Synthesis of Hydrogels and Their Progress in Environmental Remediation and Antimicrobial Application
Abstract
1. Introduction
2. Synthesis and Products
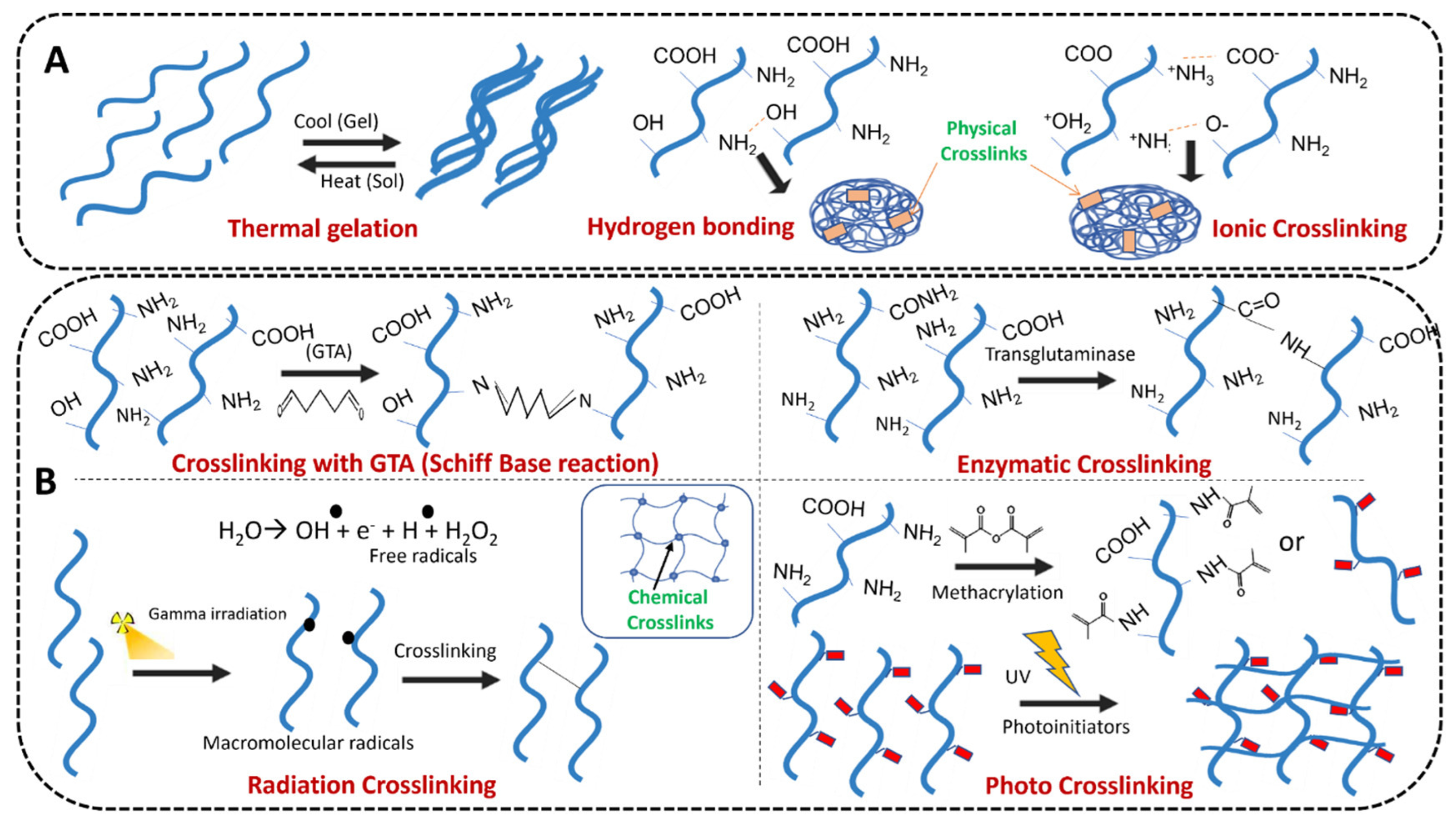
2.1. Physical Crosslinking Synthesis
2.1.1. Hydrogen Bonding
2.1.2. Crystallite Formation
2.2. Chemical Crosslinking Synthesis
2.2.1. Radiation Crosslinking
2.2.2. Crystallite Formation
2.2.3. Enzymatic Crosslinking
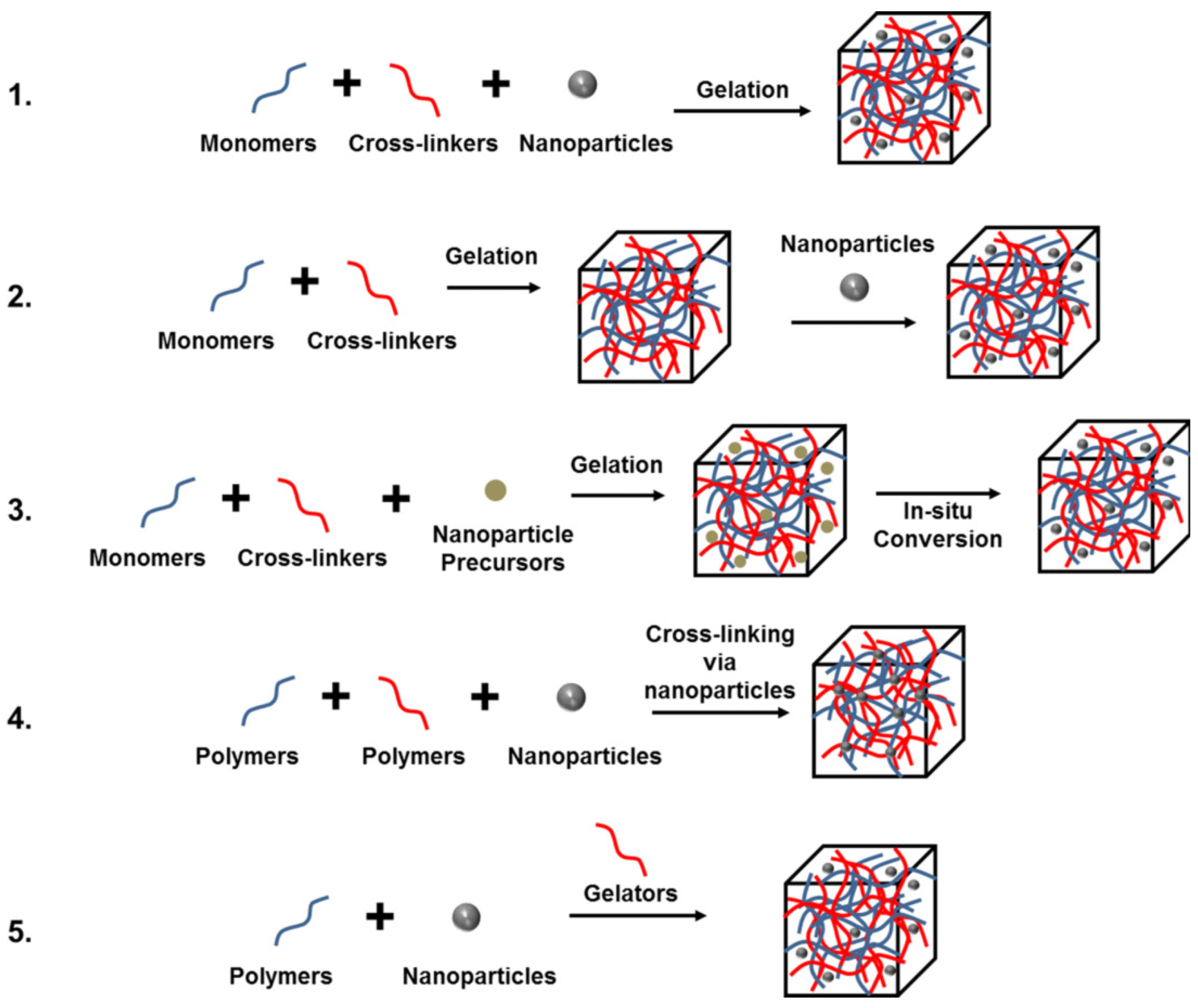
2.3. Composite Hyrogel Products
2.3.1. Nanocomposite Hydrogels
2.3.2. Interpenetrating Polymer Network Hydrogels (IPNs) and Semi-Interpenetrating Polymer Network Hydrogels (IPNs)
2.3.3. Double Network Hydrogels (DNs)
3. Application Progress of Hydrogels in Environmental Remediation (Water Sustainability/Protection)
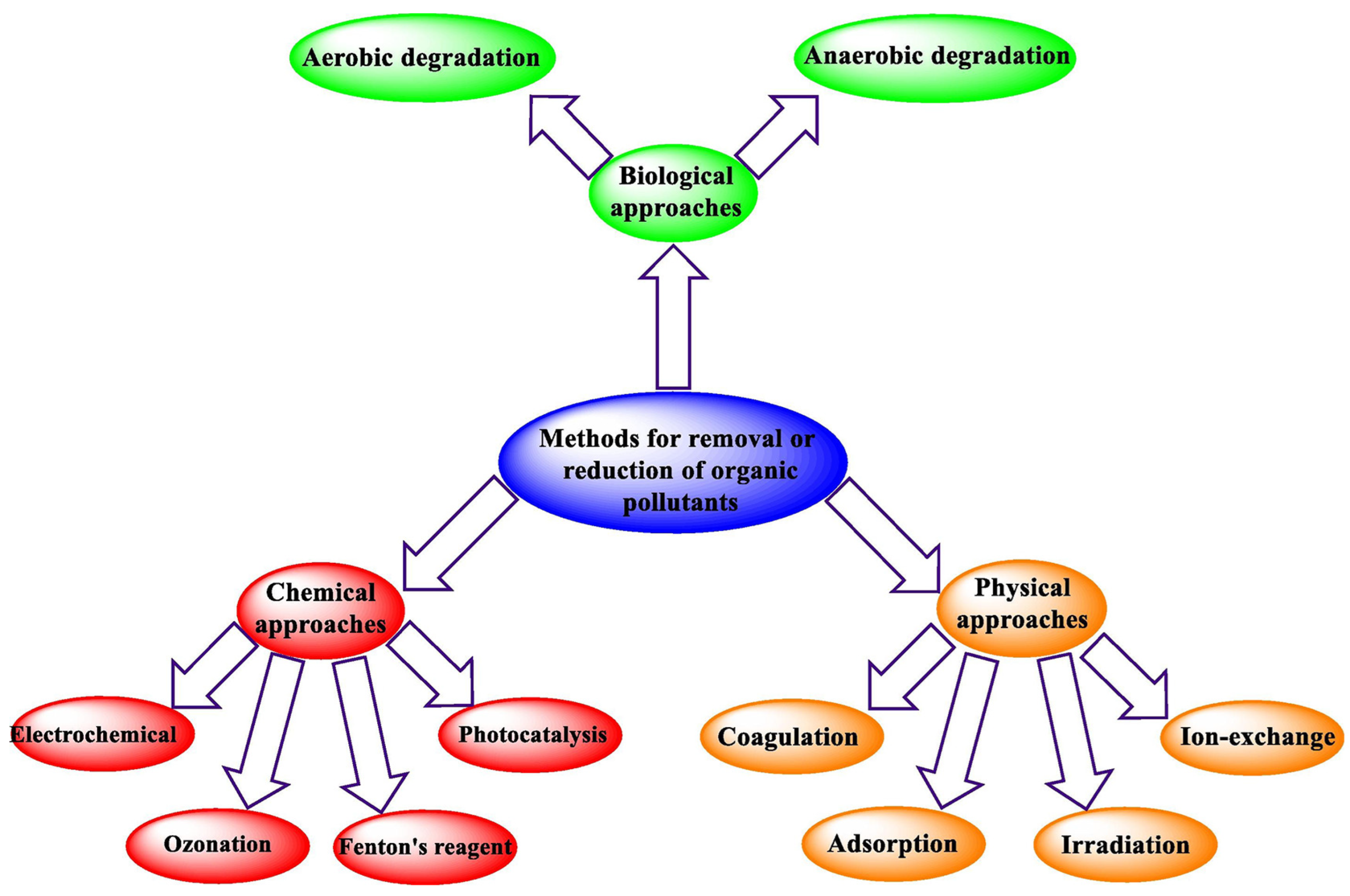
3.1. Removal of Heavy Metals, Dyes, and Organic Pollutants from Wastewater
3.1.1. Functionalized Hydrogel by N, O, S-Containing Groups
3.1.2. Composite Hydrogels
3.2. Water Sustainability
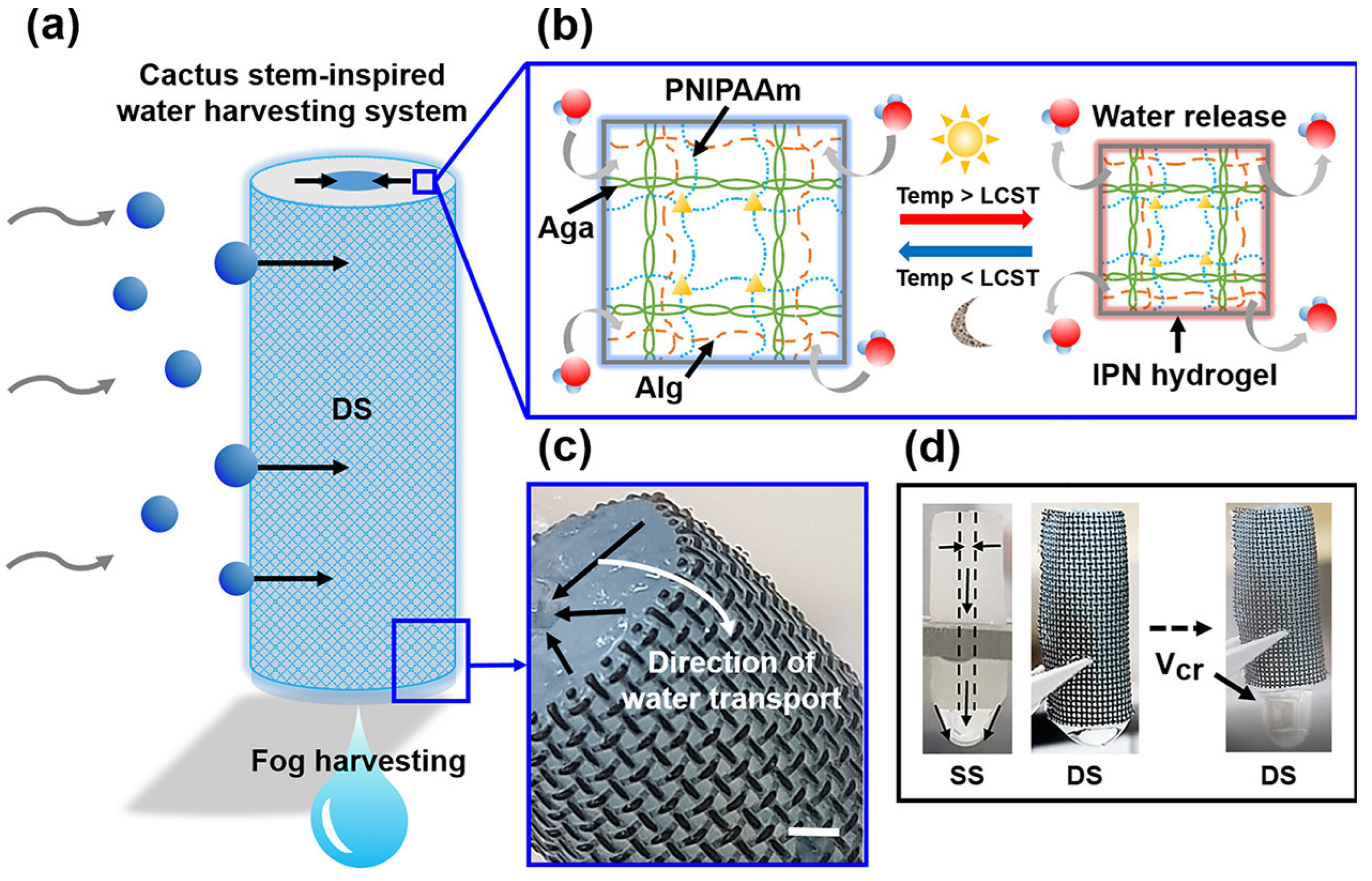
3.2.1. Atmospheric Water Harvesting
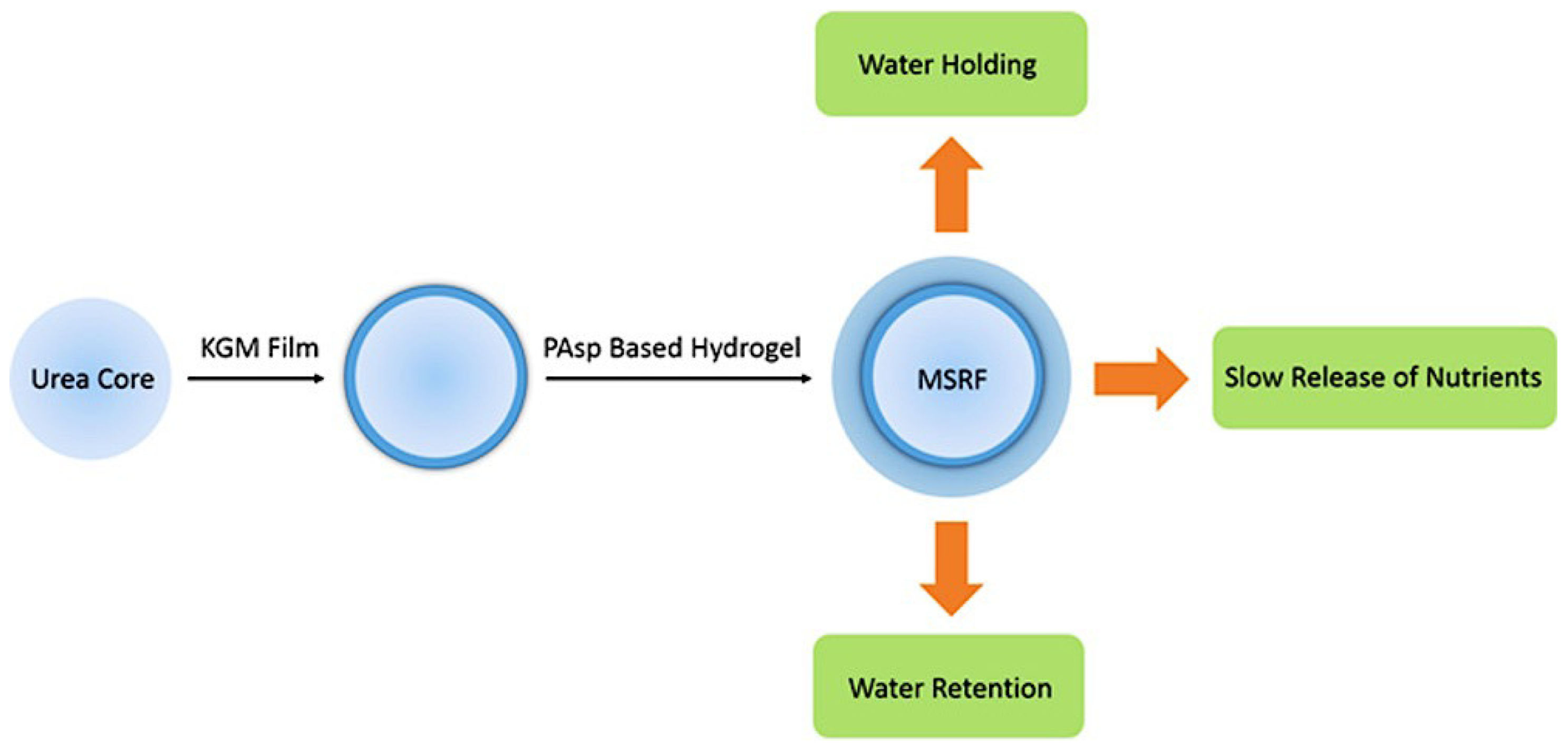
3.2.2. Agriculture Applications
4. Antimicrobial Hydrogel Application (Wound Healing)
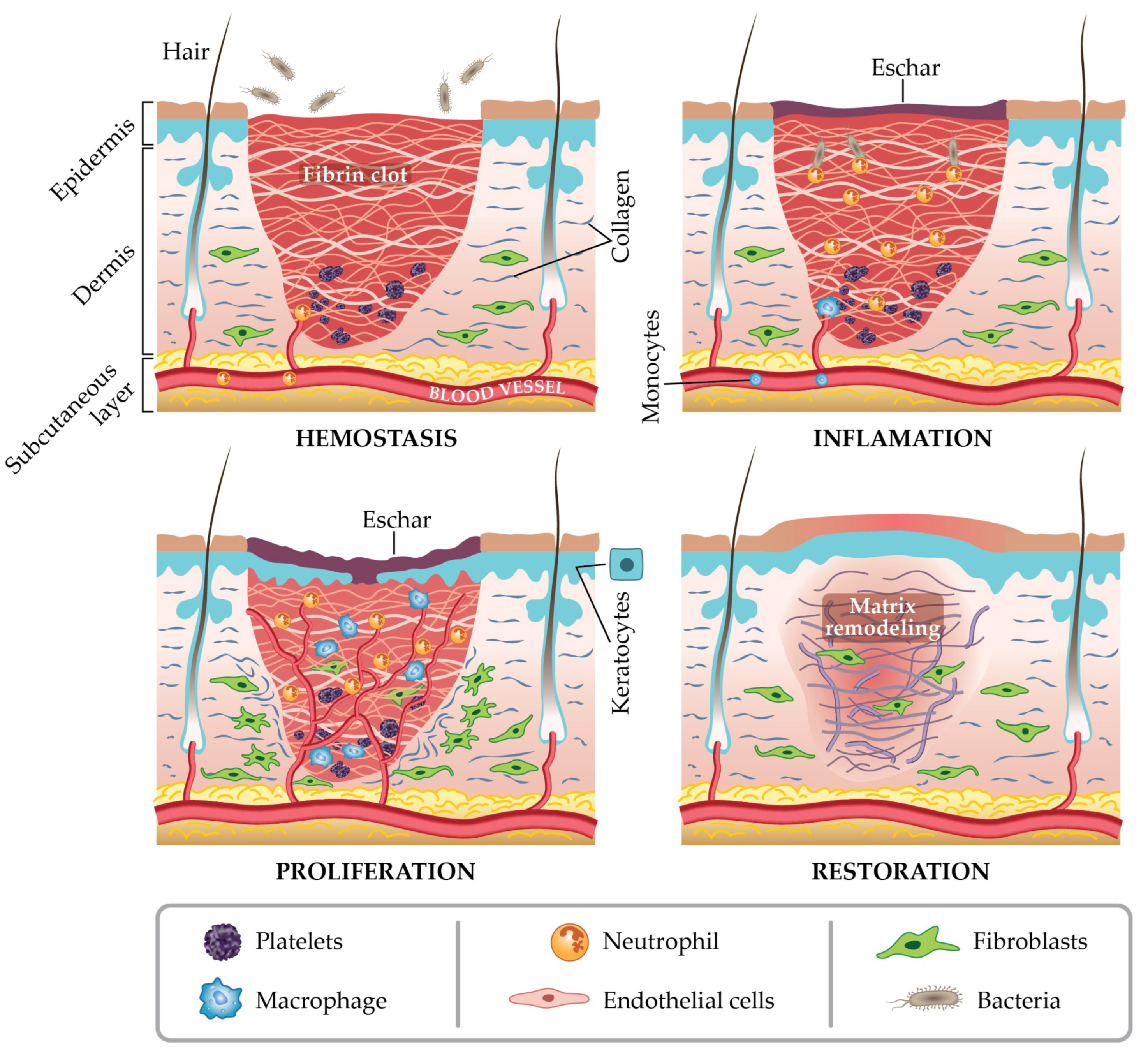
4.1. Wound Healing Mechanism with Appropriate Dressing Materials
4.2. Hydrogel Strategies for Acute Wounds
4.3. Hydrogel Strategies for Chronic Wounds
4.4. Other Wounds
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaharwar, A.; Peppas, N.; Khademhosseini, A. Nanocomposite Hydrogels for Biomedical Applications. Biotechnol. Bioeng. 2013, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Raza, Z.; Batool, S.; Zahid, M.; Onder, O.; Rafique, A.; Nazeer, M. Preparation, Properties, and Applications of Gelatin-Based Hydrogels (Ghs) in The Environmental, Technological, and Biomedical Sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Haraguchi, K. Nanocomposite Hydrogels. Curr. Opin. Solid State Mater. Sci. 2007, 11, 47–54. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M. A Review on Nanocomposite Hydrogels and Their Biomedical Applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-Based Biopolymer Hydrogels for Heavy Metal Detection and Adsorption. J. Adv. Res. 2022. [Google Scholar] [CrossRef]
- Ali, L.; Farhood, A.; Ali, F. Technique of Batch Adsorption for the Elimination of (Malachite Green) Dye from Industrial Waste Water by Exploitation Walnut Shells as Sorbent. Indones. J. Chem. 2017, 17, 211. [Google Scholar] [CrossRef]
- Yadav, A.; Rajhans, K.; Ramteke, S.; Sahu, B.; Patel, K.; Blazhev, B. Contamination of Industrial Waste Water in Central India. J. Environ. Prot. 2016, 07, 72–81. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, L.; Tan, B.; Li, J.; Gao, X.; He, Y.; Du, X.; Zhang, W.; Wang, W. Adsorption Behavior of Heavy Metal Ions from Aqueous Solution onto Composite Dextran-Chitosan Macromolecule Resin Adsorbent. Int. J. Biol. Macromol. 2019, 141, 738–746. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Petrounias, P.; Rogkala, A.; Giannakopoulou, P.; Tsikouras, B.; Lampropoulou, P.; Kalaitzidis, S.; Hatzipanagiotou, K.; Lambrakis, N.; Christopoulou, M. An Experimental Study for the Remediation of Industrial Waste Water Using a Combination of Low Cost Mineral Raw Materials. Minerals 2019, 9, 207. [Google Scholar] [CrossRef]
- Akpor, O.; Muchie, M. Remediation of Heavy Metals in Drinking Water and Wastewater Treatment Systems: Processes and Applications. Int. J. Phys. Sci. 2010, 5, 1807–1817. [Google Scholar]
- Balamurugan, P.; Vinnilavu, G. Removal of Methyl Violet Dye from Industrial Waste Water Using Neem Leaf Powder. Indian J. Ecol. 2020, 47, 442–445. [Google Scholar]
- Wu, Q.; He, H.; Zhou, H.; Xue, F.; Zhu, H.; Zhou, S.; Wang, L.; Wang, S. Multiple Active Sites Cellulose-Based Adsorbent for The Removal of Low-Level Cu(II), Pb(II) And Cr(VI) via Multiple Cooperative Mechanisms. Carbohydr. Polym. 2020, 233, 115860. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Hydrogel Adsorbent in Industrial Wastewater Treatment and Ecological Environment Protection. Environ. Technol. Innov. 2020, 20, 101107. [Google Scholar] [CrossRef]
- Behera, S.; Mahanwar, P. Superabsorbent Polymers in Agriculture and Other Applications: A Review. Polym.-Plast. Technol. Mater. 2019, 59, 341–356. [Google Scholar] [CrossRef]
- Kozicki, M.; Kołodziejczyk, M.; Szynkowska, M.; Pawlaczyk, A.; Leśniewska, E.; Matusiak, A.; Adamus, A.; Karolczak, A. Hydrogels Made from Chitosan and Silver Nitrate. Carbohydr. Polym. 2016, 140, 74–87. [Google Scholar] [CrossRef]
- Lake, G.; Thomas, A. The Strength of Highly Elastic Materials. Proc. Math. Phys. Eng. Sci. 1967, 300, 108–119. [Google Scholar]
- Zhang, G.; Lv, L.; Deng, Y.; Wang, C. Self-Healing Gelatin Hydrogels Cross-Linked by Combining Multiple Hydrogen Bonding and Ionic Coordination. Macromol. Rapid Commun. 2017, 38, 1700018. [Google Scholar] [CrossRef]
- Aristeidis, P.; Spiridon-Paraskevas, N.; Andreas, P.; Eleni, K. Physicochemical properties of electrostatically crosslinked carrageenan/chitosan hydrogels and carrageenan/chitosan/Laponite nanocomposite hydrogels. Int. J. Biol. Macromol. 2022. [Google Scholar] [CrossRef]
- Yuan, N.; Xu, L.; Xu, B.; Zhao, J.; Rong, J. Chitosan Derivative-Based Self-Healable Hydrogels with Enhanced Mechanical Properties by High-Density Dynamic Ionic Interactions. Carbohydr. Polym. 2018, 193, 259–267. [Google Scholar] [CrossRef]
- Alves, M.; Jensen, B.; Smith, A.; Zelikin, A. Poly(Vinyl Alcohol) Physical Hydrogels: New Vista on a Long Serving Biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Rebers, L.; Reichsöllner, R.; Regett, S.; Tovar, G.; Borchers, K.; Baudis, S.; Southan, A. Differentiation of Physical and Chemical Cross-Linking in Gelatin Methacryloyl Hydrogels. Sci. Rep. 2021, 11, 3256. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.; Tibbitt, M.; Webber, M.; Mattix, B.; Veiseh, O.; Langer, R. Self-Assembled Hydrogels Utilizing Polymer-Nanoparticle Interactions. Nat. Commun. 2015, 6, 6295. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Gao, J.; Awada, H.; Wang, Y. Dual Physical Dynamic Bond-Based Injectable and Biodegradable Hydrogel for Tissue Regeneration. J. Mater. Chem. B 2016, 4, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Peak, C.; Stein, J.; Gold, K.; Gaharwar, A. Nanoengineered Colloidal Inks for 3D Bioprinting. Langmuir 2017, 34, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Umashankar, P.; Kumari, T.; Mohanan, P. Glutaraldehyde Treatment Elicits Toxic Response Compared to Decellularization in Bovine Pericardium. Toxicol. Int. 2012, 19, 51. [Google Scholar] [CrossRef]
- Liu, K.; Zang, S.; Xue, R.; Yang, J.; Wang, L.; Huang, J.; Yan, Y. Coordination-Triggered Hierarchical Folate/Zinc Supramolecular Hydrogels Leading to Printable Biomaterials. ACS Appl. Mater. Interfaces 2018, 10, 4530–4539. [Google Scholar] [CrossRef]
- Avery, R.; Albadawi, H.; Akbari, M.; Zhang, Y.; Duggan, M.; Sahani, D.; Olsen, B.; Khademhosseini, A.; Oklu, R. An Injectable Shear-Thinning Biomaterial for Endovascular Embolization. Sci. Transl. Med. 2016, 8, 365. [Google Scholar] [CrossRef]
- Guvendiren, M.; Lu, H.; Burdick, J. Shear-Thinning Hydrogels for Biomedical Applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Chee, B.; Goetten de Lima, G.; Devine, D.; Nugent, M. Investigation of The Effects of Orientation on Freeze/Thawed Polyvinyl Alcohol Hydrogel Properties. Mater. Today Commun. 2018, 17, 82–93. [Google Scholar] [CrossRef]
- Wahab, A.; Saad, A.; Harun, M.; Syahrom, A.; Ramlee, M.; Sulong, M.; Kadir, M. Developing Functionally Graded PVA Hydrogel Using Simple Freeze-Thaw Method for Artificial Glenoid Labrum. J. Mech. Behav. Biomed. Mater. 2019, 91, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Zhang, D.; Yin, J.; Qian, J.; Wu, Z.; Fu, J. Interpenetrating Polymer Network Hydrogels Composed of Chitosan and Photocrosslinkable Gelatin with Enhanced Mechanical Properties for Tissue Engineering. Mater. Sci. Eng. C 2018, 92, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Badali, E.; Hosseini, M.; Mohajer, M.; Hassanzadeh, S.; Saghati, S.; Hilborn, J.; Khanmohammadi, M. Enzymatic Crosslinked Hydrogels for Biomedical Application. Polym. Sci. Ser. A 2021, 63, 1–22. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Zhang, X.; Xu, L.; Chen, X.; Wei, S. Influence of Radiation Crosslinked Carboxymethyl-Chitosan/Gelatin Hydrogel on Cutaneous Wound Healing. Mater. Sci. Eng. C 2013, 33, 4816–4824. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Yoshida, F.; Ueno, M.; Taguchi, M. Application of Radiation Crosslinking Technique to Development of Gelatin Scaffold for Tissue Engineering. Radiat. Phys. Chem. 2021, 180, 109287. [Google Scholar] [CrossRef]
- Carvalho, I.; Mansur, H. Engineered 3D-Scaffolds Oof Photocrosslinked Chitosan-Gelatin Hydrogel Hybrids for Chronic Wound Dressings and Regeneration. Mater. Sci. Eng. C. 2017, 78, 690–705. [Google Scholar] [CrossRef]
- Hu, X.; Ma, L.; Wang, C.; Gao, C. Gelatin Hydrogel Prepared by Photo-Initiated Polymerization and Loaded with TGF-β1 for Cartilage Tissue Engineering. Macromol. Biosci. 2009, 9, 1194–1201. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2018, 8, 1800465. [Google Scholar] [CrossRef]
- Tao, H.; Kaplan, D.; Omenetto, F. Silk Materials—A Road to Sustainable High Technology. Adv. Mater. 2012, 24, 2824–2837. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, C.; Sun, N.; Tan, D.; Li, Q.; Bi, S.; Song, J. Recent Progress in Multifunctional Hydrogel-Based Supercapacitors. J. Sci.-Adv. Mater. Dev. 2021, 6, 338–350. [Google Scholar] [CrossRef]
- Cui, X.; Soliman, B.; Alcala-Orozco, C.; Li, J.; Vis, M.; Santos, M.; Wise, S.; Levato, R.; Malda, J.; Woodfield, T.; et al. Rapid Photocrosslinking of Silk Hydrogels with High Cell Density and Enhanced Shape Fidelity. Adv. Healthc. Mater. 2020, 9, 1901667. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.; Lewandowska, A.; Szcześniak, K.; Przesławski, G.; Marcinkowska, A. Optimization of the Properties of Photocured Hydrogels for Use in Electrochemical Capacitors. Polymers 2021, 13, 3495. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.; Tan, B.; Freeman, A.; Gagnon, J.; Hoffman, C.; Logan, M.; Maranchi, J.; Gerasopoulos, K. UV-Cured Gel Polymer Electrolytes with Improved Stability for Advanced Aqueous Li-Ion Batteries. Chem. Comm. 2019, 55, 13085–13088. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Lai, J. Amination Degree Of Gelatin Is Critical For Establishing Structure-Property-Function Relationships of Biodegradable Thermogels as Intracameral Drug Delivery Systems. Mater. Sci. Eng. C 2019, 98, 897–909. [Google Scholar] [CrossRef]
- Ryu, S.; Park, K.; Park, K. In Situ Graphene Oxide-Gelatin Hydrogels with Enhanced Mechanical Property for Tissue Adhesive and Regeneration. Biochem. Biophys. Res. Commun. 2022, 592, 24–30. [Google Scholar] [CrossRef]
- Raia, N.; Partlow, B.; McGill, M.; Kimmerling, E.; Ghezzi, C.; Kaplan, D. Enzymatically Crosslinked Silk-Hyaluronic Acid Hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and Future Scope of Hydrogels in Wound Healing: Synthesis, Materials and Evaluation. Eur. Polym. J. 2019, 130, 109609. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Benoit, D. Degradable Poly(Ethylene Glycol) (PEG)-Based Hydrogels for Spatiotemporal Control of Sirna/Nanoparticle Delivery. J. Control Release 2018, 287, 58–66. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.; Guo, B. Degradable Conductive Injectable Hydrogels as Novel Antibacterial, Anti-Oxidant Wound Dressings for Wound Healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Ma, W.; Ma, H.; Qiu, P.; Zhang, H.; Yang, Z.; Ma, B.; Chang, J.; Shi, X.; Wu, C. Sprayable β-Fesi2 Composite Hydrogel for Portable Skin Tumor Treatment and Wound Healing. Biomaterials 2021, 279, 121225. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.; Karim, A.; Young, D.; Loh, X. Nanoparticle-Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.; Laurencin, C. Silver Nanoparticles: Synthesis and Therapeutic Applications. J. Biomed. Nanotechnol. 2007, 3, 301–316. [Google Scholar] [CrossRef]
- Murthy, P.; Murali Mohan, Y.; Varaprasad, K.; Sreedhar, B.; Mohana Raju, K. First Successful Design of Semi-IPN Hydrogel-Silver Nanocomposites: A Facile Approach for Antibacterial Application. J. Colloid Interface Sci. 2008, 318, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jon, A.; José, M.; Sáez-Martínez, V.; Benito-Cid, S.; Pérez-González, R.; Vilas-Vilela, J.; Pérez-Álvarez, L. Hyaluronic acid-based hydrogel coatings on Ti6Al4V implantable biomaterial with multifunctional antibacterial activity. Carbohydr. Polym. 2023, 301, 120366. [Google Scholar]
- Shiotani, A.; Mori, T.; Niidome, T.; Niidome, Y.; Katayama, Y. Stable Incorporation of Gold Nanorods into N-Isopropylacrylamide Hydrogels and Their Rapid Shrinkage Induced By Near-Infrared Laser Irradiation. Langmuir 2007, 23, 4012–4018. [Google Scholar] [CrossRef] [PubMed]
- Sershen, S.; Mensing, G.; Ng, M.; Halas, N.; Beebe, D.; West, J. Independent Optical Control of Microfluidic Valves Formed from Optomechanically Responsive Nanocomposite Hydrogels. Adv. Mater. 2005, 17, 1366–1368. [Google Scholar] [CrossRef]
- Shi, W.; Crews, K.; Chopra, N. Multicomponent and Hybrid Hydrogels Comprised of Carbon Nanotube-Nickel/Nickel Oxide Core/Shell Nanoparticle Heterostructures Incorporated in Polyvinyl Alcohol. Mater. Technol. 2010, 25, 149–157. [Google Scholar] [CrossRef]
- Ozay, O.; Ekici, S.; Baran, Y.; Aktas, N.; Sahiner, N. Removal of Toxic Metal Ions with Magnetic Hydrogels. Water Res. 2009, 43, 4403–4411. [Google Scholar] [CrossRef]
- Caykara, T.; Yörük, D.; Demirci, S. Preparation and Characterization of Poly(N-Tert-Butylacrylamide-Co-Acrylamide) Ferrogel. J. Appl. Polym. Sci. 2009, 112, 800–804. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, R.; Cheng, Y.; Fu, J. Super-Tough Double-Network Hydrogels Reinforced by Covalently Compositing with Silica-Nanoparticles. Soft Matter 2012, 8, 6048. [Google Scholar] [CrossRef]
- Ninh, C.; Cramer, M.; Bettinger, C. Photoresponsive Hydrogel Networks Using Melanin Nanoparticle Photothermal Sensitizers. Biomater. Sci. 2014, 2, 766. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.; Patel, A.; Dolatshahi-Pirouz, A.; Zhang, H.; Rangarajan, K.; Iviglia, G.; Shin, S.; Hussain, M.; Khademhosseini, A. Elastomeric Nanocomposite Scaffolds Made from Poly(Glycerol Sebacate) Chemically Crosslinked with Carbon Nanotubes. Biomater. Sci. 2015, 3, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Hasan, A.; Kindi, H.; Gaharwar, A.; Rao, V.; Nikkhah, M.; Shin, S.; Krafft, D.; Dokmeci, M.; Shum-Tim, D.; et al. Injectable Graphene Oxide/Hydrogel-Based Angiogenic Gene Delivery System for Vasculogenesis and Cardiac Repair. ACS Nano 2014, 8, 8050–8062. [Google Scholar] [CrossRef]
- Zhang, H.; Patel, A.; Gaharwar, A.; Mihaila, S.; Iviglia, G.; Mukundan, S.; Bae, H.; Yang, H.; Khademhosseini, A. Hyperbranched Polyester Hydrogels with Controlled Drug Release and Cell Adhesion Properties. Biomacromolecules 2013, 14, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sharma, V. Targeted Drug Delivery System: A Review. Res. J. Chem. Sci. 2011, 1, 135–138. [Google Scholar]
- Hench, L.; Splinter, R.; Allen, W.; Greenlee, T. Bonding Mechanisms at the Interface of Ceramic Prosthetic Materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hu, X.; Hao, X.; Wu, Y.; Zhang, J.; Zhang, X.; Wang, P.; Zou, G.; Liang, X. Multifunctional Hybrid Silica Nanoparticles for Controlled Doxorubicin Loading and Release with Thermal and Ph Dual Response. Mater. Chem. B 2013, 1, 1109. [Google Scholar] [CrossRef]
- Lotfibakhshaiesh, N.; Brauer, D.; Hill, R. Bioactive Glass Engineered Coatings for Ti6al4v Alloys: Influence of Strontium Substitution for Calcium on Sintering Behaviour. J. Non-Cryst. Solids 2010, 356, 2583–2590. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.; Jamshidi, A. 3-Dimensional Cell-Laden Nano-Hydroxyapatite/Protein Hydrogels for Bone Regeneration Applications. Mater. Sci. Eng. C 2015, 49, 835–843. [Google Scholar] [CrossRef]
- Straub, D. Calcium Supplementation In Clinical Practice: A Review of Forms, Doses, and Indications. Nutr. Clin. Pract. 2007, 22, 286–296. [Google Scholar] [CrossRef]
- Banerjee, S.; Ray, S.; Maiti, S.; Sen, K.; Bhattacharyya, U.; Kaity, S. Interpenetrating Polymer Network (IPN): A Novel Biomaterial. Int. J. Appl. Pharm. 2010, 2, 28–34. [Google Scholar]
- Jenkins, A.; Kratochvíl, P.; Stepto, R.; Suter, U. Glossary of Basic Terms In Polymer Science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Ishikawa, S.; Iijima, K.; Matsukuma, D.; Asawa, Y.; Hoshi, K.; Osawa, S.; Otsuka, H. Interpenetrating Polymer Network Hydrogels via a One-Pot and in Situ Gelation System Based on Peptide Self-Assembly and Orthogonal Cross-Linking for Tissue Regeneration. Chem. Mater. 2020, 32, 2353–2364. [Google Scholar] [CrossRef]
- Gong, J.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Chen, F.; Yang, K.; Zhao, D.; Yang, H. Thermal- and Salt-Activated Shape Memory Hydrogels Based on a Gelatin/Polyacrylamide Double Network. RSC Adv. 2019, 9, 18619–18626. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.; Kitamura, N.; Kurokawa, T.; Goto, K.; Wada, S.; Nonoyama, T.; Kanaya, F.; Sugahara, K.; Gong, J.P.; Yasuda, K. Fundamental Biomaterial Properties of Tough Glycosaminoglycan-Containing Double Network Hydrogels Newly Developed Using the Molecular Stent Method. Acta Biomater. 2016, 43, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Kitamura, N.; Nonoyama, T.; Kiyama, R.; Kurokawa, T.; Gong, J.P.; Yasuda, K. Hydroxyapatite-Coated Double Network Hydrogel Directly Bondable to the Bone: Biological and Biomechanical Evaluations of the Bonding Property in an Osteochondral Defect. Acta Biomater. 2016, 44, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liong Han, M.K.; Jiang, Q.; Li, B.; Feng, J.; del Campo, A. 4D Hydrogel for Dynamic Cell Culture with Orthogonal, Wavelength-Dependent Mechanical and Biochemical Cues. Mater. Horizons 2020, 7, 111–116. [Google Scholar] [CrossRef]
- Che, L.; Lei, Z.; Wu, P.; Song, D. A 3D Printable and Bioactive Hydrogel Scaffold to Treat Traumatic Brain Injury. Adv. Funct. Mater. 2019, 29, 1904450. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Application of Carboxymethyl Polysaccharides as Bio-Sorbents for the Sequestration of Heavy Metals in Aquatic Environments. Carbohydr. Polym. 2020, 237, 116142. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Zhao, C.; Wang, Q.; Zheng, J. A Robust, One-Pot Synthesis of Highly Mechanical and Recoverable Double Network Hydrogels Using Thermoreversible Sol-Gel Polysaccharide. Adv. Mater 2013, 25, 4171–4176. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D. Interfaces and the Driving Force of Hydrophobic Assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Ren, X.; Gao, G. The Role of Chemical and Physical Crosslinking in Different Deformation Stages of Hybrid Hydrogels. Eur. Polym. J. 2018, 100, 86–95. [Google Scholar] [CrossRef]
- Rodell, C.B.; Dusaj, N.N.; Highley, C.B.; Burdick, J.A. Injectable and Cytocompatible Tough Double-Network Hydrogels through Tandem Supramolecular and Covalent Crosslinking. Adv. Mater. 2016, 28, 8419–8424. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oderinde, O.; Hussain, I.; Yao, F.; Fu, G. Dual Ionic Cross-Linked Double Network Hydrogel with Self-Healing, Conductive, and Force Sensitive Properties. Polymer 2018, 144, 111–120. [Google Scholar] [CrossRef]
- Ye, Y.N.; Frauenlob, M.; Wang, L.; Tsuda, M.; Sun, T.L.; Cui, K.; Takahashi, R.; Zhang, H.J.; Nakajima, T.; Nonoyama, T.; et al. Tough and Self-Recoverable Thin Hydrogel Membranes for Biological Applications. Adv. Funct. Mater. 2018, 28, 1801489. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.; Gao, G. Robust and Flexible Strain Sensors Based on Dual Physically Cross-Linked Double Network Hydrogels for Monitoring Human-Motion. Chem. Eng. J. 2018, 354, 817–824. [Google Scholar] [CrossRef]
- Li, C.; Rowland, M.J.; Shao, Y.; Cao, T.; Chen, C.; Jia, H.; Zhou, X.; Yang, Z.; Scherman, O.A.; Liu, D. Responsive Double Network Hydrogels of Interpenetrating DNA and CB[8] Host-Guest Supramolecular Systems. Adv. Mater. 2015, 27, 3298–3304. [Google Scholar] [CrossRef]
- Bing, B.; Fan, B.; Jia, X.; Wu, H. The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar]
- Wang, N.; Xu, X.; Li, H.; Zhai, J.; Yuan, L.; Zhang, K.; Yu, H. Preparation and Application of a Xanthate-Modified Thiourea Chitosan Sponge for the Removal of Pb(Ii) from Aqueous Solutions. Ind. Eng. Chem. Res. 2016, 55, 4960–4968. [Google Scholar] [CrossRef]
- Sarkar, K.; Ansari, Z.; Sen, K. Detoxification of Hg(Ii) from Aqueous and Enzyme Media: Pristine vs. Tailored Calcium Alginate Hydrogels. Int. J. Biol. Macromol. 2016, 91, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Karbarz, M.; Khalil, A.M.; Wolowicz, K.; Kaniewska, K.; Romanski, J.; Stojek, Z. Efficient Removal of Cadmium and Lead Ions from Water by Hydrogels Modified with Cystine. J. Environ. Chem. Eng. 2018, 6, 3962–3970. [Google Scholar] [CrossRef]
- Feng, Z.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Preparation of Magnetic Biochar and Its Application in Catalytic Degradation of Organic Pollutants: A Review. Sci. Total Environ. 2021, 765, 142673. [Google Scholar] [CrossRef] [PubMed]
- Oyewo, O.A.; Elemike, E.E.; Onwudiwe, D.C.; Onyango, M.S. Metal Oxide-Cellulose Nanocomposites for the Removal of Toxic Metals and Dyes from Wastewater. Int. J. Biol. Macromol. 2020, 164, 2477–2496. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dai, R.; Chen, M.; Khan, S.J.; Wang, Z. Applications of Membrane Bioreactors for Water Reclamation: Micropollutant Removal, Mechanisms and Perspectives. Bioresour. Technol. 2018, 269, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Quintana, J.B.; Concha-Graña, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Emerging Pollutants in Sewage, Surface and Drinking Water in Galicia (NW Spain). Chemosphere 2012, 86, 1040–1049. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, G.; Ahamad, T.; Ghfar, A.A.; Pathania, D.; Naushad, M. Efficient Photocatalytic Degradation of Toxic Dyes from Aqueous Environment Using Gelatin-ZR(IV) Phosphate Nanocomposite and Its Antimicrobial Activity. Colloids Surf. B 2017, 157, 456–463. [Google Scholar] [CrossRef]
- Xing, J.; Yang, B.; Shen, Y.; Wang, Z.; Wang, F.; Shi, X.; Zhang, Z. Selective Removal of Acid Fuchsin from Aqueous Solutions by Rapid Adsorption onto Polypyrrole Crosslinked Cellulose/Gelatin Hydrogels. Dispers Sci. Technol. 2019, 40, 1591–1599. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible Applications of Coal Fly Ash in Wastewater Treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef]
- Dadashi, J.; Ali Ghasemzadeh, M.; Alipour, S.; Zamani, F. A review on catalytic reduction/degradation of organic pollution through silver-based hydrogels. Arab. J. Chem. 2022, 15, 104023. [Google Scholar] [CrossRef]
- Godiya, C.B.; Martins Ruotolo, L.A.; Cai, W. Functional Biobased Hydrogels for the Removal of Aqueous Hazardous Pollutants: Current Status, Challenges, and Future Perspectives. J. Mater. Chem. A 2020, 8, 21585–21612. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; Yin, L. Hydrogel: Diversity of Structures and Applications in Food Science. Food Rev. Int. 2021, 37, 313–372. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.M.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.K.; Zhao, B.; Pinnaratip, R.; et al. Catechol-Functionalized Hydrogels: Biomimetic Design, Adhesion Mechanism, and Biomedical Applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S. Flexible Core-Shell/Bead-like Alginate@Pei with Exceptional Adsorption Capacity, Recycling Performance toward Batch and Column Sorption of Cr(Vi). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Liu, J.; Su, D.; Yao, J.; Huang, Y.; Shao, Z.; Chen, X. Soy Protein-Based Polyethylenimine Hydrogel and Its High Selectivity for Copper Ion Removal in Wastewater Treatment. J. Mater. Chem. A 2017, 5, 4163–4171. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Ouyang, X.-K.; Ji, C.; Liu, Y.; Huang, F.; Yang, L.-Y. Fabrication of Cross-Linked Chitosan Beads Grafted by Polyethylenimine for Efficient Adsorption of Diclofenac Sodium from Water. Int. J. Biol. Macromol. 2020, 145, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Xiao, Y.; Lu, X. Amine Functionalized Sodium Alginate Hydrogel for Efficient and Rapid Removal of Methyl Blue in Water. Int. J. Biol. Macromol. 2020, 144, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Borsagli, F.G.L.; Ciminelli, V.S.T.; Ladeira, C.L.; Haas, D.J.; Lage, A.P.; Mansur, H.S. Multi-Functional Eco-Friendly 3D Scaffolds Based on N-Acyl Thiolated Chitosan for Potential Adsorption of Methyl Orange and Antibacterial Activity against Pseudomonas Aeruginosa. J. Environ. Chem. Eng. 2019, 7, 103286. [Google Scholar] [CrossRef]
- Chaudhary, J.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Thakur, V.K. Hydrogel of Gelatin in the Presence of Graphite for the Adsorption of Dye: Towards the Concept for Water Purification. J. Environ. Chem. Eng. 2021, 9, 104762. [Google Scholar] [CrossRef]
- Lone, S.; Yoon, D.H.; Lee, H.; Cheong, I.W. Gelatin-Chitosan Hydrogel Particles for Efficient Removal of Hg(Ii) from Wastewater. Environ. Sci. Water Res. Technol. 2019, 5, 83–90. [Google Scholar] [CrossRef]
- Dil, N.N.; Sadeghi, M. Free Radical Synthesis of Nanosilver/Gelatin-Poly (Acrylic Acid) Nanocomposite Hydrogels Employed for Antibacterial Activity and Removal of Cu(Ii) Metal Ions. J. Hazard. Mater. 2018, 351, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wang, R.; Zhang, Y.; Wang, J. Progress and Expectation of Atmospheric Water Harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A Multi-Structural and Multi-Functional Integrated Fog Collection System in Cactus. Nat. Commun. 2012, 3, 2147. [Google Scholar] [CrossRef]
- Lee, S.J.; Ha, N.; Kim, H. Superhydrophilic-Superhydrophobic Water Harvester Inspired by Wetting Property of Cactus Stem. ACS Sustain. Chem. Eng. 2019, 7, 10561–10569. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Medina, J.; González, A.; Diez, M.C.; Cartes, P.; Monreal, C.; Navia, R. Evaluation of Biodegradable Polymers as Encapsulating Agents for the Development of a Urea Controlled-Release Fertilizer Using Biochar as Support Material. Sci. Total Environ. 2015, 505, 446–453. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Synthesis and Swelling Properties of Ph-Sensitive Semi-IPN Superabsorbent Hydrogels Based on Sodium Alginate-g-Poly(Sodium Acrylate) and Polyvinylpyrrolidone. Carbohydr. Polym. 2010, 80, 1028–1036. [Google Scholar] [CrossRef]
- Lü, S.; Feng, C.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Gao, N.; Liu, M. Multifunctional Environmental Smart Fertilizer Based on l-Aspartic Acid for Sustained Nutrient Release. J. Agric. Food Chem. 2016, 64, 4965–4974. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why Chronic Wounds Will Not Heal: A Novel Hypothesis. Wound Repair Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef]
- Clark, R.A.F.; Ghosh, K.; Tonnesen, M.G. Tissue Engineering for Cutaneous Wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.-M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound Healing in the 21st Century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater. Sci. Eng. C 2021, 118, 111324. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; de Sousa, H.C. Recent Advances on the Development of Wound Dressings for Diabetic Foot Ulcer Treatment—A Review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef]
- Morin, R.J.; Tomaselli, N.L. Interactive Dressings and Topical Agents. Clin. Plast. Surg. 2007, 34, 643–658. [Google Scholar] [CrossRef]
- Fasiku, V.O.; Omolo, C.A.; Devnarain, N.; Ibrahim, U.H.; Rambharose, S.; Faya, M.; Mocktar, C.; Singh, S.D.; Govender, T. Chitosan-Based Hydrogel for the Dual Delivery of Antimicrobial Agents against Bacterial Methicillin-Resistant Staphylococcus Aureus Biofilm-Infected Wounds. ACS Omega 2021, 6, 21994–22010. [Google Scholar] [CrossRef]
- Carpa, R.; Remizovschi, A.; Culda, C.A.; Butiuc-Keul, A.L. Inherent and Composite Hydrogels as Promising Materials to Limit Antimicrobial Resistance. Gels 2022, 8, 70. [Google Scholar] [CrossRef]
- Suhail, M.; Wu, P.-C.; Minhas, M.U. Development and Characterization of Ph-Sensitive Chondroitin Sulfate-Co-Poly(Acrylic Acid) Hydrogels for Controlled Release of Diclofenac Sodium. J. Saudi Chem. Soc. 2021, 25, 101212. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Juby, K.A.; Dwivedi, C.; Kumar, M.; Kota, S.; Misra, H.S.; Bajaj, P.N. Silver Nanoparticle-Loaded PVA/Gum Acacia Hydrogel: Synthesis, Characterization and Antibacterial Study. Carbohydr. Polym. 2012, 89, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, X. Multiple Strategies to Activate Gold Nanoparticles as Antibiotics. Nanoscale 2013, 5, 8340. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N.; Hikmat, S.; Abu Ghith, D.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold Nanoparticles Loaded into Polymeric Hydrogel for Wound Healing in Rats: Effect of Nanoparticles’ Shape and Surface Modification. Int. J. Pharm. 2019, 565, 174–186. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Bowers, D.T.; Liu, W.; Ma, M. Functional Hydrogels for Diabetic Wound Management. APL Bioeng. 2021, 5, 031503. [Google Scholar] [CrossRef]
- Dumville, J.C.; Soares, M.O.; O’Meara, S.; Cullum, N. Systematic Review and Mixed Treatment Comparison: Dressings to Heal Diabetic Foot Ulcers. Diabetologia 2012, 55, 1902–1910. [Google Scholar] [CrossRef]
- Hoque, J.; Bhattacharjee, B.; Prakash, R.G.; Paramanandham, K.; Haldar, J. Dual Function Injectable Hydrogel for Controlled Release of Antibiotic and Local Antibacterial Therapy. Biomacromolecules 2017, 19, 267–278. [Google Scholar] [CrossRef]
- Ito, T.; Yeo, Y.; Highley, C.B.; Bellas, E.; Benitez, C.A.; Kohane, D.S. The Prevention of Peritoneal Adhesions by in Situ Cross-Linking Hydrogels of Hyaluronic Acid and Cellulose Derivatives. Biomaterials 2007, 28, 975–983. [Google Scholar] [CrossRef]
- Sun, D.; Wang, H.; Liu, J.; Wang, X.; Guo, H.; Xue, L.; Li, L.; Li, J.; Zhang, B.; Xue, Y.; et al. An Enzyme Cross-Linked Hydrogel as a Minimally Invasive Arterial Tissue Sealing and Anti-Adhesion Barrier. Nano Today 2022, 44, 101467. [Google Scholar] [CrossRef]
- Cheng, F.; Xu, L.; Dai, J.; Yi, X.; He, J.; Li, H. N, O-carboxymethyl chitosan/oxidized cellulose composite sponge containing ε-poly-L-lysine as a potential wound dressing for the prevention and treatment of postoperative adhesion. Int. J. Biol. Macromol. 2022, 209, 2151–2164. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.G.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022, 10, 5369–5390. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.W.; Zhang, X.W.; Mi, C.H.; Qi, X.Y.; Zhou, J.; Wei, D.X. Recent advances in hyaluronic acid-based hydrogels for 3D bioprinting in tissue engineering applications. Arab. J. Chem. 2023, 4, 59–68. [Google Scholar] [CrossRef]

| Types of Nanocomposite Hydrogels | Properties | Applications | |
|---|---|---|---|
| Metal Nanoparticle Hydrogels | Silver NPs | Antimicrobial properties Biocompatibility Non-toxic Low mechanical properties Low binding affinity with surfaces | Dental fillings [52] Wound dressing [53] Concentration sensors [53] Functional antibacterial coatings [54] Eye impants [54] |
| Gold NPs | Antimicrobial properties Conductivity High costs | Remote drug dilivery [55] Remote control microfluidic valve [56] | |
| Other NPs | Magnetism properties Low costs High surface/volume ratio | Catalysts [57] Liquid separation [57] | |
| Metal oxide NPs | Ferromagnetic Semiconductivity Light response | Toxic ion absorber [58] Magnetically driven actuators [59] UV protection [60] Photocatalytic [60] | |
| Carbon Nanoparticle Hydrogels | Carbon Nanotubes | Electrical, thermal- stimulation response High ductility | Photothermal drug dilivery [61] Crosslinking agent [62] |
| Graphene | Hydrophilic Conductivity | Site-specific gene delivery [63] Photothermal drug delivery [61] | |
| Polymer-based Nanoparticle Hydrogels | Dendrimers/Hyperbranched polymers | High loading efficiency High mechanical properties | Encapsulate hydrophobic drug molecules [64] |
| Liposomes | High elasticity Hydrophilic/hydrophobic within a same structure | Drug delivery [65] Wound dressing [65] | |
| Inorganic-based Nanoparticle Hydrogels | Si-NPs | Mechanical properties Antimicrobial properties | Implantable material [66] Carrier of catalysts or functional materials [67] |
| Glass ceramics | Ordered, stable structure | Induction agents for bone growth [68] | |
| Hydroxyapatite | High rigidity Low biosorption rate Poor stimulating effect on growth of new tissues | Fillers for amputation bone replacement [69] Plant coatings [69] | |
| Calcium phosphate | Calcium supply | Stimulate bone growth [70] | |
| Types of DN Hydrogels | Reinforcing Mechanism | Properties | Applications |
|---|---|---|---|
| Covalent | Sacrificial bonds | Resistance to biological contamination High strength High toughness Dimensional stability | Hard tissue replacement [76] Bone formation [77] Self-growing materials [78] |
| Non- covalent | (Hybrid) Metal ion coordination | Complete recovery rate High strength High toughness Convenient control | 3D printing Promote skull regeneration Protect brain tissue [79] |
| (Hybrid) Hydrogen bonding | Unstable in aqueous environment, stable in hydrophobic environment Biodegradable Non-toxic | Adsorption of heavy metal ions [80] Biomedical applications [81] | |
| (Hybrid) Hydrophobic interactions | High tensile strength Large fracture strain Good toughness | Biological assembly [82] Synthesis of bioinspired DN hydrogels [83] | |
| (Hybrid) Host-guest interactions | Injectable Weight-bearing Cytocompatibility Adjustable mechanical properties | Biomedical stents [84] | |
| (Physical) Metal ion coordination | High affinity Conductivity | Electrical skins Actuators [85] | |
| (Physical) Hydrogen bonding | Non-cytotoxic Salt tolerance Very low adhesion to tissues | Replacement of living tissue [86] | |
| (Physical) Hydrophobic interactions | Self-healing | Electronic sensors [87] | |
| (Physical) Host-guest interactions | Shear thinning Rapid thixotropic behavior | Vivo injection [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Wang, J.; He, J.; Kan, D.; Chen, K.; Lu, J. Synthesis of Hydrogels and Their Progress in Environmental Remediation and Antimicrobial Application. Gels 2023, 9, 16. https://doi.org/10.3390/gels9010016
Song M, Wang J, He J, Kan D, Chen K, Lu J. Synthesis of Hydrogels and Their Progress in Environmental Remediation and Antimicrobial Application. Gels. 2023; 9(1):16. https://doi.org/10.3390/gels9010016
Chicago/Turabian StyleSong, Mengshan, Jingfeng Wang, Jiabei He, Dongxiao Kan, Kaiyun Chen, and Jialu Lu. 2023. "Synthesis of Hydrogels and Their Progress in Environmental Remediation and Antimicrobial Application" Gels 9, no. 1: 16. https://doi.org/10.3390/gels9010016
APA StyleSong, M., Wang, J., He, J., Kan, D., Chen, K., & Lu, J. (2023). Synthesis of Hydrogels and Their Progress in Environmental Remediation and Antimicrobial Application. Gels, 9(1), 16. https://doi.org/10.3390/gels9010016






