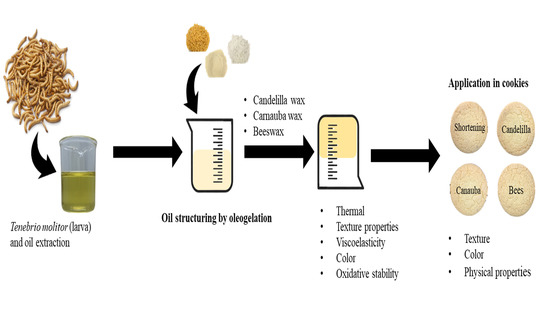
The Characteristic of Insect Oil for a Potential Component of Oleogel and Its Application as a Solid Fat Replacer in Cookies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fatty Acid Composition of Tenebrio Molitor Oil
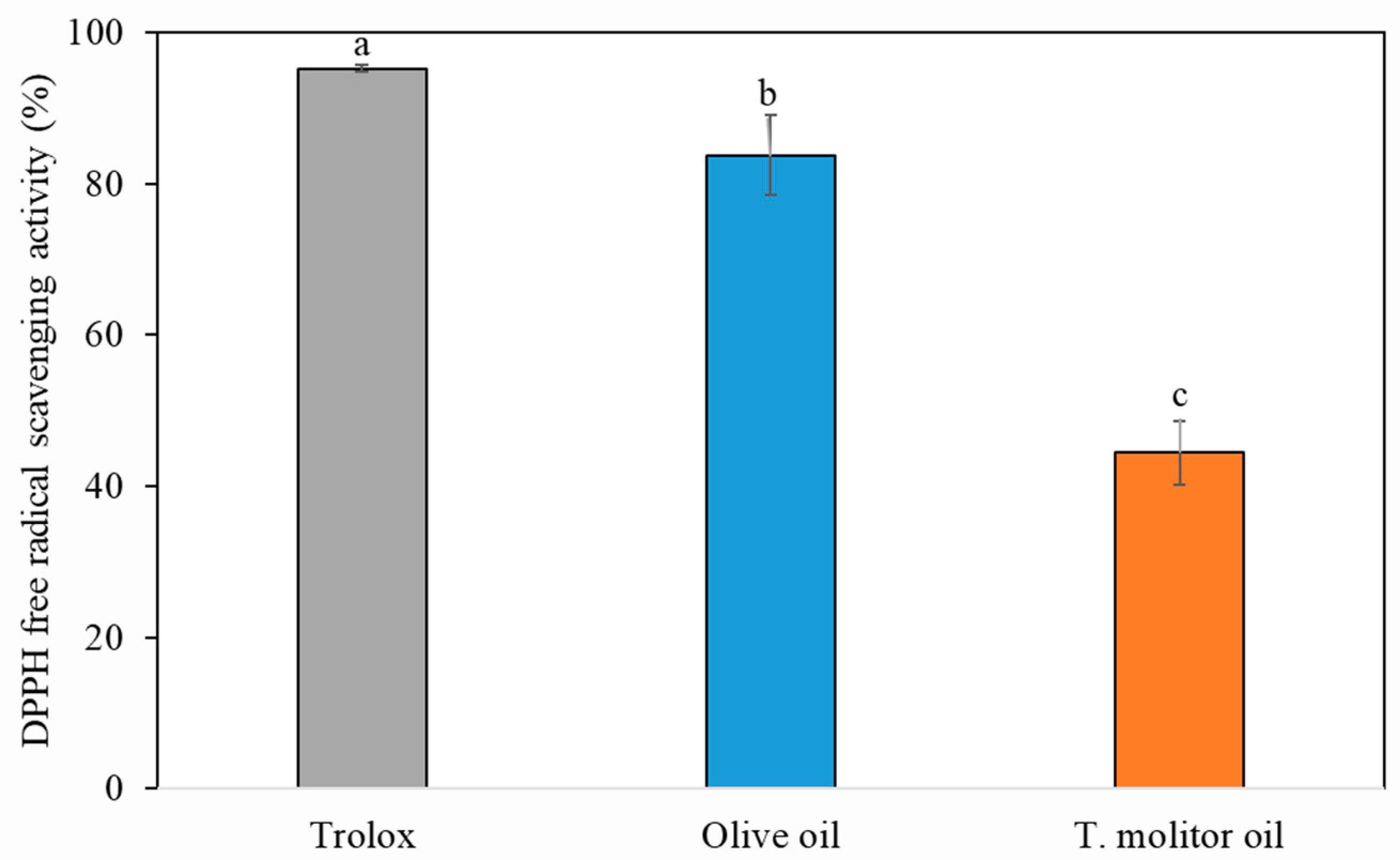
2.2. Antioxidant Activities of Tenebrio Molitor Oil
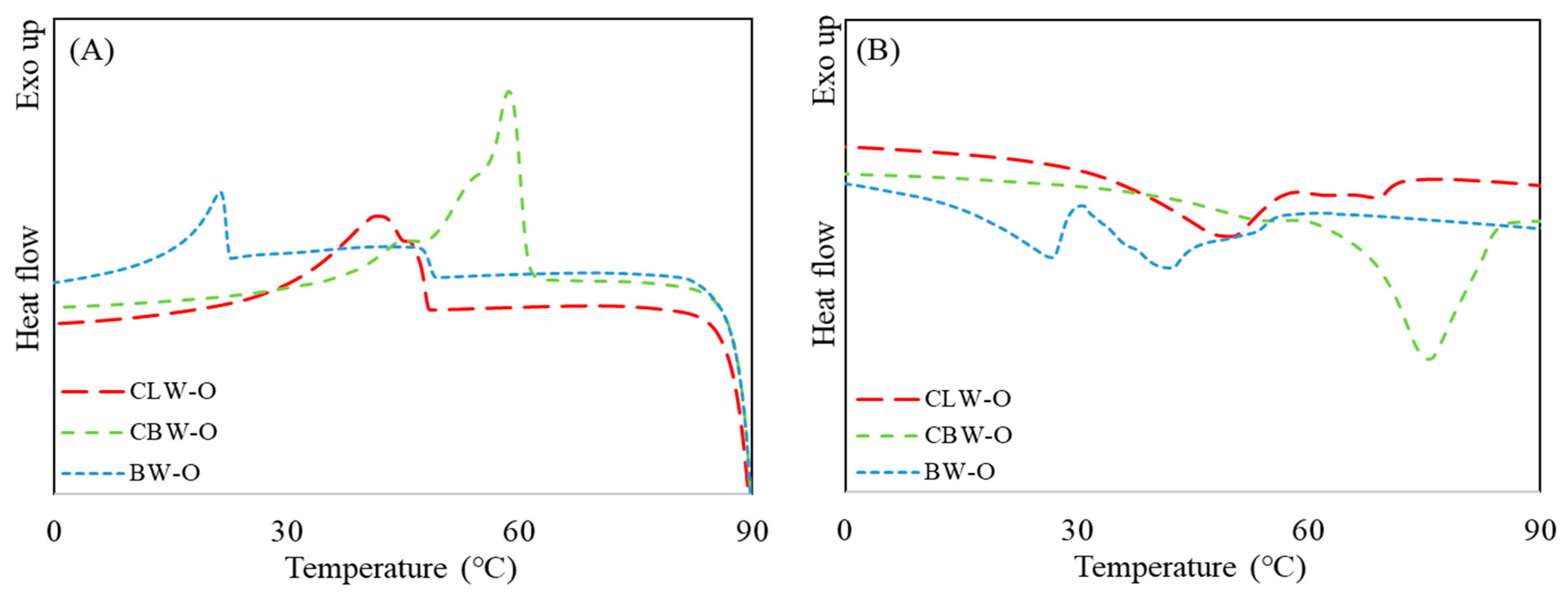
2.3. Thermal Properties of Oleogels
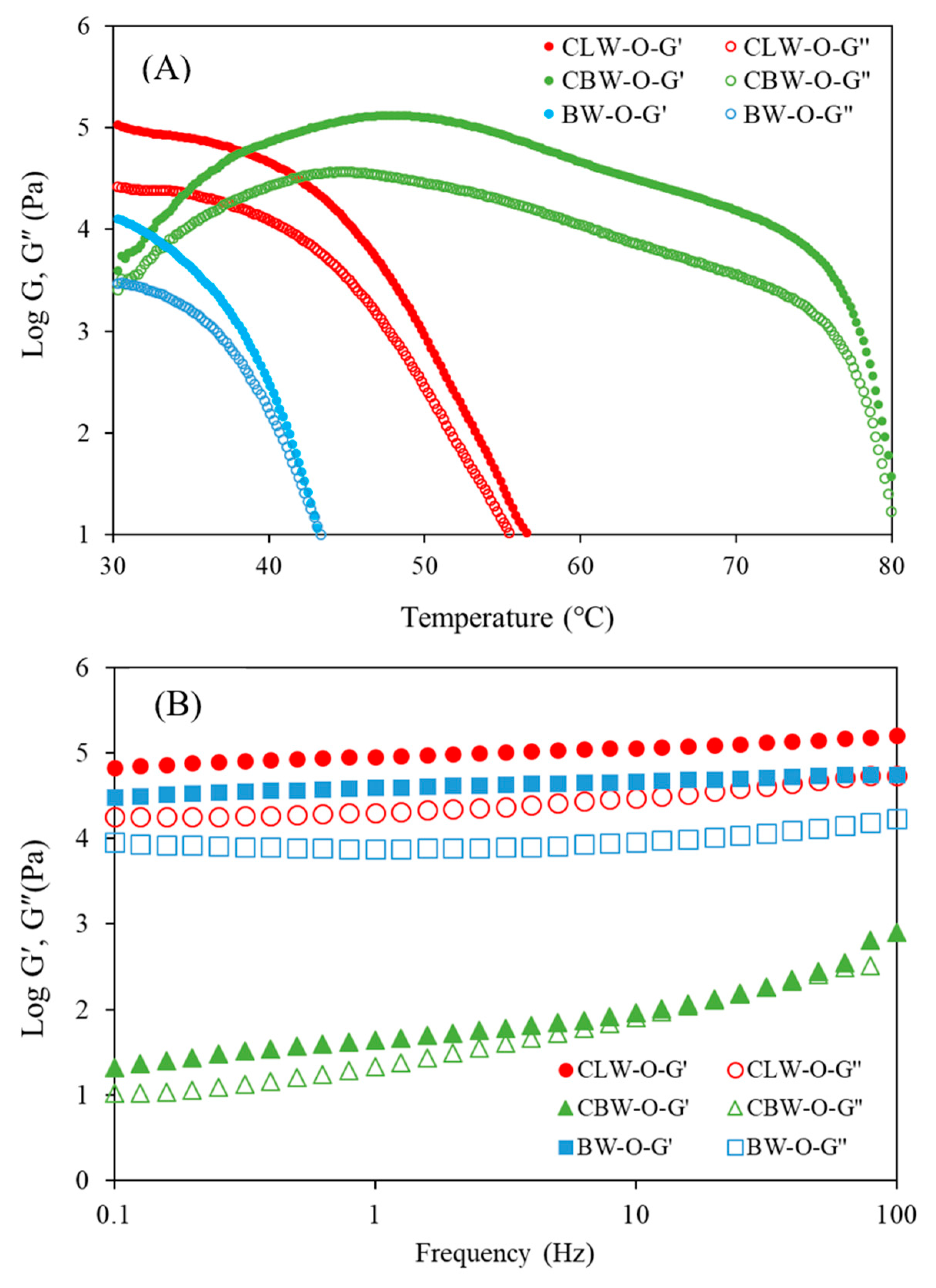
2.4. Viscoelasticity of Oleogels
2.5. Gel Strength and Oil Binding Capacities of Oleogels
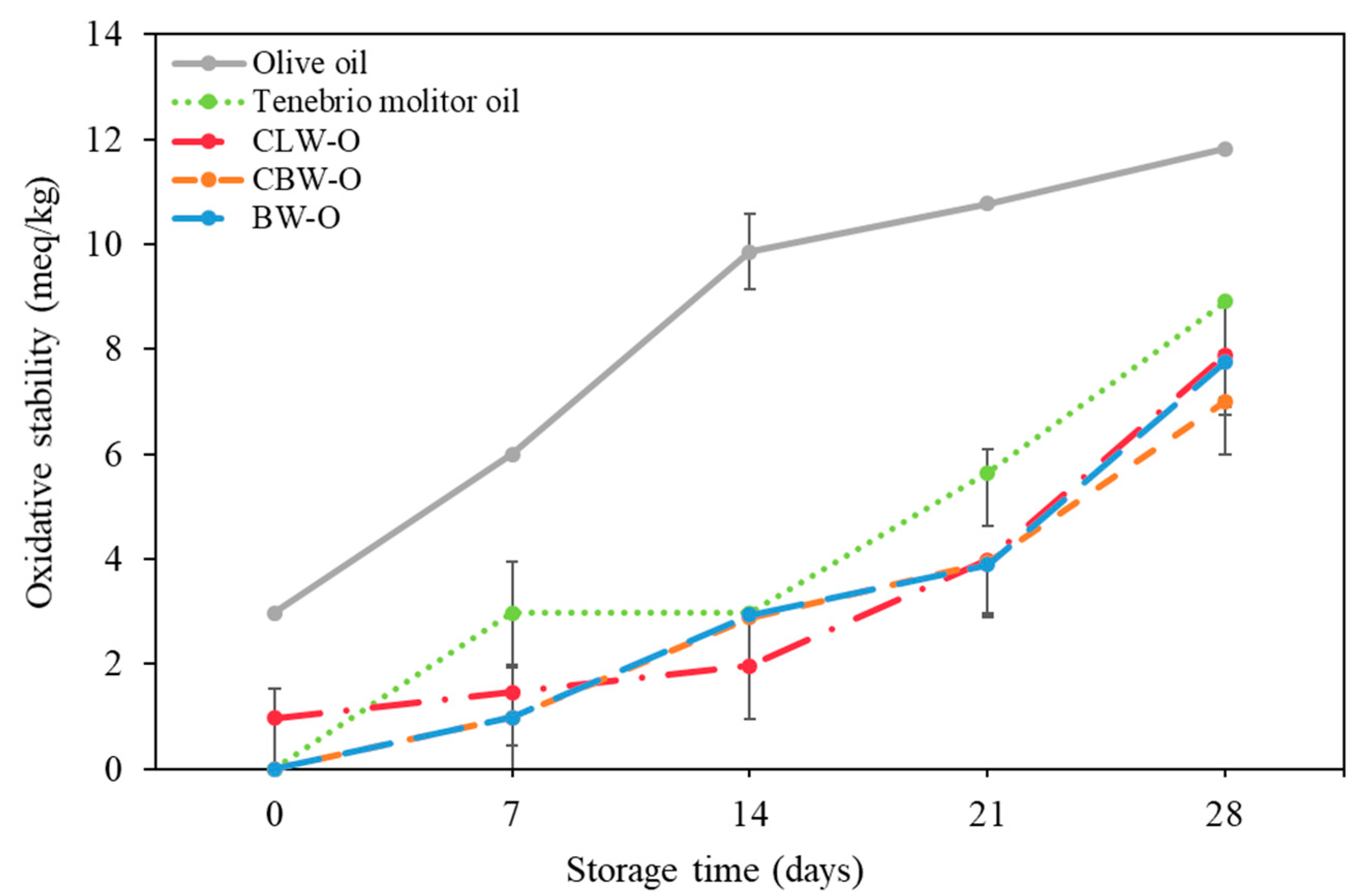
2.6. Oxidative Stability
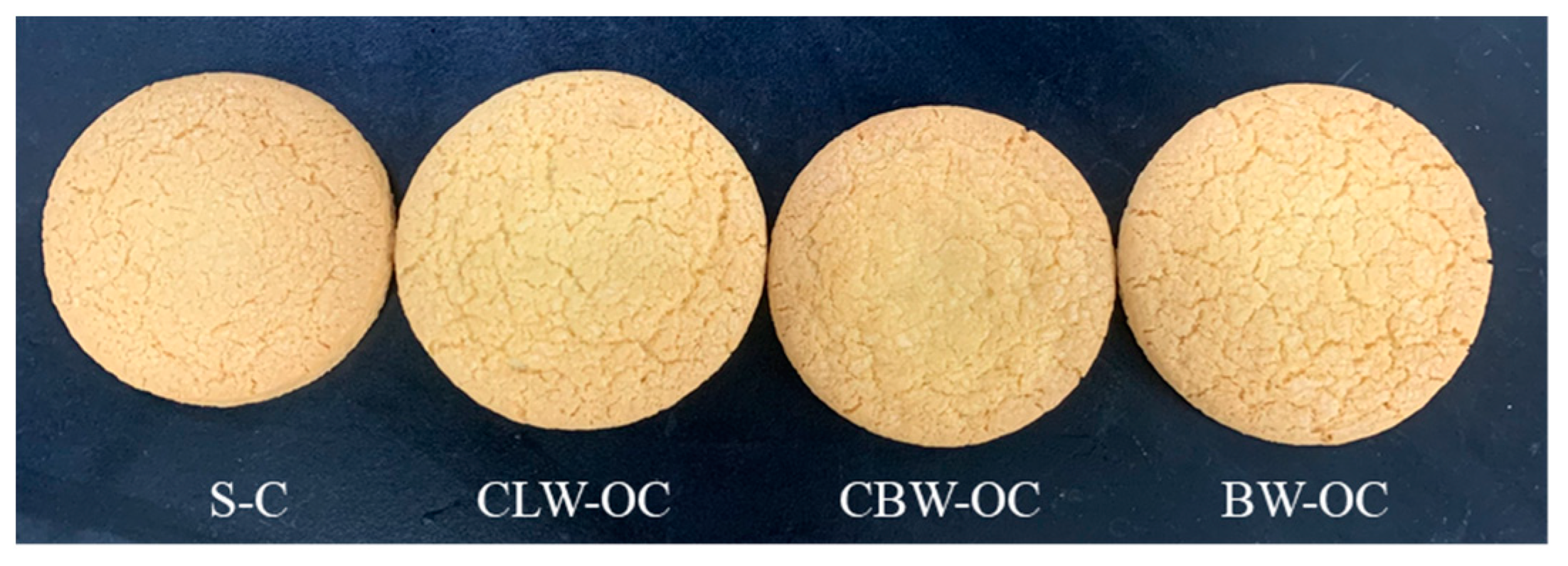
2.7. Color of Oleogel Cookies
2.8. Physical Properties of Oleogel Cookies
2.9. Texture Characteristics of Oleogel Cookies
3. Conclusions
4. Materials and Methods
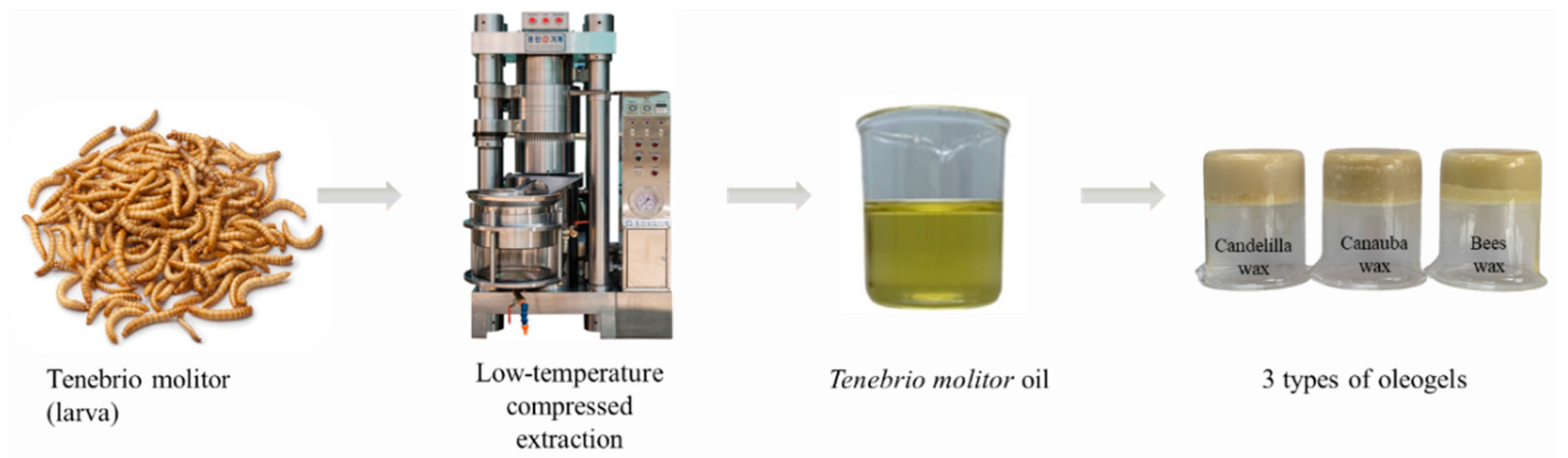
4.1. Oil Extraction from Tenebrio Molitor Larvae
4.2. Analysis of Fatty Acid Composition
4.3. Antioxidant Activity Measurement
4.4. Preparation of Oleogels
4.5. Thermal Properties of Oleogels
4.6. Dynamic Viscoelastic Properties of Oleogels
4.7. Gel Strength and Oil Binding Capacity (OBC) of Oleogels
4.8. Measurement of Peroxide Values
4.9. Preparation of Cookies Replacing Shortening with Oleogels
4.10. Color, Geometry, and Texture Measurement of Cookies
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liceaga, A.M. Processing insects for use in the food and feed industry. Curr. Opin. Insect Sci. 2021, 48, 32–36. [Google Scholar] [CrossRef]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Nat. C 2017, 72, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Marcos, A.; Nova, E.; Montero, A. Changes in the immune system are conditioned by nutrition. Eur. J. Clin. Nutr. 2003, 57, 66–69. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Cini, E. Stone milling versus roller milling: A systematic review of the effects on wheat flour quality, dough rheology, and bread characteristics. Trends Food Sci. Technol. 2020, 97, 147–155. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Martínez-Monzó, J. Physicochemical properties and consumer acceptance of bread enriched with alternative proteins. Foods 2020, 9, 933. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Fogliano, V.; Lakemond, C.; Severini, C. Effects of formulation and process conditions on microstructure, texture and digestibility of extruded insect-riched snacks. Innov. Food Sci. Emerg. Technol. 2018, 45, 344–353. [Google Scholar] [CrossRef]
- Kim, H.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Kröncke, N.; Böschen, V.; Woyzichovski, J.; Demtröder, S.; Benning, R. Comparison of suitable drying processes for mealworms Tenebrio molitor. Innov. Food Sci. Emerg. Technol. 2018, 50, 20–25. [Google Scholar] [CrossRef]
- Fombong, F.T.; Van Der Borght, M.; Vanden Broeck, J. Influence of freeze-drying and oven-drying post blanching on the nutrient composition of the edible insect Ruspolia Differens. Insects 2017, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- DeFoliart, G.R. Insect fatty acids: Similar to those of poultry and fish in their degree of unsaturation, but higher in the polyunsaturates. Food Insects Newsl. 1991, 4, 1–4. [Google Scholar]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.; van Boekel, M.A.; Lakemond, C.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Jang, A.; Bae, W.; Hwang, H.; Lee, H.G.; Lee, S. Evaluation of canola oil oleogels with candelilla wax as an alternative to shortening in baked goods. Food Chem. 2015, 187, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Garti, N. Edible Oleogels: Structure and Health Implications; AOCS Press: Champaign, IL, USA, 2018; pp. 110–127. [Google Scholar]
- Choi, K.; Hwang, H.; Jeong, S.; Kim, S.; Lee, S. The thermal, rheological, and structural characterization of grapeseed oil oleogels structured with binary blends of oleogelator. J. Food Sci. 2020, 85, 3432–3441. [Google Scholar] [CrossRef]
- Oh, I.; Amoah, C.; Lim, J.; Jeong, S.; Lee, S. Assessing the effectiveness of wax-based sunflower oil oleogels in cakes as a shortening replacer. LWT 2017, 86, 430–437. [Google Scholar] [CrossRef]
- Yılmaz, E.; Öğütcü, M. The texture, sensory properties and stability of cookies prepared with wax oleogels. Food Fun. 2015, 6, 1194–1204. [Google Scholar] [CrossRef]
- Li, S.; Wu, G.; Li, X.; Jin, Q.; Wang, X.; Zhang, H. Roles of gelator type and gelation technology on texture and sensory properties of cookies prepared with oleogels. Food Chem. 2021, 356, 129667. [Google Scholar] [CrossRef]
- Yoo, J.; Hwang, J.; Goo, T.; Yun, E. Comparative analysis of nutritional and harmful components in Korean and Chinese mealworms (Tenebrio molitor). J. Korean Soc. Food Sci. Nutr. 2013, 42, 249–254. [Google Scholar] [CrossRef]
- Son, Y.; Choi, S.Y.; Hwang, I.; Nho, C.W.; Kim, S.H. Could defatted mealworm (Tenebrio molitor) and mealworm oil be used as food ingredients? Foods 2020, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Sánchez-Muros-Lozano, M.J.; García-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of oleogelation on physical properties and oxidative stability of camellia oil-based oleogels and oleogel emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Cipolatti, E.P.; Aguieiras, E.C.; Cerqueira Pinto, M.C.; Kopsahelis, N.; Freire, D.M.; Mandala, I.; Koutinas, A.A. Development of microbial oil wax-based oleogel with potential application in food formulations. Food Bioproc. Technol. 2019, 12, 899–909. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Del Hierro, J.N.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.; Oh, I. Development of Antioxidant-Fortified Oleogel and Its Application as a Solid Fat Replacer to Muffin. Foods 2021, 10, 3059. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Nan, Y.; Liu, G. The effects of oil type and crystallization temperature on the physical properties of vitamin C-loaded oleogels prepared by an emulsion-templated approach. Food Funct. 2020, 11, 8028–8037. [Google Scholar] [CrossRef]
- Pan, H.; Xu, X.; Qian, Z.; Cheng, H.; Shen, X.; Chen, S.; Ye, X. Xanthan gum-assisted fabrication of stable emulsion-based oleogel structured with gelatin and proanthocyanidins. Food Hydrocoll. 2021, 115, 106596. [Google Scholar] [CrossRef]
- Masotta, N.E.; Höcht, C.; Contin, M.; Lucangioli, S.; Rojas, A.M.; Tripodi, V.P. Bioavailability of coenzyme Q10 loaded in an oleogel formulation for oral therapy: Comparison with a commercial-grade solid formulation. Int. J. Pharm. 2020, 582, 119315. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Pintado, T.; Jiménez-Colmenero, F.; Cofrades, S. The effect of household storage and cooking practices on quality attributes of pork burgers formulated with PUFA-and curcumin-loaded oleogels as healthy fat substitutes. LWT 2020, 119, 108909. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, H.; Lee, S. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2017, 60, 17–22. [Google Scholar] [CrossRef]
- Rocha, J.C.B.; Lopes, J.D.; Mascarenhas, M.C.N.; Arellano, D.B.; Guerreiro, L.M.R.; da Cunha, R.L. Thermal and rheological properties of organogels formed by sugarcane or candelilla wax in soybean oil. Food Res. Int. 2013, 50, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Öğütcü, M.; Yılmaz, E. Characterization of hazelnut oil oleogels prepared with sunflower and carnauba waxes. Int. J. Food Prop. 2015, 18, 1741–1755. [Google Scholar] [CrossRef]
- Hwang, H.; Winkler-Moser, J.K. Properties of margarines prepared from soybean oil oleogels with mixtures of candelilla wax and beeswax. J. Food Sci. 2020, 10, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Rapeseed oil in new application: Assessment of structure of oleogels based on their physicochemical properties and microscopic observations. Agriculture 2020, 10, 211. [Google Scholar] [CrossRef]
- Laredo, T.; Barbut, S.; Marangoni, A.G. Molecular interactions of polymer oleogelation. Soft Matter. 2011, 7, 2734–2743. [Google Scholar] [CrossRef]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and physical properties of plant wax crystal networks and their relationship to oil binding capacity. J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Li, L.; Taha, A.; Geng, M.; Zhang, Z.; Su, H.; Xu, X.; Pan, S.; Hu, H. Ultrasound-assisted gelation of β-carotene enriched oleogels based on candelilla wax-nut oils: Physical properties and in-vitro digestion analysis. Ultrason. Sonochem. 2021, 79, 105762. [Google Scholar] [CrossRef]
- Maduko, C.O.; Park, Y.W.; Akoh, C.C. Characterization and oxidative stability of structured lipids: Infant milk fat analog. J. Am. Oil Chem. Soc. 2008, 85, 197–204. [Google Scholar] [CrossRef]
- Luo, S.; Hu, X.; Jia, Y.; Pan, L.; Zheng, Z.; Zhao, Y.; Mu, D.D.; Zhong, X.Y.; Yiang, S.T. Camellia oil-based oleogels structuring with tea polyphenol-palmitate particles and citrus pectin by emulsion-templated method: Preparation, characterization and potential application. Food Hydrocoll. 2019, 95, 76–87. [Google Scholar] [CrossRef]
- Oh, I.; Lee, J.; Lee, H.G.; Lee, S. Feasibility of hydroxypropyl methylcellulose oleogel as an animal fat replacer for meat patties. Food Res. Int. 2019, 122, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Doescher, L.C.; Hoseney, R.C.; Milliken, G.A.; Rubenthaler, G.L. Effect of sugars and flours on cookie spread evaluated by time-lapse photography. Cereal Chem. 1987, 64, 163–167. [Google Scholar]
- Zhao, M.; Lan, Y.; Cui, L.; Monono, E.; Rao, J.; Chen, B. Physical properties and cookie-making performance of oleogels prepared with crude and refined soybean oil: A comparative study. Food Funct. 2020, 11, 2498–2508. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, J.; Lee, J.; Hwang, H.; Lee, S. Utilization of oleogels as a replacement for solid fat in aerated baked goods: Physicochemical, rheological, and tomographic characterization. J. Food Sci. 2017, 82, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yi, B.; Kim, M.; Lee, S.Y.; Lee, J. Physicochemical properties and oxidative stability of oleogels made of carnauba wax with canola oil or beeswax with grapeseed oil. Food Sci. Biotechnol. 2017, 26, 79–87. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Peroxide Value of Oils and Fats (965.33) Official Methods of Analysis; 2000 Methods Press: College Park, MD, USA, 1990; pp. 956–957. [Google Scholar]
- AACC American Association of Cereal Chemists. 2009 Methods and Additions; AACC Press: Saint Paul, MN, USA, 2000; pp. 10.02–52.02. [Google Scholar]
- Franco, D.; Martins, A.J.; López-Pedrouso, M.; Purriños, L.; Cerqueira, M.A.; Vicente, A.A.; Pastrana, L.M.; Zapata, C.; Lorenzo, J.M. Strategy towards replacing pork backfat with a linseed oleogel in frankfurter sausages and its evaluation on physicochemical, nutritional, and sensory characteristics. Foods 2019, 8, 366. [Google Scholar] [CrossRef] [Green Version]
| Fatty Acids | % of Total Fatty Acid |
|---|---|
| Lauric acid (c12:0) | 0.3 ± 0.0 |
| Myristic acid (c14:0) | 3.6 ± 0.0 |
| Palmitic acid (c16:0) | 17.3 ± 1.2 |
| Stearic acid (c18:0) | 2.5 ± 0.2 |
| Arachidic acid (c20:0) | 0.1 ± 0.0 |
| Saturated fatty acid content | 23.8 ± 1.3 |
| Palmitoleic acid (c16:1) | 2.3 ± 0.0 |
| Oleic acid (c18:1) | 46.1 ± 2.1 |
| Linolenic acid (c18:2) | 25.1 ± 0.5 |
| Linolenic acid(α) (c18:3) | 1.8 ± 0.2 |
| Gondoic acid (c20:1) | 0.5 ± 0.0 |
| Eicosadienic acid (c20:2) | 0.1 ± 0.0 |
| Unaturated fatty acid content | 73.6 ± 3.8 |
| CLW-O (1) | CBW-O (2) | BW-O (3) | |
|---|---|---|---|
| Hardness (N) | 2.65 ± 0.01 a | 1.21 ± 0.07 c | 2.20 ± 0.11 b |
| Oil binding capacity (%) | 94.18 ± 0.23 a | 92.94 ± 0.59 b | 93.18 ± 0.83 b |
| S-C (1) | CLW-OC | CBW-OC | BW-OC | ||
|---|---|---|---|---|---|
| Diameter (cm) | 10.46 ± 0.17 c | 10.69 ± 0.00 b | 10.63 ± 0.00 b | 10.82 ± 0.03 a | |
| Height (cm) | 1.30 ± 0.02 a | 1.16 ± 0.01 b | 1.31 ± 0.03 a | 1.13 ± 0.00 c | |
| Spread factor | 8.06 ± 0.16 c | 9.20 ± 0.14 b | 8.09 ± 0.17 c | 9.55 ± 0.12 a | |
| Snapping force (N) | 80.57 ± 2.99 a | 68.01 ± 3.59 b | 80.30 ± 2.32 a | 58.77 ± 2.28 c | |
| Hunter’s value | L* | 59.20 ± 1.79 a | 61.49 ± 1.76 a | 61.22 ± 1.99 a | 61.95 ± 1.94 a |
| a* | 11.58 ± 0.50 a | 10.30 ± 0.80 b | 10.69 ± 0.74 ab | 10.39 ± 0.65 b | |
| b* | 33.34 ± 0.74 a | 32.95 ± 0.57 a | 30.62 ± 0.49 b | 29.07 ± 0.70 c | |
| Chroma (C*) | 35.29 ± 0.54 a | 34.53 ± 0.39 b | 32.44 ± 0.29 c | 30.87 ± 0.45 d | |
| Hue angel (H°) | 70.83 ± 1.16 b | 73.91 ± 0.24 a | 70.75 ± 1.48 b | 70.32 ± 1.56 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Oh, I. The Characteristic of Insect Oil for a Potential Component of Oleogel and Its Application as a Solid Fat Replacer in Cookies. Gels 2022, 8, 355. https://doi.org/10.3390/gels8060355
Kim D, Oh I. The Characteristic of Insect Oil for a Potential Component of Oleogel and Its Application as a Solid Fat Replacer in Cookies. Gels. 2022; 8(6):355. https://doi.org/10.3390/gels8060355
Chicago/Turabian StyleKim, Doyoung, and Imkyung Oh. 2022. "The Characteristic of Insect Oil for a Potential Component of Oleogel and Its Application as a Solid Fat Replacer in Cookies" Gels 8, no. 6: 355. https://doi.org/10.3390/gels8060355
APA StyleKim, D., & Oh, I. (2022). The Characteristic of Insect Oil for a Potential Component of Oleogel and Its Application as a Solid Fat Replacer in Cookies. Gels, 8(6), 355. https://doi.org/10.3390/gels8060355