Supramolecular Rings as Building Blocks for Stimuli-Responsive Materials
Abstract
:1. Introduction
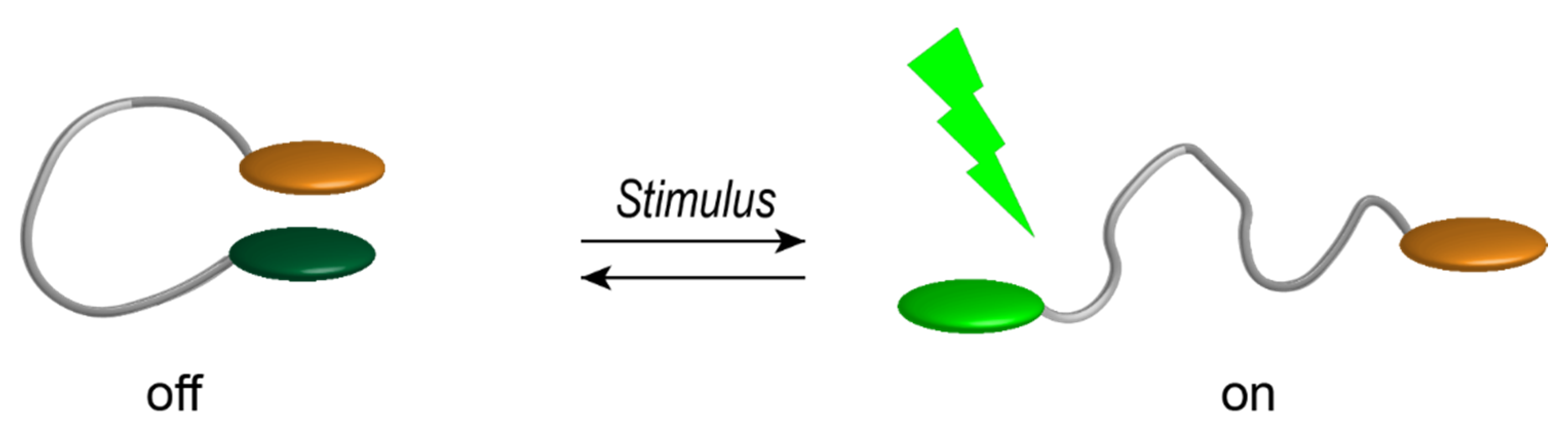
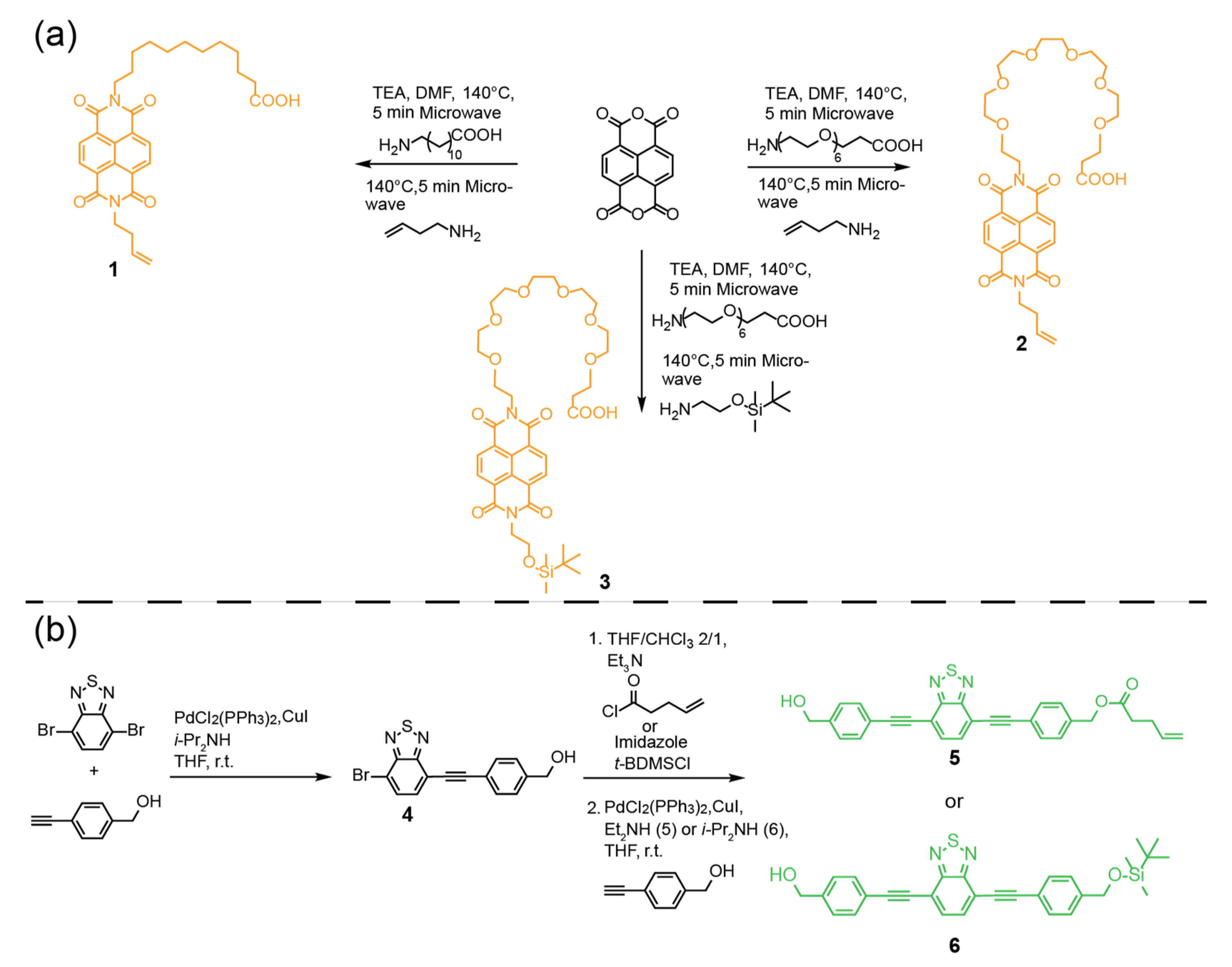
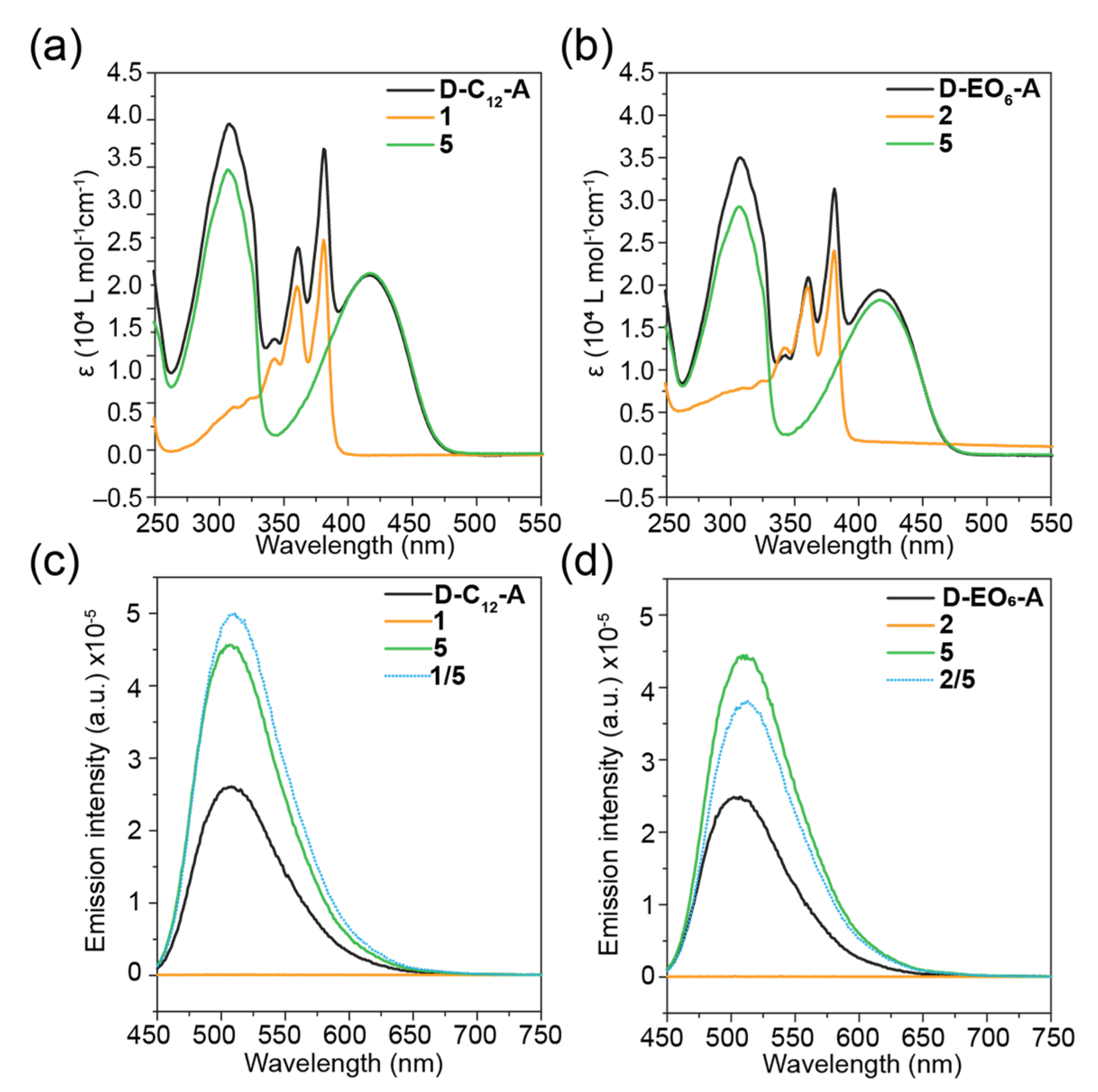
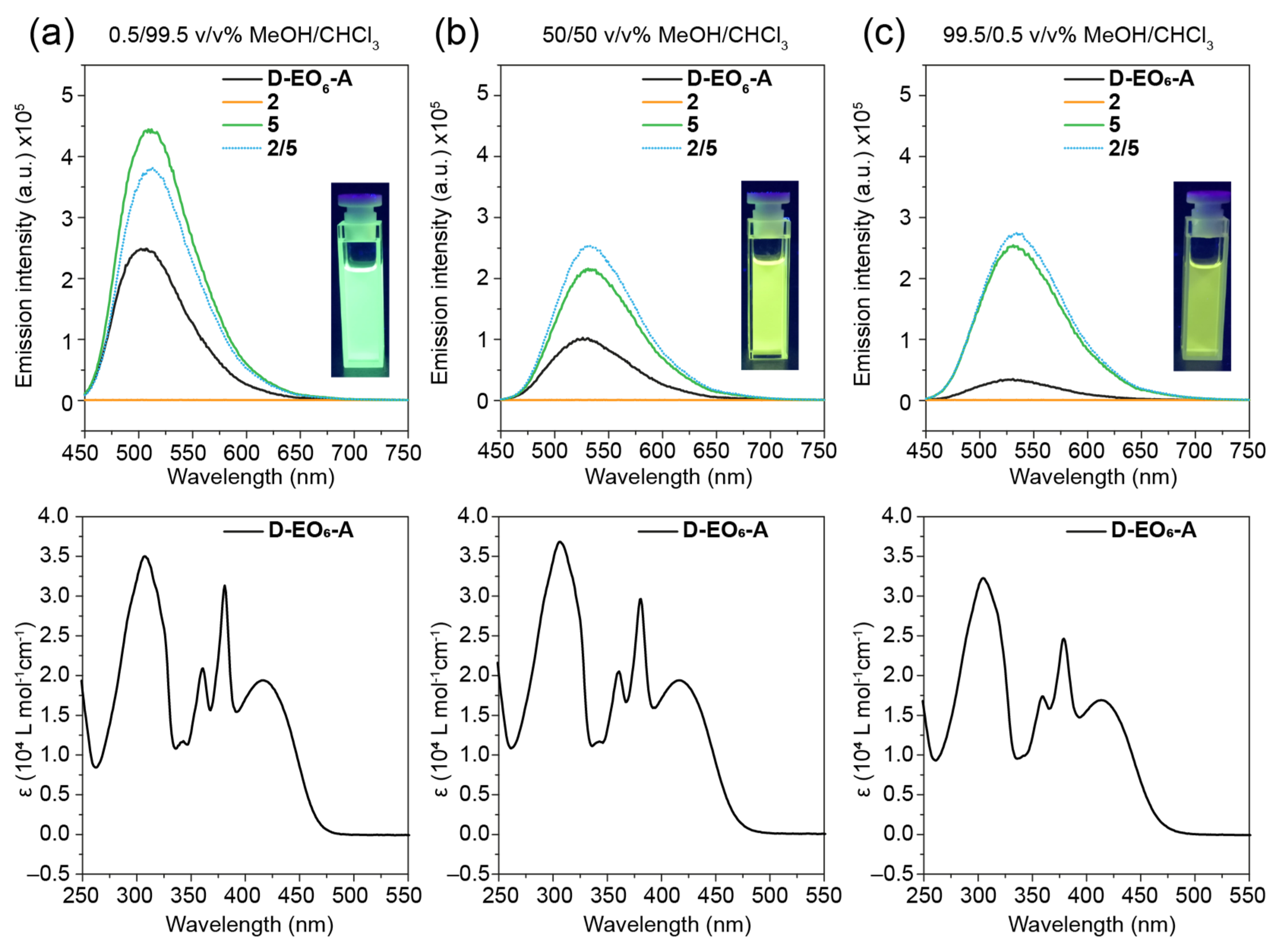
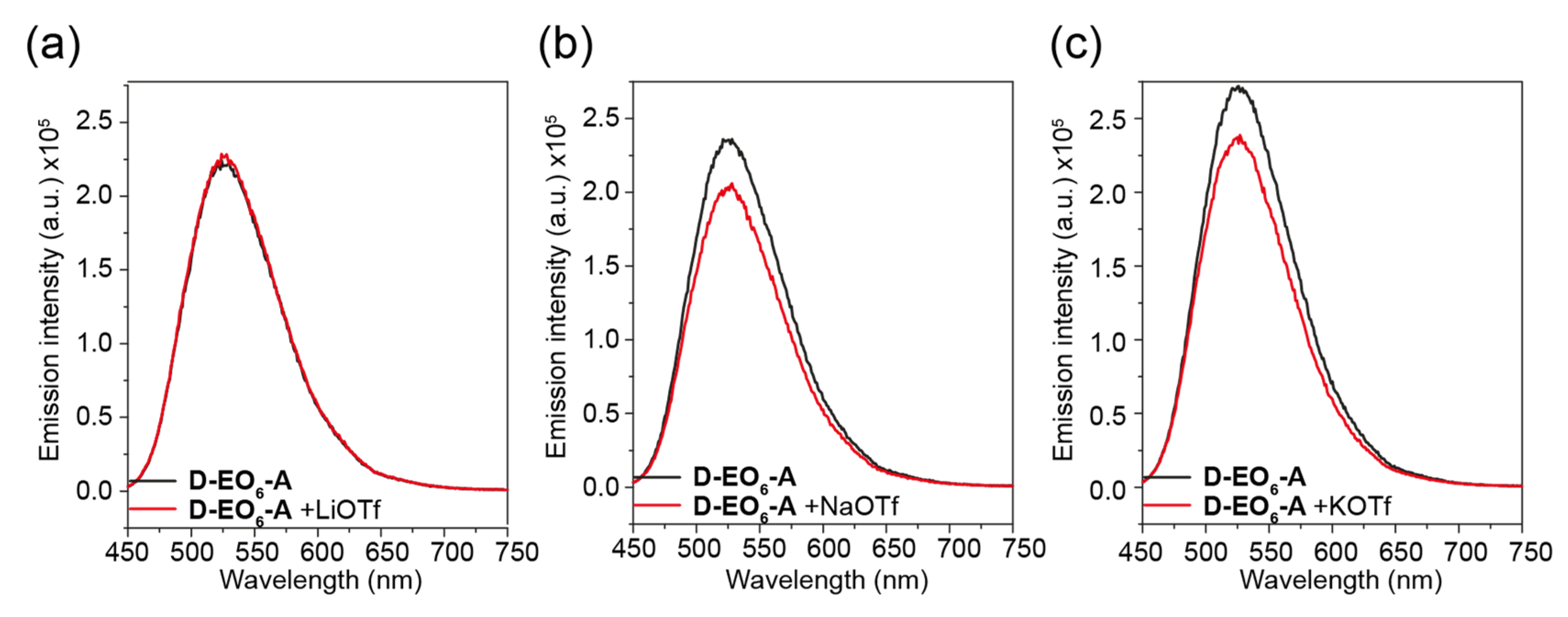
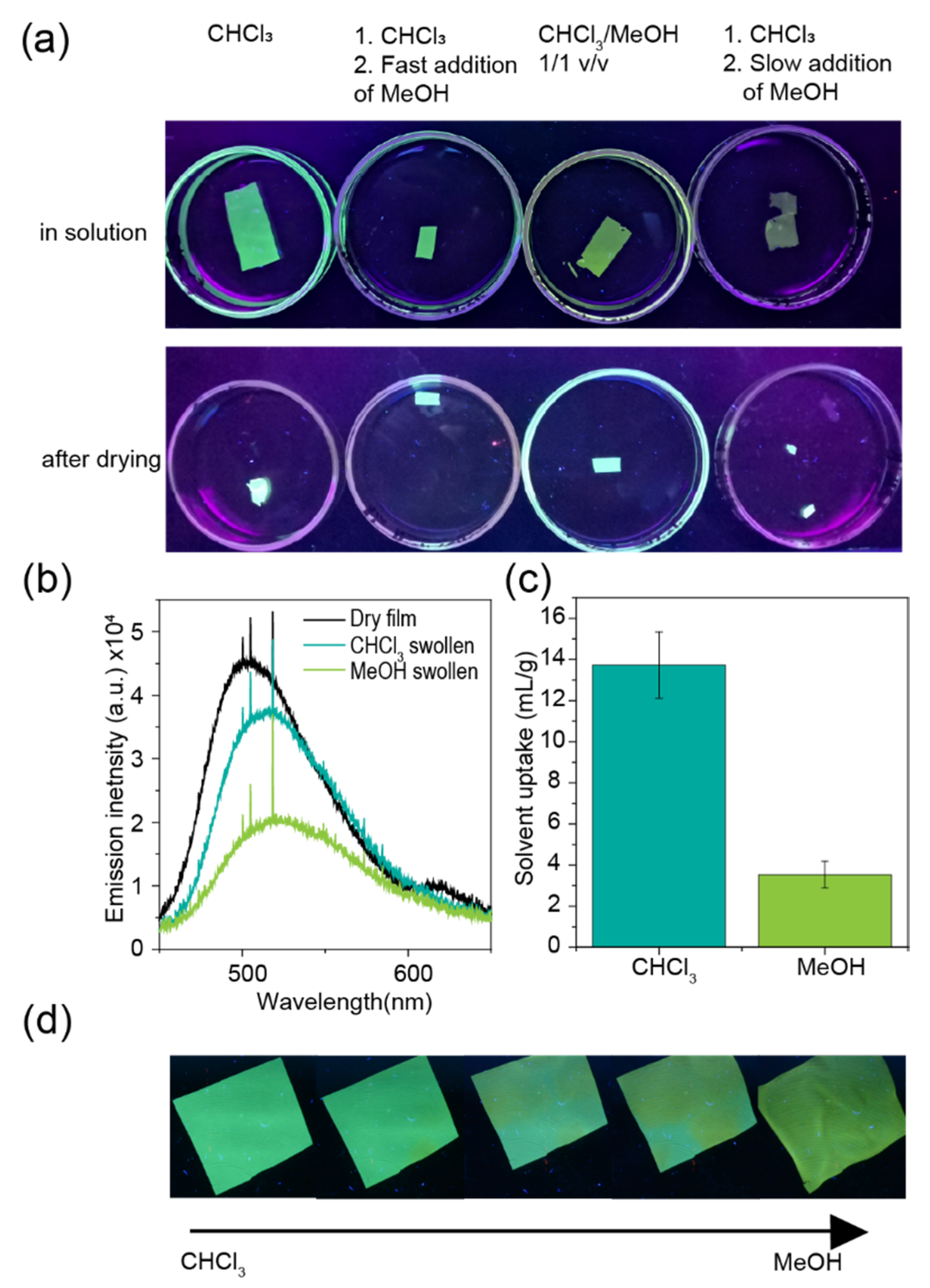
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Nuclear Magnetic Resonance (NMR)
4.3. Mass Spectrometry (MS)
4.4. UV-Vis Absorption Spectroscopy
4.5. Fluorescence Spectroscopy
4.6. Differential Scanning Calorimetry (DSC)
4.7. Thermogravimetric Analysis (TGA)
4.8. Photographs
4.9. Flash Column Chromatography
4.10. Microwave Reactor
4.11. Size-Exclusion Chromatography (SEC)
4.12. Protocol for the Preparation of PU-D/A Films
4.13. Emission Spectra of Polymer Films
4.14. Swelling Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pernici, B.D.T.S.; Colosimo, B.M.; Faravelli, T.; Paolucci, R.; Piardi, S. Materials That Change Color; Springer: Heidelberg, Germany, 2014. [Google Scholar]
- Makowski, B.; Kunzelman, J.; Weder, C. Stimuli-Driven Assembly of Chromogenic Dye Molecules: A Versatile Approach for the Design of Responsive Polymers. In Handbook of Stimuli-Responsive Materials; Urban, M.W., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 117–138. [Google Scholar]
- Traeger, H.; Kiebala, D.J.; Weder, C.; Schrettl, S. From Molecules to Polymers—Harnessing Inter- and Intramolecular Interactions to Create Mechanochromic Materials. Macromol. Rapid Commun. 2021, 42, 2000573. [Google Scholar] [CrossRef] [PubMed]
- Calvino, C.; Neumann, L.; Weder, C.; Schrettl, S. Approaches to polymeric mechanochromic materials. J. Polym. Sci. A Polym. Chem. 2017, 55, 640–652. [Google Scholar] [CrossRef]
- Liu, H.; Wei, S.; Qiu, H.; Si, M.; Lin, G.; Lei, Z.; Lu, W.; Zhou, L.; Chen, T. Supramolecular Hydrogel with Orthogonally Responsive R/G/B Fluorophores Enables Multi-Color Switchable Biomimetic Soft Skins. Adv. Funct. Mater. 2022, 32, 2108830. [Google Scholar] [CrossRef]
- Xu, J.; Luo, Y.; Chen, Y.; Guo, Z.; Zhang, Y.; Xie, S.; Li, N.; Xu, L. Tough, Self-Recoverable, Spiropyran (SP3) Bearing Polymer Beads Incorporated PAM Hydrogels with Sole Mechanochromic Behavior. Gels 2022, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Löwe, C.; Weder, C. Oligo(p-phenylene vinylene) Excimers as Molecular Probes: Deformation-Induced Color Changes in Photoluminescent Polymer Blends. Adv. Mater. 2002, 14, 1625–1629. [Google Scholar] [CrossRef]
- Sagara, Y.; Traeger, H.; Li, J.; Okado, Y.; Schrettl, S.; Tamaoki, N.; Weder, C. Mechanically Responsive Luminescent Polymers Based on Supramolecular Cyclophane Mechanophores. J. Am. Chem. Soc. 2021, 143, 5519–5525. [Google Scholar] [CrossRef] [PubMed]
- Creusen, G.; Schmidt, R.S.; Walther, A. One-Component DNA Mechanoprobes for Facile Mechanosensing in Photopolymerized Hydrogels and Elastomers. ACS Macro Lett. 2021, 10, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Imato, K.; Yamanaka, R.; Nakajima, H.; Takeda, N. Fluorescent supramolecular mechanophores based on charge-transfer interactions. Chem. Commun. 2020, 56, 7937–7940. [Google Scholar] [CrossRef]
- Sagara, Y.; Karman, M.; Verde-Sesto, E.; Matsuo, K.; Kim, Y.; Tamaoki, N.; Weder, C. Rotaxanes as Mechanochromic Fluorescent Force Transducers in Polymers. J. Am. Chem. Soc. 2018, 140, 1584–1587. [Google Scholar] [CrossRef] [Green Version]
- Crenshaw, B.R.; Weder, C. Phase Separation of Excimer-Forming Fluorescent Dyes and Amorphous Polymers: A Versatile Mechanism for Sensor Applications. Adv. Mater. 2005, 17, 1471–1476. [Google Scholar] [CrossRef]
- Kunzelman, J.; Crenshaw, B.R.; Weder, C. Self-assembly of chromogenic dyes—A new mechanism for humidity sensors. J. Mater. Chem. 2007, 17, 2989–2991. [Google Scholar] [CrossRef]
- Tang, L.; Whalen, J.; Schutte, G.; Weder, C. Stimuli-Responsive Epoxy Coatings. ACS Appl. Mater. Interfaces 2009, 1, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Karman, M.; Seki, A.; Pannipara, M.; Tamaoki, N.; Weder, C. Rotaxane-Based Mechanophores Enable Polymers with Mechanically Switchable White Photoluminescence. ACS Cent. Sci. 2019, 5, 874–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muramatsu, T.; Sagara, Y.; Traeger, H.; Tamaoki, N.; Weder, C. Mechanoresponsive Behavior of a Polymer-Embedded Red-Light Emitting Rotaxane Mechanophore. ACS Appl. Mater. Interfaces 2019, 11, 24571–24576. [Google Scholar] [CrossRef]
- Muramatsu, T.; Okado, Y.; Traeger, H.; Schrettl, S.; Tamaoki, N.; Weder, C.; Sagara, Y. Rotaxane-Based Dual Function Mechanophores Exhibiting Reversible and Irreversible Responses. J. Am. Chem. Soc. 2021, 143, 9884–9892. [Google Scholar] [CrossRef]
- Merindol, R.; Delechiave, G.; Heinen, L.; Catalani, L.H.; Walther, A. Modular Design of Programmable Mechanofluorescent DNA Hydrogels. Nat. Commun. 2019, 10, 528. [Google Scholar] [CrossRef]
- Calvino, C.; Guha, A.; Weder, C.; Schrettl, S. Self-Calibrating Mechanochromic Fluorescent Polymers Based on Encapsulated Excimer-Forming Dyes. Adv. Mater. 2018, 30, 1704603. [Google Scholar] [CrossRef]
- Crenshaw, B.R.; Weder, C. Self-Assessing Photoluminescent Polyurethanes. Macromolecules 2006, 39, 9581–9589. [Google Scholar] [CrossRef]
- Lavrenova, A.; Balkenende, D.W.R.; Sagara, Y.; Schrettl, S.; Simon, Y.C.; Weder, C. Mechano- and Thermoresponsive Photoluminescent Supramolecular Polymer. J. Am. Chem. Soc. 2017, 139, 4302–4305. [Google Scholar] [CrossRef] [Green Version]
- Kiebala, D.J.; Fan, Z.; Calvino, C.; Fehlmann, L.; Schrettl, S.; Weder, C. Mechanoresponsive Elastomers Made with Excimer-Forming Telechelics. Org. Mater. 2020, 02, 313–322. [Google Scholar] [CrossRef]
- Davis, D.A.; Hamilton, A.; Yang, J.; Cremar, L.D.; Van Gough, D.; Potisek, S.L.; Ong, M.T.; Braun, P.V.; Martínez, T.J.; White, S.R.; et al. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 2009, 459, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Patrick, J.F.; Robb, M.J.; Sottos, N.R.; Moore, J.S.; White, S.R. Polymers with autonomous life-cycle control. Nature 2016, 540, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Traeger, H.; Sagara, Y.; Kiebala, D.J.; Schrettl, S.; Weder, C. Folded Perylene Diimide Loops as Mechanoresponsive Motifs. Angew. Chem. Int. Ed. 2021, 60, 16191–16199. [Google Scholar] [CrossRef] [PubMed]
- Traeger, H.; Sagara, Y.; Berrocal, J.A.; Schrettl, S.; Weder, C. Strain-correlated mechanochromism in different polyurethanes featuring a supramolecular mechanophore. Polym. Chem. 2022, 13, 2860–2869. [Google Scholar] [CrossRef]
- Chen, J.; Ziegler, A.W.; Zhao, B.; Wan, W.; Li, A.D.Q. Chemomechanical-force-induced folding–unfolding directly controls distinct fluorescence dual-color switching. Chem. Commun. 2017, 53, 4993–4996. [Google Scholar] [CrossRef]
- Tambara, K.; Ponnuswamy, N.; Hennrich, G.; Pantoş, G.D. Microwave-Assisted Synthesis of Naphthalenemonoimides and N-Desymmetrized Naphthalenediimides. J. Org. Chem. 2011, 76, 3338–3347. [Google Scholar] [CrossRef]
- Pengo, P.; Pantoş, G.D.; Otto, S.; Sanders, J.K.M. Efficient and Mild Microwave-Assisted Stepwise Functionalization of Naphthalenediimide with α-Amino Acids. J. Org. Chem. 2006, 71, 7063–7066. [Google Scholar] [CrossRef] [Green Version]
- Neto, B.A.D.; Lapis, A.A.M.; Mancilha, F.S.; Vasconcelos, I.B.; Thum, C.; Basso, L.A.; Santos, D.S.; Dupont, J. New Sensitive Fluorophores for Selective DNA Detection. Org. Lett. 2007, 9, 4001–4004. [Google Scholar] [CrossRef]
- Neto, B.A.D.N.; Brenno, A.D.; da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2013, 2013, 228–255. [Google Scholar] [CrossRef]
- Holleman, A.; Wiberg, N. Holleman-Wiberg Lehrbuch der Anorganischen Chemie. 101. Auflage; Walter de Gruyter: Berlin, Germany, 1995. [Google Scholar]
- Valente, M.; Sousa, S.F.; Lopes Magalhães, A.; Freire, C. Crown-Ether Type Podands as Alkali Metal Cation Extractants: Influence of the Number of Oxygens in the Chain. J. Solut. Chem. 2010, 39, 1230–1242. [Google Scholar] [CrossRef]
- Ghosh, S.; Ramakrishnan, S. Aromatic Donor–Acceptor Charge-Transfer and Metal-Ion-Complexation-Assisted Folding of a Synthetic Polymer. Angew. Chem. Int. Ed. 2004, 43, 3264–3268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löhr, H.-G.; Vögtle, F. Chromoionophore, VII. Podanden mit Elektronen-Donor- und -Acceptor-Endgruppen und kationgesteuerter Charge-Transfer-Absorption. Ber. Dtsch. Chem. Ges. 1985, 118, 914–921. [Google Scholar] [CrossRef]
- Moore, J.S.; Stupp, S.I. Room temperature polyesterification. Macromolecules 1990, 23, 65–70. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Biswal, A.K.; Chang, Y.-H.; Misra, P.K.; Yang, J.-M. Synthesis and Characterization of Modified Poly(vinyl alcohol) Membrane and Study of Its Enhanced Water-Induced Shape-Memory Behavior. J. Polym. Environ. 2022. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traeger, H.; Ghielmetti, A.; Sagara, Y.; Schrettl, S.; Weder, C. Supramolecular Rings as Building Blocks for Stimuli-Responsive Materials. Gels 2022, 8, 350. https://doi.org/10.3390/gels8060350
Traeger H, Ghielmetti A, Sagara Y, Schrettl S, Weder C. Supramolecular Rings as Building Blocks for Stimuli-Responsive Materials. Gels. 2022; 8(6):350. https://doi.org/10.3390/gels8060350
Chicago/Turabian StyleTraeger, Hanna, Alyssa Ghielmetti, Yoshimitsu Sagara, Stephen Schrettl, and Christoph Weder. 2022. "Supramolecular Rings as Building Blocks for Stimuli-Responsive Materials" Gels 8, no. 6: 350. https://doi.org/10.3390/gels8060350
APA StyleTraeger, H., Ghielmetti, A., Sagara, Y., Schrettl, S., & Weder, C. (2022). Supramolecular Rings as Building Blocks for Stimuli-Responsive Materials. Gels, 8(6), 350. https://doi.org/10.3390/gels8060350






