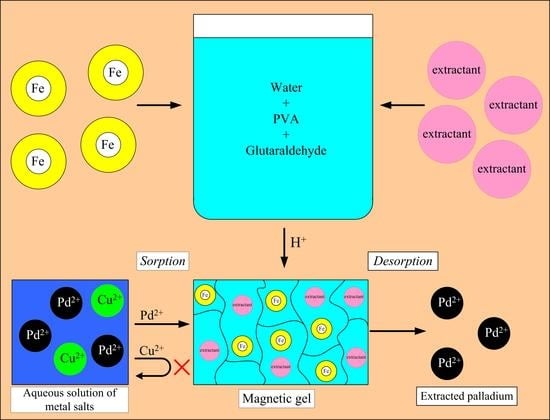
Extraction of Palladium(II) with a Magnetic Sorbent Based on Polyvinyl Alcohol Gel, Metallic Iron, and an Environmentally Friendly Polydentate Phosphazene-Containing Extractant
Abstract
:1. Introduction
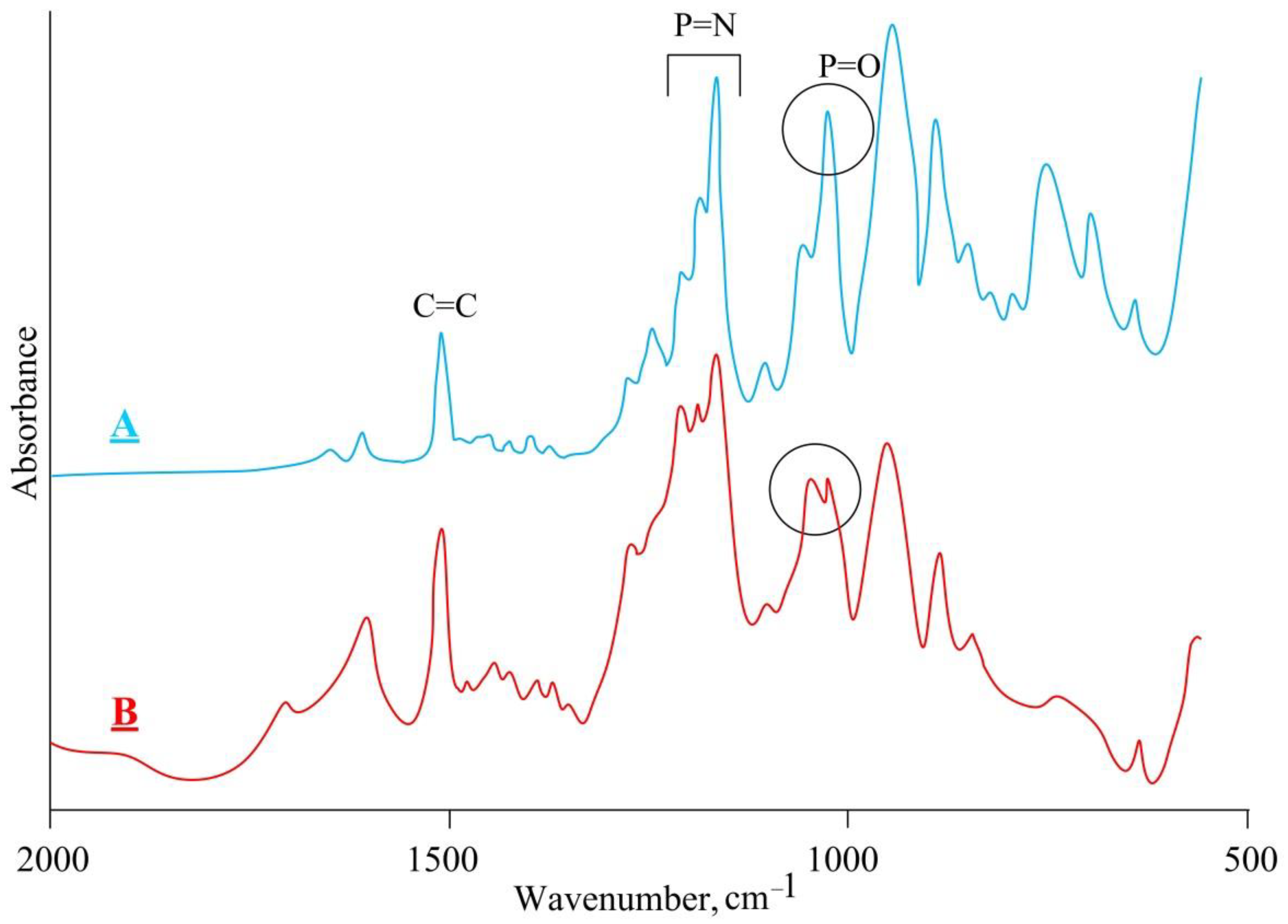
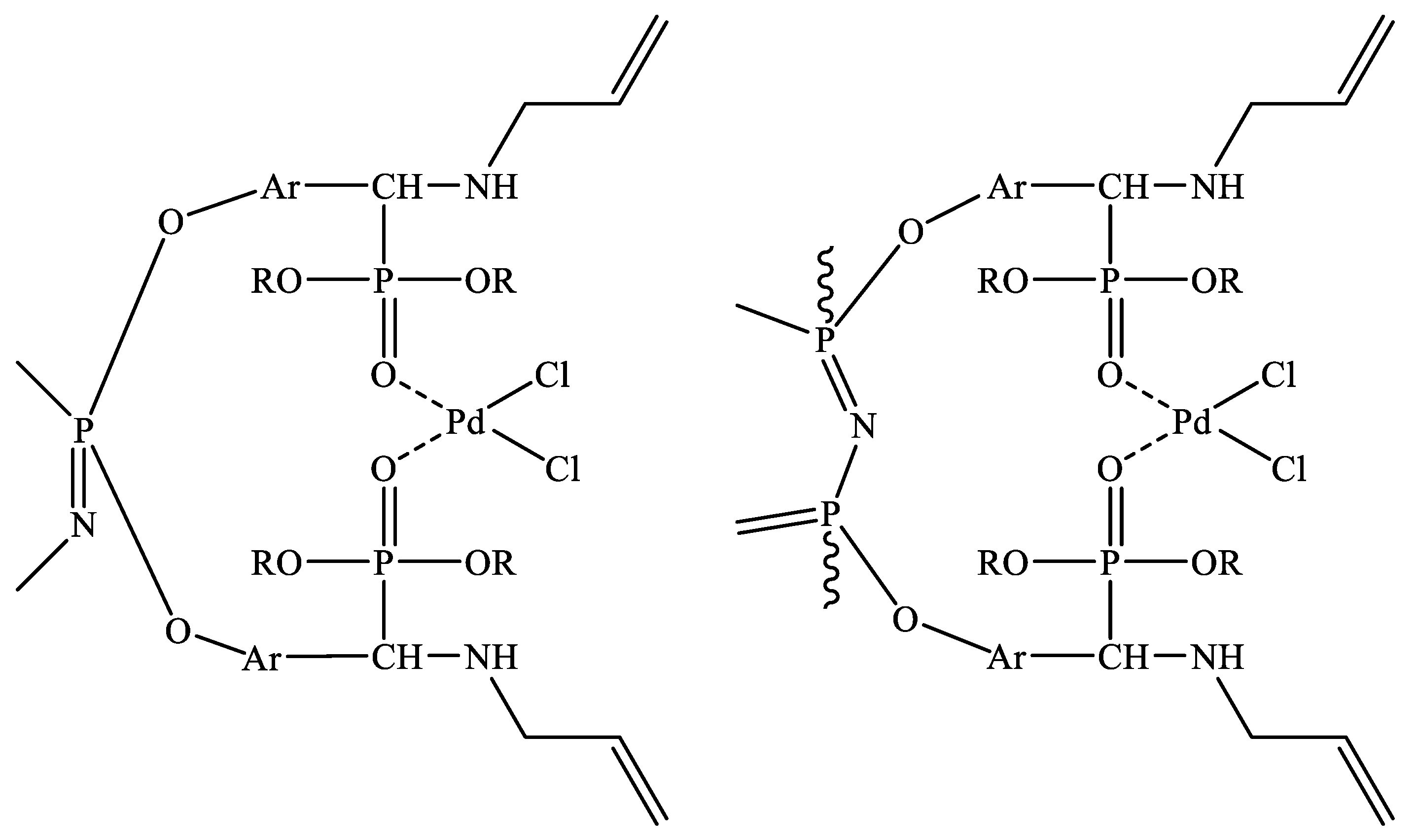
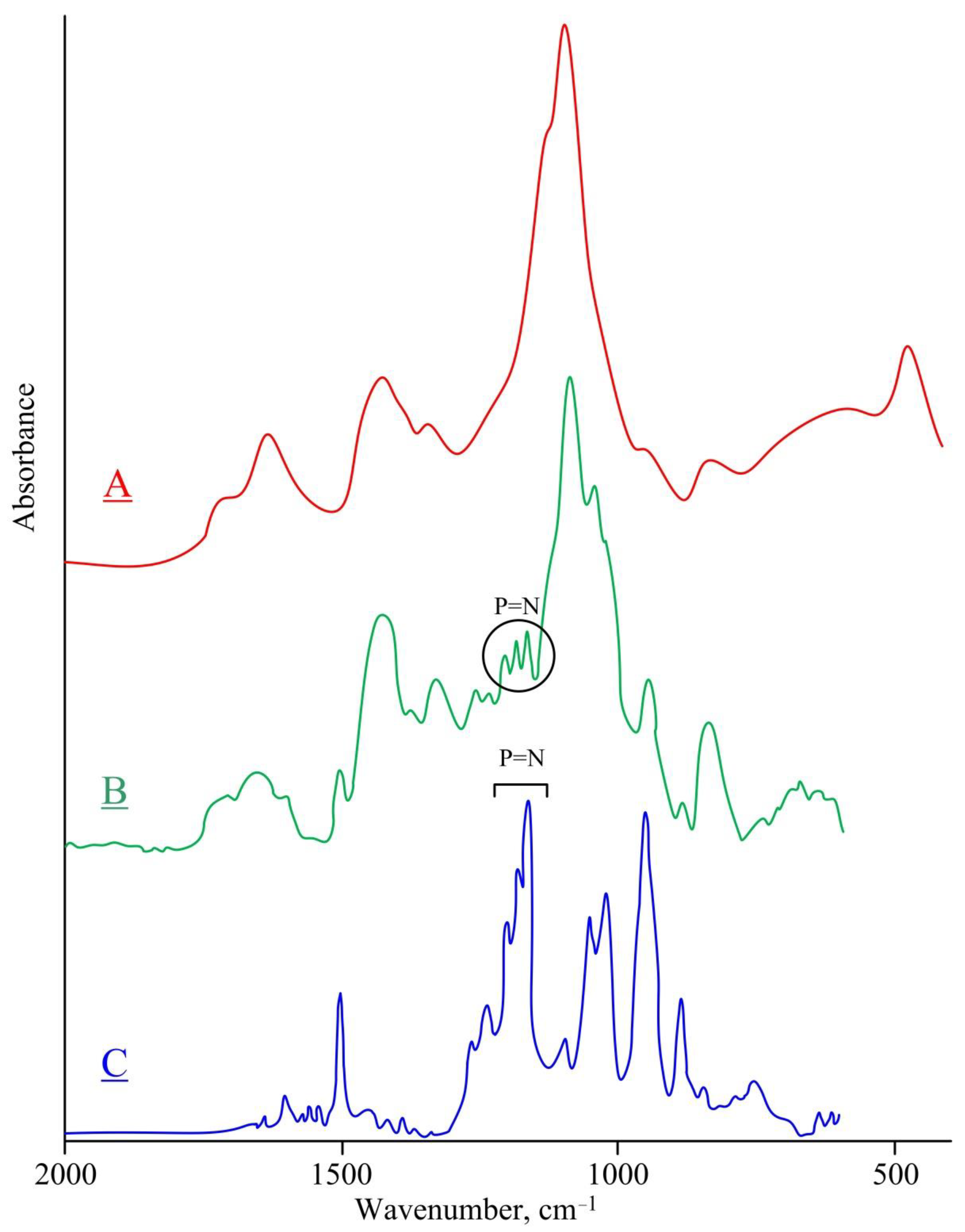
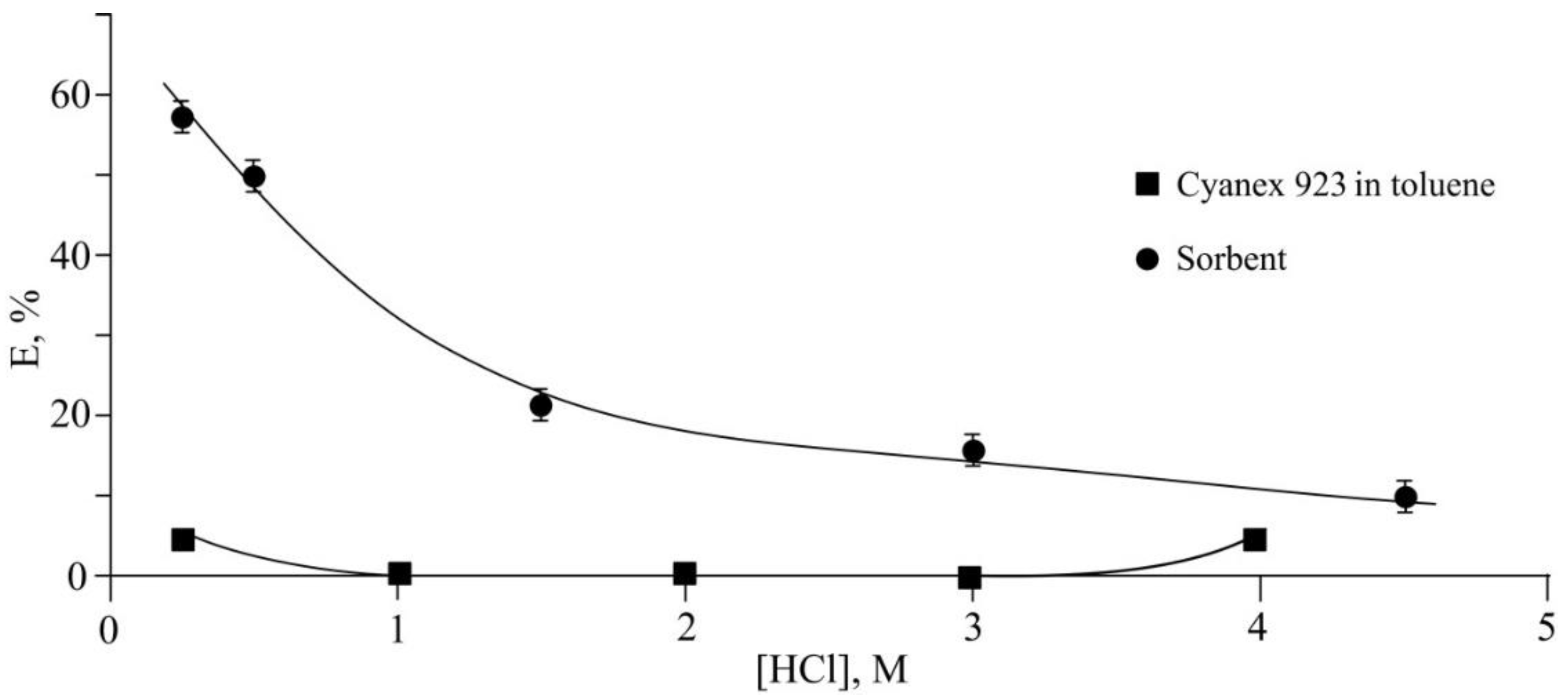
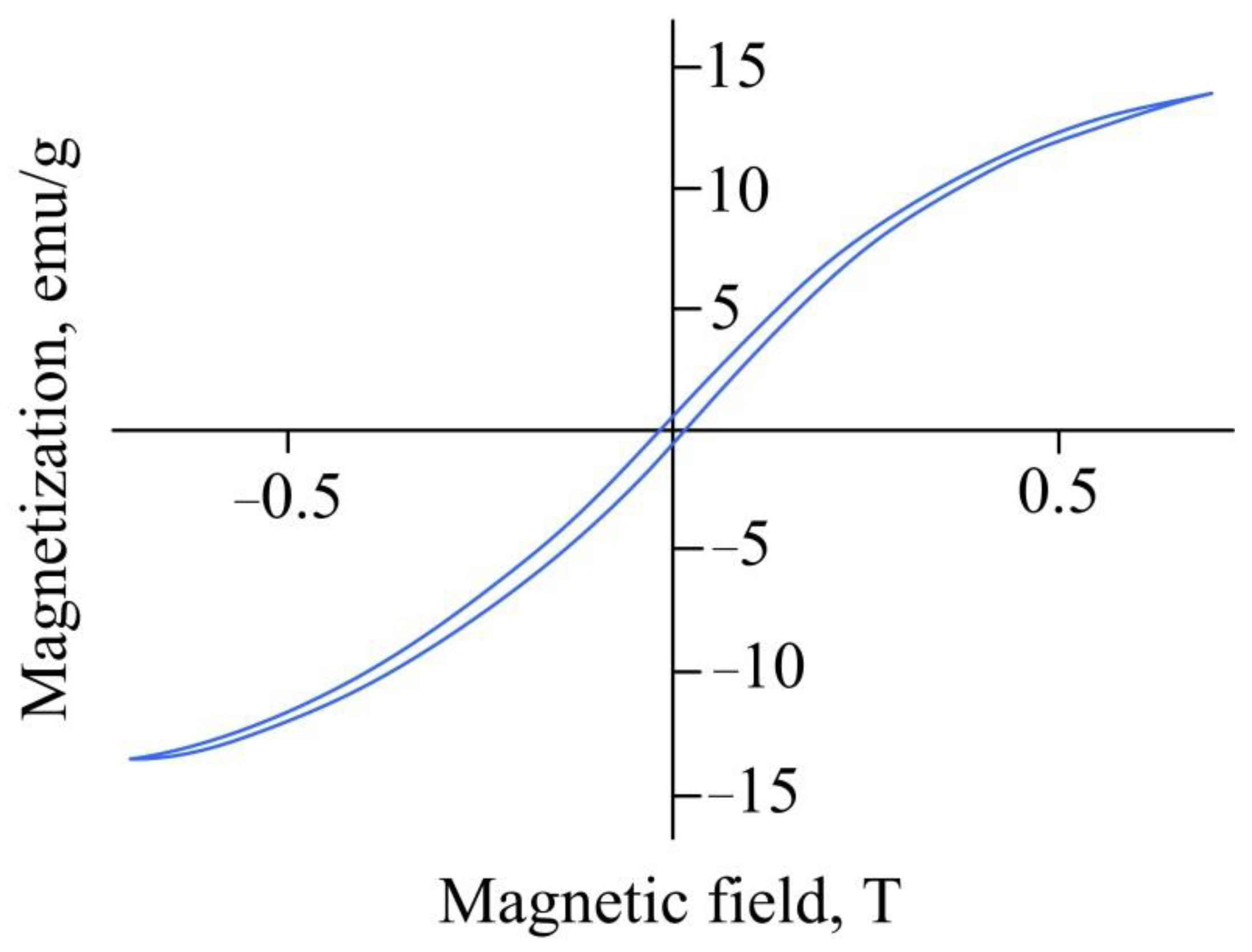
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
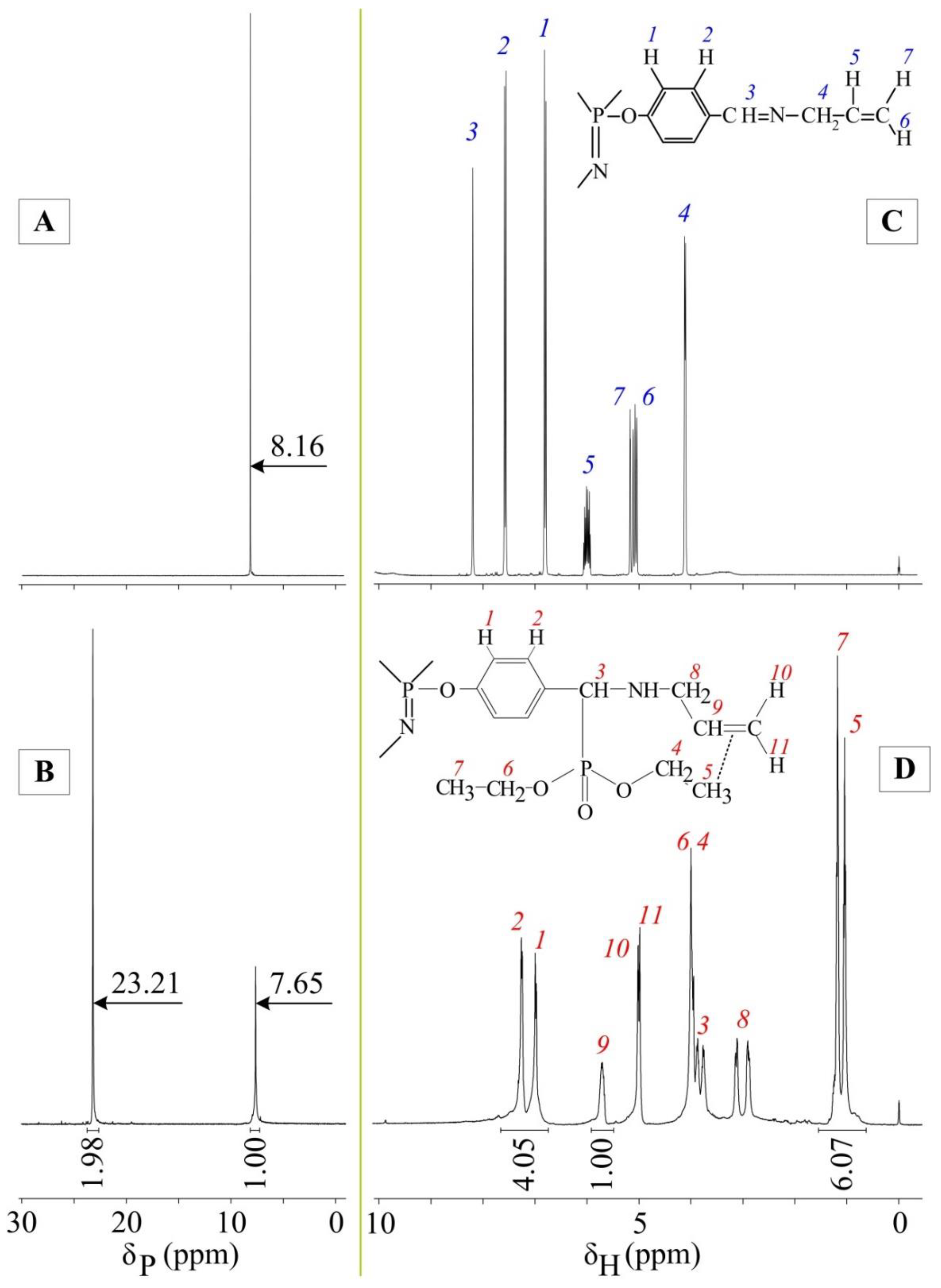
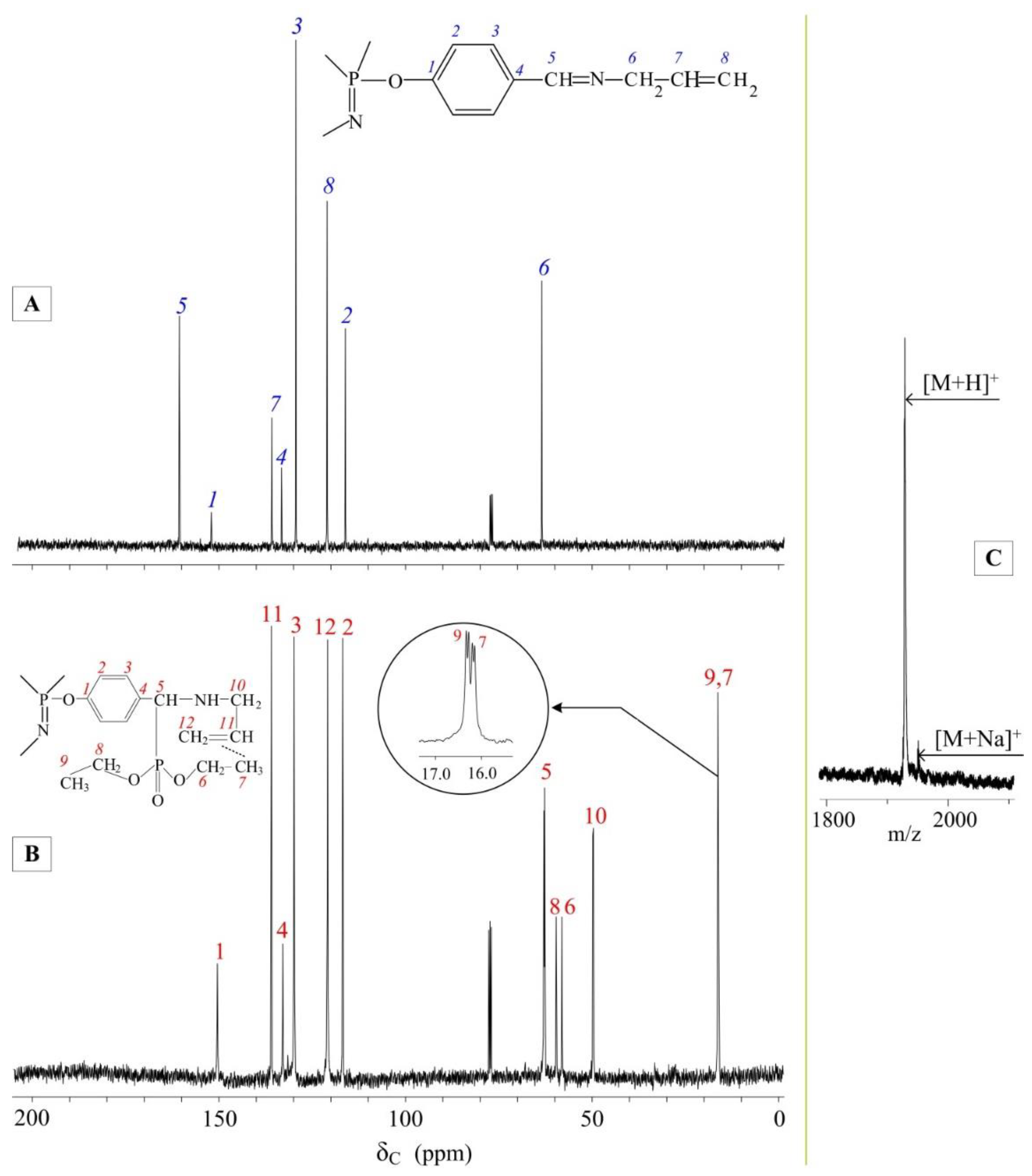
4.3. Synthesis of Hexakis-[4-{(N-allylimino)methyl}-phenoxy]-cyclotriphosphazene
4.4. Synthesis of Hexakis-[4-{α,α-(N-allylamino)(O,O-diethylphosphoryl)methylidine}phenoxy]cyclotriphosphazene (Extractant)
4.5. Extraction of Palladium with the Developed Extractant
4.6. Synthesis of Sorbents
4.7. Sorption of Palladium by the Developed Sorbent
4.8. Stripping of Palladium
4.9. Sorption of Palladium by the Developed Gel in the Presence of Copper
4.10. Synthesis of a Magnetic Sorbent Containing Acid-Resistant Iron
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bourgeois, D.; Lacanau, V.; Mastretta, R.; Contino-Pépin, C.; Meyer, D. A simple process for the recovery of palladium from wastes of printed circuit boards. Hydrometallurgy 2019, 191, 105241. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wieczorek, D. Extraction and separation of palladium(II), platinum(IV), gold(III) and rhodium(III) using piperidine-based extractants. Hydrometallurgy 2018, 175, 359–366. [Google Scholar] [CrossRef]
- Dubiella-Jackowska, A.; Namieśnik, J. Platinum Group Elements in the Environment: Emissions and Exposure. Rev. Environ. Contam. Toxicol. 2008, 199, 1–25. [Google Scholar] [CrossRef]
- Li, W.-X.; Yang, B.-W.; Na, J.-H.; Rao, W.; Chu, X.-Q.; Shen, Z.-L. Palladium-catalyzed cross-coupling of alkylindium reagent with diaryliodonium salt. Tetrahedron Lett. 2022, 95, 153729. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, D.; Chen, J.; Han, J.; Shi, F.; Li, B.; He, X.; Wang, L.; Wang, H.; Wang, Y. Unexpected electocatalytic activity of a micron-sized carbon sphere-graphene (MS-GR) supported palladium composite catalyst for ethanol oxidation reaction (EOR). Mater. Chem. Phys. 2020, 259, 124035. [Google Scholar] [CrossRef]
- Dergachev, Y.M. Hydrogen absorption by transition metals. Inorg. Mater. 2006, 42, 112–115. [Google Scholar] [CrossRef]
- Groshev, N.Y.; Rundkvist, T.V.; Karykowski, B.T.; Maier, W.D.; Korchagin, A.U.; Ivanov, A.N.; Junge, M. Low-Sulfide Platinum–Palladium Deposits of the Paleoproterozoic Fedorova–Pana Layered Complex, Kola Region, Russia. Minerals 2019, 9, 764. [Google Scholar] [CrossRef] [Green Version]
- Torres, R.; Lapidus-Lavine, G. Platinum, palladium and gold leaching from magnetite ore, with concentrated chloride solutions and ozone. Hydrometallurgy 2016, 166, 185–194. [Google Scholar] [CrossRef]
- Shi, W.; Hu, Y.; Li, Q.; Lan, T.; Zhang, X.; Cao, J. Recovery of Pd(II) in chloride solutions by solvent extraction with new vinyl sulfide ploymer extractants. Hydrometallurgy 2021, 204, 105716. [Google Scholar] [CrossRef]
- Song, L.; Wang, X.; Li, L.; Wang, Z.; Xu, H.; He, L.; Li, Q.; Ding, S. Recovery of palladium(II) from strong nitric acid solutions relevant to high-level liquid waste of PUREX process by solvent extraction with pyrazole-pyridine-based amide ligands. Hydrometallurgy 2022, 211, 105888. [Google Scholar] [CrossRef]
- Rios-Vera, R.M.; Chagnes, A.; Hernández-Perales, L.; Martínez-Rodríguez, D.E.; Navarro-Segura, D.L.; Gaillon, L.; Sirieix-Plénet, J.; Rizzi, C.; Rollet, A.L.; Avila-Rodriguez, M.; et al. Trihexyl(tetradecyl)phosphonium bis-2,4,4-(trimethylpentyl)phosphinate micellar behavior in the extraction of Ag(I) from acidic nitrate media. J. Mol. Liq. 2022, 358, 119132. [Google Scholar] [CrossRef]
- Junior, A.B.; Espinosa, D.; Vaughan, J.; Tenório, J. Recovery of scandium from various sources: A critical review of the state of the art and future prospects. Miner. Eng. 2021, 172, 107148. [Google Scholar] [CrossRef]
- Zhang, S.-M.; Wu, Q.-Y.; Yuan, L.-Y.; Wang, C.-Z.; Lan, J.-H.; Chai, Z.-F.; Liu, Z.-R.; Shi, W.-Q. Theoretical study on the extraction behaviors of MoO22+ with organophosphorous extractants. J. Mol. Liq. 2022, 355, 118969. [Google Scholar] [CrossRef]
- Sarkar, S.; Ammath, S.; Kirubananthan, S.; Suneesh, A.S. Investigation of the Phase Splitting Behaviour of U(VI) and Th(IV) loaded Trialkyl Phosphate Solvents in the Absence of Aqueous Phase. ChemistrySelect 2021, 6, 13725–13735. [Google Scholar] [CrossRef]
- Atanassova, M.; Kukeva, R.; Stoyanova, R.; Todorova, N.; Kurteva, V. Synergistic and antagonistic effects during solvent extraction of Gd(III) ion in ionic liquids. J. Mol. Liq. 2022, 353, 118818. [Google Scholar] [CrossRef]
- Atanassova, M.; Angelov, R.; Gerginova, D.; Karashanova, D. Neutral organophosphorus ligands as a molecular lab for simultaneous detecting of Ag(I) ions. J. Mol. Liq. 2021, 335, 116287. [Google Scholar] [CrossRef]
- Anderson, S.; Nilsson, M.; Kalu, E.E. Electrochemical Impedance Spectroscopy (EIS) Characterization of Water/Sodium Bis(2-Ethylhexyl) Sulfosuccinate-HDEHP/n-Dodecane Reverse Micelles for Electroextraction of Neodymium. ChemEngineering 2017, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, H.; Zeng, Z.; Gao, Y.; Liu, C.; Sun, X. Separation of lithium and transition metals from the leachate of spent lithium-ion battery by extraction-precipitation with p-tert-butylphenoxy acetic acid. Hydrometallurgy 2021, 206, 105768. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Song, S.J.; Lee, M.S. Separation of palladium and platinum metals by selective and simultaneous leaching and extraction with aqueous/non-aqueous solutions. Hydrometallurgy 2022, 208, 105814. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J.; Li, H.; Li, K.; Li, D. Further improvement for separation of heavy rare earths by mixtures of acidic organophosphorus extractants. Hydrometallurgy 2019, 188, 73–80. [Google Scholar] [CrossRef]
- Junior, A.B.B.; Espinosa, D.C.R.; Tenório, J.A.S. Selective separation of Sc(III) and Zr(IV) from the leaching of bauxite residue using trialkylphosphine acids, tertiary amine, tri-butyl phosphate and their mixtures. Sep. Purif. Technol. 2021, 279, 119798. [Google Scholar] [CrossRef]
- Kukkonen, E.; Virtanen, E.J.; Moilanen, J.O. α-Aminophosphonates, -Phosphinates, and -Phosphine Oxides as Extraction and Precipitation Agents for Rare Earth Metals, Thorium, and Uranium: A Review. Molecules 2022, 27, 3465. [Google Scholar] [CrossRef] [PubMed]
- Samiey, B.; Cheng, C.-H.; Wu, J. Organic-Inorganic Hybrid Polymers as Adsorbents for Removal of Heavy Metal Ions from Solutions: A Review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; El-Monaem, E.M.A.; Ziora, Z.M. Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab. J. Chem. 2021, 15, 103543. [Google Scholar] [CrossRef]
- Kong, D.; Foley, S.R.; Wilson, L.D. An Overview of Modified Chitosan Adsorbents for the Removal of Precious Metals Species from Aqueous Media. Molecules 2022, 27, 978. [Google Scholar] [CrossRef]
- Surgutskaia, N.S.; Di Martino, A.; Zednik, J.; Ozaltin, K.; Lovecká, L.; Bergerová, E.D.; Kimmer, D.; Svoboda, J.; Sedlarik, V. Efficient Cu2+, Pb2+ and Ni2+ ion removal from wastewater using electrospun DTPA-modified chitosan/polyethylene oxide nanofibers. Sep. Purif. Technol. 2020, 247, 116914. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Yang, A.; Zhu, Q.; Sun, H.; Sun, P.; Yao, B.; Zang, Y.; Du, X.; Dong, L. Xanthate-Modified Magnetic Fe3O4@SiO2-Based Polyvinyl Alcohol/Chitosan Composite Material for Efficient Removal of Heavy Metal Ions from Water. Polymers 2022, 14, 1107. [Google Scholar] [CrossRef]
- Jakavula, S.; Biata, N.R.; Dimpe, K.M.; Pakade, V.E.; Nomngongo, P.N. Magnetic Ion Imprinted Polymers (MIIPs) for Selective Extraction and Preconcentration of Sb(III) from Environmental Matrices. Polymers 2021, 14, 21. [Google Scholar] [CrossRef]
- Han, Q.; Du, M.; Guan, Y.; Luo, G.; Zhang, Z.; Li, T.; Ji, Y. Removal of simulated radioactive cerium (III) based on innovative magnetic trioctylamine-polystyrene composite microspheres. Chem. Phys. Lett. 2020, 741, 137092. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Ionic Liquids as Components of Systems for Metal Extraction. ChemEngineering 2022, 6, 6. [Google Scholar] [CrossRef]
- Yu, M.; Wang, L.; Hu, L.; Li, Y.; Luo, D.; Mei, S. Recent applications of magnetic composites as extraction adsorbents for determination of environmental pollutants. TrAC Trends Anal. Chem. 2019, 119, 115611. [Google Scholar] [CrossRef]
- Ozay, O.; Ozay, H. Novel hexacentered phosphazene compound as selective Fe3+ ions sensor with high quantum yield: Synthesis and application. Phosphorus Sulfur. Silicon Relat. Elem. 2018, 194, 221–228. [Google Scholar] [CrossRef]
- Çıralı, D.E.; Dayan, O. Synthesis of Tetranuclear Ruthenium (Ii) Complex of Pyridyloxy-Substituted 2,2′-Dioxybiphenyl-Cyclotriphosphazene Platform and its Catalytic Application in the Transfer Hydrogenation of Ketones. Phosphorus Sulfur. Silicon Relat. Elem. 2015, 190, 1100–1107. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Garifzyanov, A.R.; Zakharov, S.V.; Vinokurov, A.V.; Galkin, V.I. Liquid extraction of noble metal ions with bis(α-aminophosphonates). Russ. J. Gen. Chem. 2006, 76, 417–420. [Google Scholar] [CrossRef]
- Gupta, B.; Singh, I. Extraction and separation of platinum, palladium and rhodium using Cyanex 923 and their recovery from real samples. Hydrometallurgy 2013, 134–135, 11–18. [Google Scholar] [CrossRef]
- Chistyakov, E.; Kolpinskaya, N.; Yudaev, P. Magnetic polymer granules based on metallic iron and polyvinyl alcohol. Int. Multidiscip. Sci. Geoconf. SGEM 2020, 20, 507–514. [Google Scholar] [CrossRef]
- Chistyakov, E.; Yudaev, P.; Nelyubina, Y. Crystallization of Nano-Sized Macromolecules by the Example of Hexakis-[4-{(N-Allylimino)methyl}phenoxy]cyclotriphosphazene. Nanomaterials 2022, 12, 2268. [Google Scholar] [CrossRef]
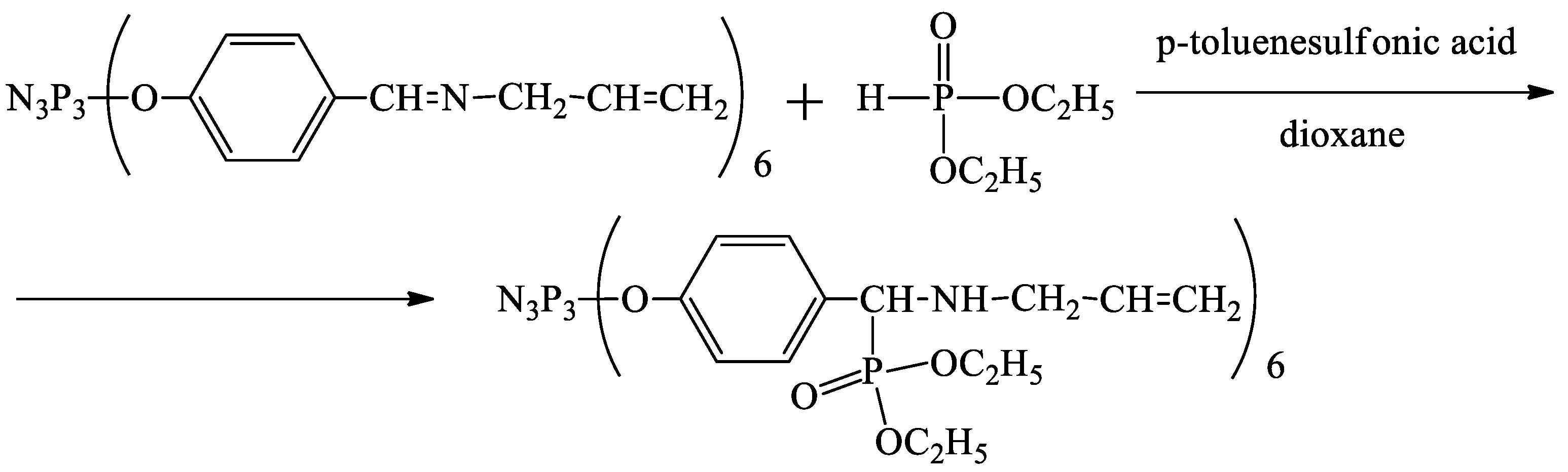

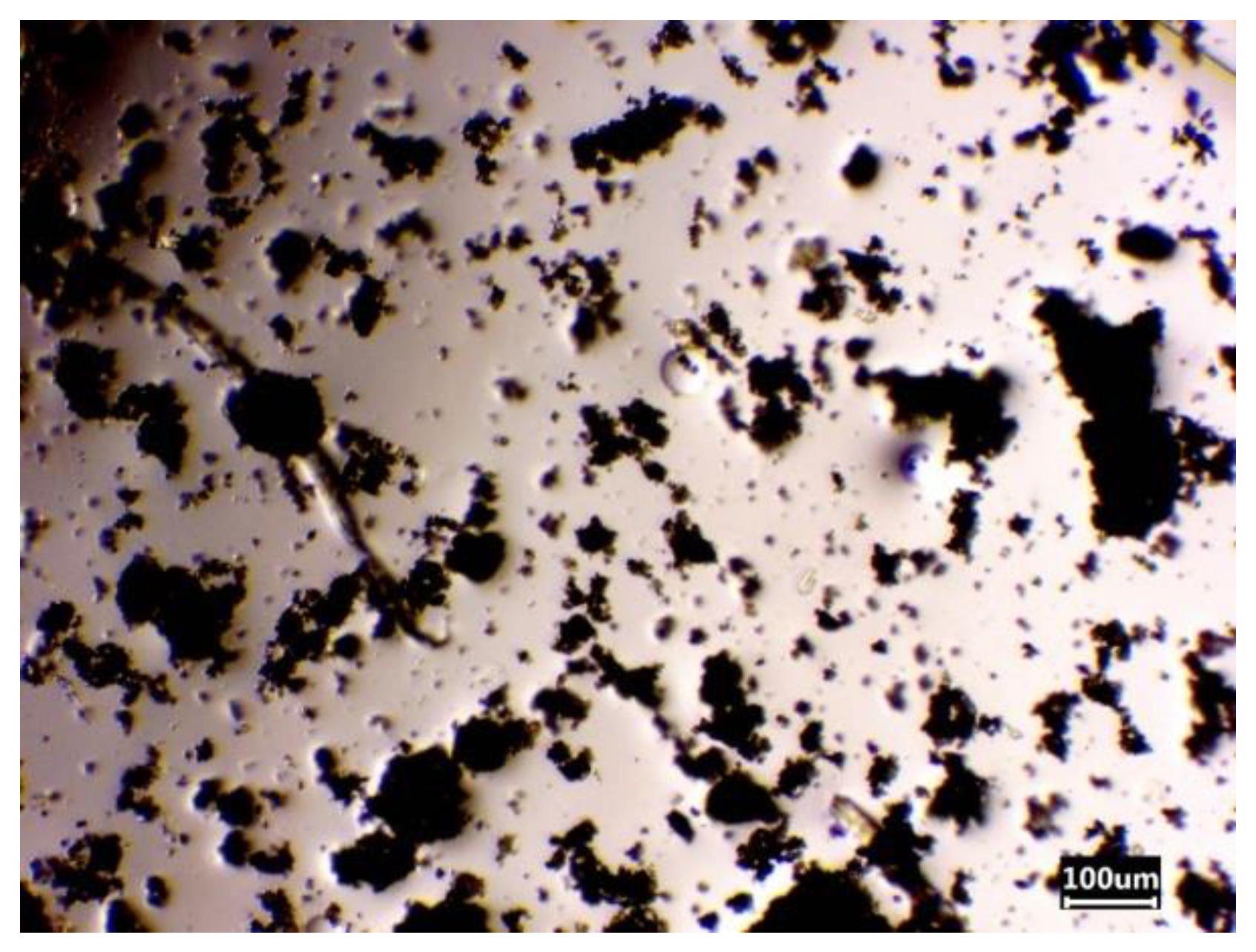
| Chemical Element | Actual Content | Theoretical Content | ||
|---|---|---|---|---|
| Weight | Atomic | Weight | Atomic | |
| C | 41.01 | 32.24 | 41.05 | 32.17 |
| N | 5.15 | 3.47 | 5.13 | 3.45 |
| O | 15.7 | 9.25 | 15.63 | 9.20 |
| P | 11.23 | 3.42 | 11.34 | 3.45 |
| Cl | 8.65 | 2.30 | 8.67 | 2.30 |
| Pd | 13.1 | 1.17 | 13.00 | 1.15 |
| H | 5.16 | 48.15 | 5.18 | 48.28 |
| Optical Density, Units | Number of Microorganisms, CFU mL−1 | ||
|---|---|---|---|
| Bacteria | Yeast and Fungi | ||
| Control | 9.03 | 8.0 × 109 | 6.0 × 105 |
| Sample | 9.29 | 1.29 × 1010 | 2.6 × 106 |
| No. | Gelation Time | Displaced Liquid, wt % | Water Uptake, wt % |
|---|---|---|---|
| 1. | 1 day | 0 | 54.7 |
| 2. | 1 day | 23.4 | 53.4 |
| 3. | 12 h | 43.1 | 45.1 |
| 4. | 7 h | 71.6 | 39.4 |
| 5. | 5 h | 75.1 | 22.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudaev, P.; Butorova, I.; Stepanov, G.; Chistyakov, E. Extraction of Palladium(II) with a Magnetic Sorbent Based on Polyvinyl Alcohol Gel, Metallic Iron, and an Environmentally Friendly Polydentate Phosphazene-Containing Extractant. Gels 2022, 8, 492. https://doi.org/10.3390/gels8080492
Yudaev P, Butorova I, Stepanov G, Chistyakov E. Extraction of Palladium(II) with a Magnetic Sorbent Based on Polyvinyl Alcohol Gel, Metallic Iron, and an Environmentally Friendly Polydentate Phosphazene-Containing Extractant. Gels. 2022; 8(8):492. https://doi.org/10.3390/gels8080492
Chicago/Turabian StyleYudaev, Pavel, Irina Butorova, Gennady Stepanov, and Evgeniy Chistyakov. 2022. "Extraction of Palladium(II) with a Magnetic Sorbent Based on Polyvinyl Alcohol Gel, Metallic Iron, and an Environmentally Friendly Polydentate Phosphazene-Containing Extractant" Gels 8, no. 8: 492. https://doi.org/10.3390/gels8080492