Collagen Matrices Mediate Glioma Cell Migration Induced by an Electrical Signal
Abstract
:1. Introduction
2. Results
2.1. Glioma Cells, Fetal Astrocytes, and Adult Astrocytes Showed High Migration Velocity in Low-Concentration Collagen Hydrogels
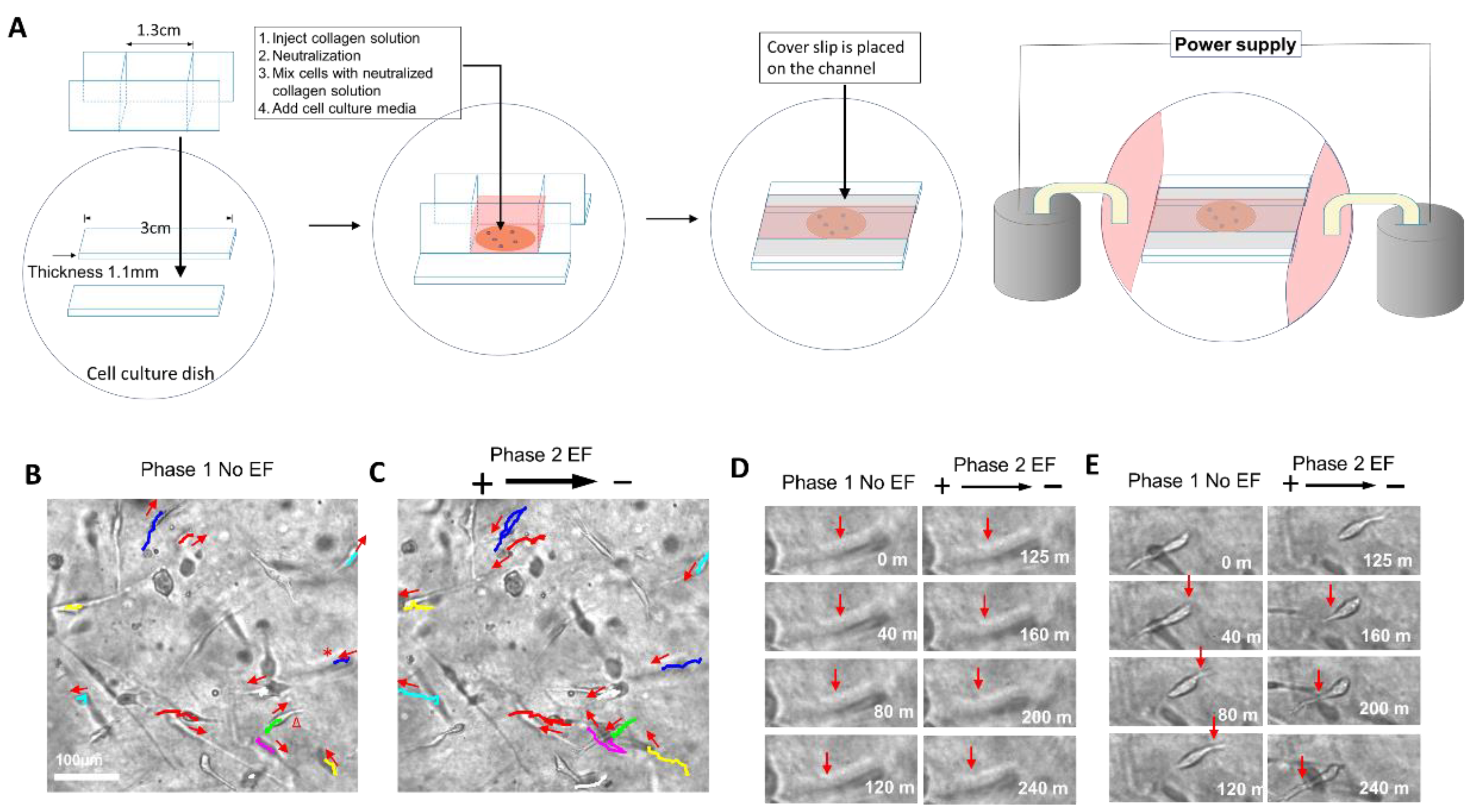
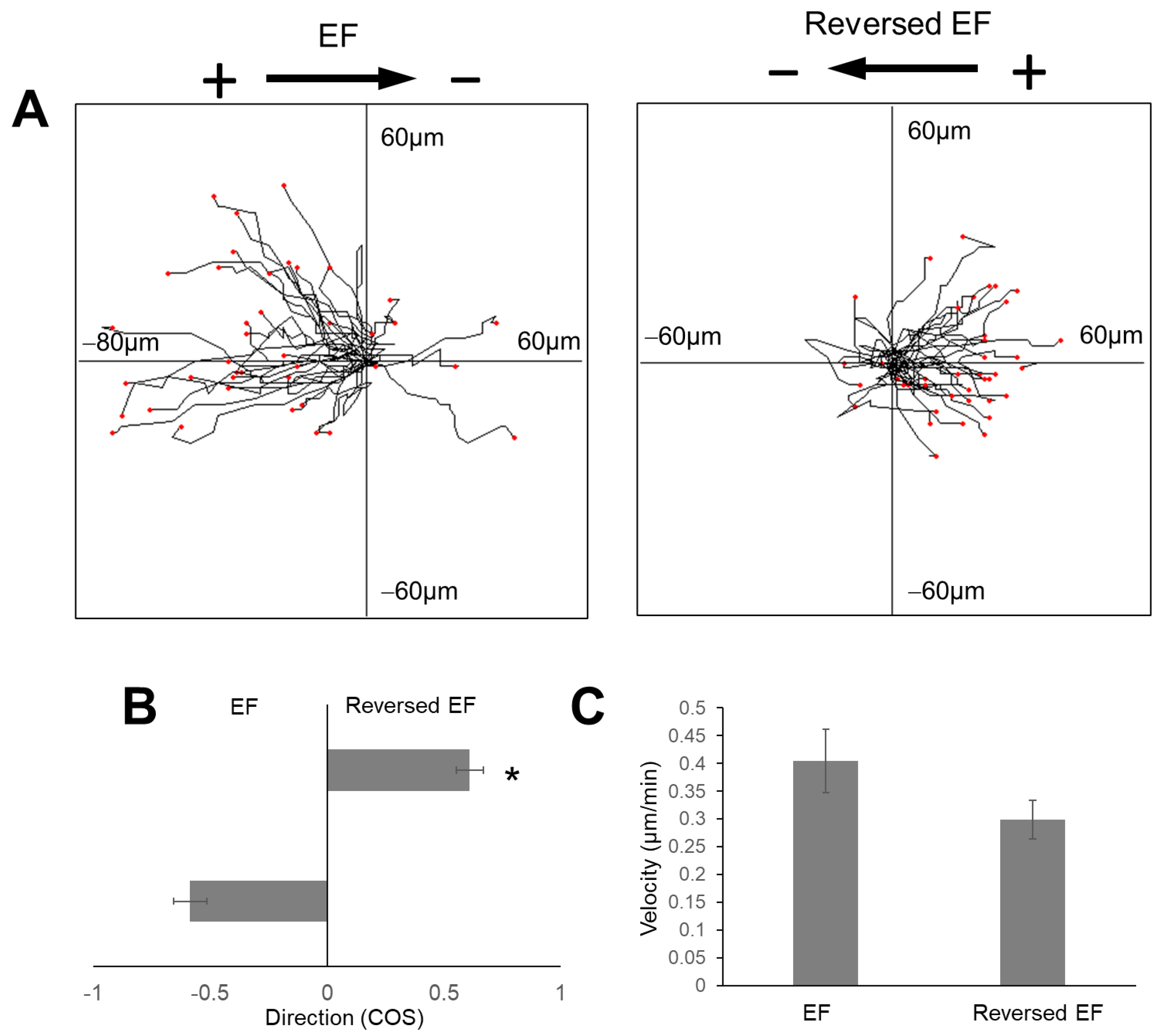
2.2. The Electric Field Directed Glioma Cell Migration in the Collagen Gels
2.3. The Increase in Field Strength Enhanced Anodal Migration Directedness
2.4. EF Stimulation Increased Glioma Cell Migration Velocity in the Collagen Hydrogels
2.5. Reversal of EF Polarity Reversed the Cell Migration Direction
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture in Collagen Hydrogels
5.2. EF Application for Glioma Cells Cultured in Collagen Hydrogels
5.3. Time-Lapse Imaging for Recording Cell Migration
5.4. Cell Migration Analysis
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mehta, S.; Lo Cascio, C. Developmentally regulated signaling pathways in glioma invasion. Cell. Mol. Life Sci. CMLS 2018, 75, 385–402. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Robel, S.; Watkins, S.; Sontheimer, H. A neurocentric perspective on glioma invasion. Nat. Rev. Neurosci. 2014, 15, 455–465. [Google Scholar] [CrossRef]
- Mahesparan, R.; Read, T.A.; Lund-Johansen, M.; Skaftnesmo, K.O.; Bjerkvig, R.; Engebraaten, O. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol. 2003, 105, 49–57. [Google Scholar] [CrossRef]
- Belousov, A.; Titov, S.; Shved, N.; Garbuz, M.; Malykin, G.; Gulaia, V.; Kagansky, A.; Kumeiko, V. The Extracellular Matrix and Biocompatible Materials in Glioblastoma Treatment. Front. Bioeng. Biotechnol. 2019, 7, 341. [Google Scholar] [CrossRef]
- Payne, L.S.; Huang, P.H. The pathobiology of collagens in glioma. Mol. Cancer Res. MCR 2013, 11, 1129–1140. [Google Scholar] [CrossRef]
- Pointer, K.B.; Clark, P.A.; Schroeder, A.B.; Salamat, M.S.; Eliceiri, K.W.; Kuo, J.S. Association of collagen architecture with glioblastoma patient survival. J. Neurosurg. 2016, 126, 1812–1821. [Google Scholar] [CrossRef]
- Velez, D.O.; Tsui, B.; Goshia, T.; Chute, C.L.; Han, A.; Carter, H.; Fraley, S.I. 3D collagen architecture induces a conserved migratory and transcriptional response linked to vasculogenic mimicry. Nat. Commun. 2017, 8, 1651. [Google Scholar] [CrossRef]
- Zaman, M.H.; Trapani, L.M.; Sieminski, A.L.; MacKellar, D.; Gong, H.; Kamm, R.D.; Wells, A.; Lauffenburger, D.A.; Matsudaira, P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 10889–10894. [Google Scholar] [CrossRef]
- Bordeleau, F.; Tang, L.N.; Reinhart-King, C.A. Topographical guidance of 3D tumor cell migration at an interface of collagen densities. Phys. Biol. 2013, 10, 065004. [Google Scholar] [CrossRef]
- Plou, J.; Juste-Lanas, Y.; Olivares, V.; Del Amo, C.; Borau, C.; García-Aznar, J.M. From individual to collective 3D cancer dissemination: Roles of collagen concentration and TGF-β. Sci. Rep. 2018, 8, 12723. [Google Scholar] [CrossRef]
- Kaufman, L.J.; Brangwynne, C.P.; Kasza, K.E.; Filippidi, E.; Gordon, V.D.; Deisboeck, T.S.; Weitz, D.A. Glioma expansion in collagen I matrices: Analyzing collagen concentration-dependent growth and motility patterns. Biophys. J. 2005, 89, 635–650. [Google Scholar] [CrossRef]
- Kaphle, P.; Li, Y.; Yao, L. The mechanical and pharmacological regulation of glioblastoma cell migration in 3D matrices. J. Cell. Physiol. 2019, 234, 3948–3960. [Google Scholar] [CrossRef]
- Lyon, J.G.; Carroll, S.L.; Mokarram, N.; Bellamkonda, R.V. Electrotaxis of Glioblastoma and Medulloblastoma Spheroidal Aggregates. Sci. Rep. 2019, 9, 5309. [Google Scholar] [CrossRef]
- Clancy, H.; Pruski, M.; Lang, B.; Ching, J.; McCaig, C.D. Glioblastoma cell migration is directed by electrical signals. Exp. Cell Res. 2021, 406, 112736. [Google Scholar] [CrossRef]
- Zhu, K.; Hum, N.R.; Reid, B.; Sun, Q.; Loots, G.G.; Zhao, M. Electric Fields at Breast Cancer and Cancer Cell Collective Galvanotaxis. Sci. Rep. 2020, 10, 8712. [Google Scholar] [CrossRef]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling cell behavior electrically: Current views and future potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef]
- Borgens, R.B.; Shi, R. Uncoupling histogenesis from morphogenesis in the vertebrate embryo by collapse of the transneural tube potential. Dev. Dyn. 1995, 203, 456–467. [Google Scholar] [CrossRef]
- Shi, R.; Borgens, R.B. Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev. Dyn. 1995, 202, 101–114. [Google Scholar] [CrossRef]
- Song, B.; Zhao, M.; Forrester, J.; McCaig, C. Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J. Cell Sci. 2004, 117, 4681–4690. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Xu, K.; Wang, Z.; Fan, X.; Zhang, C.; Li, S.; Qiu, X.; Jiang, T. Relationship between necrotic patterns in glioblastoma and patient survival: Fractal dimension and lacunarity analyses using magnetic resonance imaging. Sci. Rep. 2017, 7, 8302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Ruan, L.X.; Zhang, S. Rapid progression of glioblastoma multiforme: A case report. Oncol. Lett. 2016, 12, 4803–4806. [Google Scholar] [CrossRef]
- Aguilar, A.A.; Ho, M.C.; Chang, E.; Carlson, K.W.; Natarajan, A.; Marciano, T.; Bomzon, Z.; Patel, C.B. Permeabilizing Cell Membranes with Electric Fields. Cancers 2021, 13, 2283. [Google Scholar] [CrossRef]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef]
- Sprugnoli, G.; Golby, A.J.; Santarnecchi, E. Newly discovered neuron-to-glioma communication: New noninvasive therapeutic opportunities on the horizon? Neuro-Oncol. Adv. 2021, 3, vdab018. [Google Scholar] [CrossRef]
- Ulrich, T.A.; de Juan Pardo, E.M.; Kumar, S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009, 69, 4167–4174. [Google Scholar] [CrossRef]
- Yao, L.; Shanley, L.; McCaig, C.; Zhao, M. Small applied electric fields guide migration of hippocampal neurons. J. Cell. Physiol. 2008, 216, 527–535. [Google Scholar] [CrossRef]
- Yao, L.; Shippy, T.; Li, Y. Genetic analysis of the molecular regulation of electric fields-guided glia migration. Sci. Rep. 2020, 10, 16821. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.-S.; Lucas, G.; Li, R.; Yao, L. ARP2/3 complex is required for directional migration of neural stem cell-derived oligodendrocyte precursors in electric fields. Stem Cell Res. Ther. 2015, 6, 41. [Google Scholar] [CrossRef]
- Li, Y.; Weiss, M.; Yao, L. Directed migration of embryonic stem cell-derived neural cells in an applied electric field. Stem Cell Rev. Rep. 2014, 10, 653–662. [Google Scholar] [CrossRef]
- Lange, F.; Venus, J.; Abady, D.S.E.; Porath, K.; Einsle, A.; Sellmann, T.; Neubert, V.; Reichart, G.; Linnebacher, M.; Köhling, R.; et al. Galvanotactic Migration of Glioblastoma and Brain Metastases Cells. Life 2022, 12, 580. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, R.; Devreotes, P.N.; Mogilner, A.; Zhao, M. 3D arrays for high throughput assay of cell migration and electrotaxis. Cell Biol. Int. 2013, 37, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Calafiore, M.; Zeng, Q.; Zhang, X.; Huang, Y.; Li, R.A.; Deng, W.; Zhao, M. Electrically guiding migration of human induced pluripotent stem cells. Stem Cell Rev. Rep. 2011, 7, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Toms, S.A.; Kim, C.Y.; Nicholas, G.; Ram, Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: A subgroup analysis of the EF-14 phase III trial. J. Neuro-Oncol. 2019, 141, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Dbalý, V.; Tovarys, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Tran, K.; Nguyen, D. Collagen Matrices Mediate Glioma Cell Migration Induced by an Electrical Signal. Gels 2022, 8, 545. https://doi.org/10.3390/gels8090545
Yao L, Tran K, Nguyen D. Collagen Matrices Mediate Glioma Cell Migration Induced by an Electrical Signal. Gels. 2022; 8(9):545. https://doi.org/10.3390/gels8090545
Chicago/Turabian StyleYao, Li, Kimmy Tran, and Diana Nguyen. 2022. "Collagen Matrices Mediate Glioma Cell Migration Induced by an Electrical Signal" Gels 8, no. 9: 545. https://doi.org/10.3390/gels8090545
APA StyleYao, L., Tran, K., & Nguyen, D. (2022). Collagen Matrices Mediate Glioma Cell Migration Induced by an Electrical Signal. Gels, 8(9), 545. https://doi.org/10.3390/gels8090545




