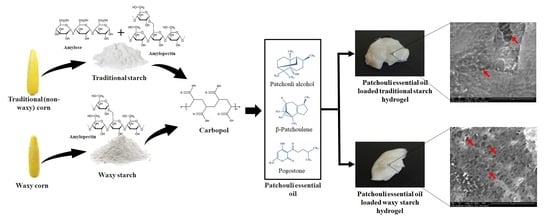
Synthesis and Characterization of Novel Patchouli Essential Oil Loaded Starch-Based Hydrogel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction and Characterization of Patchouli Essential Oil
2.2. Physical Properties of Hydrogel Samples
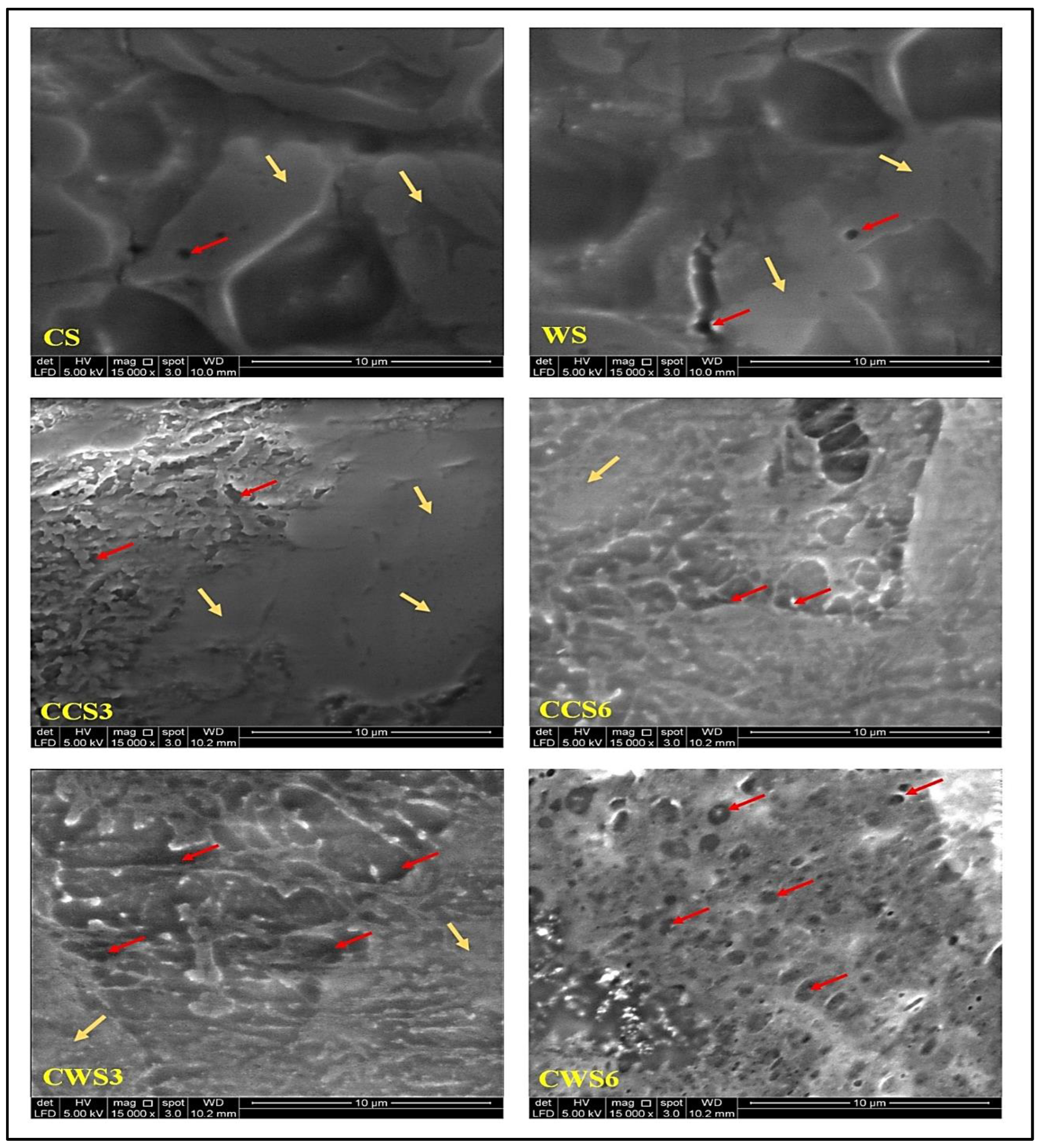
2.3. Morphological Properties of Hydrogel Samples
2.4. Fourier Transform Infrared Spectroscopy (FT-IR) Spectra
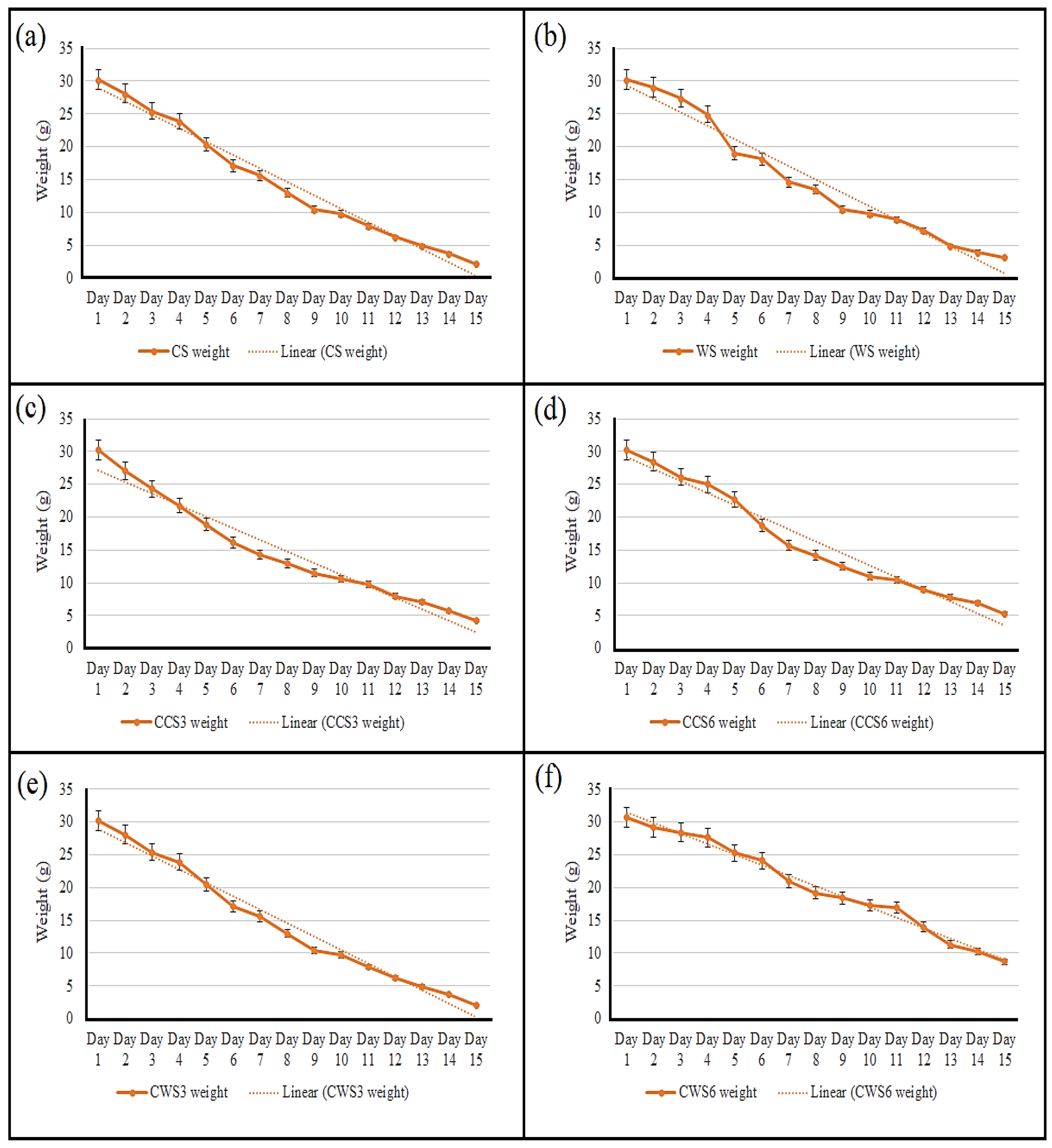
2.5. In Vitro Degradation Study
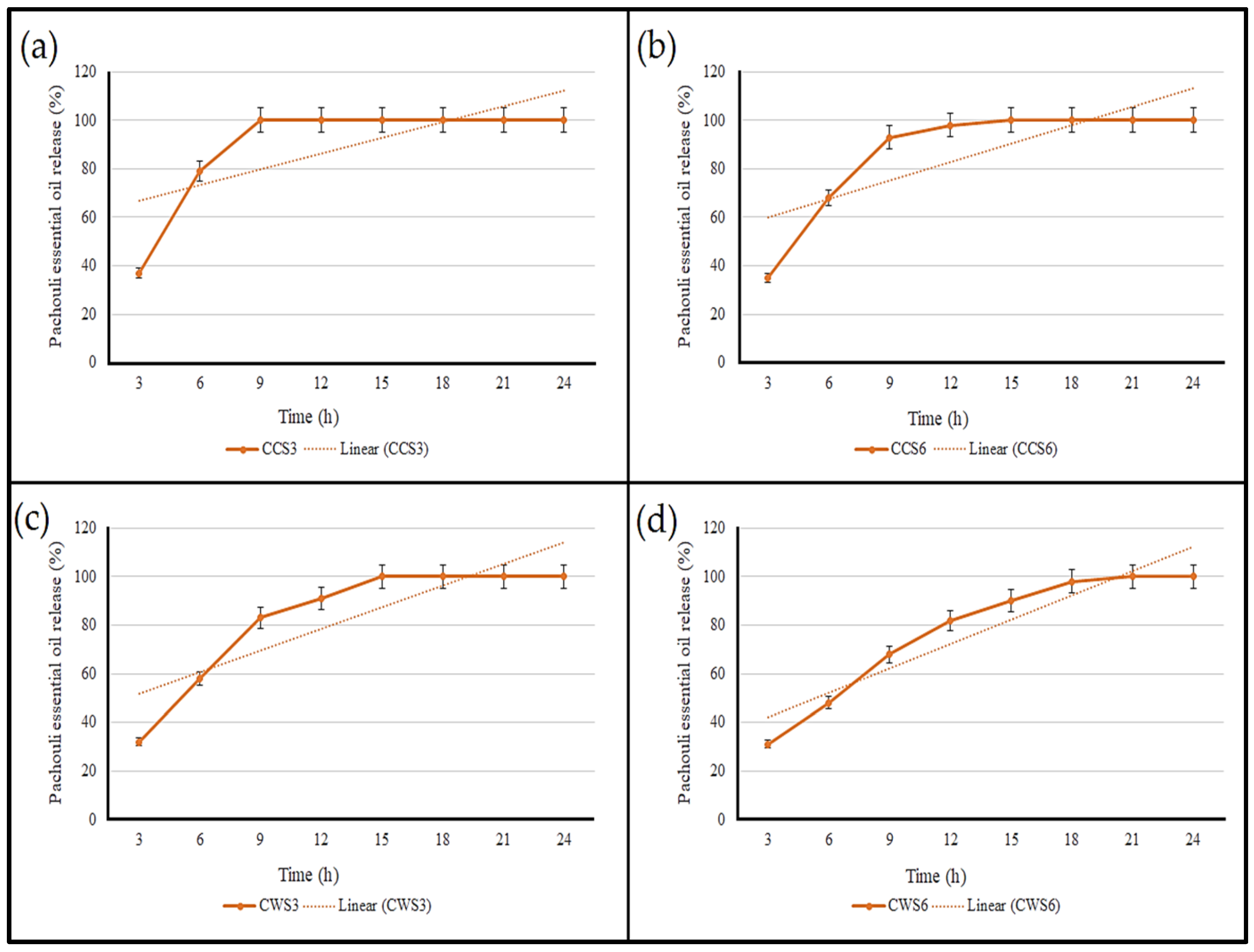
2.6. The Rate of Essential Oil Release
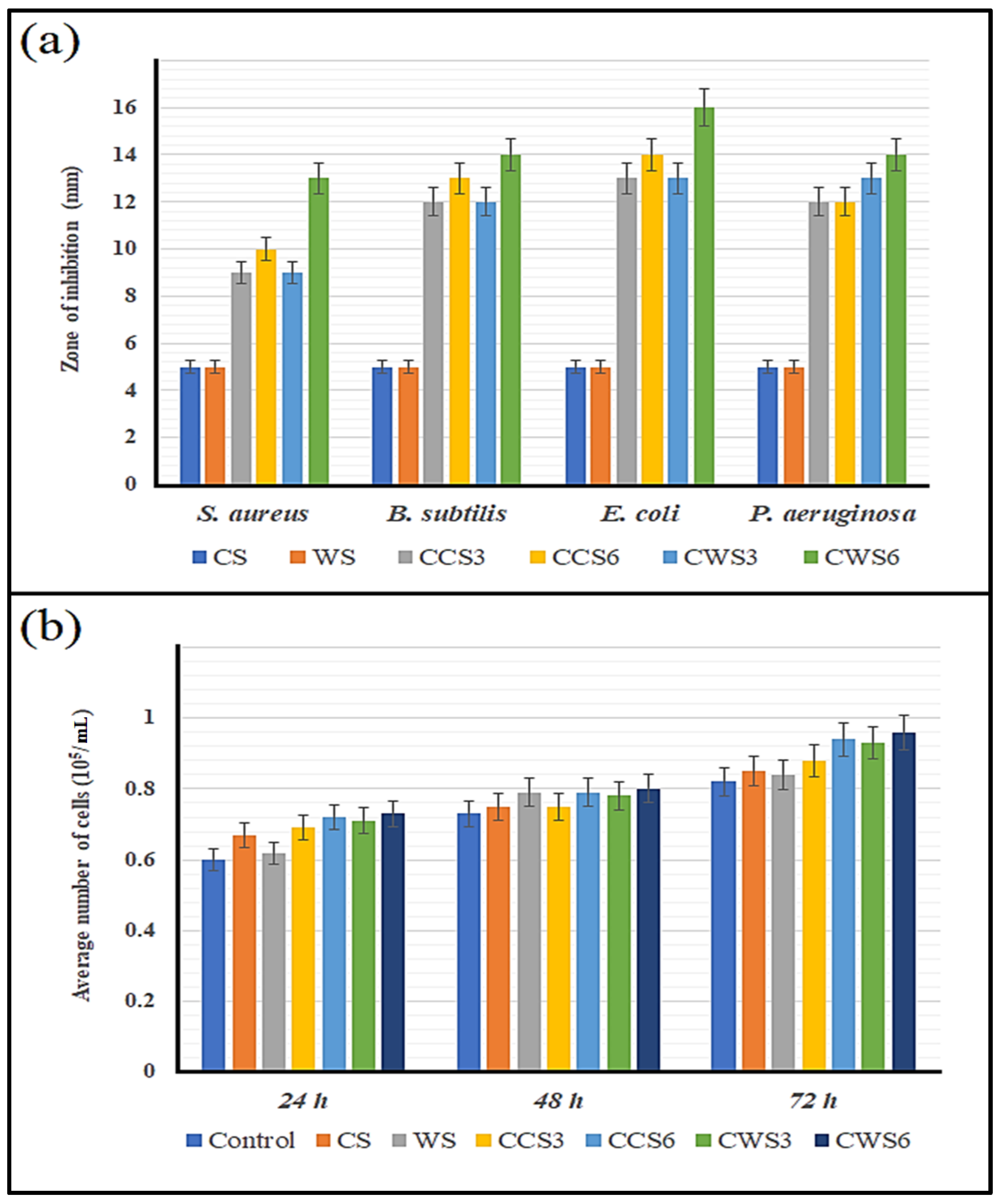
2.7. Antibacterial Activity and Biocompatability of the Hydrogel Samples
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Extraction of Patchouli Essential Oil
4.3. Preparation of Loaded and Non-Loaded Hydrogels
4.4. Antibacterial Activity, MIC and MBC of Patchouli Essential Oil
4.5. Characterization of Hydrogels
4.5.1. Physical and Morphological Analysis
4.5.2. Surface Functional Groups
4.5.3. Antibacterial Activity
4.5.4. In Vitro Biodegradation
4.5.5. In Vitro Essential Oil Release
4.5.6. Biocompatibility Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; El Halal, S.L.d.M.; Lim, L.-T.; Dias, Á.R.G.; da Rosa Zavareze, E. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Kim, T.; Suh, J.; Kim, W.J. Polymeric Aggregate-Embodied Hybrid Nitric-Oxide-Scavenging and Sequential Drug-Releasing Hydrogel for Combinatorial Treatment of Rheumatoid Arthritis. Adv. Mater. 2021, 33, 2008793. [Google Scholar] [CrossRef]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef]
- Choi, G.; Fitriasari, E.I.; Park, C. Electro-Mechanochemical Gating of a Metal–Phenolic Nanocage for Controlled Guest-Release Self-Powered Patches and Injectable Gels. ACS Nano 2021, 15, 14580–14586. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.; Ibrahim, H. An overview on starch-based sustainable hydrogels: Potential applications and aspects. J. Polym. Environ. 2021, 30, 19–50. [Google Scholar] [CrossRef]
- Garcia, M.A.V.T.; Garcia, C.F.; Faraco, A.A.G. Pharmaceutical and biomedical applications of native and modified starch: A review. Starch Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Jummaat, F.; Yahya, E.B.; Abdul Khalil, H.P.S.; Adnan, A.; Alqadhi, A.M.; Abdullah, C.; AK, A.S.; Olaiya, N.; Abdat, M. The role of biopolymer-based materials in obstetrics and gynecology applications: A review. Polymers 2021, 13, 633. [Google Scholar] [CrossRef]
- Hsieh, C.-F.; Liu, W.; Whaley, J.K.; Shi, Y.-C. Structure and functional properties of waxy starches. Food Hydrocoll. 2019, 94, 238–254. [Google Scholar] [CrossRef]
- van Beek, T.A.; Joulain, D. The essential oil of patchouli, Pogostemon cablin: A review. Flavour Fragr. J. 2018, 33, 6–51. [Google Scholar] [CrossRef] [Green Version]
- Swamy, M.K.; Mohanty, S.K.; Sinniah, U.R.; Maniyam, A. Evaluation of patchouli (Pogostemon cablin Benth.) cultivars for growth, yield and quality parameters. J. Essent. Oil Bear. Plants 2015, 18, 826–832. [Google Scholar] [CrossRef]
- Jia, J.; Duan, S.; Zhou, X.; Sun, L.; Qin, C.; Li, M.; Ge, F. Long-Term Antibacterial Film Nanocomposite Incorporated with Patchouli Essential Oil Prepared by Supercritical CO2 Cyclic Impregnation for Wound Dressing. Molecules 2021, 26, 5005. [Google Scholar] [CrossRef]
- Sufriadi, E.; Meilina, H.; Munawar, A.; Idroes, R. Fourier Transformed Infrared (FTIR) spectroscopy analysis of patchouli essential oils based on different geographical area in Aceh. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Banda Aceh, Indonesia, 15–16 October 2020; p. 012067. [Google Scholar]
- Li, Y.-Q.; Kong, D.-X.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crops Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Fahmi, Z.; Mudasir, M.; Rohman, A. Attenuated total reflectance-FTIR spectra combined with multivariate calibration and discrimination analysis for analysis of patchouli oil adulteration. Indones. J. Chem. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Almashgab, A.M.; Yahya, E.B.; Banu, A. The Cytotoxicity Effects of Outer Membrane Vesicles Isolated from Hospital and Laboratory Strains of Pseudomonas Aeruginosa on Human Keratinocyte Cell Line. Malays. J. Sci. 2020, 39, 45–53. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yang, S.-P.; Liu, W.-Q. Evaluation of the antibacterial activity of patchouli oil. Iran. J. Pharm. Res. 2013, 12, 307. [Google Scholar]
- Thoppil, J.; Tajo, A.; Minija, J.; Deena, M.; Sreeranjini, K.; Leeja, L.; Sivadasan, M.; Alfarhan, A. Antimicrobial activity of the essential oils of three species of Pogostemon. J. Environ. Biol. 2014, 35, 795. [Google Scholar]
- Peng, F.; Wan, F.; Xiong, L.; Peng, C.; Dai, M.; Chen, J. In vitro and in vivo antibacterial activity of Pogostone. Chin. Med. J. 2014, 127, 4001–4005. [Google Scholar]
- Boddupalli, A.; Zhu, L.; Bratlie, K.M. Methods for implant acceptance and wound healing: Material selection and implant location modulate macrophage and fibroblast phenotypes. Adv. Healthc. Mater. 2016, 5, 2575–2594. [Google Scholar] [CrossRef]
- Manne, A.A.; Arigela, B.; Giduturi, A.K.; Komaravolu, R.K.; Mangamuri, U.; Poda, S. Pterocarpus marsupium Roxburgh heartwood extract/chitosan nanoparticles loaded hydrogel as an innovative wound healing agent in the diabetic rat model. Mater. Today Commun. 2021, 26, 101916. [Google Scholar] [CrossRef]
- Nagam, S.P.; Jyothi, A.N.; Poojitha, J.; Aruna, S.; Nadendla, R. A comprehensive review on hydrogels. Int. J. Curr. Pharm. Res. 2016, 8, 19–23. [Google Scholar]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Pagbilao, L.M.O.; Mendoza, D.V.M.; Hipolito, M.C.; Gabriel, J.P.; Abesamis, J.R.M.M. Development of Hydrogel from Rice Bran Starch. Orient. J. Chem. 2020, 36, 493–497. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Namazi, H.; Hasani, M.; Yadollahi, M. Antibacterial oxidized starch/ZnO nanocomposite hydrogel: Synthesis and evaluation of its swelling behaviours in various pHs and salt solutions. Int. J. Biol. Macromol. 2019, 126, 578–584. [Google Scholar] [CrossRef]
- Das, T.; Manna, M.; Rudra, A. Formulation and characterization of papaya leaf gel. GSC Biol. Pharm. Sci. 2020, 10, 89–94. [Google Scholar]
- Zhang, X.; Li, Y.; Ma, Z.; He, D.; Li, H. Modulating degradation of sodium alginate/bioglass hydrogel for improving tissue infiltration and promoting wound healing. Bioact. Mater. 2021, 6, 3692–3704. [Google Scholar] [CrossRef]
- Asadi, N.; Pazoki-Toroudi, H.; Del Bakhshayesh, A.R.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Shen, C.-J.; Shen, C.-F.; Cheng, C.-M. Role of pH value in clinically relevant diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, S.; Khan, K.U.; Sohail, M.; Abdullah, O.; Khalid, I.; Malik, N.S. Synthesis and evaluation of polyethylene glycol-4000-co-poly (AMPS) based hydrogel membranes for controlled release of mupirocin for efficient wound healing. Curr. Drug Deliv. 2022, 19, 1102 –1115. [Google Scholar] [CrossRef]
- Esquivel-Castro, T.A.; Ibarra-Alonso, M.; Oliva, J.; Martínez-Luévanos, A. Porous aerogel and core/shell nanoparticles for controlled drug delivery: A review. Mater. Sci. Eng. C 2019, 96, 915–940. [Google Scholar] [CrossRef]
- Yahya, E.B.; Jummaat, F.; Amirul, A.; Adnan, A.; Olaiya, N.; Abdullah, C.; Rizal, S.; Mohamad Haafiz, M.; Abdul Khalil, H.P.S. A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef]
- Yahya, E.; Abdulsamad, M.A. In-vitro Antibacterial Activity of Carbopol-Essential Oils hydrogels. J. Appl. Sci. Process Eng. 2020, 7, 564–571. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alzalouk, M.M.; Alfallous, K.A.; Abogmaza, A.F. Antibacterial cellulose-based aerogels for wound healing application: A review. Biomed. Res. Ther. 2020, 7, 4032–4040. [Google Scholar] [CrossRef]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef]
- Moreira, L.C.; de Ávila, R.I.; Veloso, D.F.M.C.; Pedrosa, T.N.; Lima, E.S.; do Couto, R.O.; Lima, E.M.; Batista, A.C.; de Paula, J.R.; Valadares, M.C. In vitro safety and efficacy evaluations of a complex botanical mixture of Eugenia dysenterica DC. (Myrtaceae): Prospects for developing a new dermocosmetic product. Toxicol. Vitr. 2017, 45, 397–408. [Google Scholar] [CrossRef]
- Jeong, J.B.; Choi, J.; Lou, Z.; Jiang, X.; Lee, S.-H. Patchouli alcohol, an essential oil of Pogostemon cablin, exhibits anti-tumorigenic activity in human colorectal cancer cells. Int. Immunopharmacol. 2013, 16, 184–190. [Google Scholar] [CrossRef]
- Seyed Ahmadi, S.G.; Farahpour, M.R.; Hamishehkar, H. Topical application of Cinnamon verum essential oil accelerates infected wound healing process by increasing tissue antioxidant capacity and keratin biosynthesis. Kaohsiung J. Med. Sci. 2019, 35, 686–694. [Google Scholar] [CrossRef]
- Oyekanmi, A.; Abdul Khalil, H.P.S.; Rahman, A.; Mistar, E.; Olaiya, N.; Alfatah, T.; Yahya, E.B.; Mariana, M.; Hazwan, C.; Abdullah, C. Extracted supercritical CO2 cinnamon oil functional properties enhancement in cellulose nanofibre reinforced Euchema cottoni biopolymer films. J. Mater. Res. Technol. 2021, 15, 4293–4308. [Google Scholar] [CrossRef]
- Boyanova, L.; Ilieva, J.; Gergova, G.; Mitov, I. Levofloxacin susceptibility testing against Helicobacter pylori: Evaluation of a modified disk diffusion method compared to E test. Diagn. Microbiol. Infect. Dis. 2016, 84, 55–56. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, J.; Goyal, A. Carbopol 940 vs. Carbol 904: A better Polymer for Hydrogel Formulation. Res. J. Pharm. Technol. 2021, 14, 1561–1564. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.; Liang, Y.; Pan, G.; Guo, B. Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds. Chem. Eng. J. 2022, 427, 132039. [Google Scholar] [CrossRef]
- Quintanilla de Stéfano, J.C.; Abundis-Correa, V.; Herrera-Flores, S.D.; Alvarez, A.J. PH-sensitive starch-based hydrogels: Synthesis and effect of molecular components on drug release behavior. Polymers 2020, 12, 1974. [Google Scholar] [CrossRef]
- Villamizar-Sarmiento, M.G.; Moreno-Villoslada, I.; Martínez, S.; Giacaman, A.; Miranda, V.; Vidal, A.; Orellana, S.L.; Concha, M.; Pavicic, F.; Lisoni, J.G. Ionic nanocomplexes of hyaluronic acid and polyarginine to form solid materials: A green methodology to obtain sponges with biomedical potential. Nanomaterials 2019, 9, 944. [Google Scholar] [CrossRef] [Green Version]



| Sample | Physical Appearance and Odor | Homogeneity | pH | Spreadability (g·cm/s) | Viscosity (cP) | Hydrogel Photograph |
|---|---|---|---|---|---|---|
| CS | Deep white and odorless | Watery | 6.81 ± 0.2 | 7.71 ± 0.94 | 3973 ± 81 |  |
| WS | Deep white and odorless | Watery | 6.97 ± 0.2 | 6.94 ± 0.82 | 5321 ± 37 |  |
| CCS3 | Deep white with strong patchouli smell | Good | 7.13 ± 0.1 | 5.26 ± 0.64 | 10,917 ± 82 |  |
| CCS6 | Deep white with strong patchouli smell | Excellent | 7.04 ± 0.2 | 4.59 ± 0.88 | 13,008 ± 29 |  |
| CWS3 | Deep white with strong patchouli smell | Good | 7.23 ± 0.1 | 4.87 ± 0.71 | 12,411 ± 91 |  |
| CWS6 | Deep white with strong patchouli smell | Excellent | 7.19 ± 0.1 | 4.02 ± 0.34 | 15,016 ± 59 |  |
| Sample | Conventional Starch (%) | Waxy Starch (%) | Carbopol (%) | Patchouli Essential Oil (%) | Glycerol (%) | Triethanol-Amine | Water (%) |
|---|---|---|---|---|---|---|---|
| CS | 6 | - | - | - | - | - | 94 |
| WS | - | 6 | - | - | - | - | 94 |
| CCS3 | 3 | - | 0.25 | 3 | 5 | 0.05 | 88.7 |
| CCS6 | 6 | - | 0.25 | 3 | 5 | 0.05 | 85.7 |
| CWS3 | - | 3 | 0.25 | 3 | 5 | 0.05 | 88.7 |
| CWS6 | - | 6 | 0.25 | 3 | 5 | 0.05 | 85.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Khalil, H.P.S.; Muhammad, S.; Yahya, E.B.; Amanda, L.K.M.; Abu Bakar, S.; Abdullah, C.K.; Aiman, A.R.; Marwan, M.; Rizal, S. Synthesis and Characterization of Novel Patchouli Essential Oil Loaded Starch-Based Hydrogel. Gels 2022, 8, 536. https://doi.org/10.3390/gels8090536
Abdul Khalil HPS, Muhammad S, Yahya EB, Amanda LKM, Abu Bakar S, Abdullah CK, Aiman AR, Marwan M, Rizal S. Synthesis and Characterization of Novel Patchouli Essential Oil Loaded Starch-Based Hydrogel. Gels. 2022; 8(9):536. https://doi.org/10.3390/gels8090536
Chicago/Turabian StyleAbdul Khalil, H. P. S., Syaifullah Muhammad, Esam Bashir Yahya, Lee Kar Mun Amanda, Suriani Abu Bakar, C. K. Abdullah, Abd Rahim Aiman, M. Marwan, and Samsul Rizal. 2022. "Synthesis and Characterization of Novel Patchouli Essential Oil Loaded Starch-Based Hydrogel" Gels 8, no. 9: 536. https://doi.org/10.3390/gels8090536
APA StyleAbdul Khalil, H. P. S., Muhammad, S., Yahya, E. B., Amanda, L. K. M., Abu Bakar, S., Abdullah, C. K., Aiman, A. R., Marwan, M., & Rizal, S. (2022). Synthesis and Characterization of Novel Patchouli Essential Oil Loaded Starch-Based Hydrogel. Gels, 8(9), 536. https://doi.org/10.3390/gels8090536