Chlorella vulgaris Extract-Decorated Gold Nanoparticle Hybridized Antimicrobial Hydrogel as a Potential Dressing
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of AuNPs
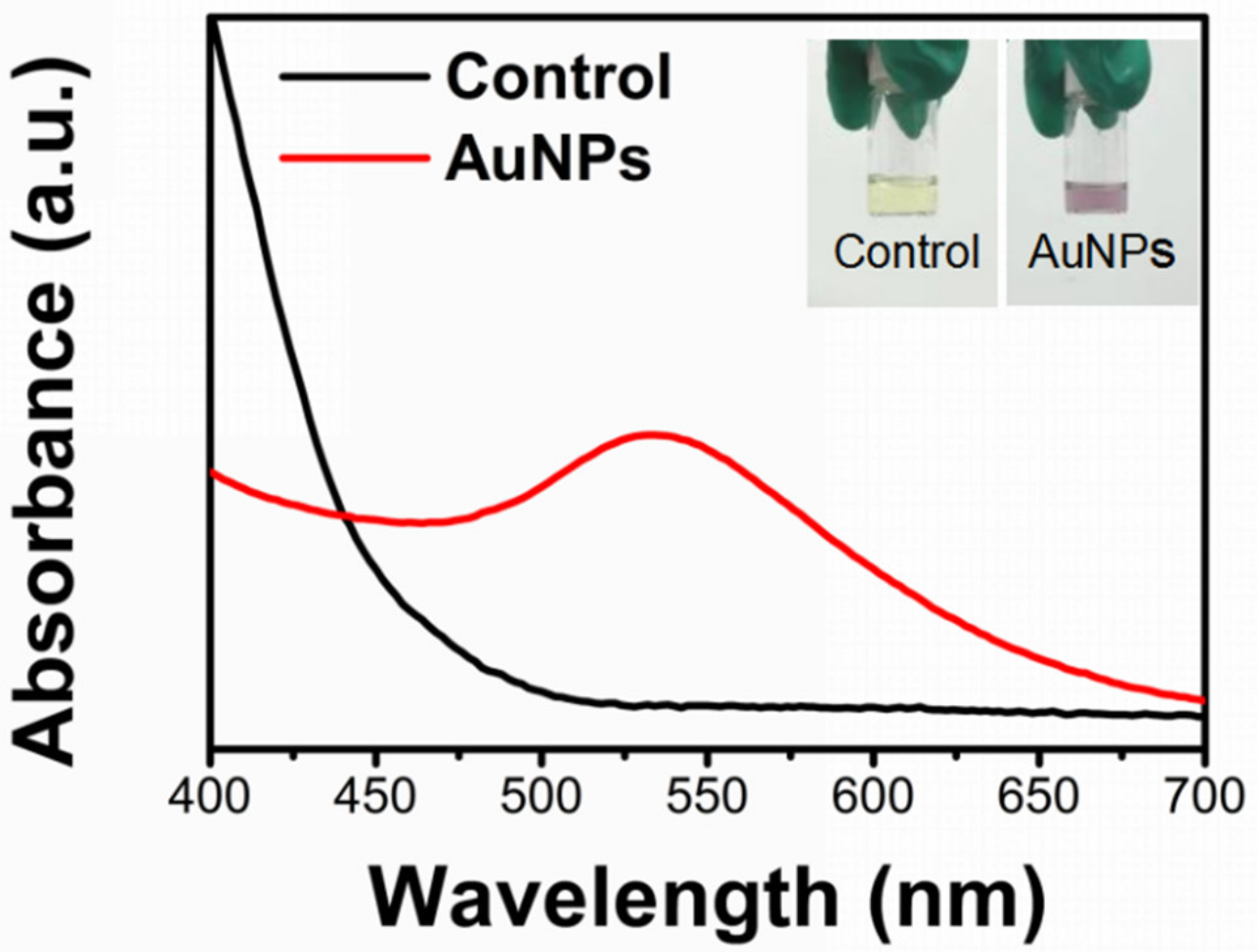
2.1.1. UV-Vis Spectroscopy Characterization of AuNPs@Chlorella
2.1.2. Characterization of the Qualitative Structure of AuNPs@Chlorella
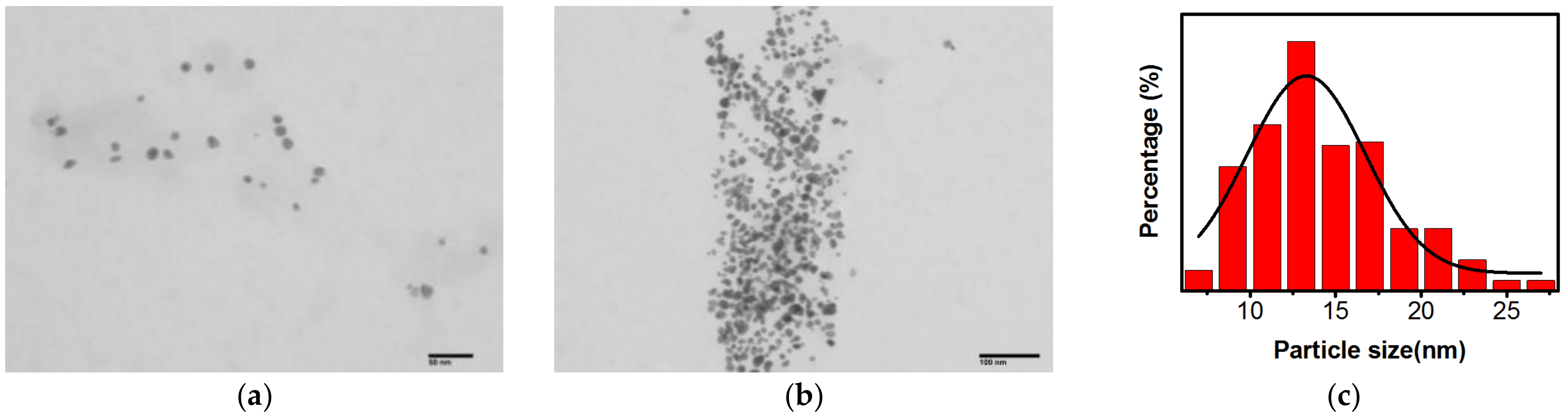
2.1.3. Characterization of the Morphology and Size of AuNPs@Chlorella
2.2. Preparation and Characterization of Hydrogels
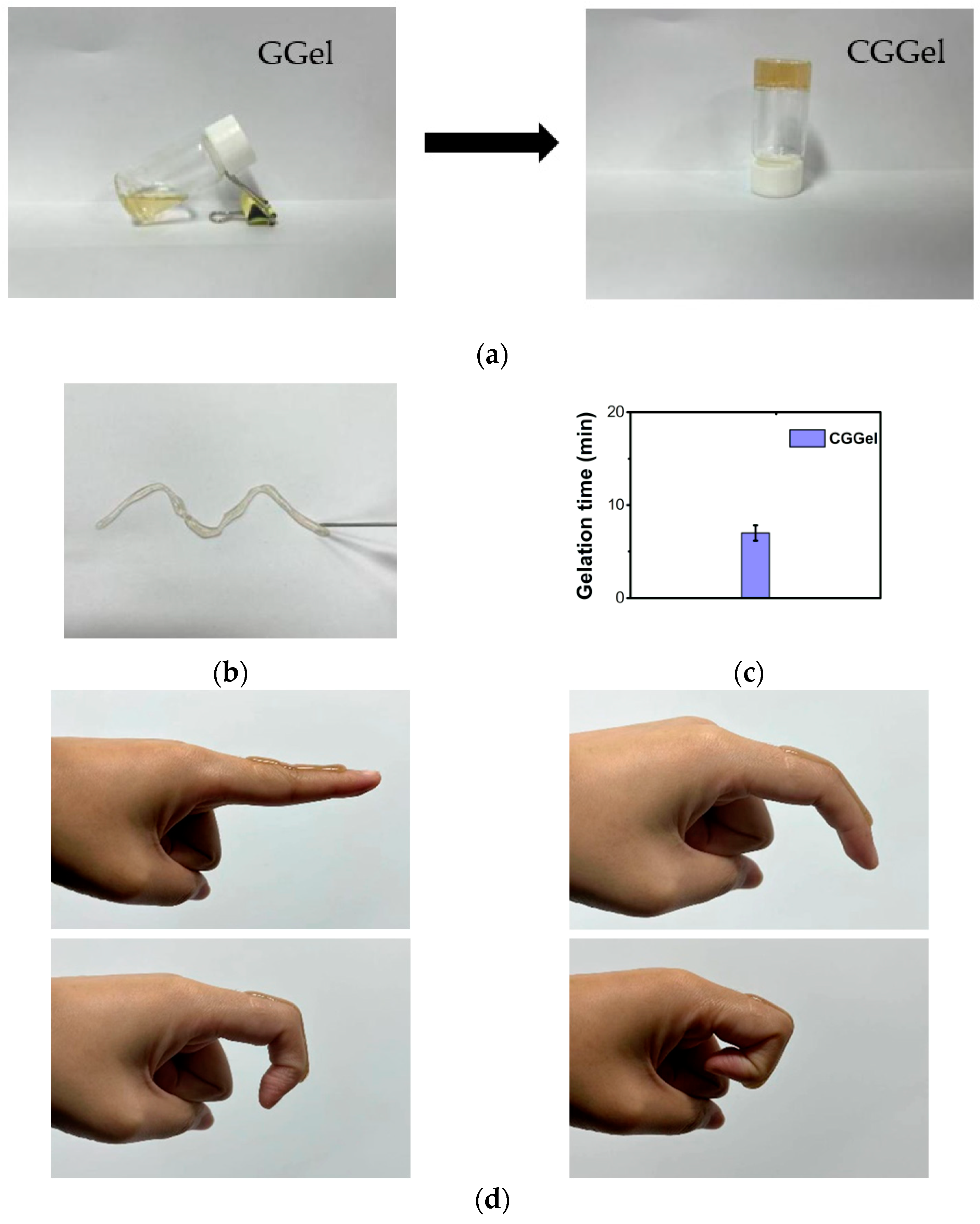
2.2.1. Gel Time of CGGel Gels
2.2.2. Adhesivity of CGGel Gels
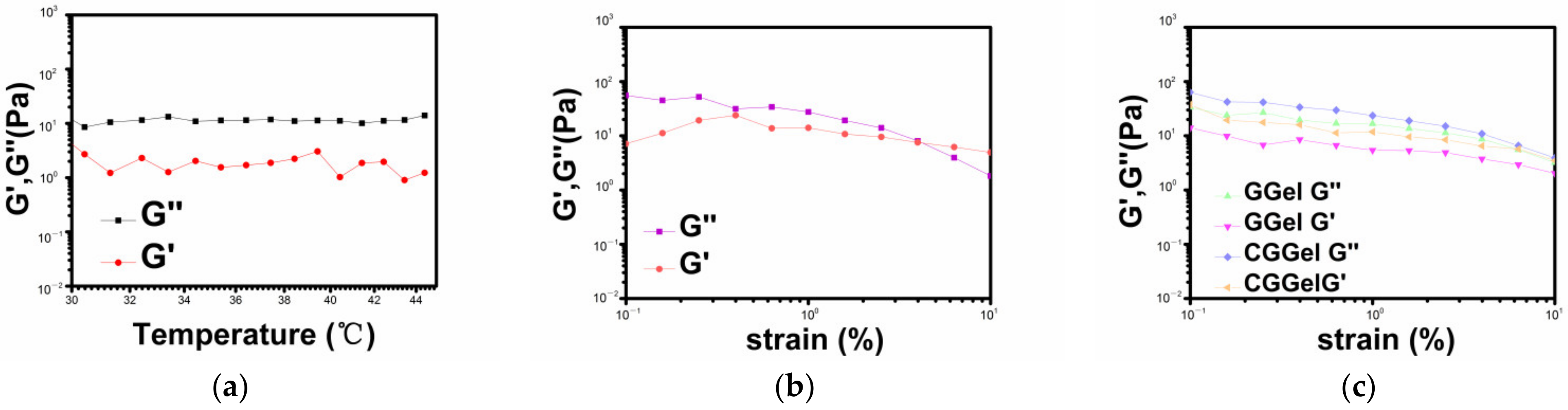
2.2.3. Stability of CGGel Gels
2.2.4. Microscopic Morphology of CGGel Gels
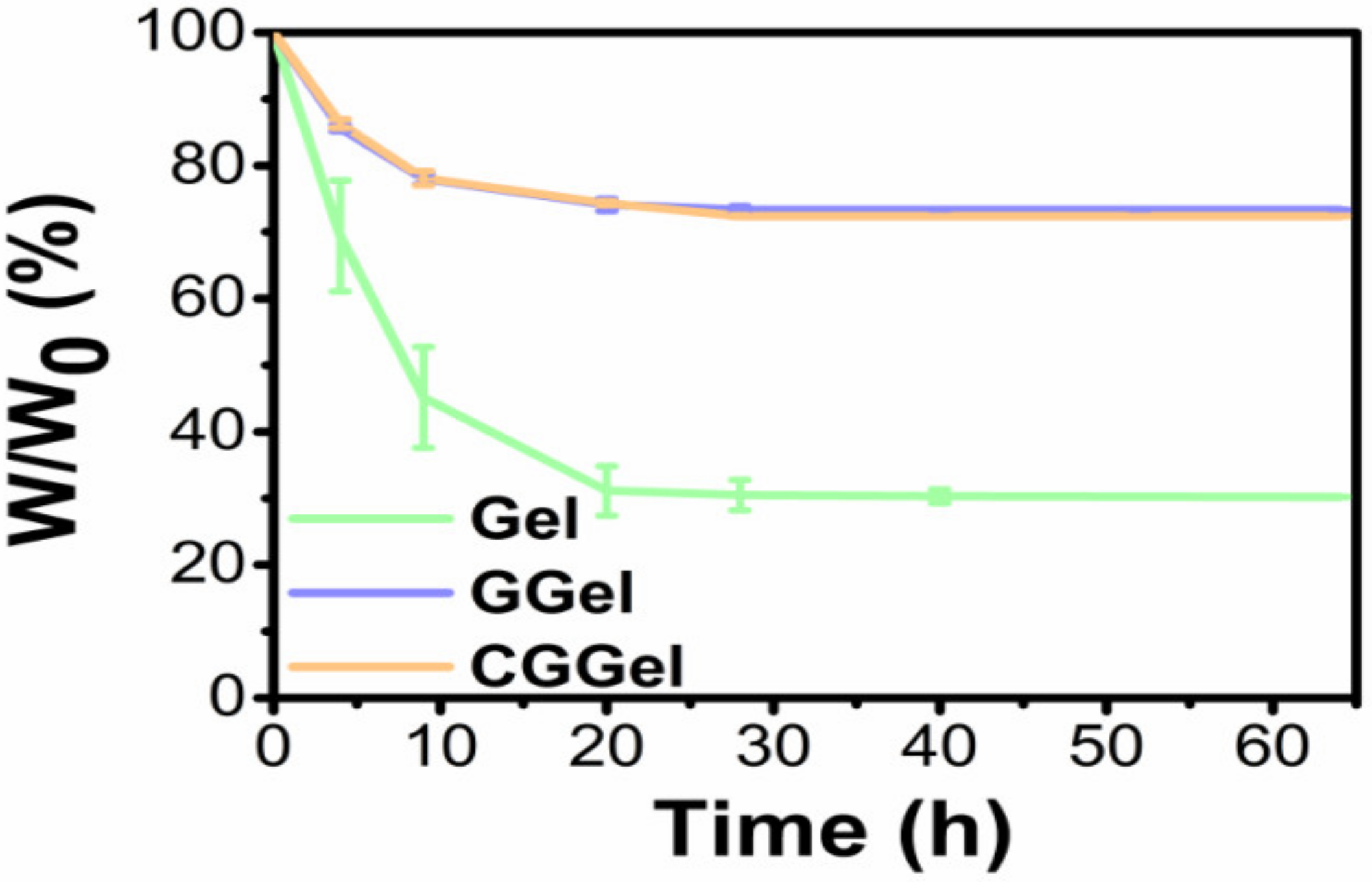
2.2.5. Long-Lasting Moisture of CGGel Gels
2.3. Antibacterial Activity of CGGel Gels
3. Conclusions
4. Materials and Methods
4.1. Synthesis of AuNPs via the Green Method
4.2. Preparation of Hydrogels
4.3. Physicochemical Characterization of AuNPs@Chlorella
4.4. Characterisation of Hydrogels
4.4.1. Determination of Gelation Time
4.4.2. The Retention Ability of the Hydrogel
4.4.3. The Adhesive Property of the Hydrogels
4.4.4. Scanning Electron Microscopy
4.5. In Vitro Antibacterial Activity Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schäfer, M.; Werner, S. The cornified envelope: A first line of defense against reactive oxygen species. J. Investig. Dermatol. 2011, 131, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- DesJardins-Park, H.E.; Gurtner, G.C.; Wan, D.C.; Longaker, M.T. From Chronic Wounds to Scarring: The Growing Health Care Burden of Under- and Over-Healing Wounds. Adv. Wound Care 2022, 11, 496–510. Available online: https://pubmed.ncbi.nlm.nih.gov/34521257/ (accessed on 10 December 2022). [CrossRef] [PubMed]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, e1901502. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13 (Suppl. 2), 5–15. Available online: https://pubmed.ncbi.nlm.nih.gov/27460943/ (accessed on 10 December 2022). [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health J. Int. Soc. Pharm. Outcomes Res. 2018, 21, 27–32. Available online: https://pubmed.ncbi.nlm.nih.gov/29304937/ (accessed on 10 December 2022). [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. Available online: https://pubmed.ncbi.nlm.nih.gov/35621561/ (accessed on 10 December 2022). [CrossRef]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, Biomedical and Pharmaceutical Applications of Fish Gelatin/Hydrolysates. Mar. Drugs 2021, 19, 145. Available online: https://pubmed.ncbi.nlm.nih.gov/33800149/ (accessed on 10 December 2022). [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. Available online: https://pubmed.ncbi.nlm.nih.gov/34502997/ (accessed on 10 December 2022). [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. Available online: https://pubmed.ncbi.nlm.nih.gov/33466924/ (accessed on 10 December 2022). [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Ćirić, A.; Lopes, E.; Molina, A.K.; Calhelha, R.C.; Soković, M.; Ferreira, O.; Ferreira, I.; Barros, L. Extraction of Aloesin from Aloe vera Rind Using Alternative Green Solvents: Process Optimization and Biological Activity Assessment. Biology 2021, 10, 951. Available online: https://pubmed.ncbi.nlm.nih.gov/34681050/ (accessed on 10 December 2022). [CrossRef]
- Dong, L.; Han, Z.; Zhang, H.; Yang, R.; Fang, J.; Wang, L.; Li, X.; Li, X. Tea polyphenol/glycerol-treated double-network hydrogel with enhanced mechanical stability and anti-drying, antioxidant and antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2022, 208, 530–543. Available online: https://pubmed.ncbi.nlm.nih.gov/35346679/ (accessed on 10 December 2022). [CrossRef]
- Shojaee Kang Sofla, M.; Mortazavi, S.; Seyfi, J. Preparation and characterization of polyvinyl alcohol/chitosan blends plasticized and compatibilized by glycerol/polyethylene glycol. Carbohydr. Polym. 2020, 232, 115784. Available online: https://pubmed.ncbi.nlm.nih.gov/31952592/ (accessed on 10 December 2022). [CrossRef]
- Wright, J.B.; Lam, K.; Burrell, R.E. Wound management in an era of increasing bacterial antibiotic resistance: A role for topical silver treatment. Am. J. Infect. Control 1998, 26, 572–577. Available online: https://pubmed.ncbi.nlm.nih.gov/9836841/ (accessed on 10 December 2022). [CrossRef]
- Mi, G.; Shi, D.; Wang, M.; Webster, T.J. Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv. Healthc. Mater. 2018, 7, 1800103. Available online: https://pubmed.ncbi.nlm.nih.gov/29790304/ (accessed on 10 December 2022). [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Anticancer, antimicrobial, antioxidant, and catalytic activities of green-synthesized silver and gold nanoparticles using Bauhinia purpurea leaf extract. Bioprocess Biosyst. Eng. 2019, 42, 305–319. Available online: https://pubmed.ncbi.nlm.nih.gov/30421171/ (accessed on 10 December 2022). [CrossRef]
- Sampath, S.; Madhavan, Y.; Muralidharan, M.; Sunderam, V.; Lawrance, A.V.; Muthupandian, S. A review on algal mediated synthesis of metal and metal oxide nanoparticles and their emerging biomedical potential. J. Biotechnol. 2022, 360, 92–109. Available online: https://pubmed.ncbi.nlm.nih.gov/36272578/ (accessed on 10 December 2022). [CrossRef]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An Overview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules 2020, 10, 1498. Available online: https://pubmed.ncbi.nlm.nih.gov/33143289/ (accessed on 10 December 2022). [CrossRef]
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Ameliorative effect of biofabricated ZnO nanoparticles of Trianthema portulacastrum Linn. on dermal wounds via removal of oxidative stress and inflammation. RSC Adv. 2018, 8, 21621–21635. Available online: https://pubmed.ncbi.nlm.nih.gov/35539937/ (accessed on 10 December 2022). [CrossRef]
- Thangaswamy, S.J.K.; Mir, M.A.; Muthu, A. Green synthesis of mono and bimetallic alloy nanoparticles of gold and silver using aqueous extract of Chlorella acidophile for potential applications in sensors. Prep. Biochem. Biotechnol. 2021, 51, 1026–1035. Available online: https://pubmed.ncbi.nlm.nih.gov/33687315/ (accessed on 10 December 2022). [CrossRef]
- Surujpaul, P.P.; Gutiérrez-Wing, C.; Ocampo-García, B.; Ramírez Fde, M.; Arteaga de Murphy, C.; Pedraza-López, M.; Camacho-López, M.A.; Ferro-Flores, G. Gold nanoparticles conjugated to [Tyr3]octreotide peptide. Biophys. Chem. 2008, 138, 83–90. Available online: https://pubmed.ncbi.nlm.nih.gov/18819743/ (accessed on 10 December 2022). [CrossRef]
- Govindaraju, S.; Ramasamy, M.; Baskaran, R.; Ahn, S.J.; Yun, K. Ultraviolet light and laser irradiation enhances the antibacterial activity of glucosamine-functionalized gold nanoparticles. Int. J. Nanomed. 2015, 10, 67–78. Available online: https://pubmed.ncbi.nlm.nih.gov/26345521/ (accessed on 10 December 2022).
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. Available online: https://pubmed.ncbi.nlm.nih.gov/35902015/ (accessed on 10 December 2022). [CrossRef] [PubMed]
- Hou, M.; Wang, X.; Yue, O.; Zheng, M.; Zhang, H.; Liu, X. Development of a multifunctional injectable temperature-sensitive gelatin-based adhesive double-network hydrogel. Biomater. Adv. 2022, 134, 112556. Available online: https://pubmed.ncbi.nlm.nih.gov/35525757/ (accessed on 10 December 2022). [CrossRef] [PubMed]
- Wang, Y.; Shang, L.; Chen, G.; Sun, L.; Zhang, X.; Zhao, Y. Bioinspired structural color patch with anisotropic surface adhesion. Sci. Adv. 2020, 6, 8258. Available online: https://pubmed.ncbi.nlm.nih.gov/32042897/ (accessed on 10 December 2022). [CrossRef] [PubMed]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. Available online: https://pubmed.ncbi.nlm.nih.gov/34940315/ (accessed on 10 December 2022). [CrossRef]
- Rey, F.; Barzaghini, B.; Nardini, A.; Bordoni, M.; Zuccotti, G.V.; Cereda, C.; Raimondi, M.T.; Carelli, S. Advances in Tissue Engineering and Innovative Fabrication Techniques for 3-D-Structures: Translational Applications in Neurodegenerative Diseases. Cells 2020, 9, 1636. Available online: https://pubmed.ncbi.nlm.nih.gov/32646008/ (accessed on 10 December 2022). [CrossRef]
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.L.; Cheng, B.; Mo, X.M.; Chen, C.; Pan, J.F. Moist-Retaining, Self-Recoverable, Bioadhesive, and Transparent in Situ Forming Hydrogels To Accelerate Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 2023–2038. Available online: https://pubmed.ncbi.nlm.nih.gov/31895528/ (accessed on 10 December 2022). [CrossRef]
- Lima, P.J.M.; da Silva, R.M.; Neto, C.; Gomes, E.S.N.C.; Souza, J.; Nunes, Y.L.; Sousa Dos Santos, J.C. An overview on the conversion of glycerol to value-added industrial products via chemical and biochemical routes. Biotechnol. Appl. Biochem. 2021, 69, 2794–2818. Available online: https://pubmed.ncbi.nlm.nih.gov/33481298/ (accessed on 10 December 2022).
- Sukhikh, S.; Prosekov, A.; Ivanova, S.; Maslennikov, P.; Andreeva, A.; Budenkova, E.; Kashirskikh, E.; Tcibulnikova, A.; Zemliakova, E.; Samusev, I.; et al. Identification of Metabolites with Antibacterial Activities by Analyzing the FTIR Spectra of Microalgae. Life 2022, 12, 1395. Available online: https://pubmed.ncbi.nlm.nih.gov/36143431/ (accessed on 10 December 2022). [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. Available online: https://pubmed.ncbi.nlm.nih.gov/32995675/ (accessed on 10 December 2022). [CrossRef]
- Nalawade, T.M.; Bhat, K.G.; Sogi, S. Antimicrobial Activity of Endodontic Medicaments and Vehicles using Agar Well Diffusion Method on Facultative and Obligate Anaerobes. Int. J. Clin. Pediatr. Dent. 2016, 9, 335–341. Available online: https://pubmed.ncbi.nlm.nih.gov/28127166/ (accessed on 10 December 2022).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Zhou, D.; Xiao, L.; Li, Y. Chlorella vulgaris Extract-Decorated Gold Nanoparticle Hybridized Antimicrobial Hydrogel as a Potential Dressing. Gels 2023, 9, 11. https://doi.org/10.3390/gels9010011
He R, Zhou D, Xiao L, Li Y. Chlorella vulgaris Extract-Decorated Gold Nanoparticle Hybridized Antimicrobial Hydrogel as a Potential Dressing. Gels. 2023; 9(1):11. https://doi.org/10.3390/gels9010011
Chicago/Turabian StyleHe, Ruiying, Dong Zhou, Lan Xiao, and Yulin Li. 2023. "Chlorella vulgaris Extract-Decorated Gold Nanoparticle Hybridized Antimicrobial Hydrogel as a Potential Dressing" Gels 9, no. 1: 11. https://doi.org/10.3390/gels9010011
APA StyleHe, R., Zhou, D., Xiao, L., & Li, Y. (2023). Chlorella vulgaris Extract-Decorated Gold Nanoparticle Hybridized Antimicrobial Hydrogel as a Potential Dressing. Gels, 9(1), 11. https://doi.org/10.3390/gels9010011







