Abstract
Metallic materials such as stainless steel (SS), titanium (Ti), magnesium (Mg) alloys, and cobalt-chromium (Co-Cr) alloys are widely used as biomaterials for implant applications. Metallic implants sometimes fail in surgeries due to inadequate biocompatibility, faster degradation rate (Mg-based alloys), inflammatory response, infections, inertness (SS, Ti, and Co-Cr alloys), lower corrosion resistance, elastic modulus mismatch, excessive wear, and shielding stress. Therefore, to address this problem, it is necessary to develop a method to improve the biofunctionalization of metallic implant surfaces by changing the materials’ surface and morphology without altering the mechanical properties of metallic implants. Among various methods, surface modification on metallic surfaces by applying coatings is an effective way to improve implant material performance. In this review, we discuss the recent developments in ceramics, polymers, and metallic materials used for implant applications. Their biocompatibility is also discussed. The recent trends in coatings for biomedical implants, applications, and their future directions were also discussed in detail.
1. Introduction
Bioimplants are defined as engineered medical devices that are developed to replace the non-functional or broken biological structural parts of the human body, providing support to the given host. Biomaterial surface modification plays a key role in determining the outcome of the interaction between human biology and materials. Substantial development in research in the field of biomaterials has increased the scope of use for a wide range of orthopedic and dental implants that include total bone replacement, fracture fixation, dental screws, joint arthrodesis, and so on [1]. Essentially, the success of bioimplants depends not only on their bulk properties but also on the properties of their surfaces, which interact with human body tissues. As a result, the evolution of bioimplants has reached a level of choice of materials based on specific properties on the basis of selected specific materials [2]. Though alloys and metallic substances meet many of the biomedical requirements, their interfacial bonding between the surrounding tissue or bone and the metallic surface ranges from poor to virtually absent. The failure of the metallic implant originates at the implant-tissue interface due to poor bonding at the interface, which leads to the formation of a nonadherent layer and movement at the tissue-implant interface [3].
Corrosion in biometallic implants can affect the surface and biocompatible behavior that induce tissue reactions, which lead to the release of corrosion byproducts from the implant surface and result in premature failure. A minimum durability of 15 to 20 years for older patients and more than 20 years for younger patients is expected from a bioimplant [4]. However, there are problems associated with the use of metallic implants due to the lack of poor implant fixation, lack of osteoconductivity, corrosion, and wear resistance leading to the formation of wear debris and release of corrosive ions [5,6,7]. These problems are mostly associated with the surface of the metallic implants. In view of this, the surface of the bioimplant plays a major role in the biological environment because the reactions occur directly on the surface of the implant after implant fixation. Hence, it is necessary to modify the surface of the metallic substrate with specific properties that are different from those in bulk [8,9]. This modification is required to accomplish good bone formability and desired biological interactions. In some applications, biocompatibility, wear, and corrosion resistance are also required.
Surface modifications of bioimplants are explored intensively with many bioactive materials to avoid adverse effects such as lack of biocompatibility, post-surgery infections, long-term survivability, and risks related to implant surface corrosion [10,11]. At first, the research in this field was focused on the improvement in biomechanical properties of metallic implants, but in recent days, it has turned towards improvement in the biological properties of these biomedical devices [12,13]. By applying the appropriate modification on the surface of the material, one can tailor and improve the biocompatibility, cell interactions, and adhesion [14]. Thus, the development and design of biomaterials rely on surface modification. For that, it is necessary to develop techniques for functionalization of the surface of metallic implants through changing the materials’ surface composition, morphology, and structure without losing their mechanical properties. By adopting this, the service life and performance of orthopedic and dental implants can be significantly increased. This can be achieved by applying suitable biocompatible coatings with a unique combination of properties.
In view of reliability and performance, the best way to functionalize the implants in direct contact with bones and tissues is ceramic coatings owing to their excellent osteoconductive properties and high stability [15,16]. Surface modification by coating can enhance the antibacterial activity of a bioimplant. The coated surfaces facilitate grafting of cell-binding peptides, directed mutations of the cellular host, protein of extracellular matrix (ECM), and growth of tissues to improve the acceptance of a bioimplant further. Ceramic coatings on bioimplants show promising results in orthopedics with improved bone regeneration and repair [17]. The overview of applications of ceramic coatings used for metallic implants is listed in Table 1.

Table 1.
Ceramic coatings used for biomedical applications [18].
The major requirements for the selection of coating materials are (a) biocompatibility and nondetrimental effects such as allergy, inflammation, and toxicity, (b) adequate fracture toughness, fatigue, and mechanical strength to withstand the forces, and (c) resistance to corrosion in the human body fluid atmosphere, which contains many constituents such as amino acids, chlorine, water, proteins, sodium, and plasma acids. The choice of coatings, by considering their degeneration and surface properties, plays a major role in terms of reliability and performance of bioimplants. The coatings for biomedical applications can be subdivided into three groups: (a) bioinert, (b) bioactive, and (c) bioresorbable coatings [19]. The coatings having a minimum interaction with the surrounding tissues after implantation in the human body are considered as bioinert coatings. The typical examples of bioinert coatings are metal oxides, nitrides, carbides, carbonitrides, and oxynitrides. Transition metal nitrides (TiN, ZrN, TiAlN, NbN), carbides (TiC), oxides (ZrO2, Al2O3, TiO2), or oxynitride (TiON) coatings find a wide range of applications in bioceramic coatings due to their remarkable properties such as wear, tear, hardness, biocompatibility, and corrosion resistance [20,21].
The current review incorporates a description of the biomaterials and coatings that are commonly used in the manufacturing of different orthopedic and dental implants.
2. Biomaterials for Biomedical Applications
Biomaterials are used to make devices that interact with the biological systems in the human body and coexist for a long time with minimal failure. The type of material used in implant applications shows specific properties that make them primary candidates for specific applications. The key requirements for the selection of biometallic materials consist of (a) cost effectiveness, (b) mechanical behavior equal to that of the human skull and bones, and (c) their biocompatibility [22,23]. In addition, the major requirement for the bioimplant materials is that it should be compatible with the human body, i.e., it should integrate with the human body without negative impacts. Moreover, it must possess corrosion and wear resistance in the human body environment. These properties will determine the effectiveness of the implant materials.
If a metallic material experiences wear and corrosion, the surrounding tissues present at the implant area can become inflamed, causing unfavorable biological reactions within the human body [24]. The ions and toxins released from the metallic substrates as a byproduct may be potentially harmful and can cause life threatening diseases and increase the risk of using metallic implants. Therefore, it is important to choose correct material for correct applications while performing bioimplants. In addition to that, the mechanical performance of the biomaterial should be close to that of the replacing material where it must sustain complicated and varying mechanical loading cycles [25]. Typical examples for implanting areas are teeth, knee joints, and hips. The selection of biomaterial based on mechanical properties is important to ensure no implant failures within the body when subjected to numerous loading cycles during service life. Moreover, the material should be biocompatible with the surrounding tissues and economically viable. Finally, it is essential that the choice of material should be cost effective, efficient, and able to integrate with the human body. Based on the requirements defined above, several materials were developed in recent years to be used as biomaterials for implant applications. Still, it is hard for a single metallic material to fulfill the desired properties. Biomaterials used for biomedical applications are broadly classified into ceramics, polymers, and metallic systems.
2.1. Ceramics
Ceramics are inorganic compounds formed at high temperatures. Typical examples are bioactive glass (BG), zirconium oxide (ZrO2), aluminum oxide (Al2O3), hydroxyapatite (HAp), and other calcium and silica-based ceramics. These ceramics are noted for their great biocompatibility, which makes them an excellent candidate for biomedical implant applications. Depending on the reactivity with the human body, ceramic implants are classified into three categories: (a) bioactive, (b) bioinert, and (c) bioresorbable ceramics [26]. Bioactive ceramics are used to interact with the surrounding cells and exhibit a higher level of reactivity within the implant sites. Typical examples for bioactive ceramics are HA and fluorapatites [27]. In an opposite trend, bioinert ceramics do not show any reactivity with the host tissues at the implant sites but form a physical bonding when implanted [28]. Bioresorbable ceramics exhibit a low level of reactivity with the host body tissues [29]. After implantation, these ceramics are gradually resorbed and finally replaced with the bone tissue. These bioresorbable ceramics are widely used in orthopedics and dentistry due to their better biocompatibility and chemical interactions [30].
No risk of transmitting disease plus immunogenicity after implantation are the major advantages of the ceramics [31]. Other remarkable advantages are higher resistance to compressive force, low toxicity, good corrosion resistance, and promotion of the formation of new hard tissues. For example, hydroxyapatite-based ceramics exhibit higher Ca/P ratios, which are desirable due to similar chemical properties of bone and teeth hard tissues [32,33,34,35]. Due to these attractive properties, ceramics are increasingly utilized for bioimplant applications.
Ceramics are known for their high hardness and stress-shielding effects due to their high elastic moduli, and slow initiation of crack growth over time, which significantly decrease the reliability of the implants [36]. In addition, brittleness, fracture toughness, and fabrication issues limit their use as bioimplants. The ceramics share the brittleness factor, which limits the performance in terms of load-bearing applications (hip implants). If the difference in mechanical properties of ceramic and bone is large, the load will not be transmitted through the bone, thus leading to failure of the bone [37].
Ceramic composite materials provide superior properties compared to single materials. The inferior mechanical properties of monolithic ceramics can be overcome by composite ceramics while diminishing the limitations of each component. The remarkable properties of composites such as the weight to strength ratio enable them to be used extensively for the restoration of bones, ligaments, and dental fillings [38]. Moreover, the composites prepared through the combination of bioactive and bioinert ceramics show better bioactivity and mechanical strength [39]. Typical examples are HA and Al2O3 composites which show better osteointegration with bone, good bioactivity, and high yield strength [40,41].
2.2. Polymers
The most widely used materials in biomedical applications are polymers. Polymers are the building blocks of small repeating units’ monomers and are classified into two categories called biodegradable and non-biodegradable. Typical examples for biodegradable polymers are polyacetal, chitosan (CS), alginate, polylactide, and polycaprolactone, whereas non-biodegradable polymers include polypropylene, polytetrafluoroethylene, polyethylene terephthalate, polymethylmethacrylate, etc. Polymer implants are mostly used in replacing heart valves, kidneys, bone, skin, contact lens, and artificial blood vessels, in addition as pacemakers [42]. Among biodegradable polymers, CS shows remarkable properties such as biocompatibility, biodegradability, wound healing, and antibacterial activity [43]. It is also environmentally friendly and hence acts as a capping agent [44,45]. Polymers show lower strength and elastic moduli as compared to metals and ceramics. Therefore, they are not generally used for load-bearing applications such as joint and knee prostheses. The polymers are also degraded in the body environment due to biochemical factors.
Polymer implants are quite interesting as bioimplants due to their low cost while offering sufficient mechanical properties. For example, Polyether ether ketone (PEEK), composed of 20% TiO2 particles and an additional ketone group results in 80% higher compressive strength and better fatigue properties than pure PEEK [46]. Depending upon the replacement anatomy to which the polymer is being applied, a wide variety of polymers can be applied. Polymers have the advantage of complete degradation over time, leaving no signs of their presence at the implant locations in a body. This was possible with the subsequent research and development in biodegradable polymer materials, where the proteins and extracellular matrix mimic the cell signaling functions of the surrounding tissue, permitting better bio-integration [47].
Though polymers show exceptional properties and are cost-efficient and easy to manufacture, they show different forms of cytotoxicity: depending on the host body conditions, inflammatory reactions can occur within the implant region. This will induce bone degeneration, abnormalities, rapid rate of corrosion, and decreases in mechanical properties over time. Moreover, the elastic modulus of polymers is extremely low compared to human bone (between 10 and 30 GPa) [48]. This will create an impact while applying load. Another major issue that is being faced is that the polymer implant degrades as the bone heals. If the process is too fast, the neighboring tissues feel more stress, which causes potential discomfort. These limitations prevent them from being widely used as bioimplants.
Polymeric Gels
Natural polymers such as collagen are the main components of natural bone due to their hydrophilic nature, enabling the formation of hydrogels with aqueous solutions that exhibit several desirable characteristics for bone-tissue engineering [49]. Polymeric gels are often referred to as hydrogels owing to their ability to hold water inside their networks [50]. These hydrogels swell upon water intake and shrink upon drying [51]. Taking advantage of this property, water soluble drugs, growth factors, and other biological entities such as proteins and even live cells can be incorporated into these hydrogels [52]. These gels can be designed for delivery systems based on certain external stimuli such as pH [53,54], temperature, or the presence of specific chemicals or target molecules [55]. Many researchers choose collagen because it is the most important organic component of human bone [56,57,58].
Hydrogels are attractive soft biomaterials because of their soft consistency (stiffness and viscoelasticity are essential in directing the immune response), high water content, porosity, and biocompatibility [59]. They are widely used in 3D cell cultures for modeling the biological extracellular matrix or as coatings for promoting cell attachment. Other natural polymer-based hydrogels used as bone tissue engineering (BTE) materials include polysaccharides (e.g., cellulose) and polypeptides (e.g., alginate). Compared with natural polymeric gels, synthetic polymeric gels offer more possibilities for molecular alterations that facilitate tailoring the candidate properties to specific requirements, i.e., tuning mechanical properties and biophysical and biochemical cues. For instance, Poly(ethylene glycol) (PEG) hydrogels, modified with adhesion ligand arginine–glycine–aspartic acid (RGD), offer tunable mechanical properties as well as improved cell attachment and cell differentiation [60]. However, generally, the poor mechanical strength of hydrogels limits their usage and needs further improvement for bone regeneration. Recent emerging technologies such as 3D printing in the manufacturing of hydrogel-based components may offer entirely new possibilities for addressing the challenges [61].
2.3. Metals and Alloys
Even though ceramics show excellent biocompatible performance, they have poor fracture toughness and exhibit brittle behavior, and their use in load-bearing applications is limited. Thus, metals and alloys are generally used for implants where high strength and load-bearing capacity are required. Most medical industrial segments rely on metallic implants. They are generally used to replace some load-bearing applications such as the hip, plates, knee prostheses, pins, dental materials, screws, and cardiovascular applications [62]. Though metals show high strength and durability, they can lose their properties under physiological conditions with a potential release of various ions and debris which may trigger a biological response. Most of the alloys release metal ions to the plasma in the blood [63]. The excessive release of ions in the blood has a high risk of accumulation in organs such as the spleen and liver that later form particulates, affecting the normal functioning of these organs. This phenomenon leads to cytotoxicity followed by organ failure upon prolonged accumulation.
Metallic materials are not fully accepted by the human body, and the tissue growth is impaired because of inadequate attachment of the implant, leading to discomfort or pain in the implant region [64]. As compared to ceramic materials, the risk of infection is higher, and the healing time is slower in the case of metallic implants. Although metallic implants have some limitations, preference should be given based on their corrosion resistance, cost effectiveness, and mechanical strength. The chemically inert platinum and gold do not show any corrosion in situ, and these materials can be used as bioimplants, but they are expensive. Hence, recent biomedical industries use Ti-based alloys and Mg-based alloys due to their better biocompatibility and good mechanical strength under human body conditions [65]. The widely used metallic materials used as biomedical devices are stainless steel and Ti- and Co-based alloys [66,67].
2.3.1. Stainless Steels (SS)
In India, SS 304 and 316L are the most used implant materials for biomedical applications due to their cost effectiveness, wide resource availability, reliability, and ease of fabrication as compared to Ti- and Co-based alloys. Among various grades of SS, the primary recommended grade for implant applications is AISI type 316L SS. The presence of chromium (minimum content of 10.5 wt. %) yields a thin and passive oxide layer and protects the implant surface against corrosion [68]. The presence of carbon (min. 0.03 wt. %) in SS increases its mechanical properties, especially fracture toughness, corrosion resistance, and tribological performance of the implants. Their load-bearing capability makes them a suitable orthopedic implant material [69]. However, almost 90% of 316L grade SS implants lose their properties due to a pitting corrosion attack and the release of nickel and chromium ions, which cause allergic reactions in the implant region. Hence, a small addition of molybdenum (2 to 4 wt. %) improves the corrosion resistance and strengthens the 316L SS grade.
The 316L SS used in biomedical devices is classified into two categories: (a) conventional SS and (b) Ni-free stainless steels [70]. The primary use of conventional stainless steels is to provide a load-bearing property to the implanted surfaces: they are often used as fracture plates, nails, screws, and stents in the implant process. In addition, the Ni-free SS provides higher corrosion resistance and biocompatibility [71]. When compared to other bioimplants, the chemical composition of SS alloys offers an advantage when good mechanical properties are desired. Moreover, they have a high cost-to-benefit ratio and exhibit a linear relationship with the manufacturing processes and final structure/properties.
Its elastic modulus (200 GPa), which is higher than that of the human bone (10–30 GPa), results in high stress-shielding effect at the tissue/implant interface leading to the failure of the implanted SS [72,73,74]. In recent days, SS was modified with hydroxyapatite (HAp) which improves its bio-integration and osteointegration properties. Typical implanted materials are screws, pins, sutures, bone plates, steel threads, and medullary nails, which are used in fracture fixation. However, the corrosion resistance, biocompatibility, and osseointegration of SS are lower compared to Ti-based alloys, where implant success rates are much higher [75].
2.3.2. Co-Cr Alloys
Co-based alloys are considered as one of the most successful materials used for implant applications. This alloy was first used in the early 1900s, where it was used as an implant material for hip replacement. Co-based alloys show better corrosion, wear, and mechanical properties and are used in bioimplant applications. The in vivo and in vitro studies confirmed that Co-based alloys show better biocompatibility and can be used for the manufacturing of surgical implants such as in the hip, knee, shoulder, and fractured bone surfaces [76,77]. The most widely used combination of Co alloys are Co-Cr-Mo owing to their unique combination of strength and ductility. By comparing with other metallic implants, this alloy shows a better elastic modulus, density as well as stiffness, becoming an ideal material for the implant process [78]. This alloy is primarily focused on permanent implant fixation procedures because these alloys maintain their initial properties for a long time after implantation. The cumulative likelihood of endurance reached 96% at 12 years for patients aged above 60 years [79]. A Co-Cr-Mo alloy combined with ultra-high molecular weight polyethylene (UHMWPE) is used in artificial ankles and knees [80,81].
Other major alloying elements of Co-based alloys include Ni, Mo, and Cr. These elements were proven to be toxic to the human body when leached out from the metal surface to the body fluid during corrosion of Co alloys and can lead to skin-related diseases. An excessive leaching of these trace elements leads to damage to organs such as the liver, kidney, blood cells, and lungs [82,83]. The addition of nickel into Co-Cr-Mo improves corrosion resistance and mechanical properties, but due to the cytotoxicity of Ni, the use of this alloy in bioimplants is limited [84]. The elastic modulus (200–250 GPa) and ultimate tensile strength (400–1000 GPa) of Co-based alloys are 10 times higher than those of the human bone. The use of these implants manufactured from Co-based alloys thus results in a stress-shielding effect at the tissue/implant interface. The surface modification of Co-Cr-Mo alloys under plasma treatment improves hardness, wear, and corrosion resistance [85,86,87]. However, they are still not recommended for joint fixtures due to their inferior frictional and tensile properties. Apart from their biocompatibility and corrosion behavior, Co-based alloys are not ideal materials for bearing and joint surfaces due to their sub-par frictional properties [88].
2.3.3. Ti Alloys
Commercially pure titanium (Ti) and its alloys (Ti-6Al-4V, Ti-6Al-7Nb, Ti-5Al-6Nb, and Ti-13Nb-13Zr) have become major assets in the biomedical field owing to their superior biocompatibility, low density, and suitable mechanical properties. At first, it was intended to be used for aerospace applications, but later in the 1970s, the discovery of its biocompatibility led to a demand for Ti and Ti alloys in biomedical applications. If commercial pure titanium (Cp Ti) is used to replace its alloys, the mechanical properties lost due to alloying elements must be compensated for [89,90]. The alloys of Ti show enhanced mechanical and biocompatibility properties in comparison to pure titanium. Depending on the presence of the iron and oxygen content in the Ti alloy, four different grades of alloys are used. The most widely used Ti alloy is Ti-6Al-4V, comprising an estimated 50% of total titanium alloys’ usage for bioimplants of this grade [91,92]. By comparing with other grades of Ti alloys, it offers excellent corrosion resistance, biocompatibility, formability, structural stability, and a better weight to strength ratio. The applications of Ti alloys as bioimplants include heart valves, dental prostheses, osteosynthesis, artificial joints, and bone replacements [93].
Biomedical grade titanium alloys are generally categorized as alpha (α, Ti-6Al-4V), near-α, α-β, and metastable β (Ti-6Al-7Nb) [94,95]. These alloys are widely used as biometallic implants, but they cause stress shielding issues at the implant-tissue interface due to their high elastic modulus values. The elastic modulus of Ti and α-β Ti-alloys (100–110 GPa) is higher than that of human bone which limits its usage in joints. The presence of vanadium and aluminum compounds results in the release of toxic ions of vanadium (oxidovanadium (IV) and vanadate (V)) and aluminum (Al3+) under the physiological environment, leading to adverse health issues [96,97,98]. Therefore, much interest has been paid to β alloys in combination with Zr, Nb, Ta, or Mo to replace V and Al in the alloy. Such alloys possess better mechanical properties, ductility, good structural stability, higher wear resistance, a lower elastic modulus, and improved corrosion resistance [99,100,101].
One of the disadvantages of using Ti alloys is their below par tribological properties, due to their high friction and abrasive wear nature [102,103]. Moreover, the formation of TiO2 during exposure protects the surface of the Ti alloy, which hinders the bioimplant-tissue relationship. The formation of titanium compounds around the surrounding tissues of the implant causes failure of the implant [104].
2.3.4. Mg Alloys
Metal-based biodegradable orthopedic implants nullify the complications associated with the long-term existence of implants inside the human body. In recent days, biodegradable metallic implants were investigated as biomedical implants [105]. Magnesium (Mg) is present in the human body as the fourth most abundant cation and is essential to the human metabolism. Mg corrodes faster in the chloride containing physiological environment; thus, it has emerged as biocompatible and biodegradable material for use as implants [106]. Moreover, Mg and its alloys have received much attention in the category of biodegradable alloys due to their leading properties such as low density, an elastic modulus close to that of bones, light weight, biocompatibility, and excellent mechanical properties [107,108]. The revision surgeries performed to remove hardware components in implants such as screws and plates from the implanted site after healing are often discomforting and expensive for the patients. The revision surgery can also lead to complications such as nosocomial infection and delay the patient’s recovery to a normal lifestyle. Mg-based biodegradable metallic implant components can overcome the revision surgery by degrading in situ, thus also eliminating the need for the procedure to remove the implant components after healing [109].
The high mechanical strength of metallic materials limits the use as bioimplants, whereas the Mg implant shows a reduced elastic modulus and prevents the mismatch between a bone and the Mg-based implant. This leads to the reduction in stress shielding at the bone/implant interface. Their mechanical and corrosion properties can be enhanced by alloying with Al, Zn, and other elements [110,111]. Current research is focused on the development of Mg-based alloys with zero or low cytotoxicity. Alloying Mg with other metals must be selected carefully to avoid metal-related toxic issues and corrosion. Different type grades of Mg alloys such as Mg-Ca and Mg-Y-Nd were studied as biodegradable bioimplants for orthopedic applications [112].
The major limitation associated with Mg and Mg-based alloys is their rapid corrosion in physiological conditions. Rapid corrosion results in quick release of byproducts such as hydrogen gases due to fast in vivo degradation. This indicates the necessity for surface modification. To overcome the rapid corrosion, alloying with various elements has been explored. For example, elements such as calcium (Ca), zinc (Zn), silver (Ag), aluminum (Al), zirconium (Zr), yttrium (Y), and Neodymium (Nd) were added to Mg to enhance the corrosion and mechanical properties [113,114,115,116,117]. Typical examples are Mg-Ca, Mg-Zn, and Mg-Zn-Ca. By carefully selecting a suitable element and its composition, the microstructure can be tailored to meet mechanical properties such as bone. This makes them ideal for bone replacement. Table 2 shows the overall comparison of materials used for biomedical applications and their applications.

Table 2.
The pros and cons of various biomaterials used in the biomedical industry [118].
3. Need for Surface Modification of Bioimplants
In an implant operation, any material inserted into the human body is treated as a foreign substance. If the foreign substances are not biocompatible, layers of fibrous tissues, also known as scar tissues, begin to develop between the tissue and implant. Eventually, due to scar tissue development, the implant fails to osteointegrate with the host bone, leading to implant failure. Therefore, the primary requirement for the successful implant process is to have a complete integration between bioimplants and human body tissues [120]. The biological responses of biomedical devices to the lifespan and performance are better controlled by their surface morphology and chemistry. To achieve better biocompatibility and osteoconductivity, surface modification on biometallic materials has been recommended to achieve the desired properties (Figure 1) to increase the success rate of implants. When the surface is effectively modified, the bulk functionality and properties of the biomedical implant device will remain unaffected for a long time [121,122]. With the advantage of bio-integration and the load-bearing capability of biomaterials, the success rate for bioimplants can be greatly increased.
In recent years, researchers tried to enhance the bio-integration of implants by modifying the implant surface that is in contact with the body environment. Two approaches are considered for modifying the surface of the implants. The first approach is to deposit organic/inorganic-based coatings on the metallic surface without modifying the implant substrate [123]. The second approach is to use conversion coatings or surface modified layers, where the chemical surface modification of a substrate results in a slight increase in thickness [124]. In this case, the substrate elements are involved in developing conversion coatings. For conversion coating, surface preparation by grinding and polishing is required to improve the surface roughness for better mechanical interlocking of coatings. This process is critical, and surface modification by depositing an overlay coating is recommended [125]. Recently, a combination of both surface modification and deposition of thin films was performed to achieve the synergy of both properties.
In a modern biomedical implant industry, surface modification of metallic implants with an appropriate coating material is used to enhance biocompatibility, corrosion resistance, antimicrobial behavior, and mechanical properties. Although there are many methods for the deposition of bioactive surface coatings, an optimal coating technique for biomedical applications has not been developed yet. Currently, the coatings on implant materials are deposited by one of the deposition techniques such as physical vapor deposition (PVD), chemical vapor deposition (CVD), electrophoretic deposition (EPD), electrodeposition (ED), or sol-gel methods [2]. Among these, PVD is recommended to deposit metal/ceramic materials over the implant surface and provide exact stoichiometry, excellent adhesion, high density, and good uniformity. Another method for surface modification other than coating methods is chemical etching to prevent bacterial adhesion and improve osseointegration [126].
The success of an implant is dependent on the stability of the coating, which provides better biocompatibility. This section is focused on the recent advancements in various types of ceramic and polymer coatings to improve bioimplant performance and reliability.
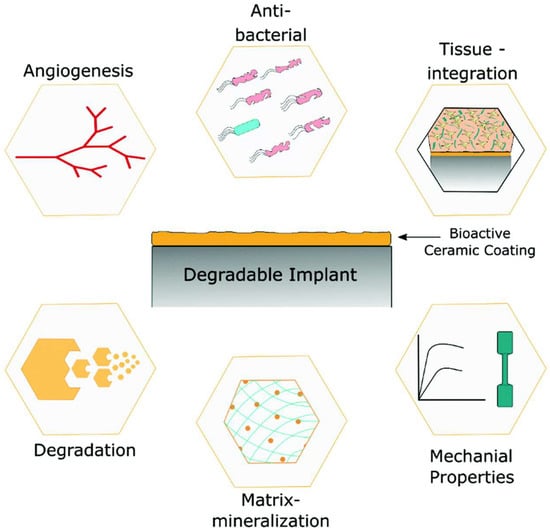
Figure 1.
The role of bioactive coated metallic implants as a potential implant material [127]. The qualities of coated implants are superior to those of uncoated metallic implants.
3.1. Polyether Ether Ketone (PEEK)
PEEK is a thermoplastic material that shows a combination of excellent stiffness, chemical and physical properties, and toughness and offers a wide range of applications [128]. Therefore, it is widely used as a bone substitute in orthopedic and dental implants, and in clamps for removable dental prostheses [129]. The PEEK coated substrates show better tribological properties, which are useful for the development of coatings on light weight alloys which lack tribological performance. Most of the sliding and bearing implant materials are coated with PEEK due to its better wear resistance and thermal stability [130,131]. Generally, PEEK coating and its composites are prepared using thermal spraying or electrophoretic processes [132,133,134,135]. PEEK coating (70–90 µm thick) deposited through electrophoretic deposition on the Ti-13Nb-13Zr titanium alloy showed excellent wear resistance, 200 times higher than the uncoated alloy [136].
PEEK in combination with other bioactive materials shows better antibacterial activity than PEEK alone [137]. Many authors reported on PEEK-based composite coatings on metallic substrates. These coatings enhance bioactivity and electrochemical corrosion resistance, especially for implant structural components. Typical examples for the composite coatings are TiO2/PEEK [138], sol-gel glass/PEEK [139], bioactive glass/PEEK [140], h-BN/PEEK [141], Ag/bioactive glass/PEEK [142], and h-BN/bioactive glass/PEEK coatings [137]. A combination of bioactive glass embedded in a polymeric matrix of PEEK makes it an interesting material for orthopedic applications as it meets biological and biomechanical requirements for the application. A cold sprayed Bioglass/PEEK composite prepared by Garrido et al. [143] showed an increase in wear resistance by more than 70%, higher hardness, and a lower coefficient of friction compared to pure PEEK. Coatings based on Bioglass/PEEK on porous Ti substrates resulted in higher adhesion between Bioglass/PEEK coating and Ti substrates [144].
Flame sprayed hexagonal boron nitride (h-BN) incorporated PEEK coating on low-carbon steel substrate increased the hardness and decreased wear and frictional coefficient values for the composite coating containing 8 wt. % h-BN due to its self-lubrication properties [145]. The coefficient of the friction value can also be reduced by the addition of alumina. The Al2O3/PEEK composite coating deposited on a Ti alloy using electrophoretic deposition showed increased corrosion resistance and significantly improved wear resistance under dry sliding conditions. The viability test revealed that the Al2O3/PEEK coating was found to be cytocompatible with MG-63 osteoblast cells [146]. The scratch resistance of PEEK coatings can be increased with the addition of amorphous Si3N4 nanoparticles. Tomasz et al. [147] performed the electrophoretic deposition of the PEEK/Si3N4 nanocomposite using a chitosan stabilizer: the coating showed higher scratch resistance than PEEK coating alone. This suggests that PEEK-based nanocomposite coatings potentially improve the bioactive as well as bio-tribological performance of Ti-based alloys used in biomedical applications. The use of PEEK with HAp as a coating can reduce the stress shielding effect. The combination of PEEK/HAp offers similar stiffness to that of the bone tissue. Recent studies suggest that the incorporation of HAp into PEEK coating improves bioactivity and mechanical properties [148]. PEEK coating prepared by different methods and their properties are summarized in Table 3.

Table 3.
Methods and properties of PEEK-based composite coatings.
3.2. Titanium Dioxide (TiO2)
TiO2 coatings are the most important materials in biomedical applications that are known for their antibacterial properties along with good mechanical properties. The applications of TiO2 coating include drug delivery systems [155], orthopedic [156], and dental applications [157]. It also shows high catalytic activity, antibacterial activity, and long-term stability under photo and chemical corrosion [158]. TiO2 promotes the formation of bone-like apatite or calcium phosphate on its surface. This property makes it a suitable candidate for reconstruction and bone replacement [159].
TiO2 coated metallic substrates show better antibacterial properties. Gartner et al. [160] observed the same biocidal effect by applying TiO2 coating on glass substrates by a sol-gel method. Photocatalytic activity of TiO2 coating received much attention as a potential material for anti-bacterial coatings. The antibacterial effects of TiO2 coating involve both a reduction in bacteria’s viability and their destruction [161]. Park et al. [162] showed that the antibacterial effect against S. aureus could be improved by adjusting the nucleation time of TiO2 film during the deposition process. The antibacterial effect of TiO2 was explained by the formation of reactive oxygen species. Apart from antibacterial properties, the antiviral properties of the TiO2 coating are also studied [163]. Table 4 summarizes the use of TiO2 and its composite coatings for bioimplant applications.
Yetim [164] prepared TiO2 coating with different concentrations of Ag using the sol-gel process on the commercially pure titanium substrate. Electrochemical corrosion properties obtained from electrochemical impedance spectroscopy measurements and potentiodynamic polarization tests in simulated body fluid (SBF) suggest that Ag doped TiO2 enhances corrosion resistance over that of the bare Ti substrates as well as undoped TiO2 coated samples [165]. The silver doped TiO2 (Ag/TiO2) nanocomposite coated glass substrate with varying Ag content synthesized by the sol-gel route showed antiviral properties against E. coli, enterovirus, and influenza A virus (H1N1) [166]. The highest level of photocatalytic degradation under irradiation with either visible or ultraviolet light was observed at an optimum Ag:TiO2 weight ratio of 1:100. The antibacterial effectiveness was greater than 99.99% against E. coli and other infectious diseases after visible light illumination.
Sol-gel derived TiO2-PTFE nanocomposite coating on stainless steel substrates was prepared by Zhang et al. [164] and their bacterial adherence were tested against two pathogens, namely S. aureus and E. coli. The bacterial adhesion and bacterial growth studies were evaluated by fluorescence microscopy after 2 h, 6 h, 12 h, and 24 h of incubation (Figure 2a,b). The TiO2-PTFE coated substrate shows the lowest bacterial adhesion when compared with the uncoated substrate. The bacterial inhibition increases with the increasing TiO2 concentration (Figure 2c,d). It is also observed that Gram-positive bacteria are less sensitive due to their cell wall thickness.
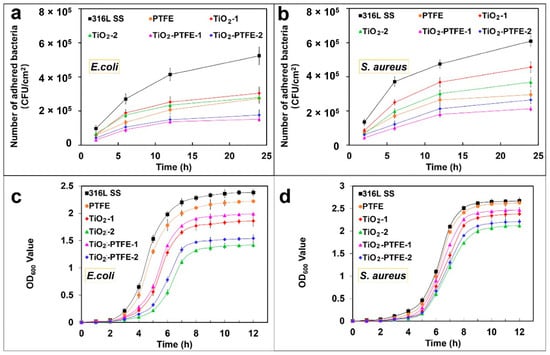
Figure 2.
Effect of bacterial adhesion (a,b) and bacterial growth of E. coli and S. aureus pathogens on TiO2-PTFE coated and uncoated substrates [164]. TiO2-PTFE coated substrates exhibit lower bacterial adherence and a significant reduction in bacterial growth (c,d) as compared to uncoated substrates.

Table 4.
Uses of TiO2 and its composite coatings in bioimplant applications.
Table 4.
Uses of TiO2 and its composite coatings in bioimplant applications.
| S. No. | Coatings | Deposition Method | Significance | Ref. |
|---|---|---|---|---|
| 1 | TiO2 coating on Ti substrates | Anodic oxidation | Potential rehabilitation to internal bone fracture | [167] |
| 2 | TiO2 coating on PEEK substrate | Dip coating | Recommended for maxillofacial and oral implants applications | [168] |
| 3 | TiO2/MoSe2/chitosan coating on Ti implants | Micro-arc oxidation process | Excellent in vivo and in vitro antibacterial property against S. mutans Better biocompatibility and hydrophilicity Better antibacterial properties | [169] |
| 4 | Poly(epsilon-caprolactone)/titania (PCL/TiO2) coating on Ti implants | Electrospinning technique | Good bioactivity against osteoblast cell Superior antibacterial against S. aureus Promoting cell attachment | [170] |
| 5 | TiO2 coating on Ti substrates | Direct lithographic anodic oxidation | Corrosion resistant | [171] |
| 6 | TiO2 nano coating | Anodizing oxidation technique | Better cell proliferation and adhesion Better osseointegration | [172] |
| 7 | Graphene/TiO2 coating on Ti substrate | Drop casting method | Better cell adhesion and proliferation behavior | [173] |
| 8 | TiO2/HAp bilayer coating on Ti substrate | MOCVD/Plasma spraying | Better hardness In vitro bioactivity | [174] |
| 9 | Y-doped TiO2 coating on Ti alloy | Plasma electrolytic oxidation method | Better antibacterial activity against E. coli and S. aureus | [175] |
| 10 | Fe3O4/TiO2 composite coating on Ti implants | Micro-arc oxidation process | Prevent inflammatory Better fibroblast response | [176] |
3.3. Transition Metal Nitrides
Earlier, transition metal nitrides and carbides were widely used to protect the metallic components against wear, tear, and corrosion, potentially offering high-temperature stability. Titanium nitride (TiN) coatings were used as decorative coatings in earlier days. In the last decade, nitride coatings for orthopedic implants were also proposed to protect the implants against wear and tear and to act as a diffusion barrier layer preventing the toxic ion release from the implant metal surfaces to the human body fluids [177,178,179,180]. The physical properties of TiN coated substrates show high scratch resistance, hardness, and low frictional coefficients. These properties make them a potential candidate for use as coatings on different metals used in arthroplasty. TiN-based coatings used for orthopedic applications show better biological properties as compared to other nitrides [181]. TiN coatings show better blood tolerability properties with a hemolysis percentage near zero [182]. TiAlN is another biocompatible nitride that has proven to be a promising alternative to TiN in biomedical applications despite its aluminum (Al) content [183].
Transition metal carbonitrides (TiCN, ZrCN) were found to increase the service life of orthopedic implants in terms of wear resistance in biological media [184,185,186]. Recently, quaternary carbonitrides-based coatings (TiAlCN, TiCrCN, TiNbCN, etc.) were found to show increased anticorrosive, mechanical, and tribological properties compared to ternary carbonitride-based coatings [187,188,189]. The tribological properties of these carbonitride coatings are very complex. However, the carbon-based carbonitride coatings show good biocompatibility, better wear resistance, and low friction [190]. Much attention has been paid to developing MeSiC-, MeSiCN-, and MeSiN- (where Me is a transition metal, and Si is an alloying element) based hard coatings [191,192,193,194]. These types of coatings show high thermal stability, a low frictional coefficient, excellent wear resistance, and good mechanical properties (hardness, Young’s modulus). Moreover, in many investigations, TiSi-based carbide and carbonitride coatings proved to be a potential candidate for a metallic implant which combines the mechanical, tribological, and anticorrosive properties of TiN and TiC with the biocompatibility behavior of SiC and SiCN [192,195,196,197].
TiN coating shows plastic deformation at the coating/surface interfaces due to dissimilarities in the hardness of the substrate and coating [198]. Thus, TiN coating cannot accommodate the fracture and deformation that creates flakes, and defects in the coatings cause deterioration of the coatings from the substrate. Therefore, chromium nitride (CrN) and chromium carbonitride (CrCN) coatings are recommended, which act as a better diffusion barrier for ion release from the alloys. These coatings also exhibit higher toughness, higher cohesive strength, and lower wear debris than TiN coatings [199].
TiN and TiCuN coatings were prepared by the axial magnetic field enhanced arc ion plating (AMFE-AIP) technique, and the in vitro angiogenic response of human umbilical vein endothelial cells was studied by Liu et al. [200]. The TiCuN coating showed better antibacterial activity, and both coatings showed no cytotoxicity to human umbilical vein endothelial cells (HUVECs). TiCuN coatings promote early cell apoptosis, which is important for vascular tissue modeling (Figure 3).
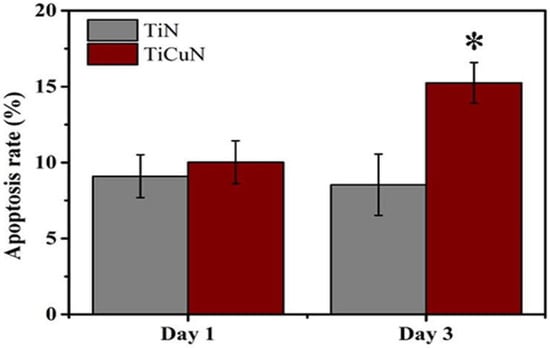
Figure 3.
Apoptosis rate of TiN and TiCuN coatings tested for Day 1 and Day 3. Annexin V-FITC/PI double staining kit was used to evaluate the apoptosis rate of these coatings [200]. TiCuN coating promoted the early cell apoptosis rate more than TiN coating. *: Denotes TiCuN coating superior performance.
Transition metal oxynitrides have been considered as interesting materials due to their known mechanical properties, chemical stability, and corrosion resistance in simulated body fluid. Zirconium oxynitride (ZrON) and titanium oxynitride (TiON) based coatings were recently used in biomedical applications for their better corrosion resistance than TiN coating and their anti-fouling ability [201,202]. The magnetron sputtered ZrON and TiON coated 316L SS specimen show better hardness and wear resistance behavior than the uncoated substrate [203]. In addition, both coatings show better anti-fouling performance against Pseudomonas aeruginosa bacterial adhesion than uncoated substrates. The coated substrates also show better corrosion protection with or without the addition of hydrogen peroxide (H2O2) in artificial blood plasma (ABP) solution [203].
Surface modified coatings prepared from ternary nitrides such as TiZrN, TiCrN, and TiAlN gained considerable attention because they retain their physiochemical properties, such as oxidation resistance, hardness, corrosion resistance, biocompatibility, and structural stability after implantation [204,205]. Magnetron sputtered TiZrN coated 316L SS substrates showed less bacterial adhesion, increased corrosion protection, and negligible human blood platelets activity than uncoated substrates [206]. Recent developments in binary, ternary, and quaternary systems of transition metal nitrides and carbide coatings are tabulated in Table 5.

Table 5.
Recent work on binary, ternary, and quaternary systems of transition metal nitride and carbide coatings for implant applications.
3.4. Carbon Based Coatings
Carbon based materials are categorized under bioinert coatings. These coatings are used in load-bearing applications and wear components to improve elevated corrosion resistance, wear, and frictional effects [219]. Besides, carbon-based coatings show minimum protein adhesion and very good biocompatibility due to the hydrophobic nature of carbon-coated surfaces. Three different types of carbon-based coatings are used for biomedical applications. They are (a) nanocrystalline diamond (NCD), (b) pyrolytic carbon (PyC), and (c) diamond-like carbon (DLC) [220]. Some of the coatings are commercially available, while others are under development.
Most of the PyC coatings in biomedical applications are found in the heart valves due to their thromboresistant qualities and biocompatibility [221]. Most of the artificial heart valves are lined with a thick PyC coating. PyC biocompatibility in heart valves is well established. PyC coatings have also been used in orthopedic applications [222]. By varying the process parameters of the PyC (such as temperature, surface area, gas flow rate, precursor) in the CVD process, a variety of the structures can be produced. The most interesting structure for biomedical applications is lamellar, isotropic, granular, and columnar [223,224,225]. PyC coated orthopedic implants are used to replace small joints such as wrist joints, knuckles, and arthroplasty of proximal interphalangeal joints [226].
Carbon coatings, including nanocrystalline diamond and DLC coating, show many remarkable biological properties and are considered as coatings for medical implants. NCD coatings deposited by the CVD process consist of sp3-hybridized carbon bonds and show grain sizes in the range of a few nanometers. NCD coatings generally show very low surface roughness and possess the properties of a diamond, such as hydrophobicity and excellent biocompatibility with blood [227,228]. This makes them an ideal coating choice for wear-resistant implant applications and cardiovascular devices. NCD coating can also be used as hard antibacterial coatings that reduce the risk of infections. The electrically active NCD coating surfaces can establish a chemical bond with the biomolecules in the surrounding environment. Medina et al. [229] observed that the NCD coating surfaces react with the cell wall or membrane of Gram-negative P. aeruginosa bacteria and establish a chemical bond that alters the bacteria morphology, hindering bacterial adhesion and colonization on the surface of the coating. The properties of NCD films are utilized in biosensing and neurochemical sensing applications [230].
More experimental studies have been reported on DLC based coatings, which are considered as the most promising materials for bioimplant applications [231,232,233,234]. Medical grade PEEK samples were coated with DLC using plasma immersion ion implantation and deposition (PIII & D) technique, and their in vitro cytocompatibility and osteogenesis studies were carried out by Mo et al. using human bone marrow mesenchymal stem cells (hBMSCs) [235]. DLC coated substrates show better surface coverage of cells and show high cell viability on the seventh day, which indicates better biocompatibility of DLC-PEEK coatings than PEEK coating (Figure 4). However, DLC suffers from residual stress arising from the substrate/coating thermal expansion mismatch and lattice misfit, which cause poor substrate adhesion and delamination of the coating from the substrate. Another major concern about DLC coatings is their instability in the aqueous environment, which promotes delamination of the coating [236]. To avoid this issue, it is recommended to use interlayers (called buffer layer) such as CrC, Ti, and Si3N4 at the interface of the substrate and DLC coating [237]. Another approach is to dope DLC coating with N, F, Ag, Zr, or Ti to avoid a thermal expansion mismatch and residual stress [238].
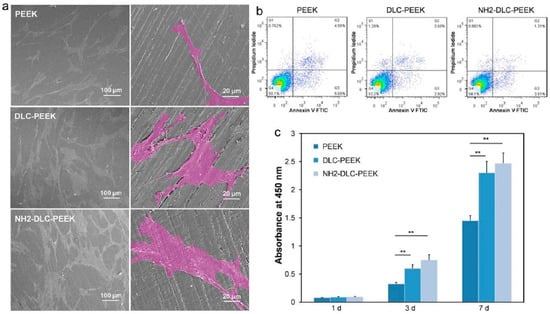
Figure 4.
Represents in vitro cytocompatibility of DLC coated PEEK substrates, (a) surface morphology of hBMSC cultured on PEEK, DLC-PEEK, and NH2-DLC-PEEK substrates for 1 day, and the enlarged cells are shown in pseudo-color, (b) cell viability for 1 day, and (c) proliferation of hBMSCs after culturing samples for 1 d, 3 d, and 7 days [235]. ** denotes p < 0.01.
The properties of DLC such as chemical inertness, surface smoothness, and hydrophobicity are important for providing better compatibility with blood, reducing platelet activation in contact with the blood, which could trigger thrombosis. DLC can act as a protective coating under the conditions of the human blood environment, which limits the release of nickel ions from metallic implants such as SS 316L. Several studies suggest that DLC coating prepared by various routes is biocompatible and does not induce any inflammation reaction both under in vivo and in vitro conditions [235,239]. Because of these remarkable features, DLC coatings found various applications as coatings in many implant devices such as cardiovascular stents, heart valves, surgery needles, medical wires, contact lenses, etc. DLC coatings can also be used as protective coating in knee replacement because of their high corrosion resistance, hardness, and low wear rate. Generally, DLC films are used to reduce the frictional coefficient and offer better wear resistance [238]. Carbon based coatings and their significance in biomedical field are summarized in Table 6.

Table 6.
Different carbon coatings and their properties.
3.5. Calcium Phosphates
Calcium phosphate (CaP) ceramics are widely used as implants since they have a chemical composition similar to the inorganic composition of the bone. By controlling the surface properties such as roughness and porosity of CaP, one can regulate the biomineral formation and cell/protein adhesion. Bioactivity properties are varied depending on the type of calcium phosphates (HAp, tricalcium phosphate (TCP)) because of the differences in crystallinity, solubility, stability, ion release, and mechanical properties. At first, CaP coatings were deposited through the vapor phase process, but in recent years, biomimetic and solution-based methods were developed. Each synthesis approach has its own intrinsic properties, but in general, CaP based coatings are promising to improve implant longevity and biocompatibility. Many studies have been focused on the development of CaP ceramic coatings on metallic substrates to achieve the biological properties identical to a bulk and to enhance the implant durability and fixation [250,251,252,253].
Presently, atmospheric plasma spraying (APS) is currently employed to develop CaP coating on implant surfaces [2]. The CaP phases in the coatings exhibit higher solubility in an aqueous medium than HAp which is desirable for activating bone formation. However, faster dissolution reduces the stability and can cause loosening of the implant. A highly crystalline HAp phase dissolves in human physiological conditions at a lower rate which provides long-term stability of the implants. Thus, for the development of implants with required properties, one must control the purity and crystallinity of the coatings. CaP coatings with a denser microstructure lower the risk of delamination of the coating during in vivo tests with human body fluids. Coating surface roughness affects its dissolution and bone apposition and growth. Porous surfaces may enhance cell attachment or formation of the extra-cellular matrix, but the accumulation of macropores at the coating/substrate interface weakens the coating adhesion [254].
CaP in the form of HAp is widely used in implant applications due to its superior biological response. The HAp composition is Ca10(PO4)6(OH)2 (Ca/P = 1.67), which resembles the chemical composition of hard tissues such as bone and teeth [255]. Hence, HAp is considered as a primary candidate material due to its exceptional biological properties such as excellent biocompatibility, osteoconductivity, osteoinductivity, and bioactivity [256]. HAp coatings release calcium and phosphate ions and regulate the activation of osteoclasts and osteoblasts, facilitating bone regeneration [257]. The use of HAp ceramics enhances the regeneration of bones, improves osteoconductivity for bone growth, and promotes mineralization through ion release control and encapsulating growth factors. HAp ceramic coating enhances bone apposition in orthopedic implants through the formation of an extremely thin bonding layer with the existing bone. Due to such tissue bonding characteristics, Hap-based ceramics are considered as bioactive-based coatings. The continuous effort to improve the durability of the HAp ceramic coatings has led to development of high-quality HAp coatings and the development of Hap-based composite coatings.
Highly porous or highly crystalline HAp coating shows poor adhesion to the substrate. Sankar et al. [258] studied the corrosion behavior of HAp coatings prepared by electrophoretic deposition (EPD) and the pulsed laser deposition (PLD) method. The corrosion results suggest that the HAp coatings show lower corrosion protection than the coatings prepared by the PLD method due to the formation of denser and pore-free coating [258]. Corrosion protection can also be enhanced by the addition of antimicrobial dopants. For example, Yugeswaran et al. [259] prepared HAp-TiO2 nanocomposite coatings by APS. The coating shows better corrosion performance in SBF medium than HAp coating without dopants due to its high compactness and the presence of TiO2 [259]. Silver (Ag) containing HAp coatings prepared by Trujillo et al. [260] show better antibacterial activity than HAp coating alone against P. aeruginosa and S. epidermidis pathogens due to the antibacterial activity of Ag. The antimicrobial activity of the Ag-doped HAp composite against E. coli and S. aureus was tested by Lett et al. [261]. The results indicated that the Ag-doped HAp composite has better inhibition of bacterial growth and shows a stronger ability against S. aureus bacteria to fight against toxic responses (Figure 5). The absence of Ag in the composite results in lower antibacterial activity of HAp composites. The variation in antibacterial activity was attributed to a thinner cell wall response of S. aureus (Figure 5b) to Ag ions than E. coli (Figure 5a) [261].
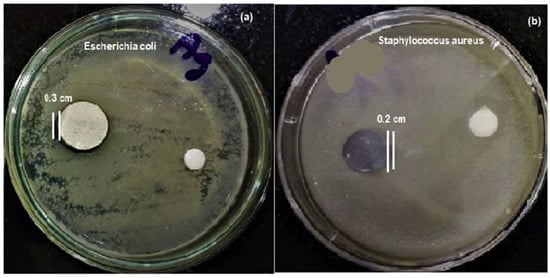
Figure 5.
Demonstration of antimicrobial activity of HAp and Ag doped HAp composites against E. coli (Gram-negative) (a) and S. aureus (Gram-positive) (b) bacteria [261]. The photograph shows that Ag-doped HAp inhibits S. aureus bacteria more effectively than E. coli.
In biomedical implants, the major challenge for the performance of implants is bacterial invasion. During surgical operation, the bacteria may enter the surface of the implants through surgical equipment or cross contamination which form a biofilm. Once the surrounding implant is infected, the infection causes implant loosening. To overcome this issue, antimicrobial agents are used as dopants in ceramics, protecting implant material from bacterial invasion and improving their durability. Zinc doped HAp composites prepared by the sol-gel route and annealed at different temperatures (500 °C and 700 °C) show higher antimicrobial activity against C. albicans fungal cells and S. aureus bacteria [262].
Multiple doping of ions into HAp coatings was also attempted to improve their structural stability, partial dissolution, and biocompatibility. Wang et al. [263] prepared Sr and F− doped hydroxyapatite and studied the properties of the coating. The addition of the dopant improves the structural stability of the HAp lattice and promotes osteogenic cell differentiation. Moreover, the addition of F− ions potentially arrests the formation of S. aureus. Dopants such as Cu, Zn, Mg, Ag added to HAp enhance antibacterial activity and decrease the toxic effects towards the human body cells [264,265,266,267]. For example, Mg-doped HAp shows better osteoblast cell adhesion than pure HAp [268].
The differences in the thermal expansion coefficient of HAp and metallic alloys result in residual thermal stress. The stress accumulation increases with the increase in the coating thickness, which promotes cracking or delamination of the coating. For a thicker coating, the outer layer may detach from the implant, whereas a thin HAp coating can prematurely resorb during bone regeneration. Various HAp composites and their biological properties are summarized in Table 7.

Table 7.
Hydroxyapatite and its composites’ coatings for implant applications.
3.6. Zirconia
Zirconia (ZrO2) is a ceramic material that can withstand high temperatures as well as higher stresses. It has widespread applications in dental implants and in the coatings on metallic implants to increase their corrosion resistance [280]. ZrO2 ceramics offer many advantages, including mechanical strength, chemical stability, biocompatibility, good aesthetics, and better wear resistance. Zirconia stabilized with yttria (YSZ) has been used as a dental implant due to its excellent mechanical strength and fracture toughness [281]. YSZ coatings show better hardness and scratch resistance than HAp coating [282]. Gobi Saravanan et al. [283] observed that the YSZ coated Ti substrates show improved hemocompatibility, activating blood platelets with pseudopods. In addition to that, superior in vitro biomineralization behavior was observed and documented through the weight gain on YSZ coating.
Zirconia stabilized with different weight fractions (0, 4, 10 wt. %) of yttria yields different phases (monoclinic, tetragonal, and cubic): zirconia ceramics with tailored mechanical properties and biocompatibility can be thus prepared. Attempts were made to deposit different phases of zirconia (m-ZrO2, t-ZrO2, and c-ZrO2) with the use of electron beam physical vapor deposition (EBPVD) [284]. All the coatings show lower surface roughness than coating prepared through the APS method and reduce pathogen bacterial invasion. Particularly, t-ZrO2 shows superior hardness over the other two zirconia phases. All the allotropes show better blood plasma protein adhesion and enhanced resistance to corrosion in comparison to uncoated medical grade stainless steel substrates in ABP solution.
Antibacterial activity of ZrO2 coating can be enhanced by the addition of Ag. Ag-ZrO2 composite coatings were prepared by Pradhaban et al. [285]. The results suggest that the coating shows antimicrobial activity against E. coli. Santos et al. [286] prepared glass ceramic composites with different concentrations of ZrO2 particles (0–50 vol. %) and carried out a ball-on-plate tribology test. ZrO2 glass ceramic composite (30 vol. % of ZrO2) shows optimal wear properties (coefficient of friction is 0.3) and is recommended for load-bearing applications. Bermi et al. [287] deposited YSZ coating through pulsed plasma deposition, and the tribological behavior of the coating in both dry and wet conditions was tested. YSZ coating deposited on a Ti6Al4V alloy ball sliding against the UHMWPE disk shows a reduction in wear rate (17% and 4% in dry and lubricated conditions) than uncoated alloy substrate.
Kaliaraj et al. [288] prepared zirconia coatings on a 316L SS substrate by electron beam physical vapor deposition (EBPVD), and a bacterial adhesion study with P. aeruginosa was carried out. Epifluorescence microscopy analysis of live/dead cells after incubation of 1, 2, 3, and 4 days showed a drastic reduction in bacterial adhesion on ZrO2 coatings, along with retardation in biofilm formation (Figure 6). This observation was attributed to the decrease in surface roughness obtained through coating deposition and the surface chemistry of ZrO2 that inhibits bacterial adhesion. Electrochemical impedance corrosion results show that ZrO2 exhibited superior corrosion resistance in the presence of H2O2 in an artificial blood plasma electrolyte solution. [288].
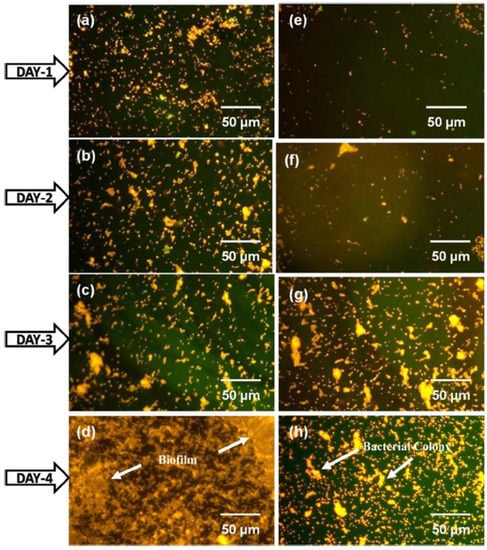
Figure 6.
Epifluorescence microscopy analysis of P. aeruginosa bacterial invasion on 316L SS (a–d) and ZrO2 film (e–h) after 1, 2, 3, and 4 days incubation [288]. The used acridine orange staining shows orange color for live cells and green color for dead cells. The reduction in bacterial adhesion was seen on ZrO2 coated substrate compared to uncoated 316L SS.
3.7. Bioactive Glass Coatings
Hench pioneered bioactive materials research and revolutionized the fields of bioactive materials and ceramics with his discovery of bioactive glass (45S5 composition), commercially known as Bioglass [289]. In the wake of Bioglass, various compositions and composites of bioactive glasses or silicates prepared both by melt quench and sol-gel techniques were investigated. Although bioactive glasses exhibit excellent bioactivity, because of their amorphous or semi-crystalline nature, they often fail as an implant material due to their poor mechanical strength. To overcome the shortage in mechanical properties, bioactive glasses are often composited with various metal oxides such as TiO2, Al2O3, ZrO2, and 2-D materials such as graphene and its derivatives (graphene oxide and reduced graphene oxide) [290]. These composites were reported to improve the corrosion resistance, antibacterial activity, and angiogenic properties of bioactive glass coatings without losing the bioactivity [291]. Similar to many ceramic materials, bioactive glasses can also be prepared in the form of particles of nano and micron size, as mesoporous particles, fibers, 3D scaffolds or monoliths, and thin films or coatings [292].
In this section, various types of coating technologies that can be used for the coating of bioactive glasses and their composites on different types of metals, alloys, and certain specific surfaces are discussed. One of the most simple and economical coating processes is the sol-gel dip-coating process. However, the coatings are often porous because of the solvent evaporation leading to poor corrosion resistance and mechanical properties. Nevertheless, this problem can be solved by incorporating metal oxides such as B2O3 as reported by Pinki Dey et al. [293]. According to their report, by replacing the silica weight percentage in the 45S5 system by 1% to 5 wt. %, they were able to decrease the porosity in the particles. Thermal spray coating, an industrial coating process, can also be employed for bioactive glass coating preparation. This process involves the coating of bioactive glasses as fine droplets or as plasma and sprayed over metal surfaces. Porous and non-porous layers with varying coating thicknesses can be achieved by the thermal spray process by tuning the deposition parameters such as velocity, size of the droplets, and temperature of the substrates [294].
Bioactive glasses can also be coated by physical deposition techniques such as radio-frequency magnetron sputtering (RF-MS) and pulsed laser deposition. In a recent study conducted by Qaisar Nawaz et al. [295], silver nanoclusters embedded in a silica matrix were deposited over the PEEK/BG layer using RF co-sputtering. They report a uniform 100 nm of the Ag-SiO2 layer that showed slower and sustained release of silver ions compared to the electrophoretically deposited coating. Although the physical deposition techniques are very robust and highly reproducible, their shortcoming is often the expensive experimental setup and precursors when compared to wet chemical sol-gel coating techniques. On the other hand, electrophoretic deposition (EPD) combines both the advantages and disadvantages of sol-gel coating and physical deposition methods. EPD is both a versatile and cost-effective method for coating ceramic materials on conducting surfaces.
Ashokraja et al. [296] reported bioactivity in simulated body fluid (SBF) and reactive oxygen production using the XTT assay for reduced graphene oxide (rGO), sol-gel derived bioactive glass rods (BGNR) followed by different methods for developing composites of rGO and BGNR such as under constant stirring (COL), under constant sonication (SOL), and with a simultaneous reduction in graphene oxide-BGNR composites (RED). In their study, they report the role of pH changes in the sol-gel process facilitating one-dimensional rod-shaped bioactive glass formation, and their immersion studies exhibited a 50-micron thick HAp layer on the seventh day for rGO/BG composites [297]. Their work also reports that the different methods employed to prepare the composites influence the HCA formation, antibacterial efficacy, hemocompatibility, and cell proliferation as shown in Figure 7.
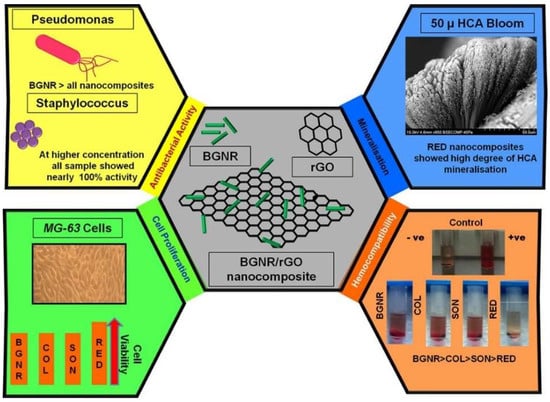
Figure 7.
Schematics for HCA formation, antibacterial activity, hemocompatibility, and cell proliferation of bioactive glass rods (BGNR) and their composites with rGO (COL, SON, and RED) [297]. Figure also shows the bioactive behavior of the BGNR-rGO composites. It is noticed that the RED composites showed better HCA layer formation, cell proliferation, and hemocompatibility.
A recent comparative study reported results between pure BG and rGO/BG thin films deposited over the anodized surface of titanium by EPD. The deposited bioactive coatings (both pure and composites) were 2 µm thick and exhibited very good HAp formation in simulated body fluids along with super hydrophilicity in pure bioactive glass coatings [298]. Table 8 summarizes a brief list of bioactive glass coatings, their compositions, coating processes: important features are elucidated.

Table 8.
Composition, the substrate used, coating process, and their salient features of bioactive glasses.
4. Summary and Future Directions
This paper reviews different biomaterials and explains their significant characteristics that influence their bioactivity. Bioimplant manufacturing involves an integrated process of selection of materials, design, fabrication, and surface modification through micro/nano texturing or coating application. Engineering of native metals by converting them into alloys yields desired properties and provides flexibility in designing the needs as per implant requirements. For a long-term application of bioimplants, surface characteristics and their biological functions are considered as key factors. Engineering the surface of the biomaterials by applying suitable coatings provides flexibility in tailoring the properties as per the requirements.
Bioceramic coatings hold great potential by tailoring the biological properties that suit our needs: the choice of the coating depends on the interaction between the cells with the coatings and substrates that are being used. Coatings on metallic implants are invaluable due to their functionality, biocompatibility, durability, and stability. Bioactive coatings are used to enhance the biological fixation between the bone and metallic implant despite their poor tribological and mechanical properties. Hence, they are often improved by developing composites with materials that possesses good mechanical strength. These improved coatings can be also used for durable load-bearing implants. All these properties lead to a better clinical success rate in long-term use in comparison to uncoated metallic implants. The bioactive ceramic coated biodegradable implants provide synergistic properties of both the implants and coating. Thus, these coatings find applications in cardiovascular stents, heart valves, orthopedic applications, tissue engineering, drug delivery, and biosensors. The current trends of ceramic coatings coated metallic implants are more focused on orthopedic applications.
Feasibility studies on complex structures, designing, fabrication of metallic alloys to form complex shapes without losing mechanical properties and surface integrity are a challenging task and should be attempted. The degradation mechanism of coatings on metallic implants changes in the human body environment. Moreover, lattice mismatch and the accumulation of residual stress cause degradation of the implant after implantation. Thus, there is a need to develop mathematical models for the prediction of degradation mechanisms. Another approach to reducing the residual stress is to deposit a functionally graded multi-layered or nanocomposite coating with multifunctional properties.
Author Contributions
Conceptualization, K.K.A.M., A.R.C. and A.D.; validation, A.P. and D.G.; writing—original draft preparation, K.K.A.M., A.R.C. and A.D.; writing—review and editing, A.P. and D.G.; supervision, A.P. and D.G.; project administration, D.G. and A.P.; funding acquisition, D.G. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 739566. This work was also created in the frame of the project Centre for Functional and Surface Functionalized Glass (CEGLASS); ITMS code is 313011R453, operational program research and innovation, co-funded from the European Regional Development Fund.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.A. Bioceramics for Implant Coatings. Mater. Today 2003, 6, 26–30. [Google Scholar] [CrossRef]
- Goharian, A. Fundamentals in Loosening and Osseointegration of Orthopedic Implants. Osseointegration Orthop. Implant. 2019, 1, 1–26. [Google Scholar] [CrossRef]
- Rodriguez-Gabella, T.; Voisine, P.; Puri, R.; Pibarot, P.; Rodés-Cabau, J. Aortic Bioprosthetic Valve Durability: Incidence, Mechanisms, Predictors, and Management of Surgical and Transcatheter Valve Degeneration. J. Am. Coll. Cardiol. 2017, 70, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Shayesteh Moghaddam, N.; Taheri Andani, M.; Amerinatanzi, A.; Haberland, C.; Huff, S.; Miller, M.; Elahinia, M.; Dean, D. Metals for Bone Implants: Safety, Design, and Efficacy. Biomanufacturing Rev. 2016, 1, 1. [Google Scholar] [CrossRef]
- Bazaka, O.; Bazaka, K.; Kingshott, P.; Crawford, R.J.; Ivanova, E.P. Chapter 1 Metallic Implants for Biomedical Applications. In Chemistry of Inorganic Biomaterials; Royal Society of Chemistry: London, UK, 2021; Volume 8, pp. 1–98. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef]
- Nedela, O.; Slepicka, P.; Švorcík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Aherwar, A.; Singh, A.K.; Patnaik, A.; Aherwar, A.; Singh, A.K.; Patnaik, A. Current and Future Biocompatibility Aspects of Biomaterials for Hip Prosthesis. AIMS Bioeng. 2015, 3, 23–43. [Google Scholar] [CrossRef]
- Wang, G.; Zreiqat, H. Functional Coatings or Films for Hard-Tissue Applications. Materials 2010, 3, 3994. [Google Scholar] [CrossRef]
- Bhat, S.; Kumar, A. Biomaterials and Bioengineering Tomorrow’s Healthcare. Biomatter 2013, 3, e24717. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive Potential of Natural Biomaterials: Identification, Retention and Assessment of Biological Properties. Signal Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, e2000395. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Akhtar, M.; Uzair, S.A.; Rizwan, M.; Ur Rehman, M.A. The Improvement in Surface Properties of Metallic Implant via Magnetron Sputtering: Recent Progress and Remaining Challenges. Front. Mater. 2022, 8, 602. [Google Scholar] [CrossRef]
- Ahirwar, H.; Zhou, Y.; Mahapatra, C.; Ramakrishna, S.; Kumar, P.; Nanda, H.S. Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials. Coatings 2020, 10, 264. [Google Scholar] [CrossRef]
- Vladescu, A.; Surmeneva, M.A.; Cotrut, C.M.; Surmenev, R.A.; Antoniac, I.V. Bioceramic Coatings for Metallic Implants. Handb. Bioceram. Biocomposites 2016, 703–733. [Google Scholar] [CrossRef]
- Sarian, M.N.; Iqbal, N.; Sotoudehbagha, P.; Razavi, M.; Ahmed, Q.U.; Sukotjo, C.; Hermawan, H. Potential Bioactive Coating System for High-Performance Absorbable Magnesium Bone Implants. Bioact. Mater. 2022, 12, 42–63. [Google Scholar] [CrossRef]
- Pana, I.; Vladescu, A.; Constantin, L.R.; Sandu, I.G.; Dinu, M.; Cotrut, C.M. In Vitro Corrosion and Tribocorrosion Performance of Biocompatible Carbide Coatings. Coatings 2020, 10, 654. [Google Scholar] [CrossRef]
- Probst, J.; Gbureck, U.; Thull, R. Binary Nitride and Oxynitride PVD Coatings on Titanium for Biomedical Applications. Surf. Coat. Technol. 2001, 148, 226–233. [Google Scholar] [CrossRef]
- Dos Santos, G.A. The Importance of Metallic Materials as Biomaterials. Adv. Tissue Eng. Regen. Med. Open Access 2017, 3, 300–302. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Biomaterials: Design, Development and Biomedical Applications. Nanotechnol. Appl. Tissue Eng. 2015, 21–44. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Prasadh, S.; Wong, R.C.W. Unraveling the Mechanical Strength of Biomaterials Used as a Bone Scaffold in Oral and Maxillofacial Defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Yamamuro, T. Bioceramics. Biomech. Biomater. Orthop. 2004, 22–33. [Google Scholar] [CrossRef]
- Shen, J.Z.; Fäldt, J. Requirements of Bioactive Ceramics for Dental Implants and Scaffolds. Adv. Ceram. Dent. 2014, 279–300. [Google Scholar] [CrossRef]
- Piconi, C.; Porporati, A.A. Bioinert Ceramics: Zirconia and Alumina. Handb. Bioceram. Biocomposites 2016, 59–89. [Google Scholar] [CrossRef]
- Barnes, D.H.; Moavenian, A.; Sharma, A.; Best, S.M. Biocompatibility of Ceramics. Mater. Med. Devices 2012, 128–134. [Google Scholar] [CrossRef]
- Elbadawi, M.; Meredith, J.; Hopkins, L. Ian Reaney Progress in Bioactive Metal and, Ceramic Implants for Load-Bearing Application. In Advanced Techniques in Bone Regeneration; InTech: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Whitehouse, M.R.; Blom, A.W. The Use of Ceramics as Bone Substitutes in Revision Hip Arthroplasty. Materials 2009, 2, 1895. [Google Scholar] [CrossRef]
- Huang, C.H.; Yoshimura, M. Direct Ceramic Coating of Calcium Phosphate Doped with Strontium via Reactive Growing Integration Layer Method on α-Ti Alloy. Sci. Rep. 2020, 10, 10602. [Google Scholar] [CrossRef]
- Lu, J.; Yu, H.; Chen, C. Biological Properties of Calcium Phosphate Biomaterials for Bone Repair: A Review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Díaz-Cuenca, A.; Rabadjieva, D.; Sezanova, K.; Gergulova, R.; Ilieva, R.; Tepavitcharova, S. Biocompatible Calcium Phosphate-Based Ceramics and Composites. Mater. Today Proc. 2022. [Google Scholar] [CrossRef]
- Kim, M.; An, S.; Huh, C.; Kim, C. Development of Zirconium-Based Alloys with Low Elastic Modulus for Dental Implant Materials. Appl. Sci. 2019, 9, 5281. [Google Scholar] [CrossRef]
- Ridzwan, M.I.Z.; Shuib, S.; Hassan, A.Y.; Shokri, A.A.; Mohammad Ibrahim, M.N. Problem of Stress Shielding and Improvement to the Hip Implant Designs: A Review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cicciù, M.; Tozum, T.F.; Saccucci, M.; Orlando, C.; Romano, G.L.; D’amico, C.; Cervino, G. Endosseous Dental Implant Materials and Clinical Outcomes of Different Alloys: A Systematic Review. Materials 2022, 15, 1979. [Google Scholar] [CrossRef]
- Abbas, Z.; Dapporto, M.; Tampieri, A.; Sprio, S. Toughening of Bioceramic Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 259. [Google Scholar] [CrossRef]
- Díez-Pascual, M.; Li, M.; Komasa, S.; Hontsu, S.; Hashimoto, Y.; Okazaki, J. Structural Characterization and Osseointegrative Properties of Pulsed Laser-Deposited Fluorinated Hydroxyapatite Films on Nano-Zirconia for Implant Applications. Int. J. Mol. Sci. 2022, 23, 2416. [Google Scholar] [CrossRef]
- Smargiassi, A.; Bertacchini, J.; Checchi, M.; Cavani, F.; Ferretti, M.; Palumbo, C. Biocompatibility Analyses of Al2O3-Treated Titanium Plates Tested with Osteocyte and Fibroblast Cell Lines. Biomedicines 2017, 5, 32. [Google Scholar] [CrossRef]
- Lam, M.T.; Wu, J.C. Biomaterial Applications in Cardiovascular Tissue Repair and Regeneration. Expert Rev. Cardiovasc. Ther. 2012, 10, 1039. [Google Scholar] [CrossRef]
- Ohta, S.; Mitsuhashi, K.; Chandel, A.K.S.; Qi, P.; Nakamura, N.; Nakamichi, A.; Yoshida, H.; Yamaguchi, G.; Hara, Y.; Sasaki, R.; et al. Silver-Loaded Carboxymethyl Cellulose Nonwoven Sheet with Controlled Counterions for Infected Wound Healing. Carbohydr. Polym. 2022, 286, 119289. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 19. [Google Scholar] [CrossRef]
- Cevik, P.; Schimmel, M.; Yilmaz, B. New Generation CAD-CAM Materials for Implant-Supported Definitive Frameworks Fabricated by Using Subtractive Technologies. BioMed Res. Int. 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Vigier, S.; Fülöp, T. Exploring the Extracellular Matrix to Create Biomaterials. In Composition and Function of the Extracellular Matrix in the Human Body; InTech: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Polyetheretherketone and Its Composites for Bone Replacement and Regeneration. Polymers 2020, 12, 2858. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-Assembly of Partially Alkylated Dextran-Graft-Poly[(2-Dimethylamino)Ethyl Methacrylate] Copolymer Facilitating Hydrophobic/Hydrophilic Drug Delivery and Improving Conetwork Hydrogel Properties. Biomacromolecules 2018, 19, 1142–1153. [Google Scholar] [CrossRef]
- Nutan, B.; Chandel, A.K.S.; Jewrajka, S.K. Liquid Prepolymer-Based in Situ Formation of Degradable Poly(Ethylene Glycol)-Linked-Poly(Caprolactone)-Linked-Poly(2-Dimethylaminoethyl)Methacrylate Amphiphilic Conetwork Gels Showing Polarity Driven Gelation and Bioadhesion. ACS Appl. Bio Mater. 2018, 1, 1606–1619. [Google Scholar] [CrossRef]
- Tallet, L.; Gribova, V.; Ploux, L.; Vrana, N.E.; Lavalle, P. New Smart Antimicrobial Hydrogels, Nanomaterials, and Coatings: Earlier Action, More Specific, Better Dosing? Adv. Healthc. Mater. 2021, 10, 2001199. [Google Scholar] [CrossRef]
- Paroha, S.; Chandel, A.K.S.; Dubey, R.D. Nanosystems for Drug Delivery of Coenzyme Q10. Environ. Chem. Lett. 2018, 16, 71–77. [Google Scholar] [CrossRef]
- Paroha, S.; Chandel, A.K.S.; Dubey, R.D. Nanotechnology Delivery Systems of Coenzyme Q10: Pharmacokinetic and Clinical Implications; Springer: Cham, Switzerland, 2017; pp. 213–228. [Google Scholar]
- Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Effect of Polyethylene Glycol on Properties and Drug Encapsulation-Release Performance of Biodegradable/Cytocompatible Agarose-Polyethylene Glycol-Polycaprolactone Amphiphilic Co-Network Gels. ACS Appl. Mater. Interfaces 2016, 8, 3182–3192. [Google Scholar] [CrossRef] [PubMed]
- Dupin, D.; Deng, Z.; Guo, Y.; Zhao, X.; Du, T.; Zhu, J.; Xie, Y.; Wu, F.; Wang, Y.; Guan, M. Poly(N-Isopropylacrylamide) Based Electrically Conductive Hydrogels and Their Applications. Gels 2022, 8, 280. [Google Scholar] [CrossRef]
- Romischke, J.; Scherkus, A.; Saemann, M.; Krueger, S.; Bader, R.; Kragl, U.; Meyer, J. Swelling and Mechanical Characterization of Polyelectrolyte Hydrogels as Potential Synthetic Cartilage Substitute Materials. Gels 2022, 8, 296. [Google Scholar] [CrossRef]
- Liu, S.; Khan, R.; Zaman, M.; Salawi, A.; Khan, M.A.; Iqbal, M.O.; Riaz, R.; Ahmed, M.M.; Butt, M.H.; Alvi, M.N.; et al. Synthesis of Chemically Cross-Linked PH-Sensitive Hydrogels for the Sustained Delivery of Ezetimibe. Gels 2022, 8, 281. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Yang, D.H.; Ha, M.Y.; Kim, H.J.; Kim, C.H.; Kim, S.H.; Choi, J.W.; Chun, H.J. 3D Printing of Cell-Laden Visible Light Curable Glycol Chitosan Bioink for Bone Tissue Engineering. Carbohydr. Polym. 2022, 287, 119328. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying Design of Biodegradable Zinc as Promising Bone Implants for Load-Bearing Applications. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Filgueira, L. Metal Is Not Inert: Role of Metal Ions Released by Biocorrosion in Aseptic Loosening—Current Concepts. J. Biomed. Mater. Res. A 2009, 91, 1252–1262. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, H.; Tandan, A. Technical Complications of Implant-Causes and Management: A Comprehensive Review. Natl. J. Maxillofac. Surg. 2015, 6, 3. [Google Scholar] [CrossRef]
- Siti Nur Hazwani, M.R.; LIM, L.X.; Lockman, Z.; Zuhailawati, H. Fabrication of Titanium-Based Alloys with Bioactive Surface Oxide Layer as Biomedical Implants: Opportunity and Challenges. Trans. Nonferrous Met. Soc. China 2022, 32, 1–44. [Google Scholar] [CrossRef]
- Hermawan, H.; Ramdan, D.; Djuansjah, J.R.P. Metals for Biomedical Applications. In Biomedical Engineering—From Theory to Applications; InTech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Hanawa, T. Research and Development of Metals for Medical Devices Based on Clinical Needs. Sci. Technol. Adv. Mater. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wiame, F.; Maurice, V.; Marcus, P. Origin of Nanoscale Heterogeneity in the Surface Oxide Film Protecting Stainless Steel against Corrosion. npj Mater. Degrad. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Marcolongo, M.; Sarkar, S.; Ganesh, N. 7.11 Trends in Materials for Spine Surgery. Compr. Biomater. II 2017, 175–198. [Google Scholar] [CrossRef]
- Yan, X.; Cao, W.; Li, H. Biomedical Alloys and Physical Surface Modifications: A Mini-Review. Materials 2021, 15, 66. [Google Scholar] [CrossRef]
- Li, M.; Yin, T.; Wang, Y.; Du, F.; Zou, X.; Gregersen, H.; Wang, G. Study of Biocompatibility of Medical Grade High Nitrogen Nickel-Free Austenitic Stainless Steel in Vitro. Mater. Sci. Eng. C 2014, 43, 641–648. [Google Scholar] [CrossRef]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical Titanium Alloys with Young’s Moduli Close to That of Cortical Bone. Regen. Biomater. 2016, 3, 173. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709. [Google Scholar] [CrossRef]
- Noyama, Y.; Miura, T.; Ishimoto, T.; Itaya, T.; Niinomi, M.; Nakano, T. Bone Loss and Reduced Bone Quality of the Human Femur after Total Hip Arthroplasty under Stress-Shielding Effects by Titanium-Based Implant. Mater. Trans. 2012, 53, 565–570. [Google Scholar] [CrossRef]
- Plecko, M.; Sievert, C.; Andermatt, D.; Frigg, R.; Kronen, P.; Klein, K.; Stübinger, S.; Nuss, K.; Bürki, A.; Ferguson, S.; et al. Osseointegration and Biocompatibility of Different Metal Implants—A Comparative Experimental Investigation in Sheep. BMC Musculoskelet. Disord. 2012, 13, 32. [Google Scholar] [CrossRef]
- Garcia-Mendez, M.C.; Urrutia-Baca, V.H.; Cuao-Moreu, C.A.; Lorenzo-Bonet, E.; Alvarez-Vera, M.; Ortiz-Martinez, D.M.; de la Garza-Ramos, M.A. In Vitro Biocompatibility Evaluation of a New Co-Cr-B Alloy with Potential Biomedical Application. Metals 2021, 11, 1267. [Google Scholar] [CrossRef]
- Marti, A. Cobalt-Base Alloys Used in Surgery. Injury 2000, 31. [Google Scholar] [CrossRef]
- Yu, W.; Li, X.; Ma, X.; Xu, X. Biomechanical Analysis of Inclined and Cantilever Design with Different Implant Framework Materials in Mandibular Complete-Arch Implant Restorations. J. Prosthet. Dent. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kaivosoja, E.; Tiainen, V.-M.; Takakubo, Y.; Rajchel, B.; Sobiecki, J.; Konttinen, Y.T.; Takagi, M. Materials Used for Hip and Knee Implants. Wear Orthop. Implant. Artif. Jt. 2013, 178–218. [Google Scholar] [CrossRef]
- Nine, M.J.; Choudhury, D.; Hee, A.C.; Mootanah, R.; Osman, N.A.A. Wear Debris Characterization and Corresponding Biological Response: Artificial Hip and Knee Joints. Materials 2014, 7, 980. [Google Scholar] [CrossRef] [PubMed]
- González-Mora, V.A.; Hoffmann, M.; Stroosnijder, R.; Espinar, E.; Llamas, J.M.; Fernández-Fairén, M.; Gil, F.J. Influence of Different CoCrMo Counterfaces on Wear in UHMWPE for Artificial Joints. J. Biomed. Sci. Eng. 2011, 4, 375–382. [Google Scholar] [CrossRef][Green Version]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Guo, Z.; Pang, X.; Yan, Y.; Gao, K.; Volinsky, A.A.; Zhang, T.Y. CoCrMo Alloy for Orthopedic Implant Application Enhanced Corrosion and Tribocorrosion Properties by Nitrogen Ion Implantation. Appl. Surf. Sci. 2015, 347, 23–34. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Hu, X.; Dong, H. Surface Modification of a Medical Grade Co-Cr-Mo Alloy by Low-Temperature Plasma Surface Alloying with Nitrogen and Carbon. Surf. Coat. Technol. 2013, 232, 906–911. [Google Scholar] [CrossRef]
- Lourenço, M.L.; Cardoso, G.C.; Sousa, K.d.S.J.; Donato, T.A.G.; Pontes, F.M.L.; Grandini, C.R. Development of Novel Ti-Mo-Mn Alloys for Biomedical Applications. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J. Metallic Biomaterials: State of the Art and New Challenges. Fundam. Biomater. Met. 2018, 1–33. [Google Scholar] [CrossRef]
- Sarraf, M.; Rezvani Ghomi, E.; Alipour, S.; Ramakrishna, S.; Liana Sukiman, N. A State-of-the-Art Review of the Fabrication and Characteristics of Titanium and Its Alloys for Biomedical Applications. Bio-Des. Manuf. 2021, 1, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Estrin, Y.; Kim, H.E.; Lapovok, R.; Ng, H.P.; Jo, J.H. Mechanical Strength and Biocompatibility of Ultrafine-Grained Commercial Purity Titanium. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Mughal, M.P.; Ahmed, N.; Mufti, N.A.; Al-Ahmari, A.M.; He, Y. On the Investigation of Surface Integrity of Ti6Al4V ELI Using Si-Mixed Electric Discharge Machining. Materials 2020, 13, 1549. [Google Scholar] [CrossRef]
- Singh, N.; Hameed, P.; Ummethala, R.; Manivasagam, G.; Prashanth, K.G.; Eckert, J. Selective Laser Manufacturing of Ti-Based Alloys and Composites: Impact of Process Parameters, Application Trends, and Future Prospects. Mater. Today Adv. 2020, 8, 100097. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical Applications of Titanium and Its Alloys. Jom 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Lampman, S. Titanium and Its Alloys for Biomedical Implants. Mater. Med. Devices 2012, 223–236. [Google Scholar] [CrossRef]
- Martin, J.W. Metals and Alloys. Mater. Eng. 2006, 71–132. [Google Scholar] [CrossRef]
- Khadija, G.; Saleem, A.; Akhtar, Z.; Naqvi, Z.; Gull, M.; Masood, M.; Mukhtar, S.; Batool, M.; Saleem, N.; Rasheed, T.; et al. Short Term Exposure to Titanium, Aluminum and Vanadium (Ti 6Al 4V) Alloy Powder Drastically Affects Behavior and Antioxidant Metabolites in Vital Organs of Male Albino Mice. Toxicol. Rep. 2018, 5, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium Ionic Species from Degradation of Ti-6Al-4V Metallic Implants: In Vitro Cytotoxicity and Speciation Evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ji, X.; Zhao, M.; Li, D. Mg/Ag Ratios Induced in Vitro Cell Adhesion and Preliminary Antibacterial Properties of TiN on Medical Ti-6Al-4V Alloy by Mg and Ag Implantation. Surf. Coat. Technol. 2020, 397, 126020. [Google Scholar] [CrossRef]
- Chlebus, E.; Kuźnicka, B.; Kurzynowski, T.; Dybała, B. Microstructure and Mechanical Behaviour of Ti-6Al-7Nb Alloy Produced by Selective Laser Melting. Mater. Charact. 2011, 62, 488–495. [Google Scholar] [CrossRef]
- Challa, V.S.A.; Mali, S.; Misra, R.D.K. Reduced Toxicity and Superior Cellular Response of Preosteoblasts to Ti-6Al-7Nb Alloy and Comparison with Ti-6Al-4V. J. Biomed. Mater. Res. Part A 2013, 101A, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Lyczkowska, E.; Szymczyk, P.; Dybała, B.; Chlebus, E. Chemical Polishing of Scaffolds Made of Ti-6Al-7Nb Alloy by Additive Manufacturing. Arch. Civ. Mech. Eng. 2014, 14, 586–594. [Google Scholar] [CrossRef]
- Niu, Q.L.; Zheng, X.H.; Ming, W.W.; Chen, M. Friction and Wear Performance of Titanium Alloys against Tungsten Carbide under Dry Sliding and Water Lubrication. Tribol. Trans. 2013, 56, 101–108. [Google Scholar] [CrossRef]
- Fellah, M.; Labaïz, M.; Assala, O.; Dekhil, L.; Taleb, A.; Rezag, H.; Iost, A. Tribological Behavior of Ti-6Al-4V and Ti-6Al-7Nb Alloys for Total Hip Prosthesis. Adv. Tribol. 2014, 2014. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.; Romanos, G. Potential Causes of Titanium Particle and Ion Release in Implant Dentistry: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef]
- Shuai, C.; Li, S.; Peng, S.; Feng, P.; Lai, Y.; Gao, C. Biodegradable Metallic Bone Implants. Mater. Chem. Front. 2019, 3, 544–562. [Google Scholar] [CrossRef]
- Harrison, R.; Maradze, D.; Lyons, S.; Zheng, Y.; Liu, Y. Corrosion of Magnesium and Magnesium–Calcium Alloy in Biologically-Simulated Environment. Prog. Nat. Sci. Mater. Int. 2014, 24, 539–546. [Google Scholar] [CrossRef]
- Amukarimi, S.; Mozafari, M. Biodegradable Magnesium-based Biomaterials: An Overview of Challenges and Opportunities. MedComm 2021, 2, 123. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg Alloys for Orthopedic Implants—A Review. J. Magnes. Alloy. 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Alam, M.E.; Pal, S.; Decker, R.; Ferreri, N.C.; Knezevic, M.; Beyerlein, I.J. Rare-Earth- and Aluminum-Free, High Strength Dilute Magnesium Alloy for Biomedical Applications. Sci. Rep. 2020, 10, 15839. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The Role and Significance of Magnesium in Modern Day Research—A Review. J. Magnes. Alloy. 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Loukil, N. Alloying Elements of Magnesium Alloys: A Literature Review. In Magnesium Alloys [Working Title]; InTech: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of Magnesium-Based Biomaterials and Their Applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Wang, Y.; Zeng, X.; Ding, W. Effect of Nd and Y Addition on Microstructure and Mechanical Properties of As-Cast Mg-Zn-Zr Alloy. J. Alloys Compd. 2007, 427, 115–123. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Qi, M.; Liao, L.; Gao, Z. Enhanced Mechanical Properties of Mg-Zn-Y-Zr Alloy by Low-Speed Indirect Extrusion. J. Mater. Res. Technol. 2020, 9, 9856–9867. [Google Scholar] [CrossRef]
- Wang, S.; Han, Z.; Nie, Y.; Dai, J.; Li, X.; Cheng, J.; Zhang, X. Modified Mechanical Properties of Mg-Nd-Zn-Ag-Zr Alloy by Solution Treatment for Cardiovascular Stent Application. Mater. Res. Express 2019, 6, 085416. [Google Scholar] [CrossRef]
- Jamel, M.M.; Jamel, M.M.; Lopez, H.F. Designing Advanced Biomedical Biodegradable Mg Alloys: A Review. Metals 2022, 12, 85. [Google Scholar] [CrossRef]
- Ralls, A.; Kumar, P.; Misra, M.; Menezes, P.L. Material Design and Surface Engineering for Bio-Implants. JOM 2020, 72, 684–696. [Google Scholar] [CrossRef]
- Hussain, M.; Askari Rizvi, S.H.; Abbas, N.; Sajjad, U.; Shad, M.R.; Badshah, M.A.; Malik, A.I. Recent Developments in Coatings for Orthopedic Metallic Implants. Coatings 2021, 11, 791. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Migonney, V.; Falentin-Daudre, C. Review of Silicone Surface Modification Techniques and Coatings for Antibacterial/Antimicrobial Applications to Improve Breast Implant Surfaces. Acta Biomater. 2021, 121, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Sikder, P.; Ren, Y.; Bhaduri, S.B. Synthesis and Evaluation of Protective Poly(Lactic Acid) and Fluorine-Doped Hydroxyapatite–Based Composite Coatings on AZ31 Magnesium Alloy. J. Mater. Res. 2019, 34, 3766–3776. [Google Scholar] [CrossRef]
- Metroke, T.L.; Parkhill, R.L.; Knobbe, E.T. Passivation of Metal Alloys Using Sol–Gel-Derived Materials—A Review. Prog. Org. Coat. 2001, 41, 233–238. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface Modification of Stainless Steel for Biomedical Applications: Revisiting a Century-Old Material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef]
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Nilawar, S.; Uddin, M.; Chatterjee, K. Surface Engineering of Biodegradable Implants: Emerging Trends in Bioactive Ceramic Coatings and Mechanical Treatments. Mater. Adv. 2021, 2, 7820–7841. [Google Scholar] [CrossRef]
- Friedrich, K.; Zhang, Z.; Schlarb, A.K. Effects of Various Fillers on the Sliding Wear of Polymer Composites. Compos. Sci. Technol. 2005, 65, 2329–2343. [Google Scholar] [CrossRef]
- Almasi, D.; Iqbal, N.; Sadeghi, M.; Sudin, I.; Abdul Kadir, M.R.; Kamarul, T. Preparation Methods for Improving PEEK’s Bioactivity for Orthopedic and Dental Application: A Review. Int. J. Biomater. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.P.; Friedrich, K. On Sliding Friction and Wear of PEEK and Its Composites. Wear 1995, 181–183, 624–631. [Google Scholar] [CrossRef]
- Vande Voort, J.; Bahadur, S. The Growth and Bonding of Transfer Film and the Role of CuS and PTFE in the Tribological Behavior of PEEK. Wear 1995, 181–183, 212–221. [Google Scholar] [CrossRef]
- Zhang, G.; Leparoux, S.; Liao, H.; Coddet, C. Microwave Sintering of Poly-Ether-Ether-Ketone (PEEK) Based Coatings Deposited on Metallic Substrate. Scr. Mater. 2006, 55, 621–624. [Google Scholar] [CrossRef]
- Normand, B.; Takenouti, H.; Keddam, M.; Liao, H.; Monteil, G.; Coddet, C. Electrochemical Impedance Spectroscopy and Dielectric Properties of Polymer: Application to PEEK Thermally Sprayed Coating. Electrochim. Acta 2004, 49, 2981–2986. [Google Scholar] [CrossRef]
- Zhang, G.; Liao, H.; Yu, H.; Ji, V.; Huang, W.; Mhaisalkar, S.G.; Coddet, C. Correlation of Crystallization Behavior and Mechanical Properties of Thermal Sprayed PEEK Coating. Surf. Coat. Technol. 2006, 200, 6690–6695. [Google Scholar] [CrossRef]
- Fiołek, A.; Zimowski, S.; Kopia, A.; Łukaszczyk, A.; Moskalewicz, T. Electrophoretic Co-Deposition of Polyetheretherketone and Graphite Particles: Microstructure, Electrochemical Corrosion Resistance, and Coating Adhesion to a Titanium Alloy. Materials 2020, 13, 3251. [Google Scholar] [CrossRef]
- Sak, A.; Moskalewicz, T.; Zimowski, S.; Cieniek, Ł.; Dubiel, B.; Radziszewska, A.; Kot, M.; Łukaszczyk, A. Influence of Polyetheretherketone Coatings on the Ti-13Nb-13Zr Titanium Alloy’s Bio-Tribological Properties and Corrosion Resistance. Mater. Sci. Eng. C 2016, 63, 52–61. [Google Scholar] [CrossRef]
- Virk, R.S.; Ur Rehman, M.A.; Boccaccini, A.R. PEEK Based Biocompatible Coatings Incorporating H-BN and Bioactive Glass by Electrophoretic Deposition. ECS Trans. 2018, 82, 89–95. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Wei, J.; Ma, J.; Deng, F.; Wei, S. Nano-TiO2/PEEK Bioactive Composite as a Bone Substitute Material: In Vitro and in Vivo Studies. Int. J. Nanomed. 2012, 7, 1215–1225. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Zych, A.; Łukaszczyk, A.; Cholewa-Kowalska, K.; Kruk, A.; Dubiel, B.; Radziszewska, A.; Berent, K.; Gajewska, M. Electrophoretic Deposition, Microstructure, and Corrosion Resistance of Porous Sol–Gel Glass/Polyetheretherketone Coatings on the Ti-13Nb-13Zr Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 2660–2673. [Google Scholar] [CrossRef][Green Version]
- Atiq Ur Rehman, M.; Bastan, F.E.; Haider, B.; Boccaccini, A.R. Electrophoretic Deposition of PEEK/Bioactive Glass Composite Coatings for Orthopedic Implants: A Design of Experiments (DoE) Study. Mater. Des. 2017, 130, 223–230. [Google Scholar] [CrossRef]
- Lebga-Nebane, J.L.; Sankarasubramanian, M.; Chojecki, G.; Ning, B.; Yuya, P.A.; Moosbrugger, J.C.; Rasmussen, D.H.; Krishnan, S. Polyetheretherketone, Hexagonal Boron Nitride, and Tungsten Carbide Cobalt Chromium Composite Coatings: Mechanical and Tribological Properties. J. Appl. Polym. Sci. 2021, 138, 50504. [Google Scholar] [CrossRef]
- Seuss, S.; Heinloth, M.; Boccaccini, A.R. Development of Bioactive Composite Coatings Based on Combination of PEEK, Bioactive Glass and Ag Nanoparticles with Antibacterial Properties. Surf. Coat. Technol. 2016, 301, 100–105. [Google Scholar] [CrossRef]
- Garrido, B.; Albaladejo-Fuentes, V.; Cano, I.G.; Dosta, S. Development of Bioglass/PEEK Composite Coating by Cold Gas Spray for Orthopedic Implants. J. Therm. Spray Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- Torres, Y.; Romero, C.; Chen, Q.; Pérez, G.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; Álvarez, L.; Arévalo, C.; Boccaccini, A.R. Electrophoretic Deposition of PEEK/45S5 Bioactive Glass Coating on Porous Titanium Substrate: Influence of Processing Conditions and Porosity Parameters. Key Eng. Mater. 2016, 704, 343–350. [Google Scholar] [CrossRef]
- Tharajak, J.; Palathai, T.; Sombatsompop, N. Morphological and Physical Properties and Friction/Wear Behavior of h-BN Filled PEEK Composite Coatings. Surf. Coat. Technol. 2015, 273, 20–29. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Zimowski, S.; Zych, A.; Łukaszczyk, A.; Reczyńska, K.; Pamuła, E. Electrophoretic Deposition, Microstructure and Selected Properties of Composite Alumina/Polyetheretherketone Coatings on the Ti-13Nb-13Zr Alloy. J. Electrochem. Soc. 2018, 165, D116–D128. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Zych, A.; Kruk, A.; Kopia, A.; Zimowski, S.; Sitarz, M.; Cieniek, Ł. Electrophoretic Deposition and Microstructure Development of Si3N4/Polyetheretherketone Coatings on Titanium Alloy. Surf. Coat. Technol. 2018, 350, 633–647. [Google Scholar] [CrossRef]
- Baştan, F.E.; Atiq Ur Rehman, M.; Avcu, Y.Y.; Avcu, E.; Üstel, F.; Boccaccini, A.R. Electrophoretic Co-Deposition of PEEK-Hydroxyapatite Composite Coatings for Biomedical Applications. Colloids Surf. B Biointerfaces 2018, 169, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jang, H.L.; Lee, K.M.; Baek, H.R.; Jin, K.; Noh, J.H. Cold-Spray Coating of Hydroxyapatite on a Three-Dimensional Polyetheretherketone Implant and Its Biocompatibility Evaluated by in Vitro and in Vivo Minipig Model. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2017, 105, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Kadiyala, A.K.; Bijwe, J.; Kalappa, P. Investigations on Influence of Nano and Micron Sized Particles of SiC on Performance Properties of PEEK Coatings. Surf. Coat. Technol. 2018, 334, 124–133. [Google Scholar] [CrossRef]
- Abdulkareem, M.H.; Abdalsalam, A.H.; Bohan, A.J. Influence of Chitosan on the Antibacterial Activity of Composite Coating (PEEK /HAp) Fabricated by Electrophoretic Deposition. Prog. Org. Coat. 2019, 130, 251–259. [Google Scholar] [CrossRef]
- Chen, X.; Ma, R.; Min, J.; Li, Z.; Yu, P.; Yu, H. Effect of PEEK and PTFE Coatings in Fatigue Performance of Dental Implant Retaining Screw Joint: An in Vitro Study. J. Mech. Behav. Biomed. Mater. 2020, 103. [Google Scholar] [CrossRef]
- Song, J.; Liao, Z.; Shi, H.; Xiang, D.; Xu, L.; Liu, Y.; Mu, X.; Liu, W. Blood Compatibility of ZrO2 Particle Reinforced PEEK Coatings on Ti6Al4V Substrates. Polymers 2017, 9, 589. [Google Scholar] [CrossRef]
- Song, J.; Liao, Z.; Wang, S.; Liu, Y.; Liu, W.; Tyagi, R. Study on the Tribological Behaviors of Different PEEK Composite Coatings for Use as Artificial Cervical Disk Materials. J. Mater. Eng. Perform. 2016, 25, 116–129. [Google Scholar] [CrossRef]
- Xie, C.; Li, P.; Liu, Y.; Luo, F.; Xiao, X. Preparation of TiO2 Nanotubes/Mesoporous Calcium Silicate Composites with Controllable Drug Release. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 433–439. [Google Scholar] [CrossRef]
- Pradhan, D.; Wren, A.W.; Misture, S.T.; Mellott, N.P. Investigating the Structure and Biocompatibility of Niobium and Titanium Oxides as Coatings for Orthopedic Metallic Implants. Mater. Sci. Eng. C 2016, 58, 918–926. [Google Scholar] [CrossRef]
- Mirak, M.; Alizadeh, M.; Ashtiani, M.N. Characterization, Mechanical Properties and Corrosion Resistance of Biocompatible Zn-HA/TiO2 Nanocomposite Coatings. J. Mech. Behav. Biomed. Mater. 2016, 62, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Q.; Zi, T.Q.; Zhao, X.R.; Liu, C.; Ren, Q.; Fang, J.B.; Li, W.M.; Li, A.D. Enhanced Visible Light Photocatalytic Activity of Fe2O3 Modified TiO2 Prepared by Atomic Layer Deposition. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, G.; Zhang, H.; Hang, R.; Huang, X.; Yao, X.; Zhang, X. Cu and Si Co-Doped Microporous TiO2 Coating for Osseointegration by the Coordinated Stimulus Action. Appl. Surf. Sci. 2020, 503, 144072. [Google Scholar] [CrossRef]
- Gartner, M.; Trapalis, C.; Todorova, N.; Giannakopoulou, T.; Dobrescu, G.; Anastasescu, M.; Osiceanu, P.; Ghita, A.; Enache, M.; Dumitru, L.; et al. Doped Sol-Gel TiO2 Films for Biological Applications. Bull. Korean Chem. Soc. 2008, 29, 1038–1042. [Google Scholar] [CrossRef]
- Radeka, M.; Markov, S.; Lončar, E.; Rudić, O.; Vučetić, S.; Ranogajec, J. Photocatalytic Effects of TiO2 Mesoporous Coating Immobilized on Clay Roofing Tiles. J. Eur. Ceram. Soc. 2014, 34, 127–136. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Heo, J.; Hong, B.Y.; Hong, J. Growth Behaviors and Biocidal Properties of Titanium Dioxide Films Depending on Nucleation Duration in Liquid Phase Deposition. Appl. Surf. Sci. 2017, 425, 547–552. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Gobouri, A.A.; Al-Yasi, H.M.; Al-Talhi, T.A.; El-Megharbel, S.M. A New Sterilization Strategy Using TiO2 Nanotubes for Production of Free Radicals That Eliminate Viruses and Application of a Treatment Strategy to Combat Infections Caused by Emerging SARS-CoV-2 during the COVID-19 Pandemic. Coatings 2021, 11, 680. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. Advanced Titanium Dioxide-Polytetrafluorethylene (TiO2-PTFE) Nanocomposite Coatings on Stainless Steel Surfaces with Antibacterial and Anti-Corrosion Properties. Appl. Surf. Sci. 2019, 490, 231–241. [Google Scholar] [CrossRef]
- Yetim, T. Corrosion Behavior of Ag-Doped TiO2 Coatings on Commercially Pure Titanium in Simulated Body Fluid Solution. J. Bionic Eng. 2016, 13, 397–405. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Chien, M.-Y.; Chen, Y.-W. Antiviral and Antibacterial Effects of Silver-Doped TiO 2 Prepared by the Peroxo Sol-Gel Method. J. Nanosci. Nanotechnol. 2019, 19, 7356–7362. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, C.; Xu, Y.; Jia, H.; Chen, X.; Bai, Y.; Wei, B. Efficiently Reduced Heat Rise in TiO 2 Coating Ti-Based Metallic Implants Using Anodic Oxidation Method. Surf. Coat. Technol. 2019, 363, 75–79. [Google Scholar] [CrossRef]
- Xian, P.; Chen, Y.; Gao, S.; Qian, J.; Zhang, W.; Udduttula, A.; Huang, N.; Wan, G. Polydopamine (PDA) Mediated Nanogranular-Structured Titanium Dioxide (TiO2) Coating on Polyetheretherketone (PEEK) for Oral and Maxillofacial Implants Application. Surf. Coat. Technol. 2020, 401. [Google Scholar] [CrossRef]
- Chai, M.; An, M.; Zhang, X. Construction of a TiO2/MoSe2/CHI Coating on Dental Implants for Combating Streptococcus Mutans Infection. Mater. Sci. Eng. C 2021, 129. [Google Scholar] [CrossRef] [PubMed]
- Kiran, A.S.K.; Kumar, T.S.S.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and Bioactive Surface Modifications of Titanium Implants by PCL/TiO2 Nanocomposite Coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef] [PubMed]
- Doll, P.W.; Ahrens, R.; Guber, A.E. Etch-Less Microfabrication of Structured TiO2 Implant Coatings on Bulk Titanium Grade 23 by Direct Lithographic Anodic Oxidation. J. Micromech. Microeng. 2021, 31. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, T.; Wen, L.M.; Li, R.; Zhang, Y.B.; Bi, W.J.; Feng, X.J.; Qi, M.C. Osteogenic Capability of Strontium and Icariin-Loaded TiO2 Nanotube Coatings in Vitro and in Osteoporotic Rats. J. Biomater. Appl. 2021, 35, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Duan, L.; Weng, W.; Cheng, K.; Wang, D.; Ouyang, H. Light-Induced Osteogenic Differentiation of BMSCs with Graphene/TiO2 Composite Coating on Ti Implant. Colloids Surf. B Biointerfaces 2021, 207. [Google Scholar] [CrossRef]
- Visentin, F.; el Habra, N.; Fabrizio, M.; Brianese, N.; Gerbasi, R.; Nodari, L.; Zin, V.; Galenda, A. TiO2-HA Bi-Layer Coatings for Improving the Bioactivity and Service-Life of Ti Dental Implants. Surf. Coat. Technol. 2019, 378. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Gao, S.; Li, Y.; Cao, R.; Cheng, J.; Li, R.; Wang, E.; Guo, Y.; Zhang, K.; et al. Y-Doped TiO2 Coating with Superior Bioactivity and Antibacterial Property Prepared via Plasma Electrolytic Oxidation. Mater. Des. 2020, 192. [Google Scholar] [CrossRef]
- Li, K.; Liu, S.; Xue, Y.; Zhang, L.; Han, Y. A Superparamagnetic Fe3O4-TiO2 Composite Coating on Titanium by Micro-Arc Oxidation for Percutaneous Implants. J. Mater. Chem. B 2019, 7, 5265–5276. [Google Scholar] [CrossRef]
- Gobbi, S.J. Orthopedic Implants: Coating with TiN. Biomed. J. Sci. Tech. Res. 2019, 16. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Kong, C.H.; Webster, R.F.; Tilley, R.D.; Takeda, Y.; Oyoshi, K.; Bondar, O.V.; Buranich, V.V.; Konstantinov, S.V.; Baimoldanova, L.S.; et al. Antibacterial Effect of Au Implantation in Ductile Nanocomposite Multilayer (TiAlSiY)N/CrN Coatings. ACS Appl. Mater. Interfaces 2019, 11, 48540–48550. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, C.Y.; Kim, D.W.; Lee, I.S.; Lee, G.H.; Park, J.C.; Lee, S.J.; Lee, K.Y. Wear Performance of Self-Mating Contact Pairs of TiN and TiAlN Coatings on Orthopedic Grade Ti-6Al-4V. Biomed. Mater. 2010, 5, 044108. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Muraleedharan, C.V.; Ananthakumar, R.; Jayachandran, M. A Comparative Study of Titanium Nitride (TiN), Titanium Oxy Nitride (TiON) and Titanium Aluminum Nitride (TiAlN), as Surface Coatings for Bio Implants. Surf. Coat. Technol. 2011, 205, 5014–5020. [Google Scholar] [CrossRef]
- Van Hove, R.P.; Sierevelt, I.N.; van Royen, B.J.; Nolte, P.A. Titanium-Nitride Coating of Orthopaedic Implants: A Review of the Literature. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Dion, I.; Baquey, C.; Candelon, B.; Monties, J.R. Hemocompatibility of Titanium Nitride. Int. J. Artif. Organs 1992, 15, 617–621. [Google Scholar] [CrossRef]
- Çomaklı, O. Improved Structural, Mechanical, Corrosion and Tribocorrosion Properties of Ti45Nb Alloys by TiN, TiAlN Monolayers, and TiAlN/TiN Multilayer Ceramic Films. Ceram. Int. 2021, 47, 4149–4156. [Google Scholar] [CrossRef]
- Dinu, M.; Pana, I.; Scripca, P.; Sandu, I.G.; Vitelaru, C.; Vladescu, A. Improvement of CoCr Alloy Characteristics by Ti-Based Carbonitride Coatings Used in Orthopedic Applications. Coatings 2020, 10, 495. [Google Scholar] [CrossRef]
- Ul-Hamid, A. Microstructure, Properties and Applications of Zr-Carbide, Zr-Nitride and Zr-Carbonitride Coatings: A Review. Mater. Adv. 2020, 1, 1012–1037. [Google Scholar] [CrossRef]
- Ghufran, M.; Uddin, G.M.; Arafat, S.M.; Jawad, M.; Rehman, A. Development and Tribo-Mechanical Properties of Functional Ternary Nitride Coatings: Applications-Based Comprehensive Review. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2020, 235, 196–232. [Google Scholar] [CrossRef]
- Chen, S.N.; Zhao, Y.M.; Zhang, Y.F.; Chen, L.; Liao, B.; Zhang, X.; Ouyang, X.P. Influence of Carbon Content on the Structure and Tribocorrosion Properties of TiAlCN/TiAlN/TiAl Multilayer Composite Coatings. Surf. Coat. Technol. 2021, 411, 126886. [Google Scholar] [CrossRef]
- Hovsepian, P.E.; Ehiasarian, A.P.; Petrov, I. TiAlCN/VCN Nanolayer Coatings Suitable for Machining of Al and Ti Alloys Deposited by Combined High Power Impulse Magnetron Sputtering/Unbalanced Magnetron Sputtering. Surf. Eng. 2013, 26, 610–614. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Amaya, C.; Yate, L.; Gómez, M.E.; Zambrano, G.; Alvarado-Rivera, J.; Muñoz-Saldaña, J.; Prieto, P. TiCN/TiNbCN Multilayer Coatings with Enhanced Mechanical Properties. Appl. Surf. Sci. 2010, 256, 5898–5904. [Google Scholar] [CrossRef]
- Rajak, D.K.; Kumar, A.; Behera, A.; Menezes, P.L. Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Appl. Sci. 2021, 11, 4445. [Google Scholar] [CrossRef]
- Moritz, Y.; Saringer, C.; Tkadletz, M.; Stark, A.; Schell, N.; Letofsky-Papst, I.; Czettl, C.; Pohler, M.; Schalk, N. Oxidation Behavior of Arc Evaporated TiSiN Coatings Investigated by In-Situ Synchrotron X-Ray Diffraction and HR-STEM. Surf. Coat. Technol. 2020, 404, 126632. [Google Scholar] [CrossRef]
- Parau, A.C.; Vitelaru, C.; Balaceanu, M.; Braic, V.; Constantin, L.R.; Braic, M.; Vladescu, A. TiSiC, TiSiC-Zr, and TiSiC-Cr Coatings—Corrosion Resistance and Tribological Performance in Saline Solution. Tribol. Trans. 2015, 59, 72–79. [Google Scholar] [CrossRef]
- Xie, X.; Li, J.; Dong, M.; Zhang, H.; Wang, L. Structure and Properties of TiSiCN Coatings with Different Bias Voltages by Arc Ion Plating. Surf. Topogr. Metrol. Prop. 2018, 6, 014003. [Google Scholar] [CrossRef]
- Lin, J.; Wei, R.; Ge, F.; Li, Y.; Zhang, X.; Huang, F.; Lei, M. TiSiCN and TiAlVSiCN Nanocomposite Coatings Deposited from Ti and Ti-6Al-4V Targets. Surf. Coat. Technol. 2018, 336, 106–116. [Google Scholar] [CrossRef]
- Varghese, V.; Ramesh, M.R.; Chakradhar, D.; Shaik, H. Characterisation and Performance Evaluation of TiSiN &TiAlSiN Coatings by RF Magnetron Sputtering Deposition during End Milling of Maraging Steel. Mater. Res. Express 2020, 6, 126440. [Google Scholar] [CrossRef]
- Vitu, T.; Polcar, T.; Cvrcek, L.; Novak, R.; Macak, J.; Vyskocil, J.; Cavaleiro, A. Structure and Tribology of Biocompatible Ti-C:H Coatings. Surf. Coat. Technol. 2008, 202, 5790–5793. [Google Scholar] [CrossRef]
- Wan, Q.; Liu, N.; Yang, B.; Liu, H.; Chen, Y. Influence of Si Content on Properties of Ti(1-x)SixN Coatings. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2019, 34, 774–780. [Google Scholar] [CrossRef]
- Yuan, Z.; Han, Y.; Zang, S.; Chen, J.; He, G.; Chai, Y.; Yang, Z.; Fu, Q. Damage Evolution Behavior of TiN/Ti Multilayer Coatings under High-Speed Impact Conditions. Surf. Coat. Technol. 2021, 426, 127807. [Google Scholar] [CrossRef]
- Zhu, L.; Feng, C.; Zhu, S.; Wang, F.; Yuan, J.; Wang, P. Comparison of CrN, AlN and TiN Diffusion Barriers on the Interdiffusion and Oxidation Behaviors of Ni+CrAlYSiN Nanocomposite Coatings. Crystals 2021, 11, 1333. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Jin, S.; Zhao, Y.; Ren, L.; Yang, K. Effect of Copper-Doped Titanium Nitride Coating on Angiogenesis. Mater. Lett. 2020, 269, 127634. [Google Scholar] [CrossRef]
- Castro, J.D.; Lima, M.J.; Carvalho, I.; Henriques, M.; Carvalho, S. Cu Oxidation Mechanism on Cu-Zr(O)N Coatings: Role on Functional Properties. Appl. Surf. Sci. 2021, 555. [Google Scholar] [CrossRef]
- Pana, I.; Braic, V.; Dinu, M.; Massima Mouele, E.S.; Parau, A.C.; Petrik, L.F.; Braic, M. In Vitro Corrosion of Titanium Nitride and Oxynitride-Based Biocompatible Coatings Deposited on Stainless Steel. Coatings 2020, 10, 710. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Kumar, N. Oxynitrides Decorated 316L SS for Potential Bioimplant Application. Mater. Res. Express 2018, 5, 036403. [Google Scholar] [CrossRef]
- Lee, D.B.; Lee, Y.C.; Kwon, S.C. High Temperature Oxidation of TiCrN Coatings Deposited on a Steel Substrate by Ion Plating. Surf. Coat. Technol. 2001, 141, 232–239. [Google Scholar] [CrossRef]
- Chiu, K.A.; Fu, C.W.; Fang, Y.S.; Do, T.H.; Shih, F.H.; Chang, L. Heteroepitaxial Growth and Microwave Plasma Annealing of DC Reactive Sputtering Deposited TiZrN Film on Si (100). Surf. Coat. Technol. 2020, 394, 125873. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Vishwakarma, V.; Ramadoss, A.; Ramachandran, D.; Rabel, A.M. Corrosion, Haemocompatibility and Bacterial Adhesion Behavior of TiZrN-Coated 316L SS for Bioimplants. Bull. Mater. Sci. 2015, 38, 951–955. [Google Scholar] [CrossRef]
- Cui, W.; Qin, G.; Duan, J.; Wang, H. A Graded Nano-TiN Coating on Biomedical Ti Alloy: Low Friction Coefficient, Good Bonding and Biocompatibility. Mater. Sci. Eng. C 2017, 71, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.; Adesina, A.Y.; Kumar, A.M.; Sorour, A.A.; Ankah, N.; Al-Aqeeli, N. Mechanical, in-Vitro Corrosion, and Tribological Characteristics of TiN Coating Produced by Cathodic Arc Physical Vapor Deposition on Ti20Nb13Zr Alloy for Biomedical Applications. Thin Solid Films 2020, 709. [Google Scholar] [CrossRef]
- Uddin, G.M.; Jawad, M.; Ghufran, M.; Saleem, M.W.; Raza, M.A.; Rehman, Z.U.; Arafat, S.M.; Irfan, M.; Waseem, B. Experimental Investigation of Tribo-Mechanical and Chemical Properties of TiN PVD Coating on Titanium Substrate for Biomedical Implants Manufacturing. Int. J. Adv. Manuf. Technol. 2019, 102, 1391–1404. [Google Scholar] [CrossRef]
- Beshchasna, N.; Ho, A.Y.K.; Saqib, M.; Kraśkiewicz, H.; Wasyluk, Ł.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Trusca, R.D.; Ficai, A.; et al. Surface Evaluation of Titanium Oxynitride Coatings Used for Developing Layered Cardiovascular Stents. Mater. Sci. Eng. C 2019, 99, 405–416. [Google Scholar] [CrossRef]
- Banakh, O.; Moussa, M.; Matthey, J.; Pontearso, A.; Cattani-Lorente, M.; Sanjines, R.; Fontana, P.; Wiskott, A.; Durual, S. Sputtered Titanium Oxynitride Coatings for Endosseous Applications: Physical and Chemical Evaluation and First Bioactivity Assays. Appl. Surf. Sci. 2014, 317, 986–993. [Google Scholar] [CrossRef]
- Banaszek, K.; Klimek, L.; Zgorzynska, E.; Swarzynska, A.; Walczewska, A. Cytotoxicity of Titanium Carbonitride Coatings for Prostodontic Alloys with Different Amounts of Carbon and Nitrogen. Biomed. Mater. 2018, 13. [Google Scholar] [CrossRef]
- Huang, H.L.; Chang, Y.Y.; Liu, J.X.; Tsai, M.T.; Lai, C.H. Antibacterial Activity and Cell Compatibility of TiZrN, TiZrCN, and TiZr-Amorphous Carbon Coatings. Thin Solid Films 2015, 596, 111–117. [Google Scholar] [CrossRef]
- Constantin, L.; Braic, M.; Dinu, M.; Balaceanu, M.; Braic, V.; Farcau, C.; Vladescu, A. Effects of Zr, Nb, or Si Addition on the Microstructural, Mechanical, and Corrosion Resistance of TiCN Hard Coatings. Mater. Corros. 2016, 67, 929–938. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, L.; Xiong, W. Tribological Properties of TiAlN-Coated Cermets. Rare Met. 2009, 28, 57–62. [Google Scholar] [CrossRef]
- Miletić, A.; Panjan, P.; Čekada, M.; Kovačević, L.; Terek, P.; Kovač, J.; Dražič, G.; Škorić, B. Nanolayer CrAlN/TiSiN Coating Designed for Tribological Applications. Ceram. Int. 2021, 47, 2022–2033. [Google Scholar] [CrossRef]
- Sampath Kumar, T.; Vinoth Jebaraj, A.; Shankar, E.; Tamiloli, N.; Sivakumar, K. Metallurgical and Mechanical Characterization of TiCN/TiAlN and TiAlN/TiCN Bilayer Nitride Coatings. Surf. Interfaces 2019, 15, 256–264. [Google Scholar] [CrossRef]
- Chetcuti, R.; Dearnley, P.A.; Mazzonello, A.; Buhagiar, J.; Mallia, B. Tribocorrosion Response of Duplex Layered CoCrMoC/CrN and CrN/CoCrMoC Coatings on Implant Grade 316LVM Stainless Steel. Surf. Coat. Technol. 2020, 384. [Google Scholar] [CrossRef]
- Rathmann, L.; Rusche, T.; Hasselbruch, H.; Mehner, A.; Radel, T. Friction and Wear Characterization of LIPSS and TiN / DLC Variants. Appl. Surf. Sci. 2022, 584, 152654. [Google Scholar] [CrossRef]
- Islam, M.; Díaz Lantada, A.; Mager, D.; Korvink, J.G.; Islam, M.; Mager, D.; Korvink, J.G.; Lantada, A.D. Carbon-Based Materials for Articular Tissue Engineering: From Innovative Scaffolding Materials toward Engineered Living Carbon. Adv. Healthc. Mater. 2022, 11, 2101834. [Google Scholar] [CrossRef] [PubMed]
- More, R.B.; Haubold, A.D.; Bokros, J.C. Pyrolytic Carbon for Long-Term Medical Implants. In Biomaterials Science: An Introduction to Materials, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 209–222. [Google Scholar] [CrossRef]
- Ross, M.; Williams, D.; Couzens, G.; Klawitter, J. Pyrocarbon for Joint Replacement. Jt. Replace. Technol. 2021, 145–163. [Google Scholar] [CrossRef]
- Victoria Cabañas, M. Bioceramic Coatings for Medical Implants. Bio-Ceram. Clin. Appl. 2014, 249–289. [Google Scholar] [CrossRef]
- Ren, J.; Lv, C.; Duan, Y.; Zhang, Y.; Zhang, J. Microstructure and Ablation Performance of HfC/PyC Core-Shell Structure Nanowire-Reinforced Hf1-XZrxC Coating. J. Eur. Ceram. Soc. 2021, 41, 7450–7463. [Google Scholar] [CrossRef]
- Feng, S.L.; Yang, Y.G.; Bai, S.; Xu, L.; Yang, X.M.; Xia, H.H.; Zhou, X.T. Microstructure of a Pyrolytic Carbon Coating on a Nuclear Graphite Substrate IG-110. Xinxing Tan Cailiao/New Carbon Mater. 2015, 30, 275–281. [Google Scholar] [CrossRef]
- Adkinson, J.M.; Chung, K.C. Advances in Small Joint Arthroplasty of the Hand. Plast. Reconstr. Surg. 2014, 134, 1260. [Google Scholar] [CrossRef]
- Nistor, P.A.; May, P.W. Diamond Thin Films: Giving Biomedical Applications a New Shine. J. R. Soc. Interface 2017, 14. [Google Scholar] [CrossRef]
- Catledge, S.A.; Thomas, V.; Vohra, Y.K. Nanostructured Diamond Coatings for Orthopaedic Applications. Woodhead Publ. Ser. Biomater. 2013, 2013, 105. [Google Scholar] [CrossRef] [PubMed]
- Medina, O.; Nocua, J.; Mendoza, F.; Gómez-Moreno, R.; Ávalos, J.; Rodríguez, C.; Morell, G. Bactericide and Bacterial Anti-Adhesive Properties of the Nanocrystalline Diamond Surface. Diam. Relat. Mater. 2012, 22, 77–81. [Google Scholar] [CrossRef]
- Siddiqui, S.; Dutta, G.; Tan, C.; Arumugam, P.U. Nanocrystalline Diamond Electrodes: Enabling Electrochemical Microsensing Applications with High Reliability and Stability. IEEE Nanotechnol. Mag. 2016, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.K.; Lee, K.R. Biomedical Applications of Diamond-like Carbon Coatings: A Review. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83, 72–84. [Google Scholar] [CrossRef]
- Peng, F.; Lin, Y.; Zhang, D.; Ruan, Q.; Tang, K.; Li, M.; Liu, X.; Chu, P.K.; Zhang, Y. Corrosion Behavior and Biocompatibility of Diamond-like Carbon-Coated Zinc: An in Vitro Study. ACS Omega 2021, 6, 9843–9851. [Google Scholar] [CrossRef]
- Bean, P.A.; Evans, M.D.; Bendavid, A. Biomineralization of Osteoblasts on DLC Coated Surfaces for Bone Implants. Biointerphases 2018, 13, 041002. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Wu, B.J.; Zhang, T.F.; Leng, Y.X.; Huang, N. Tribocorrosion Behavior of DLC-Coated CoCrMo Alloy in Simulated Biological Environment. Vacuum 2013, 92, 39–43. [Google Scholar] [CrossRef]
- Mo, S.; Zhao, F.; Gao, A.; Wu, Y.; Liao, Q.; Xie, L.; Pan, H.; Tong, L.; Chu, P.K.; Wang, H. Simultaneous Application of Diamond-like Carbon Coating and Surface Amination on Polyether Ether Ketone: Towards Superior Mechanical Performance and Osseointegration. Smart Mater. Med. 2021, 2, 219–228. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, K.R.; Ahn, S.H.; Kim, J.G. Instability of Diamond-like Carbon (DLC) Films during Sliding in Aqueous Environment. Diam. Relat. Mater. 2008, 17, 247–251. [Google Scholar] [CrossRef]
- Lung, B.H.; Chiang, M.J.; Hon, M.H. Effect of Gradient A-SiCx Interlayer on Adhesion of DLC Films. Mater. Chem. Phys. 2001, 72, 163–166. [Google Scholar] [CrossRef]
- Kumar, P.; Babu, P.D.; Mohan, L.; Anandan, C.; Grips, V.K.W. Wear and Corrosion Behavior of Zr-Doped DLC on Ti-13Zr-13Nb Biomedical Alloy. J. Mater. Eng. Perform. 2013, 22, 283–293. [Google Scholar] [CrossRef]
- Hauert, R. DLC Films in Biomedical Applications. Tribol. Diam.-Like Carbon Film. Fundam. Appl. 2008, 494–509. [Google Scholar] [CrossRef]
- Hatem, A.; Lin, J.; Wei, R.; Torres, R.D.; Laurindo, C.; Soares, P. Tribocorrosion Behavior of DLC-Coated Ti-6Al-4V Alloy Deposited by PIID and PEMS + PIID Techniques for Biomedical Applications. Surf. Coat. Technol. 2017, 332, 223–232. [Google Scholar] [CrossRef]
- Joshi, P.; Riley, P.R.; Denning, W.; Shukla, S.; Khosla, N.; Narayan, J.; Narayan, R. Laser-Patterned Carbon Coatings on Flexible and Optically Transparent Plastic Substrates for Advanced Biomedical Sensing and Implant Applications. J. Mater. Chem. C 2022. [Google Scholar] [CrossRef]
- López, R.; Menéndez, M.; Fernández, C.; Chmiela, A.; Bernardo-Sánchez, A. The Influence of Carbon Coatings on the Functional Properties of X39CR13 and 316LVM Steels Intended for Biomedical Applications. Metals 2019, 9, 815. [Google Scholar] [CrossRef]
- Awaja, F.; Cools, P.; Lohberger, B.; Nikiforov, A.Y.; Speranza, G.; Morent, R. Functionalized, Biocompatible, and Impermeable Nanoscale Coatings for PEEK. Mater. Sci. Eng. C 2017, 76, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Lackner, J.; Fleming, R.A.; Goss, J.; Chen, J.; Zou, M. Diamond-like Carbon Coatings with Zirconium-Containing Interlayers for Orthopedic Implants. J. Mech. Behav. Biomed. Mater. 2017, 68, 51–61. [Google Scholar] [CrossRef]
- Bociaga, D.; Sobczyk-Guzenda, A.; Komorowski, P.; Balcerzak, J.; Jastrzebski, K.; Przybyszewska, K.; Kaczmarek, A. Surface Characteristics and Biological Evaluation of Si-DLC Coatings Fabricated Using Magnetron Sputtering Method on Ti6Al7Nb Substrate. Nanomaterials 2019, 9, 812. [Google Scholar] [CrossRef]
- Rothammer, B.; Neusser, K.; Marian, M.; Bartz, M.; Krauß, S.; Böhm, T.; Thiele, S.; Merle, B.; Detsch, R.; Wartzack, S. Amorphous Carbon Coatings for Total Knee Replacements—Part I: Deposition, Cytocompatibility, Chemical and Mechanical Properties. Polymers 2021, 13, 1952. [Google Scholar] [CrossRef]
- Bociaga, D.; Sobczyk-Guzenda, A.; Szymanski, W.; Jedrzejczak, A.; Jastrzebska, A.; Olejnik, A.; Swiatek, L.; Jastrzebski, K. Diamond like Carbon Coatings Doped by Si Fabricated by a Multi-Target DC-RF Magnetron Sputtering Method—Mechanical Properties, Chemical Analysis and Biological Evaluation. Vacuum 2017, 143, 395–406. [Google Scholar] [CrossRef]
- Derakhshandeh, M.R.; Eshraghi, M.J.; Hadavi, M.M.; Javaheri, M.; Khamseh, S.; Sari, M.G.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Diamond-like Carbon Thin Films Prepared by Pulsed-DC PE-CVD for Biomedical Applications. Surf. Innov. 2018, 6, 167–175. [Google Scholar] [CrossRef]
- Wachesk, C.C.; Seabra, S.H.; dos Santos, T.A.T.; Trava-Airoldi, V.J.; Lobo, A.O.; Marciano, F.R. In Vivo Biocompatibility of Diamond-like Carbon Films Containing TiO2 Nanoparticles for Biomedical Applications. J. Mater. Sci. Mater. Med. 2021, 32. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Chen, W.; Zheng, M.; Lu, G.; Tang, W.; Huang, H.; Qu, D. Facile Fabrication of 3D-Printed Porous Ti6Al4V Scaffolds with a Sr-CaP Coating for Bone Regeneration. ACS Omega 2022, 7, 8391–8402. [Google Scholar] [CrossRef] [PubMed]
- Taranu, B.-O.; Ianasi, P.; Rus, S.F.; Bucur, A.I. Simultaneous Precipitation and Electrodeposition of Hydroxyapatite Coatings at Different Temperatures on Various Metal Substrates. Coatings 2022, 12, 288. [Google Scholar] [CrossRef]
- Feddes, B.; Vredenberg, A.M.; Wehner, M.; Wolke, J.C.G.; Jansen, J.A. Laser-Induced Crystallization of Calcium Phosphate Coatings on Polyethylene (PE). Biomaterials 2005, 26, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Azem, F.A.; Delice, T.K.; Ungan, G.; Cakir, A. Investigation of Duty Cycle Effect on Corrosion Properties of Electrodeposited Calcium Phosphate Coatings. Mater. Sci. Eng. C 2016, 68, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Heimann, R.B.; Lehmann, H.D. Deposition, Structure, Properties and Biological Function of Plasma-Sprayed Bioceramic Coatings. Bioceram. Coat. Med. Implant. 2015, 253–308. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Siva, T.; Ramadoss, A. Surface Functionalized Bioceramics Coated on Metallic Implants for Biomedical and Anticorrosion Performance—A Review. J. Mater. Chem. B 2021, 9, 9433–9460. [Google Scholar] [CrossRef]
- Al-Amin, M.; Abdul Rani, A.M.; Abdu Aliyu, A.A.; Bryant, M.G.; Danish, M.; Ahmad, A. Bio-Ceramic Coatings Adhesion and Roughness of Biomaterials through PM-EDM: A Comprehensive Review. Mater. Manuf. Processes 2020, 35, 1157–1180. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 1–11. [Google Scholar] [CrossRef]
- Sankar, M.; Suwas, S.; Balasubramanian, S.; Manivasagam, G. Comparison of Electrochemical Behavior of Hydroxyapatite Coated onto WE43 Mg Alloy by Electrophoretic and Pulsed Laser Deposition. Surf. Coat. Technol. 2017, 309, 840–848. [Google Scholar] [CrossRef]
- Yugeswaran, S.; Kobayashi, A.; Ucisik, A.H.; Subramanian, B. Characterization of Gas Tunnel Type Plasma Sprayed Hydroxyapatite–Nanostructure Titania Composite Coatings. Appl. Surf. Sci. 2015, 347, 48–56. [Google Scholar] [CrossRef]
- Trujillo, N.A.; Oldinski, R.A.; Ma, H.; Bryers, J.D.; Williams, J.D.; Popat, K.C. Antibacterial Effects of Silver-Doped Hydroxyapatite Thin Films Sputter Deposited on Titanium. Mater. Sci. Eng. C 2012, 32, 2135–2144. [Google Scholar] [CrossRef]
- Lett, J.A.; Sagadevan, S.; Paiman, S.; Mohammad, F.; Schirhagl, R.; Léonard, E.; Alshahateet, S.F.; Oh, W.C. Exploring the Thumbprints of Ag-Hydroxyapatite Composite as a Surface Coating Bone Material for the Implants. J. Mater. Res. Technol. 2020, 9, 12824–12833. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.; Predoi, M.; Buton, N.; Motelica-Heino, M. Zinc Doped Hydroxyapatite Thin Films Prepared by Sol–Gel Spin Coating Procedure. Coatings 2019, 9, 156. [Google Scholar] [CrossRef]
- Wang, Q.; Li, P.; Tang, P.; Ge, X.; Ren, F.; Zhao, C.; Fang, J.; Wang, K.; Fang, L.; Li, Y.; et al. Experimental and Simulation Studies of Strontium/Fluoride-Codoped Hydroxyapatite Nanoparticles with Osteogenic and Antibacterial Activities. Colloids Surf. B Biointerfaces 2019, 182, 110359. [Google Scholar] [CrossRef]
- Sutha, S.; Dhineshbabu, N.R.; Prabhu, M.; Rajendran, V. Mg-Doped Hydroxyapatite/Chitosan Composite Coated 316L Stainless Steel Implants for Biomedical Applications. J. Nanosci. Nanotechnol. 2015, 15, 4178–4187. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and Characterization of Zn-Doped Hydroxyapatite: Scaffold Application, Antibacterial and Bioactivity Studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef]
- Chi, W.; Zou, J.; Ai, F.; Lin, Y.; Li, W.; Cao, C.; Yang, K.; Zhou, K. Research of Cu-Doped Hydroxyapatite Microbeads Fabricated by Pneumatic Extrusion Printing. Materials 2019, 12, 1769. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Predoi, M.V.; Predoi, D.; Motelica-Heino, M.; Turculet, C.S.; Beuran, M. Silver-Doped Hydroxyapatite Thin Layers Obtained by Sol-Gel Spin Coating Procedure. Coatings 2019, 10, 14. [Google Scholar] [CrossRef]
- Mishra, V.K.; Bhattacharjee, B.N.; Parkash, O.; Kumar, D.; Rai, S.B. Mg-Doped Hydroxyapatite Nanoplates for Biomedical Applications: A Surfactant Assisted Microwave Synthesis and Spectroscopic Investigations. J. Alloys Compd. 2014, 614, 283–288. [Google Scholar] [CrossRef]
- Sun, T.W.; Zhu, Y.J. Solvothermal Growth of Ultralong Hydroxyapatite Nanowire Coating on Glass Substrate. Chem. Lett. 2019, 48, 1462–1464. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, S.A.; Buton, N.; Megier, C.; Beuran, M. Development of Iron-Doped Hydroxyapatite Coatings. Coatings 2021, 11, 186. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M. Cerium-Doped Hydroxyapatite/Collagen Coatings on Titanium for Bone Implants. Ceram. Int. 2019, 45, 2852–2857. [Google Scholar] [CrossRef]
- Golovanova, O.A.; Zaits, A.V. Biomimetic Coating of a Titanium Substrate with Silicon-Substituted Hydroxyapatite. Inorg. Mater. 2018, 54, 1124–1130. [Google Scholar] [CrossRef]
- Zhang, L. Surface Modification of Titanium by Hydroxyapatite/CaSiO3/Chitosan Porous Bioceramic Coating. Int. J. Electrochem. Sci. 2020, 15. [Google Scholar] [CrossRef]
- Dhinasekaran, D.; Kaliaraj, G.S.; Jagannathan, M.; Rajendran, A.R.; Prakasarao, A.; Ganesan, S.; Subramanian, B. Pulsed Laser Deposition of Nanostructured Bioactive Glass and Hydroxyapatite Coatings: Microstructural and Electrochemical Characterization. Mater. Sci. Eng. C 2021, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pei, L.; Li, H.; Zhu, F. Design and Fabrication of Pyrolytic Carbon-SiC-Fluoridated Hydroxyapatite-Hydroxyapatite Multilayered Coating on Carbon Fibers. Appl. Surf. Sci. 2019, 473, 571–577. [Google Scholar] [CrossRef]
- Batebi, K.; Abbasi Khazaei, B.; Afshar, A. Characterization of Sol-Gel Derived Silver/Fluor-Hydroxyapatite Composite Coatings on Titanium Substrate. Surf. Coat. Technol. 2018, 352, 522–528. [Google Scholar] [CrossRef]
- Melero, H.C.; Sakai, R.T.; Vignatti, C.A.; Benedetti, A.V.; Fernández, J.; Guilemany, J.M.; Suegama, P.H. Corrosion Resistance Evaluation of HVOF Produced Hydroxyapatite and TiO2-Hydroxyapatite Coatings in Hanks’ Solution. Mater. Res. 2018, 21. [Google Scholar] [CrossRef]
- Evcin, A.; Buyukleblebici, B. Ti6A14V Coating with B2O3 and Al2O3 Containing Hydroxyapatite by HVOF Technique. Sci. Iran. 2019, 26, 1980–1989. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M. Electrophoretic Deposition of Fiber Hydroxyapatite/Titania Nanocomposite Coatings. Ceram. Int. 2018, 44, 622–630. [Google Scholar] [CrossRef]
- Ionescu, R.N.; Totan, A.R.; Imre, M.M.; Țâncu, A.M.C.; Pantea, M.; Butucescu, M.; Farcașiu, A.T. Prosthetic Materials Used for Implant-Supported Restorations and Their Biochemical Oral Interactions: A Narrative Review. Materials 2022, 15, 1016. [Google Scholar] [CrossRef] [PubMed]
- Treccani, L.; Yvonne Klein, T.; Meder, F.; Pardun, K.; Rezwan, K. Functionalized Ceramics for Biomedical, Biotechnological and Environmental Applications. Acta Biomater. 2013, 9, 7115–7150. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Khor, K.A.; Lim, J.P. Yttria Stabilized Zirconia Reinforced Hydroxyapatite Coatings. Surf. Coat. Technol. 2000, 127, 66–75. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Muthaiah, B.; Alagarsamy, K.; Vishwakarma, V.; Kirubaharan, A.M.K. Role of Bovine Serum Albumin in the Degradation of Zirconia and Its Allotropes Coated 316L SS for Potential Bioimplants. Mater. Chem. Phys. 2021, 258, 123859. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Vishwakarma, V.; Alagarsamy, K.; Kamalan Kirubaharan, A.M. Biological and Corrosion Behavior of M-ZrO2 and t-ZrO2 Coated 316L SS for Potential Biomedical Applications. Ceram. Int. 2018, 44, 14940–14946. [Google Scholar] [CrossRef]
- Pradhaban, G.; Kaliaraj, G.S.; Vishwakarma, V. Antibacterial Effects of Silver–Zirconia Composite Coatings Using Pulsed Laser Deposition onto 316L SS for Bio Implants. Prog. Biomater. 2014, 3, 123–130. [Google Scholar] [CrossRef]
- Santos, R.L.P.; Buciumeanu, M.; Silva, F.S.; Souza, J.C.M.; Nascimento, R.M.; Motta, F.V.; Henriques, B. Tribological Behavior of Zirconia-Reinforced Glass–Ceramic Composites in Artificial Saliva. Tribol. Int. 2016, 103, 379–387. [Google Scholar] [CrossRef]
- Berni, M.; Lopomo, N.; Marchiori, G.; Gambardella, A.; Boi, M.; Bianchi, M.; Visani, A.; Pavan, P.; Russo, A.; Marcacci, M. Tribological Characterization of Zirconia Coatings Deposited on Ti6Al4V Components for Orthopedic Applications. Mater. Sci. Eng. C 2016, 62, 643–655. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Vishwakarma, V.; Kirubaharan, A.M.K. Biocompatible Zirconia-Coated 316 Stainless Steel with Anticorrosive Behavior for Biomedical Application. Ceram. Int. 2018, 44, 9780–9786. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef] [PubMed]
- Pachaiappan, R.; Rajendran, S.; Show, P.L.; Manavalan, K.; Naushad, M. Metal/Metal Oxide Nanocomposites for Bactericidal Effect: A Review. Chemosphere 2021, 272. [Google Scholar] [CrossRef] [PubMed]
- Crush, J.; Hussain, A.; Seah, K.T.M.; Khan, W.S. Bioactive Glass: Methods for Assessing Angiogenesis and Osteogenesis. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Dey, P.; Pal, S.K.; Banerjee, I.; Sarkar, R. Effect of Addition of B2O3 to the Sol-Gel Synthesized 45S5 Bioglass. J. Aust. Ceram. Soc. 2020, 56, 1309–1322. [Google Scholar] [CrossRef]
- Henao, J.; Poblano-Salas, C.; Monsalve, M.; Corona-Castuera, J.; Barceinas-Sanchez, O. Bio-Active Glass Coatings Manufactured by Thermal Spray: A Status Report. J. Mater. Res. Technol. 2019, 8, 4965–4984. [Google Scholar] [CrossRef]
- Nawaz, Q.; Fastner, S.; Rehman, M.A.U.; Ferraris, S.; Perero, S.; di Confiengo, G.G.; Yavuz, E.; Ferraris, M.; Boccaccini, A.R. Multifunctional Stratified Composite Coatings by Electrophoretic Deposition and RF Co-Sputtering for Orthopaedic Implants. J. Mater. Sci. 2021, 56, 7920–7935. [Google Scholar] [CrossRef]
- Ashok raja, C.; Balakumar, S.; Durgalakshmi, D.; George, R.P.; Anandkumar, B.; Kamachi Mudali, U. Reduced Graphene Oxide/Nano-Bioglass Composites: Processing and Super-Anion Oxide Evaluation. RSC Adv. 2016, 6, 19657–19661. [Google Scholar] [CrossRef]
- Balakumar, S.; Bargavi, P.; Rajashree, P.; Anandkumar, B.; George, R.P. Decoration of 1-D Nano Bioactive Glass on Reduced Graphene Oxide Sheets: Strategies and in Vitro Bioactivity Studies. Mater. Sci. Eng. C 2018, 90, 85–94. [Google Scholar] [CrossRef]
- Ashok raja, C.; Balakumar, S.; Anandkumar, B.; George, R.P.; Kamachi Mudali, U. Formation of Bioactive Nano Hybrid Thin Films on Anodized Titanium via Electrophoretic Deposition Intended for Biomedical Applications. Mater. Today Commun. 2020, 25, 101666. [Google Scholar] [CrossRef]
- Chalisgaonkar, V.; Das, M.; Balla, V.K. Laser Processing of Ti Composite Coatings Reinforced with Hydroxyapatite and Bioglass. Addit. Manuf. 2018, 20, 134–143. [Google Scholar] [CrossRef]
- López-Cuevas, J.; Rendón-Angeles, J.C.; Méndez-Nonell, J.; Barrientos-Rodríguez, H. In Vitro Bioactivity of AISI 316L Stainless Steel Coated with Hydroxyapatite-Seeded 58S Bioglass. MRS Adv. 2019, 4, 3133–3142. [Google Scholar] [CrossRef]
- Estrada-Cabrera, E.; Torres-Ferrer, L.R.; Aztatzi-Aguilar, O.G.; de Vizcaya-Ruiz, A.; Meraz-Rios, M.A.; Zarate-Triviño, D.G.; Arizmendi-Morquecho, A.; de Luna Bugallo, A.; Prokhorov, E.; Luna-Barcenas, G. Chitosan-Bioglass Coatings on Partially Nanostructured Anodized Ti-6Al-4V Alloy for Biomedical Applications. Surf. Coat. Technol. 2019, 375, 468–476. [Google Scholar] [CrossRef]
- Nino, D.; Bayona, M.; Güiza, V.; Córdoba, E. Approach for Fabricating Bioglass Coatings on Reticulated Vitreous Carbon Foams for Tissue Engineering Applications. J. Phys. Conf. Ser. 2019, 1159, 012007. [Google Scholar] [CrossRef]
- Hosseini, S.; Farnoush, H. Characterization and in Vitro Bioactivity of Electrophoretically Deposited Mn-Modified Bioglass-Alginate Nanostructured Composite Coatings. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Bala, N. Analysis of in Vitro Corrosion Behavior and Hemocompatibility of Electrophoretically Deposited Bioglass-Chitosan-Iron Oxide Coating for Biomedical Applications. J. Mater. Res. 2020, 35, 1749–1761. [Google Scholar] [CrossRef]
- Say, Y.; Aksakal, B. Enhanced Corrosion Properties of Biological NiTi Alloy by Hydroxyapatite and Bioglass Based Biocomposite Coatings. J. Mater. Res. Technol. 2020, 9, 1712–1749. [Google Scholar] [CrossRef]
- Rojas, O.; Prudent, M.; López, M.E.; Vargas, F.; Ageorges, H. Influence of Atmospheric Plasma Spraying Parameters on Porosity Formation in Coatings Manufactured from 45S5 Bioglass® Powder. J. Therm. Spray Technol. 2020, 29, 185–198. [Google Scholar] [CrossRef]
- Li, X.; Zhitomirsky, I. Deposition of Poly(Methyl Methacrylate) and Composites Containing Bioceramics and Bioglass by Dip Coating Using Isopropanol-Water Co-Solvent. Prog. Org. Coat. 2020, 148. [Google Scholar] [CrossRef]
- Shafiee, B.M.; Torkaman, R.; Mahmoudi, M.; Emadi, R.; Derakhshan, M.; Karamian, E.; Tavangarian, F. Surface Modification of 316l Ss Implants by Applying Bioglass/Gelatin/Polycaprolactone Composite Coatings for Biomedical Applications. Coatings 2020, 10, 1220. [Google Scholar] [CrossRef]
- Haftbaradaran-Esfahani, M.; Ahmadian, M.; Nassajpour-Esfahani, A. Fabrication and Characterization of Porous Biomedical Vitallium Alloy with 58S Bioglass Coating Prepared by Sol-Gel Method. Appl. Surf. Sci. 2020, 506. [Google Scholar] [CrossRef]
- Azzouz, I.; Faure, J.; Khlifi, K.; Larbi, A.C.; Benhayoune, H. Electrophoretic Deposition of 45s5 Bioglass® Coatings on the Ti6al4v Prosthetic Alloy with Improved Mechanical Properties. Coatings 2020, 10, 1192. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Bala, N. Characterization, Electrochemical Behavior and in Vitro Hemocompatibility of Hydroxyapatite-Bioglass-Iron Oxide-Chitosan Composite Coating by Electrophoretic Deposition. Surf. Coat. Technol. 2021, 405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).