Graphene Oxide/Polyethylenimine Aerogels for the Removal of Hg(II) from Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sorbents Synthesis and Structure
2.2. Hg(II) Sorption Kinetics on rGO and rGO/PEI Sorbent
2.3. Isotherm of rGO/PEI Sorbent for Hg(II) Sorption
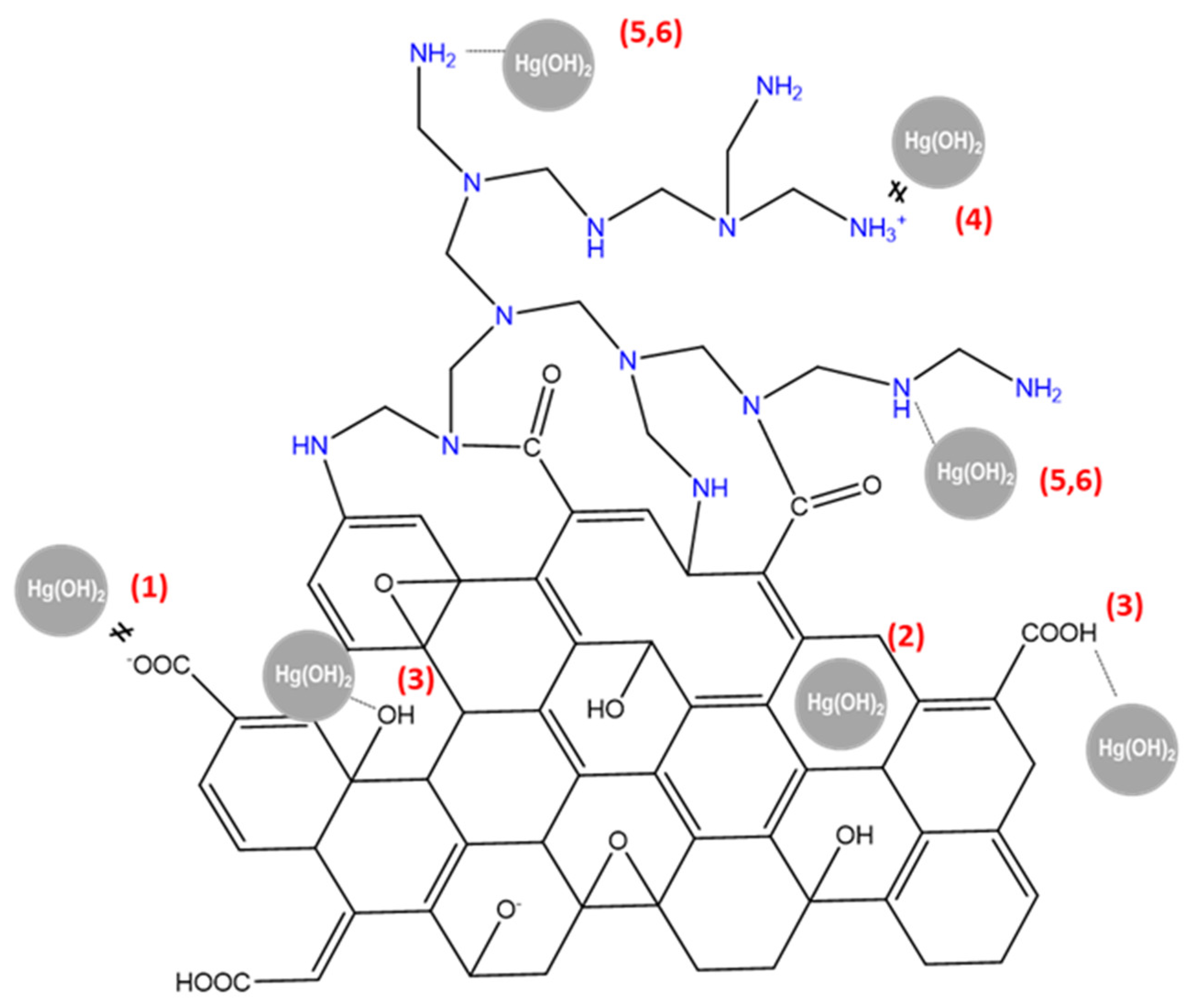
2.4. Mechanism for Mercury Sorption by rGO/PEI
- Electrostatic/complexation:
- Acid/basic aromatic π interaction:
- Hydrogen bonding:
- Electrostatic/complexation/chelation:
- Hydrogen bonding:
- Lewis base/acid coordination:
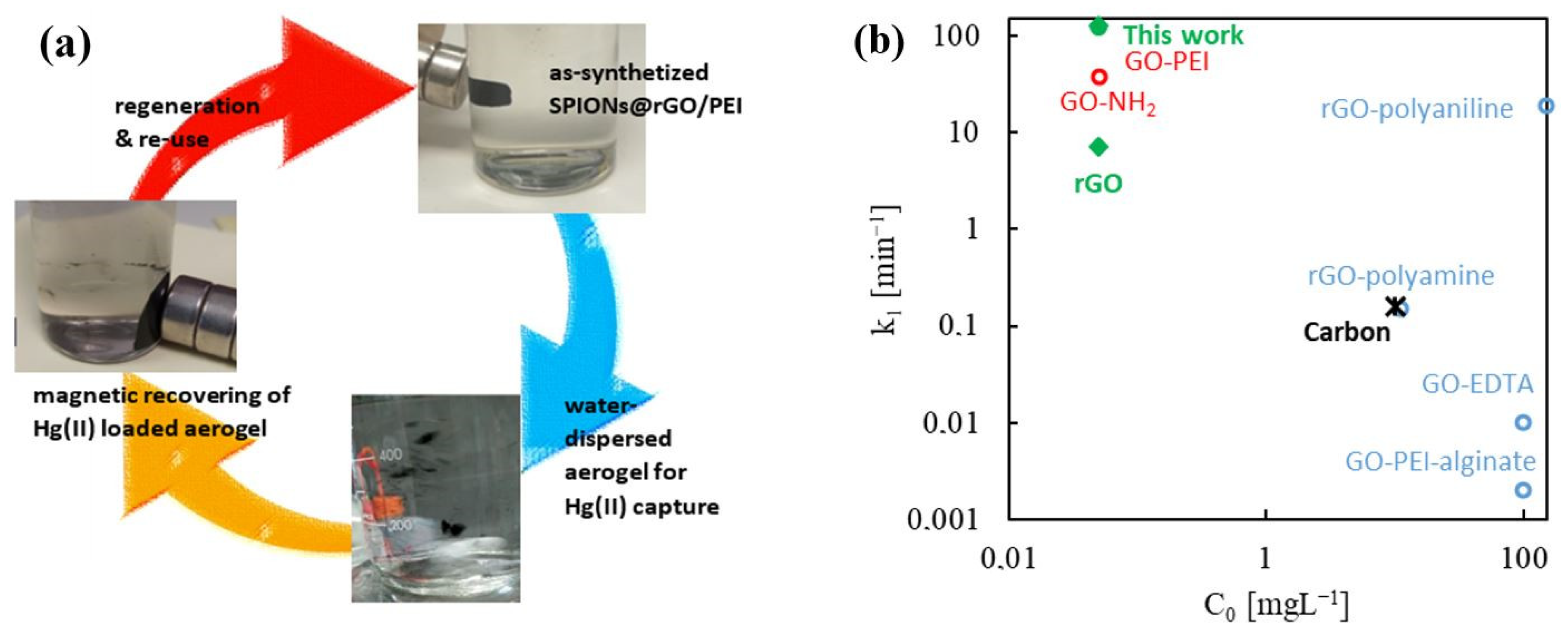
2.5. Design of Magnetic Sorbents
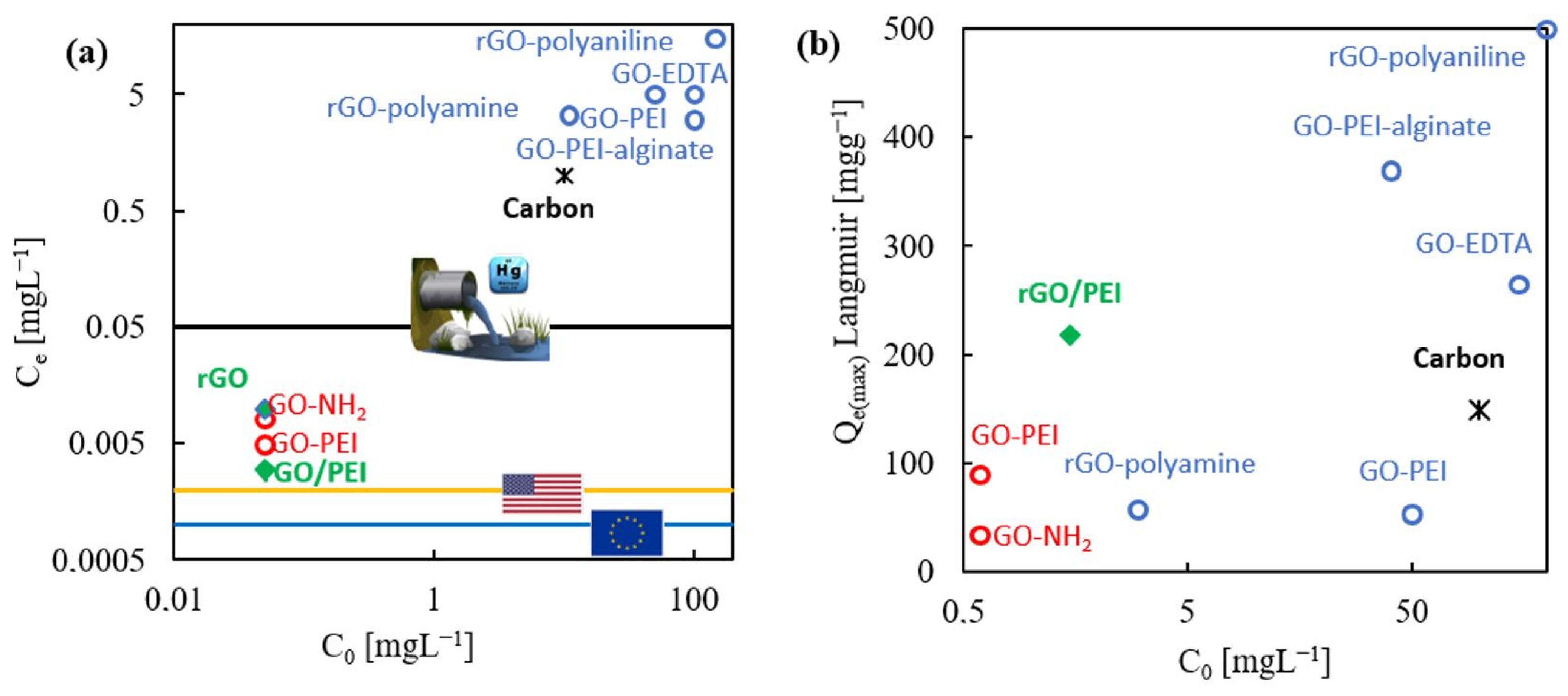
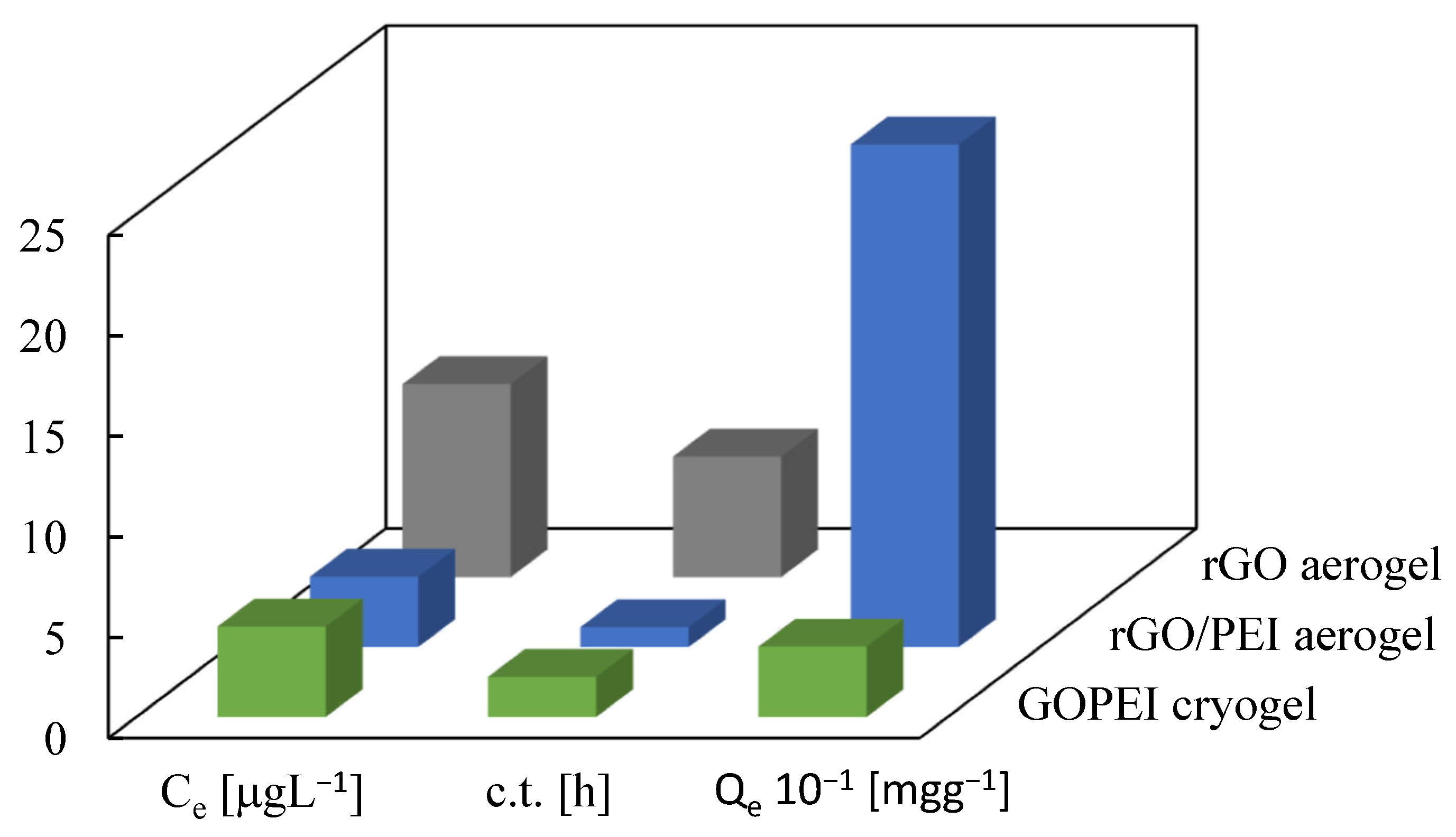
2.6. Contrasting Literature Data
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. scCO2 Synthesis of the Aerogels
4.3. Solid State Characterization
4.4. Heavy Metal Batch Sorption Studies
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, F.; He, S.; Huang, F.; Peng, Z. Adsorption Behaviour of Reduced Graphene Oxide for Removal of Heavy Metal Ions. Asian J. Chem. 2014, 26, 4901–4906. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Salleh, W.N.W.; Ismail, A.F.; Yusof, N.; Yusop, M.Z.M.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K.; et al. MOFs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Qian, H.; Wang, J.; Yan, L. Synthesis of lignin-poly(N-methylaniline)-reduced graphene oxide hydrogel for organic dye and lead ions removal. J. Bioresour. Bioprod. 2020, 5, 204–210. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef]
- Yang, K.; Wang, J.; Chen, X.; Zhao, Q.; Ghaffar, A.; Chen, B. Application of graphene-based materials in water purification: From the nanoscale to specific devices. Environ. Sci. Nano 2018, 5, 1264–1297. [Google Scholar] [CrossRef]
- Lim, J.Y.; Mubarak, N.; Abdullah, E.; Nizamuddin, S.; Khalid, M.; Inamuddin. Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals—A review. J. Ind. Eng. Chem. 2018, 66, 29–44. [Google Scholar] [CrossRef]
- Yang, S.-T.; Chang, Y.; Wang, H.; Liu, G.; Chen, S.; Wang, Y.; Liu, Y.; Cao, A. Folding/aggregation of graphene oxide and its application in Cu2+ removal. J. Colloid Interface Sci. 2010, 351, 122–127. [Google Scholar] [CrossRef]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.; Hersam, M.C.; Bouchard, D. Colloidal Properties and Stability of Graphene Oxide Nanomaterials in the Aquatic Environment. Environ. Sci. Technol. 2013, 47, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Li, T.; Li, J.; Ren, H.; Meng, F. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Part B Eng. 2021, 211, 1108642. [Google Scholar] [CrossRef]
- Seal, S.; Karn, B. Safety aspects of nanotechnology based activity. Saf. Sci. 2014, 63, 217–225. [Google Scholar] [CrossRef]
- Rezaei, F.; Webley, P. Structured adsorbents in gas separation processes. Sep. Purif. Technol. 2010, 70, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Kostoglou, N.; Tzitzios, V.; Kontos, A.G.; Giannakopoulos, K.; Tampaxis, C.; Papavasiliou, A.; Charalambopoulou, G.; Steriotis, T.; Li, Y.; Liao, K.; et al. Synthesis of nanoporous graphene oxide adsorbents by freeze-drying or microwave radiation: Characterization and hydrogen storage properties. Int. J. Hydrogen Energy 2015, 40, 6844–6852. [Google Scholar] [CrossRef]
- Bessa, A.; Henriques, B.; Gonçalves, G.; Irurueta, G.; Pereira, E.; Marques, P.A. Graphene oxide/polyethyleneimine aerogel for high-performance mercury sorption from natural waters. Chem. Eng. J. 2020, 398, 125587. [Google Scholar] [CrossRef]
- Borrás, A.; Gonçalves, G.; Marbán, G.; Sandoval, S.; Pinto, S.; Marques, P.A.A.P.; Fraile, J.; Tobias, G.; López-Periago, A.M.; Domingo, C. Preparation and Characterization of Graphene Oxide Aerogels: Exploring the Limits of Supercritical CO2 Fabrication Methods. Chem. Eur. J. 2018, 24, 15903–15911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosado, A.; Borrás, A.; Fraile, J.; Navarro, J.A.R.; Suárez-García, F.; Stylianou, K.C.; López-Periago, A.M.; Planas, J.G.; Domingo, C.; Yazdi, A. HKUST-1 Metal–Organic Framework Nanoparticle/Graphene Oxide Nanocomposite Aerogels for CO2 and CH4 Adsorption and Separation. ACS Appl. Nano Mater. 2021, 4, 12712–12725. [Google Scholar] [CrossRef]
- Borras, A.; Rosado, A.; Fraile, J.; Lopez-Periago, A.M.; Planas, J.G.; Yazdi, A.; Domingo, C. Meso/microporous MOF@graphene oxide composite aerogels prepared by generic supercritical CO2 technology. Microporous Mesoporous Mater. 2022, 335, 111825. [Google Scholar] [CrossRef]
- Guastaferro, M.; Baldino, L.; Reverchon, E.; Cardea, S. Production of Porous Agarose-Based Structures: Freeze-Drying vs. Supercritical CO2 Drying. Gels 2021, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent progress on nanocellulose aerogels: Preparation, modification, composite fabrication, applications. Adv.Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.; Gurikov, P.; Subrahmanyam, R. Aerogels, Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Nassar, G.; Daou, E.; Najjar, R.; Bassil, M.; Habchi, R. A review on the current research on graphene-based aerogels and their applications. Carbon Trends 2021, 4, 100065. [Google Scholar] [CrossRef]
- Abu-Nada, A.; McKay, G.; Abdala, A. Recent Advances in Applications of Hybrid Graphene Materials for Metals Removal from Wastewater. Nanomaterials 2020, 10, 595. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Feng, J.; Wu, P. Highly elastic graphene oxide–epoxy composite aerogels via simple freeze-drying and subsequent routine curing. J. Mater. Chem. A 2013, 1, 3495–3502. [Google Scholar] [CrossRef]
- Wang, Z.; Song, L.; Wang, Y.; Zhang, X.-F.; Yao, J. Construction of a hybrid graphene oxide/nanofibrillated cellulose aerogel used for the efficient removal of methylene blue and tetracycline. J. Phys. Chem. Solids 2021, 150, 109839. [Google Scholar] [CrossRef]
- Bessa, A.; Gonçalves, G.; Henriques, B.; Domingues, E.M.; Pereira, E.; Marques, P.A.A.P. Green Graphene–Chitosan Sorbent Materials for Mercury Water Remediation. Nanomaterials 2020, 10, 1474. [Google Scholar] [CrossRef]
- Sui, Z.-Y.; Cui, Y.; Zhu, J.-H.; Han, B.-H. Preparation of Three-Dimensional Graphene Oxide–Polyethylenimine Porous Materials as Dye and Gas Adsorbents. ACS Appl. Mater. Interfaces 2013, 5, 9172–9179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, X.; Chen, B. Stable graphene oxide/poly(ethyleneimine) 3D aerogel with tunable surface charge for high performance selective removal of ionic dyes from water. Chem. Eng. J. 2018, 334, 1119–1127. [Google Scholar] [CrossRef]
- Xu, J.; Du, P.; Bi, W.; Yao, G.; Li, S.; Liu, H. Graphene oxide aerogels co-functionalized withpolydopamine and polyethylenimine for theadsorption of anionic dyes and organic solvents. Chem. Eng. Res. Des. 2020, 15, 192–202. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Liu, J.; Liu, X.; Chen, C.; Li, G.; Meng, Y. Graphene oxides cross-linked with hyperbranched polyethylenimines: Preparation, characterization and their potential as recyclable and highly efficient adsorption materials for lead(II) ions. Chem. Eng. J. 2016, 285, 698–708. [Google Scholar] [CrossRef] [Green Version]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. Crosslinked hydrogels of polyethylenimine and graphene oxide to treat Cr(VI) contaminated wastewater. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 630, 127533. [Google Scholar] [CrossRef]
- Pakulski, D.; Czepa, W.; Witomska, S.; Aliprandi, A.; Pawluć, P.; Patroniak, V.; Ciesielski, A.; Samorì, P. Graphene oxide-branched polyethylenimine foams for efficient removal of toxic cations from water. J. Mater. Chem. A 2018, 6, 9384–9390. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.K.; Kumar, V.; Singh, V.K.; Hasan, S.H. Modeling of adsorption behavior of the amine-rich GOPEI aerogel for the removal of As(iii) and As(v) from aqueous media. RSC Adv. 2016, 6, 56684–56697. [Google Scholar] [CrossRef]
- Wu, A.; Jia, J.; Luan, S. Amphiphilic PMMA/PEI core–shell nanoparticles as polymeric adsorbents to remove heavy metal pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 180–185. [Google Scholar] [CrossRef]
- Domingues, E.M.; Gonçalves, G.A.B.; Henriques, B.; Pereira, E.; Marques, P.A.A.P. Effective and simple removal of Hg from real waters by a robust bio-nanocomposite. Environ. Sci. Nano 2022, 9, 1156–1167. [Google Scholar] [CrossRef]
- Arshad, F.; Selvaraj, M.; Zain, J.; Banat, F.; Abu Haija, M. Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep. Purif. Technol. 2019, 209, 870–880. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Zheng, X.; Lv, W.; Wang, M.; Yang, Q.-H.; Kang, F. Adsorption of Lead(II) Ions from Aqueous Solution on Low-Temperature Exfoliated Graphene Nanosheets. Langmuir 2011, 27, 7558–7562. [Google Scholar] [CrossRef] [PubMed]
- The Council of the European Union. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. J. Eur. Communities 1998, 330, 32–54. [Google Scholar]
- Kumar, M.; Puri, A. A review of permissible limits of drinking water. Indian J. Occup. Environ. Med. 2012, 16, 40–44. [Google Scholar] [CrossRef] [Green Version]
- El-Khaiary, M.I.; Malash, G.F. Common data analysis errors in batch adsorption studies. Hydrometallurgy 2011, 105, 314–320. [Google Scholar] [CrossRef]
- Gupta, N.K.; Gupta, A. 2D and 3D carbon-based adsorbents for an efficient removal of HgII ions: A review. FlatChem 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A review on heavy metal ions adsorption from water by graphene oxide and its composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Daneshmand, M.; Outokesh, M.; Akbari, A.; Kosari, M.; Tayyebi, A. Synthesis of “L-cysteine–graphene oxide” hybrid by new methods and elucidation of its uptake properties for Hg(II) ion. Sep. Sci. Technol. 2018, 53, 843–855. [Google Scholar] [CrossRef]
- Farrukh, A.; Akram, A.; Ghaffar, A.; Hanif, S.; Hamid, A.; Duran, H.; Yameen, B. Design of Polymer-Brush-Grafted Magnetic Nanoparticles for Highly Efficient Water Remediation. ACS Appl. Mater. Interfaces 2013, 5, 3784–3793. [Google Scholar] [CrossRef] [PubMed]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Y.; Wang, Q.; Wu, J.; Duan, G.; Xu, W.; Jian, S. Magnetically separable and recyclable Fe3O4@PDA covalent grafted by l-cysteine core-shell nanoparticles toward efficient removal of Pb2+. Vacuum 2021, 189, 110229. [Google Scholar] [CrossRef]
- Borrás, A.; Fraile, J.; Rosado, A.; Marbán, G.; Tobias, G.; López-Periago, A.M.; Domingo, C. Green and Solvent-Free Supercritical CO2-Assisted Production of Superparamagnetic Graphene Oxide Aerogels: Application as a Superior Contrast Agent in MRI. ACS Sustain. Chem. Eng. 2020, 8, 4877–4888. [Google Scholar] [CrossRef]
- Henriques, B.; Goncalves, G.; Emamie, N.; Pereira, E.; Vila, M.; Marques, P.A.A.P. Optimized graphene oxide foam with enhanced performance andhigh selectivity for mercury removal from water. J. Hazard. Mater. 2016, 301, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.L.; Tung, T.T.; Kabiri, S.; Matulick, N.; Tran, D.N.; Losic, D. Polyamine-modified reduced graphene oxide: A new and cost-effective adsorbent for efficient removal of mercury in waters. Sep. Purif. Technol. 2019, 238, 116441. [Google Scholar] [CrossRef]
- Zabihi, M.; Asl, A.H.; Ahmadpour, A. Studies on adsorption of mercury from aqueous solution on activated carbons prepared from walnut shell. J. Hazard. Mater. 2010, 174, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, F.; Wang, D.; Liu, X.; Jin, J. Polydopamine-mediated surface-functionalization of graphene oxide for heavy metal ions removal. J. Solid State Chem. 2015, 224, 88–93. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Yang, F. Preparation of polyaniline/reduced graphene oxide nanocomposite and its application in adsorption of aqueous Hg(II). Chem. Eng. J. 2013, 229, 460–468. [Google Scholar] [CrossRef]
- Korhonena, O.; Budtova, T. All-cellulose composite aerogels and cryogels. Compos. A 2020, 137, 106027. [Google Scholar] [CrossRef]
- Kim, I.; Pascal, T.A.; Park, S.-J.; Diallo, M.; Goddard, W.A.; Jung, Y. pH-Dependent Conformations for Hyperbranched Poly(ethylenimine) from All-Atom Molecular Dynamics. Macromolecules 2018, 51, 2187–2194. [Google Scholar] [CrossRef] [Green Version]
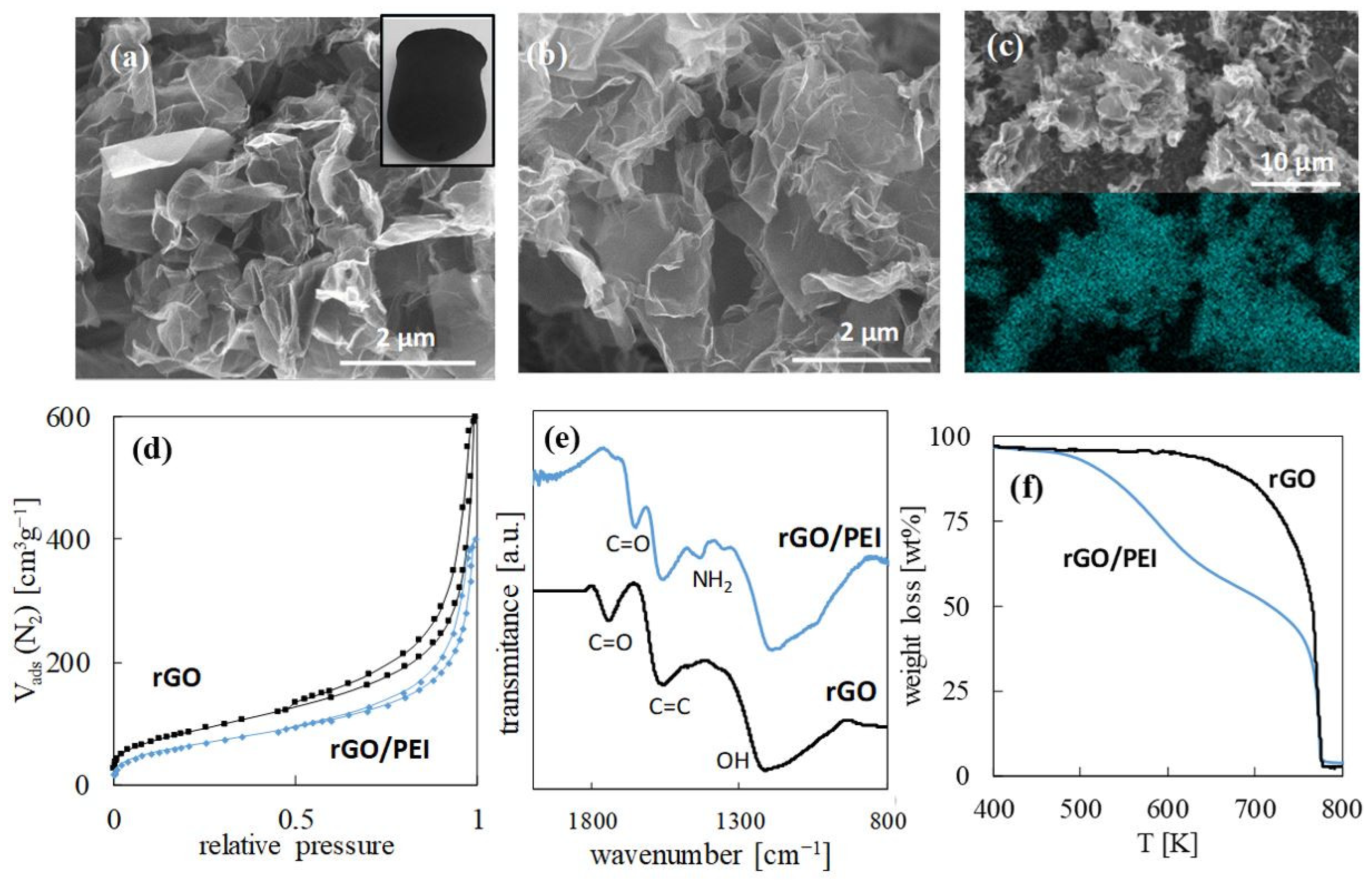
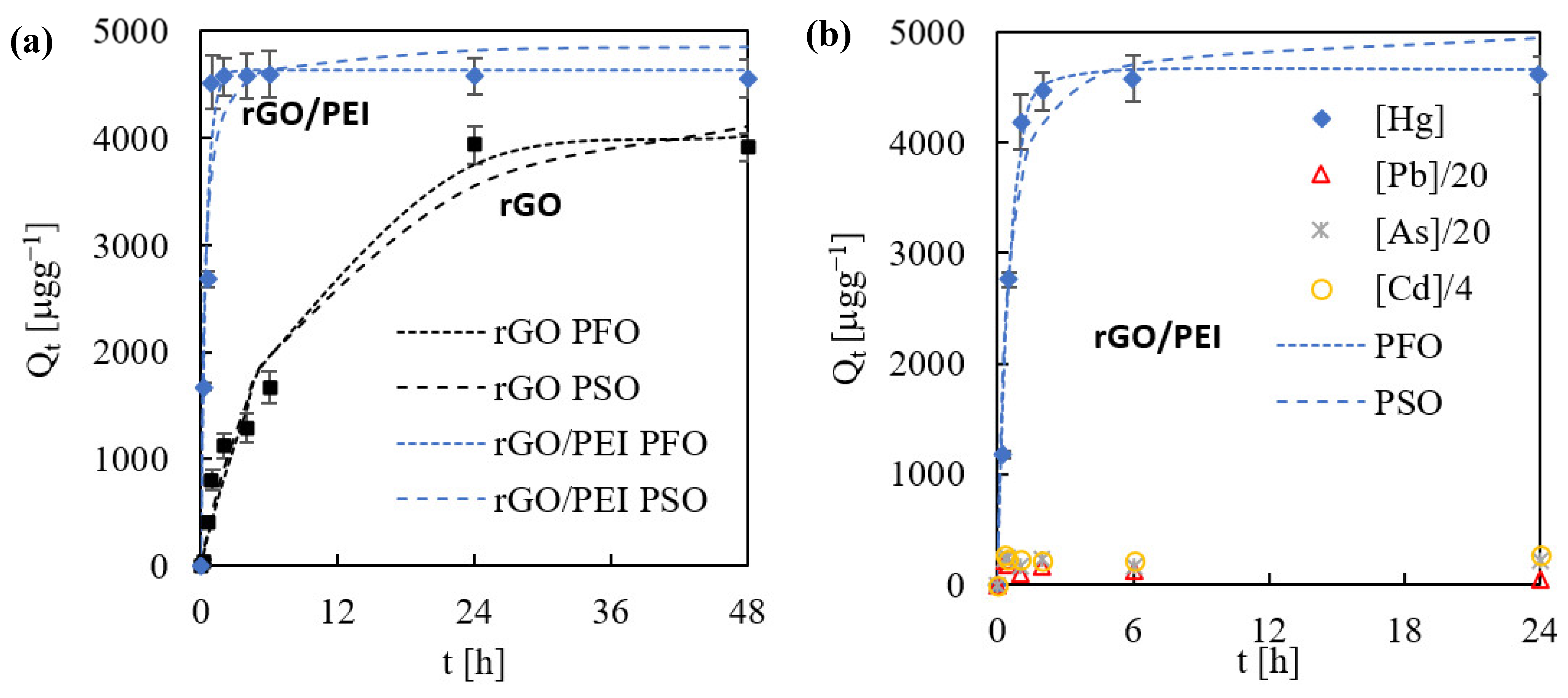
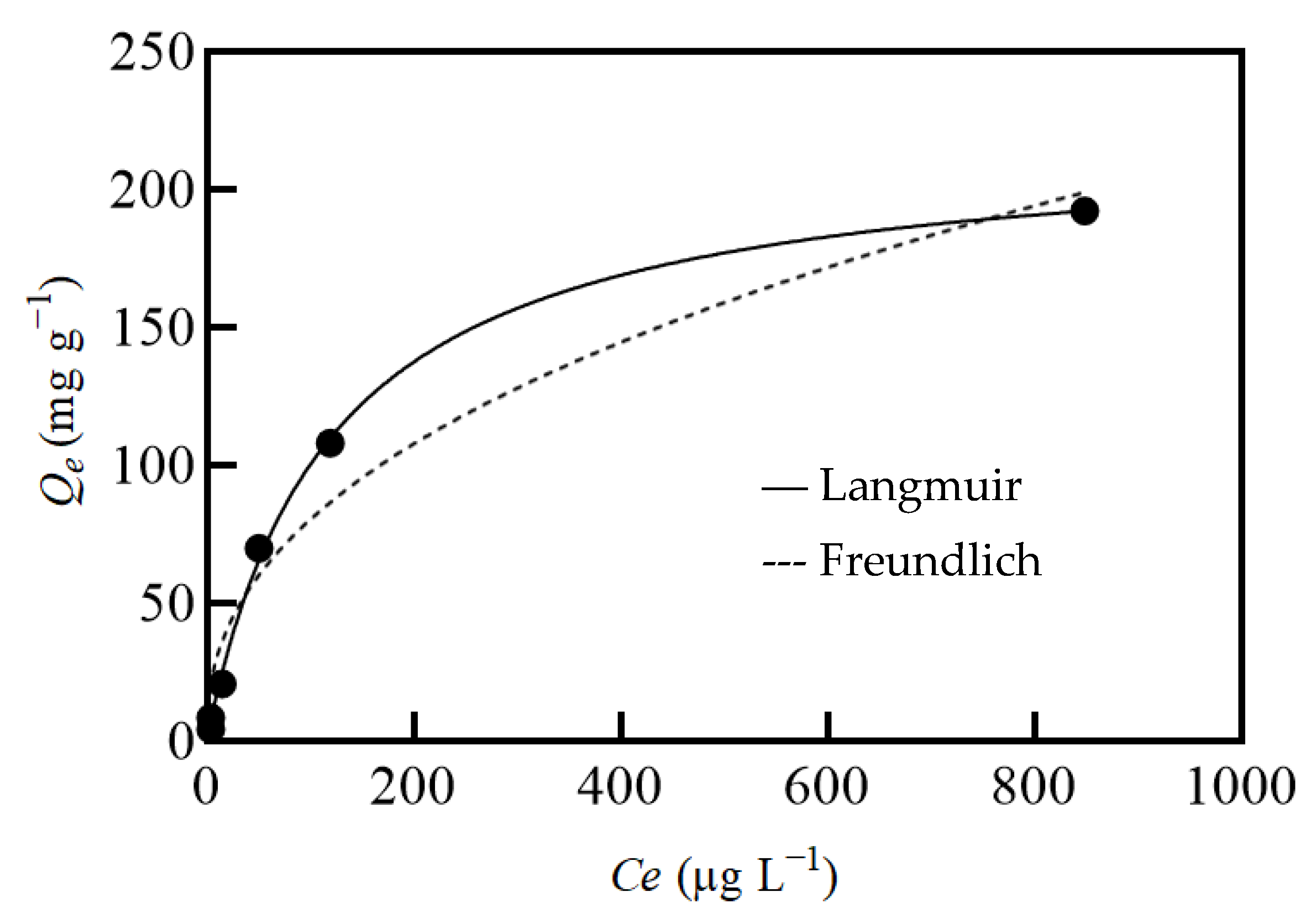

| Sample | BET Sa (m2 g−1) | BJH Vp (cm3 g−1) |
|---|---|---|
| rGO | 310 | 0.91 |
| rGO/PEI | 227 | 0.60 |
| Sample | Single Metal | Multicomponent | ||||||
|---|---|---|---|---|---|---|---|---|
| RE (wt%) | c.t. (h) | k1 (h−1) | Ce (µg L−1) | RE (wt%) | c.t. (h) | k1 (h−1) | Ce (µg L−1) | |
| rGO | 80 | 24 | 0.11 ± 0.03 | 9.6 ± 0.5 | -- | -- | -- | -- |
| rGO/PEI | 95 | 1 | 2.0 ± 0.04 | 3.6 ± 0.2 | 95 | 2 | 1.7 ± 0.09 | 3.4 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrás, A.; Henriques, B.; Gonçalves, G.; Fraile, J.; Pereira, E.; López-Periago, A.M.; Domingo, C. Graphene Oxide/Polyethylenimine Aerogels for the Removal of Hg(II) from Water. Gels 2022, 8, 452. https://doi.org/10.3390/gels8070452
Borrás A, Henriques B, Gonçalves G, Fraile J, Pereira E, López-Periago AM, Domingo C. Graphene Oxide/Polyethylenimine Aerogels for the Removal of Hg(II) from Water. Gels. 2022; 8(7):452. https://doi.org/10.3390/gels8070452
Chicago/Turabian StyleBorrás, Alejandro, Bruno Henriques, Gil Gonçalves, Julio Fraile, Eduarda Pereira, Ana M. López-Periago, and Concepción Domingo. 2022. "Graphene Oxide/Polyethylenimine Aerogels for the Removal of Hg(II) from Water" Gels 8, no. 7: 452. https://doi.org/10.3390/gels8070452
APA StyleBorrás, A., Henriques, B., Gonçalves, G., Fraile, J., Pereira, E., López-Periago, A. M., & Domingo, C. (2022). Graphene Oxide/Polyethylenimine Aerogels for the Removal of Hg(II) from Water. Gels, 8(7), 452. https://doi.org/10.3390/gels8070452










