1. Dermal Fibrosis
3.2. Cardiac Fibrosis
3.3. Intrauterine Adhesions
3.4. Challenges and Future Directions
4. Wound Healing Using Bioprinted Hydrogels
4.1. Acute Wounds
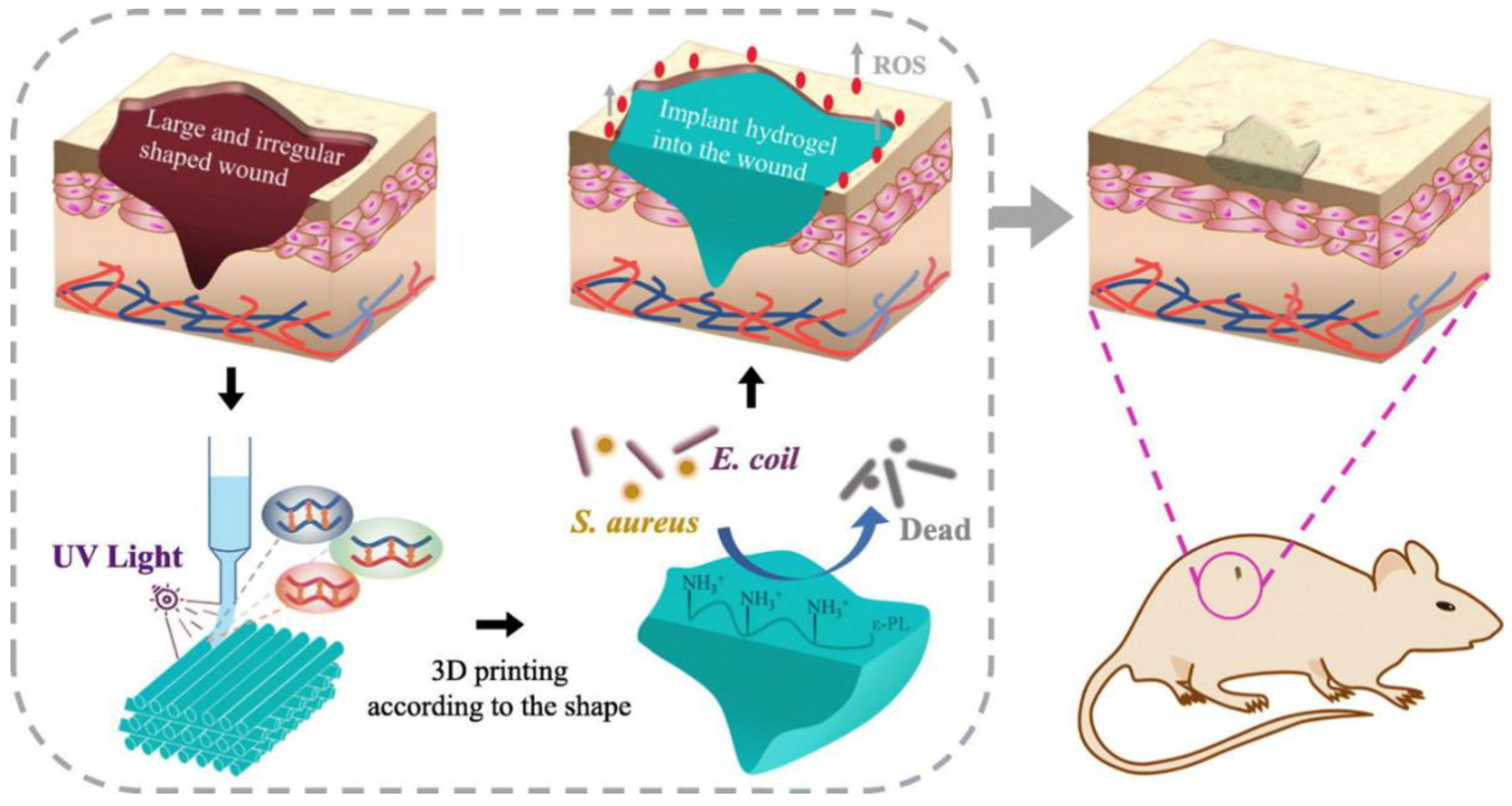
4.2. Chronic Wounds
4.3. Challenges and Future Directions
5. In Vitro Modeling of Fibrosis Using Bioprinted Hydrogels
5.1. Dermal Models
5.2. Cardiac Models
5.3. Liver Models
5.4. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- desJardins-Park, H.E.; Mascharak, S.; Chinta, M.S.; Wan, D.C.; Longaker, M.T. The Spectrum of Scarring in Craniofacial Wound Repair. Front. Physiol. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.K.; Lee, J.; Beatty, G.L. Paracrine and Cell Autonomous Signalling in Pancreatic Cancer Progression and Metastasis. EBioMedicine 2020, 53, 102662. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.-G.; Liu, X.; Shen, H. Electrospun Hierarchical Structural Films for Effective Wound Healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, H.; Wang, H.; Zaldivar-Silva, D.; Agüero, L.; Liu, Y.; Zhang, Z.; Yin, Y.; Qiu, B.; Zhao, J. An Injectable Anti-Microbial and Adhesive Hydrogel for the Effective Noncompressible Visceral Hemostasis and Wound Repair. Mater. Sci. Eng. C 2021, 129, 112422. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, X.; Fu, J.; Yu, D.-G.; Liu, Y. Recent Progress in Electrospun Polyacrylonitrile Nanofiber-Based Wound Dressing. Polymers 2022, 14, 3266. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Gorkin Iii, R. An Overview of the Suitability of Hydrogel-Forming Polymers for Extrusion-Based 3D-Printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef]
- Jang, T.-S.; Jung, H.-D.; Pan, H.M.; Han, W.T.; Chen, S.; Song, J. 3D Printing of Hydrogel Composite Systems: Recent Advances in Technology for Tissue Engineering. Int. J. Bioprinting 2018, 4, 126. [Google Scholar] [CrossRef]
- Bittner, S.M.; Guo, J.L.; Mikos, A.G. Spatiotemporal Control of Growth Factors in Three-Dimensional Printed Scaffolds. Bioprinting 2018, 12, e00032. [Google Scholar] [CrossRef]
- Bittner, S.M.; Pearce, H.A.; Hogan, K.J.; Smoak, M.M.; Guo, J.L.; Melchiorri, A.J.; Scott, D.W.; Mikos, A.G. Swelling Behaviors of 3D Printed Hydrogel and Hydrogel-Microcarrier Composite Scaffolds. Tissue Eng. Part A 2021, 27, 665–678. [Google Scholar] [CrossRef]
- Bittner, S.M.; Guo, J.L.; Melchiorri, A.; Mikos, A.G. Three-Dimensional Printing of Multilayered Tissue Engineering Scaffolds. Mater. Today 2018, 21, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D. Melt Electrowriting with Additive Manufacturing Principles. Curr. Opin. Biomed. Eng. 2017, 2, 49–57. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-Dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Diaz-Gomez, L.; Xie, V.Y.; Bittner, S.M.; Jiang, E.Y.; Wang, B.; Mikos, A.G. Three-Dimensional Printing of Click Functionalized, Peptide Patterned Scaffolds for Osteochondral Tissue Engineering. Bioprinting 2021, 22, e00136. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A.; de la Guardia, M. Hydrogel-based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol. J. 2020, 15, 2000095. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the Mechanical Enhancement of Hydrogels: Fabrication Strategies and Underlying Mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Tang, D.; Tare, R.S.; Yang, L.-Y.; Williams, D.F.; Ou, K.-L.; Oreffo, R.O. Biofabrication of Bone Tissue: Approaches, Challenges and Translation for Bone Regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef]
- Mascharak, S.; desJardins-Park, H.E.; Davitt, M.F.; Griffin, M.; Borrelli, M.R.; Moore, A.L.; Chen, K.; Duoto, B.; Chinta, M.; Foster, D.S. Preventing Engrailed-1 Activation in Fibroblasts Yields Wound Regeneration without Scarring. Science 2021, 372, eaba2374. [Google Scholar] [CrossRef]
- Mascharak, S.; Talbott, H.E.; Januszyk, M.; Griffin, M.; Chen, K.; Davitt, M.F.; Demeter, J.; Henn, D.; Bonham, C.A.; Foster, D.S. Multi-Omic Analysis Reveals Divergent Molecular Events in Scarring and Regenerative Wound Healing. Cell Stem Cell 2022, 29, 315–327.e6. [Google Scholar] [CrossRef]
- Joshi, N.; Watanabe, S.; Verma, R.; Jablonski, R.P.; Chen, C.-I.; Cheresh, P.; Markov, N.S.; Reyfman, P.A.; McQuattie-Pimentel, A.C.; Sichizya, L. A Spatially Restricted Fibrotic Niche in Pulmonary Fibrosis Is Sustained by M-CSF/M-CSFR Signalling in Monocyte-Derived Alveolar Macrophages. Eur. Respir. J. 2020, 55, 1900646. [Google Scholar] [CrossRef]
- Chen, L.; Li, Z.; Zheng, Y.; Zhou, F.; Zhao, J.; Zhai, Q.; Zhang, Z.; Liu, T.; Chen, Y.; Qi, S. 3D-Printed Dermis-Specific Extracellular Matrix Mitigates Scar Contraction via Inducing Early Angiogenesis and Macrophage M2 Polarization. Bioact. Mater. 2022, 10, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, R.I.; Do Amaral, R.J.; Reis, R.L.; Marques, A.P.; Murphy, C.M.; O’Brien, F.J. 3D-Printed Gelatin Methacrylate Scaffolds with Controlled Architecture and Stiffness Modulate the Fibroblast Phenotype towards Dermal Regeneration. Polymers 2021, 13, 2510. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, R.I.; do Amaral, R.J.; Simpson, C.R.; Casey, S.M.; Reis, R.L.; Marques, A.P.; Murphy, C.M.; O’Brien, F.J. 3D Printed Scaffolds Incorporated with Platelet-Rich Plasma Show Enhanced Angiogenic Potential While Not Inducing Fibrosis. Adv. Funct. Mater. 2022, 32, 2109915. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The Pathogenesis of Cardiac Fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Huo, D.; Li, Y.; Zhao, X.; Li, Y.; Qin, Z.; Sun, D.; Yang, G.; Yang, M.; Tan, J. Engineering HiPSC-CM and HiPSC-EC Laden 3D Nanofibrous Splenic Hydrogel for Improving Cardiac Function through Revascularization and Remuscularization in Infarcted Heart. Bioact. Mater. 2021, 6, 4415–4429. [Google Scholar] [CrossRef]
- Melhem, M.R.; Park, J.; Knapp, L.; Reinkensmeyer, L.; Cvetkovic, C.; Flewellyn, J.; Lee, M.K.; Jensen, T.W.; Bashir, R.; Kong, H. 3D Printed Stem-Cell-Laden, Microchanneled Hydrogel Patch for the Enhanced Release of Cell-Secreting Factors and Treatment of Myocardial Infarctions. ACS Biomater. Sci. Eng. 2017, 3, 1980–1987. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H. 3D Printed Complex Tissue Construct Using Stem Cell-Laden Decellularized Extracellular Matrix Bioinks for Cardiac Repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Deans, R.; Abbott, J. Review of Intrauterine Adhesions. J. Minim. Invasive Gynecol. 2010, 17, 555–569. [Google Scholar] [CrossRef]
- Feng, M.; Hu, S.; Qin, W.; Tang, Y.; Guo, R.; Han, L. Bioprinting of a Blue Light-Cross-Linked Biodegradable Hydrogel Encapsulating Amniotic Mesenchymal Stem Cells for Intrauterine Adhesion Prevention. ACS Omega 2021, 6, 23067–23075. [Google Scholar] [CrossRef]
- Wen, J.; Hou, B.; Lin, W.; Guo, F.; Cheng, M.; Zheng, J.; He, P.; Ji, W. 3D-Printed Hydrogel Scaffold-Loaded Granulocyte Colony-Stimulating Factor Sustained-Release Microspheres and Their Effect on Endometrial Regeneration. Biomater. Sci. 2022, 10, 3346–3358. [Google Scholar] [CrossRef]
- Ji, W.; Hou, B.; Lin, W.; Wang, L.; Zheng, W.; Li, W.; Zheng, J.; Wen, X.; He, P. 3D Bioprinting a Human IPSC-Derived MSC-Loaded Scaffold for Repair of the Uterine Endometrium. Acta Biomater. 2020, 116, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Rustad, K.C.; Akaishi, S.; Sorkin, M.; Glotzbach, J.P.; Januszyk, M.; Nelson, E.R.; Levi, K.; Paterno, J.; Vial, I.N. Focal Adhesion Kinase Links Mechanical Force to Skin Fibrosis via Inflammatory Signaling. Nat. Med. 2012, 18, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin Tissue Engineering—In Vivo and in Vitro Applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Clohessy, R.M.; Holder, R.C.; Gabard, A.R.; Herendeen, G.J.; Christy, R.J.; Burnett, L.R.; Fisher, J.P. In Vivo Evaluation of Three-Dimensional Printed, Keratin-Based Hydrogels in a Porcine Thermal Burn Model. Tissue Eng. Part A 2020, 26, 265–278. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Zhao, B.; Xie, X.; Mohamed, E.-N.; Hany, E.-H.; Morsi, Y.; Li, J.; Pan, J.; Mo, X. A Multifunctional Nanofiber Reinforced Photo-Crosslinking Hydrogel for Skin Wound Healing. Compos. Part B Eng. 2022, 247, 110294. [Google Scholar] [CrossRef]
- Daikuara, L.Y.; Yue, Z.; Skropeta, D.; Wallace, G.G. In Vitro Characterisation of 3D Printed Platelet Lysate-Based Bioink for Potential Application in Skin Tissue Engineering. Acta Biomater. 2021, 123, 286–297. [Google Scholar] [CrossRef]
- Jang, M.J.; Bae, S.K.; Jung, Y.S.; Kim, J.C.; Kim, J.S.; Park, S.K.; Suh, J.S.; Yi, S.J.; Ahn, S.H.; Lim, J.O. Enhanced Wound Healing Using a 3D Printed VEGF-Mimicking Peptide Incorporated Hydrogel Patch in a Pig Model. Biomed. Mater. 2021, 16, 045013. [Google Scholar] [CrossRef]
- Cleetus, C.M.; Primo, F.A.; Fregoso, G.; Raveendran, N.L.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B. Alginate Hydrogels with Embedded ZnO Nanoparticles for Wound Healing Therapy. Int. J. Nanomed. 2020, 15, 5097. [Google Scholar] [CrossRef]
- Wu, Z.; Hong, Y. Combination of the Silver–Ethylene Interaction and 3D Printing to Develop Antibacterial Superporous Hydrogels for Wound Management. ACS Appl. Mater. Interfaces 2019, 11, 33734–33747. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, J.; Zhang, W.; Pu, Y.; Yang, R.; Wang, P.; Liu, S.; Tan, X.; Chi, B. 3D-Printed Antioxidant Antibacterial Carboxymethyl Cellulose/ε-Polylysine Hydrogel Promoted Skin Wound Repair. Int. J. Biol. Macromol. 2021, 187, 91–104. [Google Scholar] [CrossRef] [PubMed]
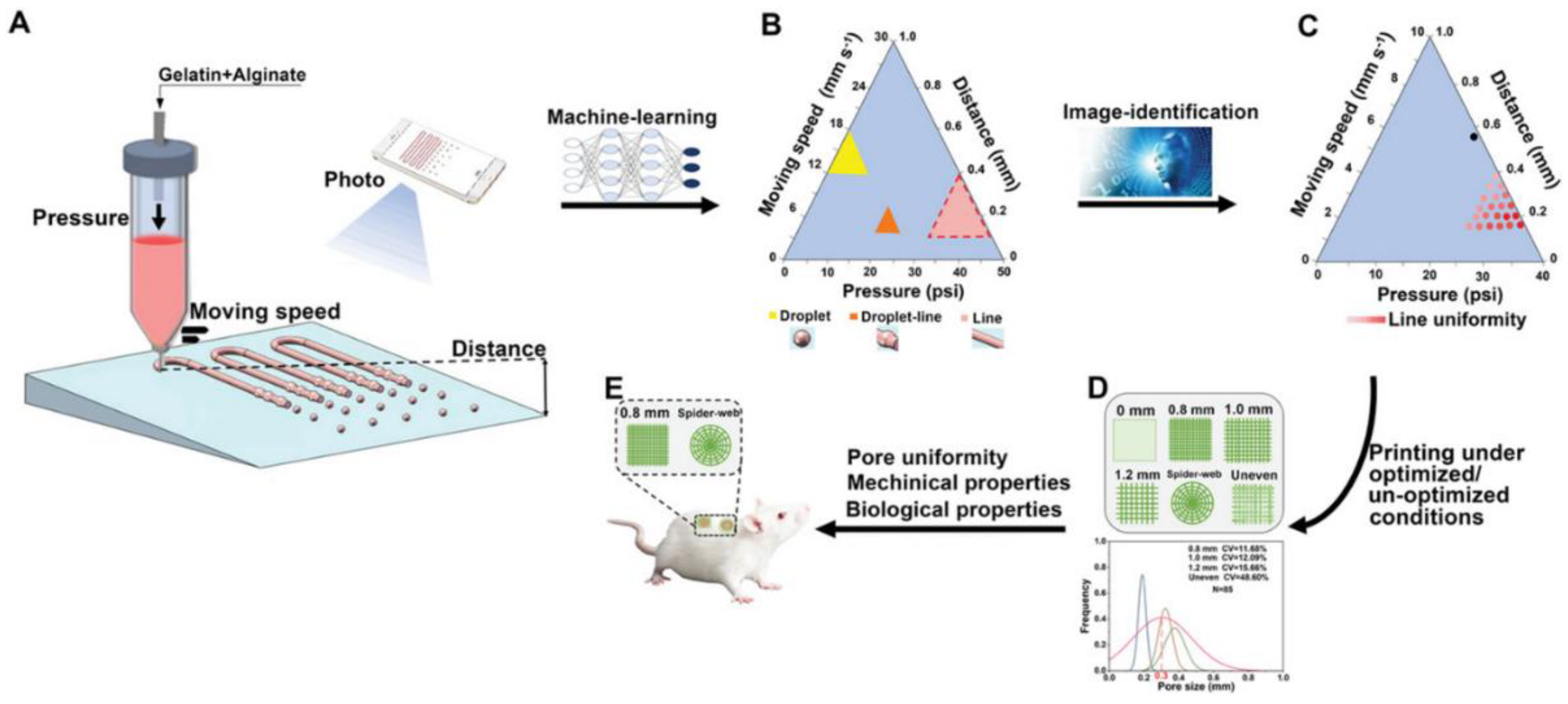
- Chen, B.; Dong, J.; Ruelas, M.; Ye, X.; He, J.; Yao, R.; Fu, Y.; Liu, Y.; Hu, J.; Wu, T. Artificial Intelligence-Assisted High-Throughput Screening of Printing Conditions of Hydrogel Architectures for Accelerated Diabetic Wound Healing. Adv. Funct. Mater. 2022, 32, 2201843. [Google Scholar] [CrossRef]
- Chae, H.J.; Lee, S.; Son, H.; Han, S.; Lim, T. Generating 3D Bio-Printable Patches Using Wound Segmentation and Reconstruction to Treat Diabetic Foot Ulcers. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, New Orleans, LA, USA, 18–24 June 2022; pp. 2539–2549. [Google Scholar]
- Guo, J.L.; Januszyk, M.; Longaker, M.T. Machine Learning in Tissue Engineering. Tissue Eng. Part A 2022. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, M.; Bansal, R.; Rouwkema, J. Bioengineered 3D Models to Recapitulate Tissue Fibrosis. Trends Biotechnol. 2020, 38, 623–636. [Google Scholar] [CrossRef]
- Davidson, M.D.; Burdick, J.A.; Wells, R.G. Engineered Biomaterial Platforms to Study Fibrosis. Adv. Healthc. Mater. 2020, 9, 1901682. [Google Scholar] [CrossRef]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef]
- Shin, Y.J.; Shafranek, R.T.; Tsui, J.H.; Walcott, J.; Nelson, A.; Kim, D.-H. 3D Bioprinting of Mechanically Tuned Bioinks Derived from Cardiac Decellularized Extracellular Matrix. Acta Biomater. 2021, 119, 75–88. [Google Scholar] [CrossRef]
- Norona, L.M.; Nguyen, D.G.; Gerber, D.A.; Presnell, S.C.; LeCluyse, E.L. Editor’s Highlight: Modeling Compound-Induced Fibrogenesis in Vitro Using Three-Dimensional Bioprinted Human Liver Tissues. Toxicol. Sci. 2016, 154, 354–367. [Google Scholar] [CrossRef]
- de Hilster, R.H.J.; Sharma, P.K.; Jonker, M.R.; White, E.S.; Gercama, E.A.; Roobeek, M.; Timens, W.; Harmsen, M.C.; Hylkema, M.N.; Burgess, J.K. Human Lung Extracellular Matrix Hydrogels Resemble the Stiffness and Viscoelasticity of Native Lung Tissue. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L698–L704. [Google Scholar] [CrossRef]
- Bersini, S.; Gilardi, M.; Ugolini, G.S.; Sansoni, V.; Talo, G.; Perego, S.; Zanotti, S.; Ostano, P.; Mora, M.; Soncini, M. Engineering an Environment for the Study of Fibrosis: A 3D Human Muscle Model with Endothelium Specificity and Endomysium. Cell Rep. 2018, 25, 3858–3868.e4. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.J.; Jones, J.D.; Woessner, A.E.; Quinn, K.P. Skin Structure–Function Relationships and the Wound Healing Response to Intrinsic Aging. Adv. Wound Care 2020, 9, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, J.-S.; Gao, G.; Cho, D.-W. Direct 3D Cell-Printing of Human Skin with Functional Transwell System. Biofabrication 2017, 9, 025034. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.; Dongzhen, Z.; Xiaoli, C.; Wei, S.; Zhao, L.; Tian, H.; Ping, Z.; Jianjun, L.; Yuzhen, W.; Yijie, Z. Modeling Human Hypertrophic Scars with 3D Preformed Cellular Aggregates Bioprinting. Bioact. Mater. 2022, 10, 247–254. [Google Scholar] [CrossRef]
- Daly, A.C.; Davidson, M.D.; Burdick, J.A. 3D Bioprinting of High Cell-Density Heterogeneous Tissue Models through Spheroid Fusion within Self-Healing Hydrogels. Nat. Commun. 2021, 12, 753. [Google Scholar] [CrossRef]
- Valverde, I.; Gomez-Ciriza, G.; Hussain, T.; Suarez-Mejias, C.; Velasco-Forte, M.N.; Byrne, N.; Ordoñez, A.; Gonzalez-Calle, A.; Anderson, D.; Hazekamp, M.G. Three-Dimensional Printed Models for Surgical Planning of Complex Congenital Heart Defects: An International Multicentre Study. Eur. J. Cardio-Thorac. Surg. 2017, 52, 1139–1148. [Google Scholar] [CrossRef]
- Soldatow, V.Y.; LeCluyse, E.L.; Griffith, L.G.; Rusyn, I. In Vitro Models for Liver Toxicity Testing. Toxicol. Res. 2013, 2, 23–39. [Google Scholar] [CrossRef]
- Nguyen, D.G.; Funk, J.; Robbins, J.B.; Crogan-Grundy, C.; Presnell, S.C.; Singer, T.; Roth, A.B. Bioprinted 3D Primary Liver Tissues Allow Assessment of Organ-Level Response to Clinical Drug Induced Toxicity in Vitro. PLoS ONE 2016, 11, e0158674. [Google Scholar] [CrossRef]
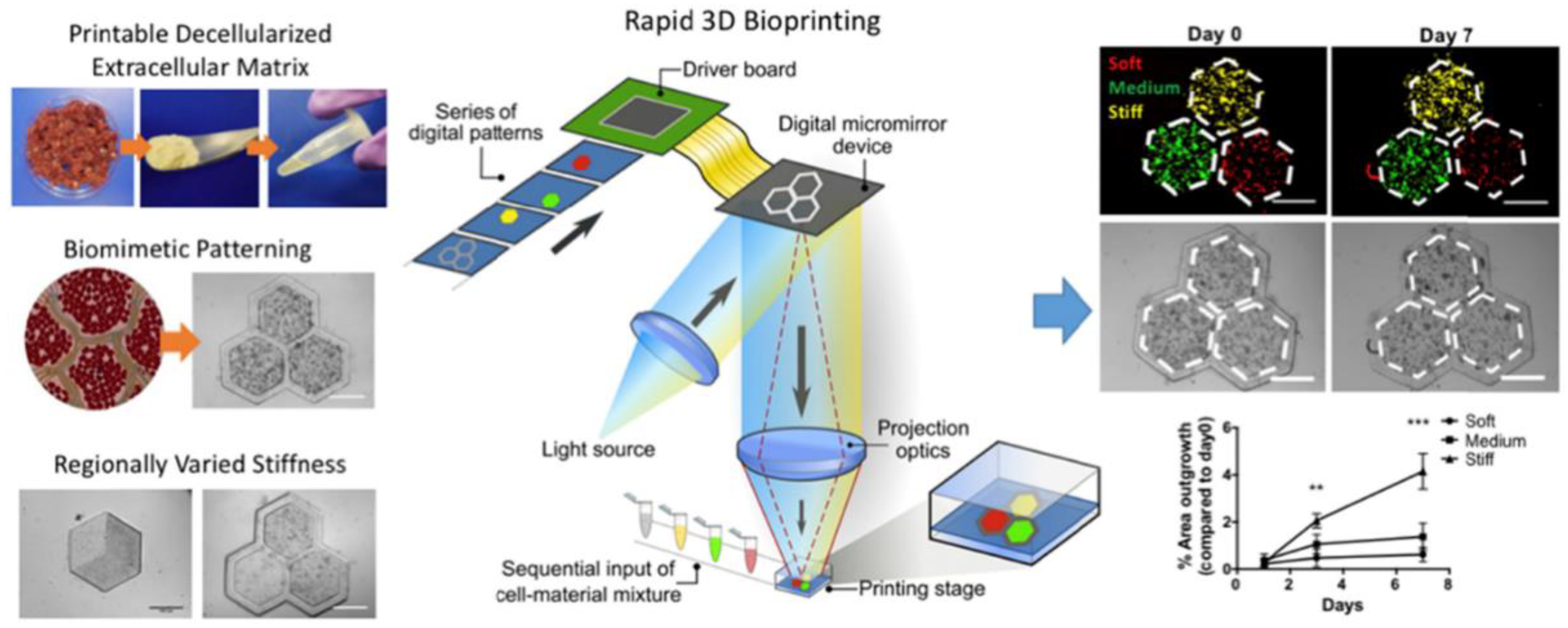
- Ma, X.; Yu, C.; Wang, P.; Xu, W.; Wan, X.; Lai, C.S.E.; Liu, J.; Koroleva-Maharajh, A.; Chen, S. Rapid 3D Bioprinting of Decellularized Extracellular Matrix with Regionally Varied Mechanical Properties and Biomimetic Microarchitecture. Biomaterials 2018, 185, 310–321. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Wang, R.; Ding, Z. Spatial Transcriptomics and Proteomics Technologies for Deconvoluting the Tumor Microenvironment. Biotechnol. J. 2021, 16, 2100041. [Google Scholar] [CrossRef] [PubMed]
| 3D Printing Technique | Common Advantages | Common Limitations |
|---|---|---|
| Extrusion printing | + Facile printing process and setup + Compatibility with many common hydrogel materials + Precise control of individual printheads/bioink conditions | - Lower resolution than some other techniques - Shear stress on bioink components - Potential for printhead clogging - Difficulties in printing overhanging parts |
| Stereolithography | + Very high resolution + Ability to achieve complex architectures + High consistency enabled by control of light source settings | - Requirement for photocrosslinkable material - Potential cytotoxicity of reagents - Greater difficulty in achieving horizontal gradients |
| Inkjet printing | + High speed of printing + Low cost + Precise control of individual printheads/bioink conditions | - Requirement for more specific, low-viscosity materials - Potential cytotoxicity of piezoelectric or thermal conditions - Potential for printhead clogging |
| Laser-assisted bioprinting | + High resolution + Precise horizontal patterning of cells and/or biomolecules + Cytocompatible conditions due to absorption of laser by donor substrate | - High cost - Requirement for specific bioinks adsorbable to donor substrate - Limitations in scale of printed construct |
| Melt electrowriting | + Ability to produce highly porous constructs and thin fibers + Replication of fibrillar structures found in native ECM + Low cost | - More extensive trial-and-error in determining printing parameters - Greater susceptibility to environmental conditions - Less predictability in achieving precise fiber deposition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.L.; Longaker, M.T. Bioprinted Hydrogels for Fibrosis and Wound Healing: Treatment and Modeling. Gels 2023, 9, 19. https://doi.org/10.3390/gels9010019
Guo JL, Longaker MT. Bioprinted Hydrogels for Fibrosis and Wound Healing: Treatment and Modeling. Gels. 2023; 9(1):19. https://doi.org/10.3390/gels9010019
Chicago/Turabian StyleGuo, Jason L., and Michael T. Longaker. 2023. "Bioprinted Hydrogels for Fibrosis and Wound Healing: Treatment and Modeling" Gels 9, no. 1: 19. https://doi.org/10.3390/gels9010019
APA StyleGuo, J. L., & Longaker, M. T. (2023). Bioprinted Hydrogels for Fibrosis and Wound Healing: Treatment and Modeling. Gels, 9(1), 19. https://doi.org/10.3390/gels9010019