Evaluation of the Adsorption Efficiency of Graphene Oxide Hydrogels in Wastewater Dye Removal: Application of Principal Component Analysis
Abstract
:1. Introduction
2. Methodology
- δ: eigenvalue of cov(M)
- U: eigenvector of cov(M)
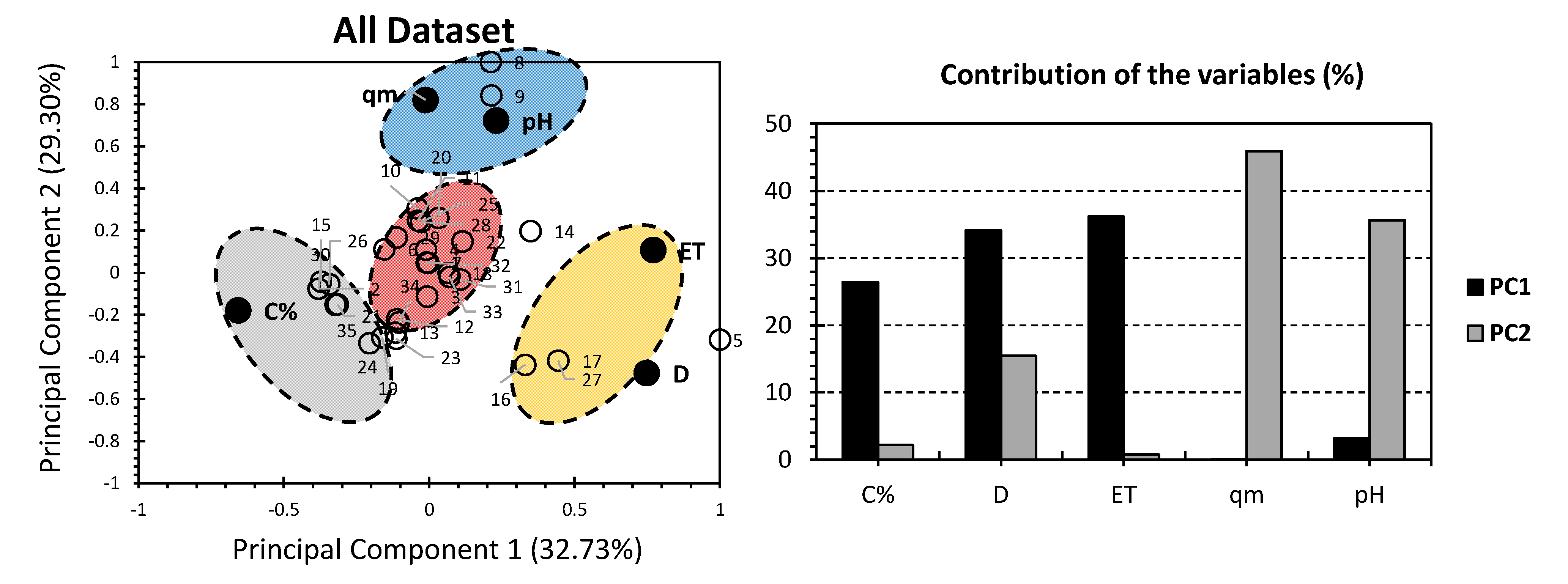
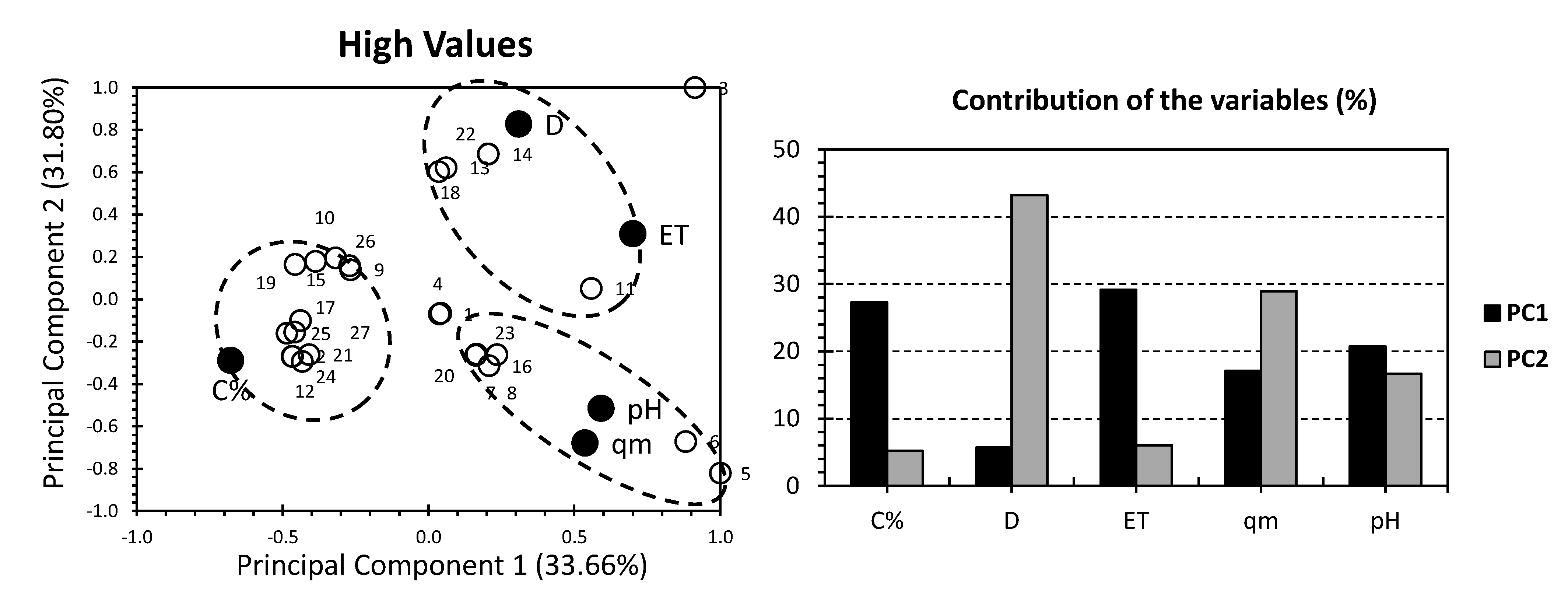
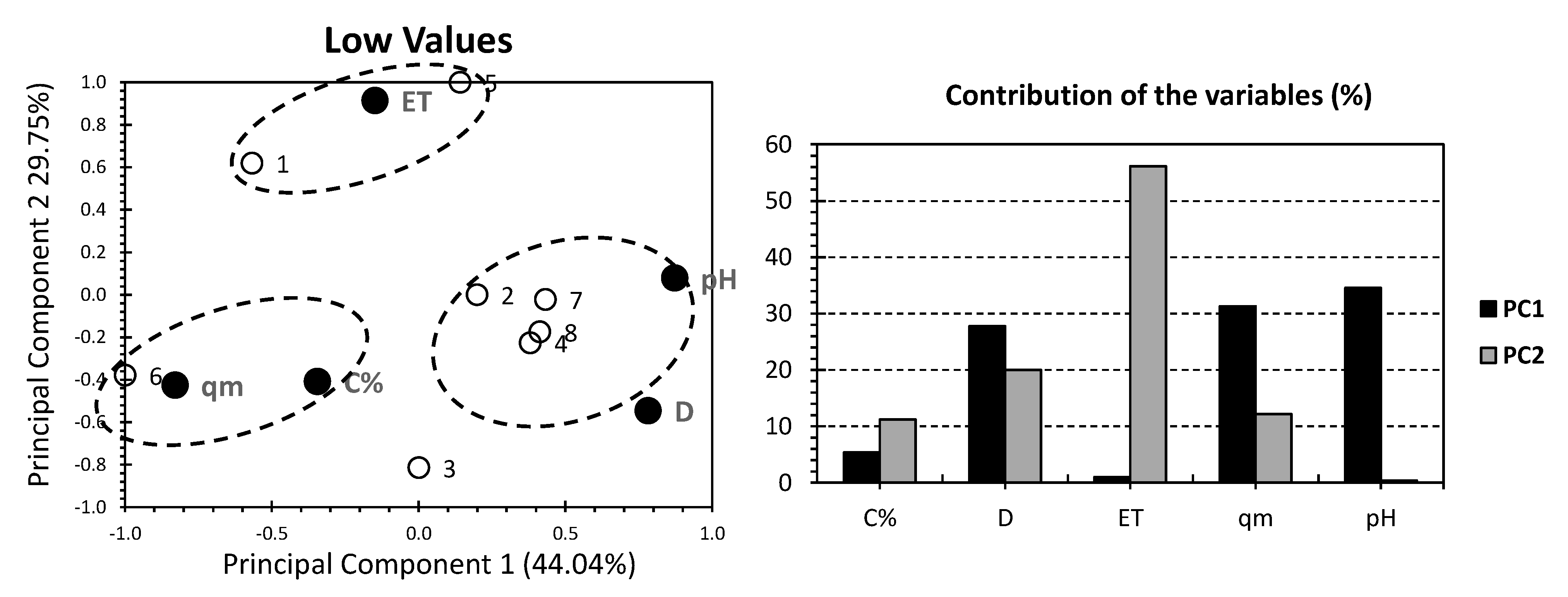
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Q. Pollution and Treatment of Dye Waste-Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 052001. [Google Scholar] [CrossRef]
- Guaratini, C.C.I.; Zanoni, M.V.B. Corantes têxteis. Quím. Nova 2000, 23, 71–78. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; Makhfouk, M.E. Removal of Methylene Blue and Eriochrome Black T from Aqueous Solutions by Biosorption on Scolymus hispanicus, L.: Kinetics, Equilibrium and Thermodynamics. J. Taiwan Inst. Chem. Eng. 2011, 42, 320–326. [Google Scholar] [CrossRef]
- Khurana, I.; Saxena, A.; Bharti; Khurana, J.M.; Rai, P.K. Removal of Dyes Using Graphene-Based Composites: A Review. Water Air Soil Pollut. 2017, 228, 180. [Google Scholar] [CrossRef]
- Carneiro, P.A.; Umbuzeiro, G.A.; Oliveira, D.P.; Zanoni, M.V.B. Assessment of Water Contamination Caused by a Mutagenic Textile Effluent/Dyehouse Effluent Bearing Disperse Dyes. J. Hazard. Mater. 2010, 174, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.B.; Rodrigues, F.H.A.; Paulino, A.T.; Martins, A.F.; Fajardo, A.R. Recent Advances on Composite Hydrogels Designed for the Remediation of Dye-Contaminated Water and Wastewater: A Review. J. Clean. Prod. 2021, 284, 124703. [Google Scholar] [CrossRef]
- Malaviya, P.; Singh, A. Physicochemical Technologies for Remediation of Chromium-Containing Waters and Wastewaters. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1111–1172. [Google Scholar] [CrossRef]
- Bharathi, K.S.; Ramesh, S.T. Removal of Dyes Using Agricultural Waste as Low-Cost Adsorbents: A Review. Appl. Water Sci. 2013, 3, 773–790. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene Oxide–Chitosan Composite Hydrogels as Broad-Spectrum Adsorbents for Water Purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Cui, W.; Ji, J.; Cai, Y.-F.; Li, H.; Ran, R. Robust, Anti-Fatigue, and Self-Healing Graphene Oxide/Hydrophobically Associated Composite Hydrogels and Their Use as Recyclable Adsorbents for Dye Wastewater Treatment. J. Mater. Chem. A 2015, 3, 17445–17458. [Google Scholar] [CrossRef]
- Lü, K.; Zhao, G.; Wang, X. A Brief Review of Graphene-Based Material Synthesis and Its Application in Environmental Pollution Management. Chin. Sci. Bull. 2012, 57, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Carrott, P.J.M.; Carrott, M.M.L.R.; Roberts, R.A. Physical Adsorption of Gases by Microporous Carbons. Colloids Surf. 1991, 58, 385–400. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Sci. New Ser. 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.-Q.; Xiao, B.; Feng, L.-R.; Zhou, S.-S.; Chen, Z.-G.; Liu, C.-B.; Chen, F.; Wu, Z.-Y.; Xu, N.; Oh, W.-C.; et al. Graphene Oxide Enhances the Fenton-like Photocatalytic Activity of Nickel Ferrite for Degradation of Dyes under Visible Light Irradiation. Carbon 2013, 64, 197–206. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.; Pang, L.; Li, M.; Song, X.; Wen, J.; Zhao, H. Preparation of Reduced Graphene Oxide/Poly(Acrylamide) Nanocomposite and its Adsorption of Pb(II) and Methylene Blue. Langmuir 2013, 29, 10727–10736. [Google Scholar] [CrossRef]
- Haubner, K.; Murawski, J.; Olk, P.; Eng, L.M.; Ziegler, C.; Adolphi, B.; Jaehne, E. The Route to Functional Graphene Oxide. Chem. Eur. J. Chem. Phys. 2010, 11, 2131–2139. [Google Scholar] [CrossRef]
- Kemp, K.C.; Seema, H.; Saleh, M.; Le, N.H.; Mahesh, K.; Chandra, V.; Kim, K.S. Environmental Applications Using Graphene Composites: Water Remediation and Gas Adsorption. Nanoscale 2013, 5, 3149. [Google Scholar] [CrossRef] [Green Version]
- Ramesha, G.K.; Vijaya Kumara, A.; Muralidhara, H.B.; Sampath, S. Graphene and Graphene Oxide as Effective Adsorbents toward Anionic and Cationic Dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef]
- Younes, K.; Grasset, L. The Application of DFRC Method for the Analysis of Carbohydrates in a Peat Bog: Validation and Comparison with Conventional Chemical and Thermochemical Degradation Techniques. Chem. Geol. 2020, 545, 119644. [Google Scholar] [CrossRef]
- Younes, K.; Laduranty, J.; Descostes, M.; Grasset, L. Molecular Biomarkers Study of an Ombrotrophic Peatland Impacted by an Anthropogenic Clay Deposit. Org. Geochem. 2017, 105, 20–32. [Google Scholar] [CrossRef]
- Younes, K.; Grasset, L. Analysis of Molecular Proxies of a Peat Core by Thermally Assisted Hydrolysis and Methylation-Gas Chromatography Combined with Multivariate Analysis. J. Anal. Appl. Pyrolysis 2017, 124, 726–732. [Google Scholar] [CrossRef]
- Korichi, W.; Ibrahimi, M.; Loqman, S.; Ouhdouch, Y.; Younes, K.; Lemée, L. Assessment of Actinobacteria Use in the Elimination of Multidrug-Resistant Bacteria of Ibn Tofail Hospital Wastewater (Marrakesh, Morocco): A Chemometric Data Analysis Approach. Environ. Sci. Pollut. Res. 2021, 28, 26840–26848. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Zhang, Y.; Chen, J.; Chu, G.; Shao, L. Preparation of CeO2 Nano-Support in a Novel Rotor–Stator Reactor and Its Use in Au-Based Catalyst for CO Oxidation. Powder Technol. 2015, 273, 191–196. [Google Scholar] [CrossRef]
- Yang, Y.; Song, S.; Zhao, Z. Graphene Oxide (GO)/Polyacrylamide (PAM) Composite Hydrogels as Efficient Cationic Dye Adsorbents. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 315–324. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Sadiku, E.R. Removal of Dye by Carboxymethyl Cellulose, Acrylamide and Graphene Oxide via a Free Radical Polymerization Process. Carbohydr. Polym. 2017, 164, 186–194. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, K.; Jiao, T.; Xing, R.; Hong, W.; Zhang, L.; Zhang, Q.; Peng, Q. Preparation of Graphene Oxide-Polymer Composite Hydrogels via Thiol-Ene Photopolymerization as Efficient Dye Adsorbents for Wastewater Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 668–676. [Google Scholar] [CrossRef]
- Zhao, H.; Jiao, T.; Zhang, L.; Zhou, J.; Zhang, Q.; Peng, Q.; Yan, X. Preparation and Adsorption Capacity Evaluation of Graphene Oxide-Chitosan Composite Hydrogels. Sci. China Mater. 2015, 58, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Omidi, S.; Kakanejadifard, A. Eco-Friendly Synthesis of Graphene–Chitosan Composite Hydrogel as Efficient Adsorbent for Congo Red. RSC Adv. 2018, 8, 12179–12189. [Google Scholar] [CrossRef] [Green Version]
- Atyaa, A.I.; Radhy, N.D.; Jasim, L.S. Synthesis and Characterization of Graphene Oxide/Hydrogel Composites and Their Applications to Adsorptive Removal Congo Red from Aqueous Solution. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1234, p. 012095. [Google Scholar]
- Zhuang, Y.; Yu, F.; Chen, J.; Ma, J. Batch and Column Adsorption of Methylene Blue by Graphene/Alginate Nanocomposite: Comparison of Single-Network and Double-Network Hydrogels. J. Environ. Chem. Eng. 2016, 4, 147–156. [Google Scholar] [CrossRef]
- Kong, Y.; Zhuang, Y.; Han, Z.; Yu, J.; Shi, B.; Han, K.; Hao, H. Dye Removal by Eco-Friendly Physically Cross-Linked Double Network Polymer Hydrogel Beads and Their Functionalized Composites. J. Environ. Sci. 2019, 78, 81–91. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Shi, Z.; Lian, M.; Tian, M.; Yin, J. Preparation and Characterization of Sulfonated Graphene-Enhanced Poly (Vinyl Alcohol) Composite Hydrogel and Its Application as Dye Absorbent. Polymer 2015, 60, 96–106. [Google Scholar] [CrossRef]
- Balkız, G.; Pingo, E.; Kahya, N.; Kaygusuz, H.; Bedia Erim, F. Graphene Oxide/Alginate Quasi-Cryogels for Removal of Methylene Blue. Water Air Soil Pollut. 2018, 229, 1–9. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, Y.; Tang, S.; Yang, J.; Chen, Y.; Chen, S.; Li, P.; Yang, Z. Construction of Chitosan/Polyacrylate/Graphene Oxide Composite Physical Hydrogel by Semi-Dissolution/Acidification/Sol-Gel Transition Method and Its Simultaneous Cationic and Anionic Dye Adsorption Properties. Carbohydr. Polym. 2020, 229, 115431. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, M.; Liu, Y.; Meng, L.; Ma, M. Functionalized Electrospun Nanofiber Membranes for Water Treatment: A Review. Sci. Total Environ. 2020, 739, 139944. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, K.; Tehrani, A.D.; Adeli, M. Bioconjugated Graphene Oxide Hydrogel as an Effective Adsorbent for Cationic Dyes Removal. Ecotoxicol. Environ. Saf. 2018, 147, 34–42. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Huang, H. Eco-Friendly Polyvinyl Alcohol/Carboxymethyl Cellulose Hydrogels Reinforced with Graphene Oxide and Bentonite for Enhanced Adsorption of Methylene Blue. Carbohydr. Polym. 2018, 185, 1–11. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Qi, Y.; Sun, W.; Men, Y. Preparation of κ-Carrageenan/Graphene Oxide Gel Beads and Their Efficient Adsorption for Methylene Blue. J. Colloid Interface Sci. 2017, 506, 669–677. [Google Scholar] [CrossRef]
- Halouane, F.; Oz, Y.; Meziane, D.; Barras, A.; Juraszek, J.; Singh, S.K.; Kurungot, S.; Shaw, P.K.; Sanyal, R.; Boukherroub, R. Magnetic Reduced Graphene Oxide Loaded Hydrogels: Highly Versatile and Efficient Adsorbents for Dyes and Selective Cr (VI) Ions Removal. J. Colloid Interface Sci. 2017, 507, 360–369. [Google Scholar] [CrossRef]
- Thompson, L.; Fu, L.; Wang, J.; Yu, A. Impact of Graphene Oxide on Dye Absorption in Composite Hydrogels. Fuller. Nanotub. Carbon Nanostructures 2018, 26, 649–653. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Shahrousvand, M.; Shojaei, S.; Khonakdar, H.A.; Asefnejad, A.; Goodarzi, V. Preparation of Superabsorbent Eco-Friendly Semi-Interpenetrating Network Based on Cross-Linked Poly Acrylic Acid/Xanthan Gum/Graphene Oxide (PAA/XG/GO): Characterization and Dye Removal Ability. Int. J. Biol. Macromol. 2020, 152, 884–893. [Google Scholar] [CrossRef]
- Guo, H.; Jiao, T.; Zhang, Q.; Guo, W.; Peng, Q.; Yan, X. Preparation of Graphene Oxide-Based Hydrogels as Efficient Dye Adsorbents for Wastewater Treatment. Nanoscale Res. Lett. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite Modified Sodium Alginate Hydrogel Composite for Efficient Removal of Malachite Green Dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Makhado, E.; Pandey, S.; Ramontja, J. Microwave Assisted Synthesis of Xanthan Gum-Cl-Poly (Acrylic Acid) Based-Reduced Graphene Oxide Hydrogel Composite for Adsorption of Methylene Blue and Methyl Violet from Aqueous Solution. Int. J. Biol. Macromol. 2018, 119, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; He, M.; Liu, Y.; Xiong, L.; Zhang, Q.; Wei, L.; Li, L.; Yu, X. Strong Alginate/Reduced Graphene Oxide Composite Hydrogels with Enhanced Dye Adsorption Performance. Polym. Bull. 2020, 77, 6609–6623. [Google Scholar] [CrossRef]
| Composite Hydrogel | C% a | D b | ET c | qm d | pH e | References | |
|---|---|---|---|---|---|---|---|
| 1 | PMPTC/GO | 0.3 | - | 150 | 13 | Wang et al. [23] | |
| 2 | PAAm/GO | 50 | 0.2 | 20 | 293 | Yang et al. [24] | |
| 3 | CMC/Aam/GO | 10 | 4 | 720 | 185 | 6 | Varaprasad et al. [25] |
| 4 | Chitin/TA/GO | 7 | 400 | 231 | 7 | Liu et al. [26] | |
| 5 | CTS/GO | 4000 | 10 | Zhao et al. [27] | |||
| 6 | CTS/amino-functionalized-GO | 20 | 5 | 385 | 7 | Omidi and Kakanejadifard [28] | |
| 7 | PVP/Aac/GO | 0.2 | 5 | 40 | 78 | 7 | Atyaa et al. [29] |
| 8 | Double ALG/GO network | 1 | 1200 | 2300 | 8 | Zhuang et al. [30] | |
| 9 | Single ALG/GO network | 1 | 1200 | 1800 | 8 | Zhuang et al. [30] | |
| 10 | Double ALG/PVA/GO network | 5 | 0.1 | 480 | 1437 | 6 | Kong et al. [31] |
| 11 | Single ALG/PVA/GO network | 5 | 0.1 | 480 | 1256 | 6 | Kong et al. [31] |
| 12 | ALG/immobilized GO network | 5 | 0.2 | 200 | 181 | 5.4 | Li et al. [32] |
| 13 | ALG/GO | 5 | 60 | 122 | 5.3 | Balkız et al. [33] | |
| 14 | CTA/PAAc/GO | 0.5 | 1 | 2250 | 297 | 7 | Chang et al. [34] |
| 15 | CTS/GO | 50 | 0.13 | 70 | 390 | 6.5 | Chen et al. [35] |
| 16 | CTS/GO | 50 | 3.5 | Zhao et al. [27] | |||
| 17 | PVA/sulfonated-GO | 1 | 80 | 720 | 5.1 | 6.2 | Li et al. [32] |
| 18 | Cellulose/GO | 0.5 | 20 | 20 | 123 | 7 | Soleimani et al. [36] |
| 19 | Cellulose/GO | 10 | 2 | 70 | 46 | Liu et al. [26] | |
| 20 | CMC/PVA/GO | 0.7 | 1.5 | 80 | 89 | 8 | Dai et al. [37] |
| 21 | k-CARR/GO | 30 | 6 | 658 | 5.3 | Yang et al. [38] | |
| 22 | PEGDMA-rGO | 1 | 2.5 | 720 | 60 | 7.4 | Halouane et al. [39] |
| 23 | PAMm/GO | 5 | 75 | 26 | Thompson et al. [40] | ||
| 24 | PEGD/thiolated-GO | 17 | 75 | 6 | Liu et al. [26] | ||
| 25 | PAAc-g-XG/GO | 0.5 | 0.25 | 7 | Hosseini et al. [41] | ||
| 26 | PEI/GO | 240 | 334 | Guo et al. [42] | |||
| 27 | PVA/sulfonated-GO | 1 | 80 | 4.4 | 6.2 | Li et al. [32] | |
| 28 | ALG/PAAc/Graphite | 60 | 629 | 7 | Verma et al. [43] | ||
| 29 | XG-g-PAAc/rGO | 5 | 0.5 | 30 | 1052 | 6 | Makhado et al. [44] |
| 30 | PAMm/GO | 50 | 0.025 | 20 | 288 | Yang et al. [24] | |
| 31 | CTS/GO | 250 | 1.9 | Zhao et al. [27] | |||
| 32 | PMPTC/GO | 0.3 | 150 | 12 | Wang et al. [23] | ||
| 33 | Cellulose/GO | 0.5 | 20 | 40 | 62 | 7 | Soleimani et al. [36] |
| 34 | PEI/GO | 240 | 132 | Guo et al. [42] | |||
| 35 | ALG-Fe3+/rGO | 50 | 360 | 18.4 | Xiao et al. [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouhtady, O.; Obeid, E.; Abu-samha, M.; Younes, K.; Murshid, N. Evaluation of the Adsorption Efficiency of Graphene Oxide Hydrogels in Wastewater Dye Removal: Application of Principal Component Analysis. Gels 2022, 8, 447. https://doi.org/10.3390/gels8070447
Mouhtady O, Obeid E, Abu-samha M, Younes K, Murshid N. Evaluation of the Adsorption Efficiency of Graphene Oxide Hydrogels in Wastewater Dye Removal: Application of Principal Component Analysis. Gels. 2022; 8(7):447. https://doi.org/10.3390/gels8070447
Chicago/Turabian StyleMouhtady, Omar, Emil Obeid, Mahmoud Abu-samha, Khaled Younes, and Nimer Murshid. 2022. "Evaluation of the Adsorption Efficiency of Graphene Oxide Hydrogels in Wastewater Dye Removal: Application of Principal Component Analysis" Gels 8, no. 7: 447. https://doi.org/10.3390/gels8070447
APA StyleMouhtady, O., Obeid, E., Abu-samha, M., Younes, K., & Murshid, N. (2022). Evaluation of the Adsorption Efficiency of Graphene Oxide Hydrogels in Wastewater Dye Removal: Application of Principal Component Analysis. Gels, 8(7), 447. https://doi.org/10.3390/gels8070447