Modulation of the Structure and Stability of Novel Camel Lens Alpha-Crystallin by pH and Thermal Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Purification of Alpha-Crystallin from Camel Lens
2.2. Equilibration of Alpha-Crystallin at Different pH Values
2.3. Far-UV CD Spectroscopy of Alpha-Crystallin at Different pH Values
2.4. Intrinsic Fluorescence Spectroscopy of Alpha-Crystallin at Different pH Values
2.5. Dynamic Multimode Spectroscopy of Alpha-Crystallin at Different pH Values
2.6. ANS (8-Anilino-1-naphthalene sulfonate) Fluorescence Measurements of Alpha-Crystallin at Different Temperatures and pH Values
3. Results
3.1. Effect of pH on the Secondary Structure of Camel Lens Alpha-Crystallin
3.2. Effect of pH on the Tertiary Structure of Alpha-Crystallin
3.3. Changes in Surface Hydrophobicity at Selected pH Values
3.4. Thermodynamic and Spectroscopic Properties of Alpha-Crystallin at Different pH Values as Determined by Dynamic Multimode Spectroscopy
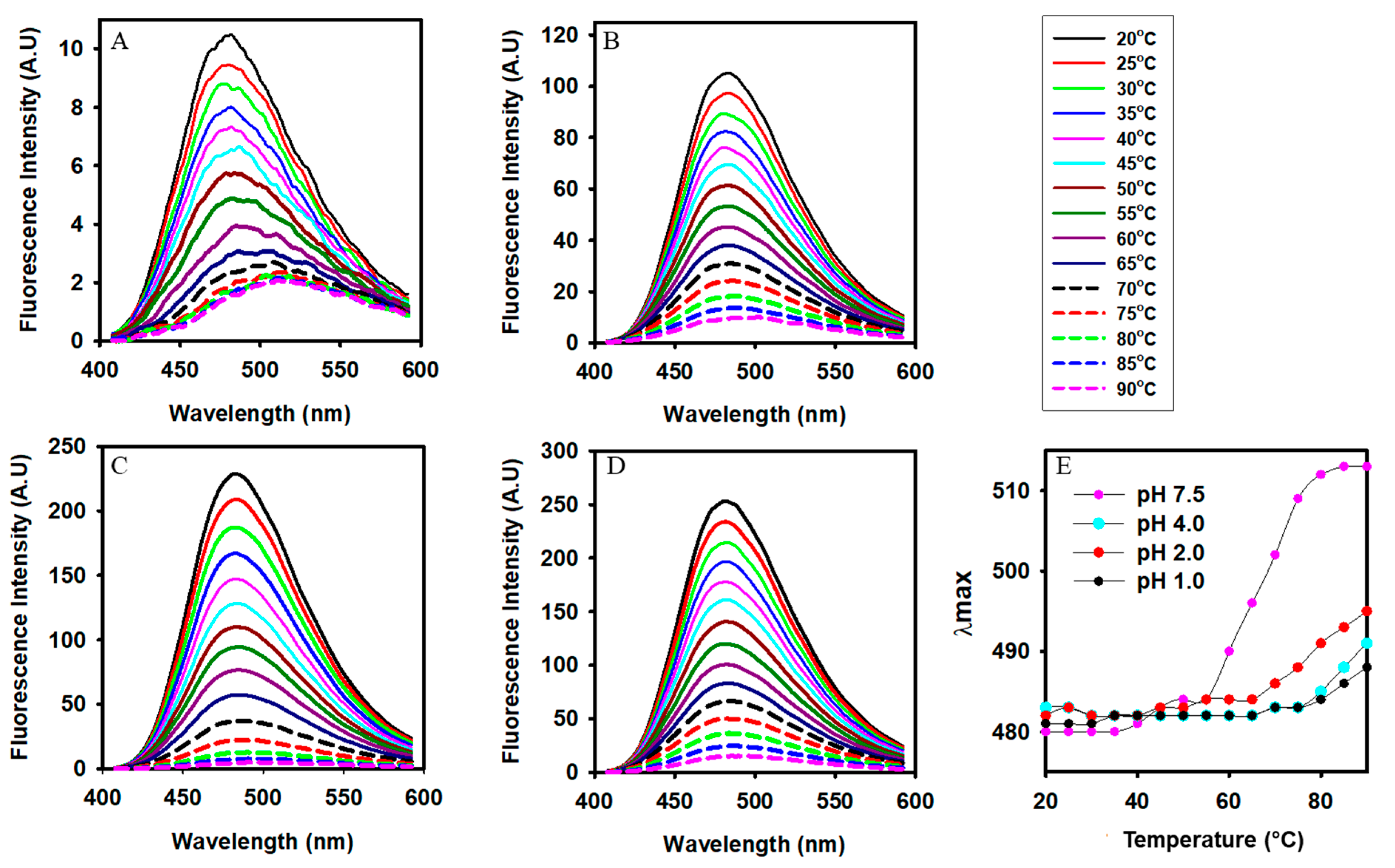
3.5. Changes in the Surface Hydrophobicity of Alpha-Crystallin at Different Temperatures and pH Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horwitz, J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 1992, 89, 10449–10453. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadyan, A.A.; Cotlier, E. Rise in lens temperature on exposure to sunlight or high ambient temperature. Br. J. Ophthalmol. 1986, 70, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Purdie, J.L.; Hirst, L.W.; Green, A.C. Sun exposure as a risk factor for nuclear cataract. Epidemiology 2003, 14, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Heys, K.R.; Friedrich, M.G.; Truscott, R.J. Presbyopia and heat: Changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell 2007, 6, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J. Alpha-crystallin. Exp. Eye Res. 2003, 76, 145–153. [Google Scholar] [CrossRef]
- Augusteyn, R.C. Alpha-crystallin: A review of its structure and function. Clin. Exp. Optom. 2004, 87, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Almaharfi, H.A.; Khan, J.M.; Hisamuddin, M.; Alamery, S.F.; Haq, S.H.; Ahmed, M.Z. Protection of zeta-crystallin by alpha-crystallin under thermal stress. Int. J. Biol. Macromol. 2021, 167, 289–298. [Google Scholar] [CrossRef]
- Srinivasan, A.N.; Nagineni, C.N.; Bhat, S.P. Alpha A-crystallin is expressed in non-ocular tissues. J. Biol. Chem. 1992, 267, 23337–23341. [Google Scholar] [CrossRef]
- Bhat, S.P.; Nagineni, C.N. Alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem. Biophys. Res. Commun. 1989, 158, 319–325. [Google Scholar] [CrossRef]
- Selcen, D.; Engel, A.G. Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann. Neurol. 2003, 54, 804–810. [Google Scholar] [CrossRef]
- Fischer, D.; Matten, J.; Reimann, J.; Bonnemann, C.; Schroder, R. Expression, localization and functional divergence of alphaB-crystallin and heat shock protein 27 in core myopathies and neurogenic atrophy. Acta Neuropathol. 2002, 104, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Tang, M.; Fu, N.; Shao, W.; Zhang, S.; Yin, Y.; Zeng, R.; Wang, X.; Hu, G.; et al. Upregulation of alphaB-crystallin expression in the substantia nigra of patients with Parkinson’s disease. Neurobiol. Aging 2015, 36, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Klettner, A.; Richert, E.; Kuhlenbaumer, G.; Nolle, B.; Bhatia, K.P.; Deuschl, G.; Roider, J.; Schneider, S.A. Alpha synuclein and crystallin expression in human lens in Parkinson’s disease. Mov. Disord. 2016, 31, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Kamps, B.; Boelens, W.C.; Reif, B. AlphaB-crystallin competes with Alzheimer’s disease beta-amyloid peptide for peptide-peptide interactions and induces oxidation of Abeta-Met35. FEBS Lett. 2006, 580, 5941–5946. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Katayama, S.; Watanabe, C.; Harada, Y.; Noda, K.; Yamamura, Y.; Nakamura, S. The relationship between alphaB-crystallin and neurofibrillary tangles in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2001, 27, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, J.; Xu, Y.; Ren, K.; Xie, W.L.; Yan, Y.E.; Zhang, B.Y.; Shi, Q.; Liu, Y.; Dong, X.P. Abnormally upregulated alphaB-crystallin was highly coincidental with the astrogliosis in the brains of scrapie-infected hamsters and human patients with prion diseases. J. Mol. Neurosci. 2013, 51, 734–748. [Google Scholar] [CrossRef]
- Renkawek, K.; de Jong, W.W.; Merck, K.B.; Frenken, C.W.; van Workum, F.P.; Bosman, G.J. Alpha B-crystallin is present in reactive glia in Creutzfeldt-Jakob disease. Acta Neuropathol. 1992, 83, 324–327. [Google Scholar] [CrossRef]
- van Noort, J.M.; Bsibsi, M.; Nacken, P.J.; Verbeek, R.; Venneker, E.H. Therapeutic Intervention in Multiple Sclerosis with Alpha B-Crystallin: A Randomized Controlled Phase IIa Trial. PLoS ONE 2015, 10, e0143366. [Google Scholar] [CrossRef]
- Stoevring, B.; Vang, O.; Christiansen, M. (Alpha)B-crystallin in cerebrospinal fluid of patients with multiple sclerosis. Clin. Chim. Acta 2005, 356, 95–101. [Google Scholar] [CrossRef]
- Malin, D.; Petrovic, V.; Strekalova, E.; Sharma, B.; Cryns, V.L. AlphaB-crystallin: Portrait of a malignant chaperone as a cancer therapeutic target. Pharmacol. Ther. 2016, 160, 1–10. [Google Scholar] [CrossRef]
- Malin, D.; Strekalova, E.; Petrovic, V.; Deal, A.M.; Al Ahmad, A.; Adamo, B.; Miller, C.R.; Ugolkov, A.; Livasy, C.; Fritchie, K.; et al. AlphaB-crystallin: A novel regulator of breast cancer metastasis to the brain. Clin. Cancer Res. 2014, 20, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Marini, I.; Moschini, R.; Del Corso, A.; Mura, U. Alpha-crystallin: An ATP-independent complete molecular chaperone toward sorbitol dehydrogenase. Cell Mol. Life Sci. 2005, 62, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Siezen, R.J.; Bindels, J.G.; Hoenders, H.J. The quaternary structure of bovine alpha-crystallin. Size and charge microheterogeneity: More than 1000 different hybrids? Eur. J. Biochem. 1978, 91, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Siezen, R.J.; Bindels, J.G.; Hoenders, H.J. The quaternary structure of bovine alpha-crystallin. Effects of variation in alkaline pH, ionic strength, temperature and calcium ion concentration. Eur. J. Biochem. 1980, 111, 435–444. [Google Scholar] [CrossRef]
- Das, K.P.; Surewicz, W.K. Temperature-induced exposure of hydrophobic surfaces and its effect on the chaperone activity of alpha-crystallin. FEBS Lett. 1995, 369, 321–325. [Google Scholar] [CrossRef]
- Raman, B.; Ramakrishna, T.; Rao, C.M. Temperature dependent chaperone-like activity of alpha-crystallin. FEBS Lett. 1995, 365, 133–136. [Google Scholar] [CrossRef]
- Gesierich, U.; Pfeil, W. The conformational stability of alpha-crystallin is rather low: Calorimetric results. FEBS Lett. 1996, 393, 151–154. [Google Scholar] [CrossRef][Green Version]
- Surewicz, W.K.; Olesen, P.R. On the thermal stability of alpha-crystallin: A new insight from infrared spectroscopy. Biochemistry 1995, 34, 9655–9660. [Google Scholar] [CrossRef]
- Walsh, M.T.; Sen, A.C.; Chakrabarti, B. Micellar subunit assembly in a three-layer model of oligomeric alpha-crystallin. J. Biol. Chem. 1991, 266, 20079–20084. [Google Scholar] [CrossRef]
- Lee, J.S.; Satoh, T.; Shinoda, H.; Samejima, T.; Wu, S.H.; Chiou, S.H. Effect of heat-induced structural perturbation of secondary and tertiary structures on the chaperone activity of alpha-crystallin. Biochem. Biophys. Res. Commun. 1997, 237, 277–282. [Google Scholar] [CrossRef]
- Raman, B.; Rao, C.M. Chaperone-like activity and temperature-induced structural changes of alpha-crystallin. J. Biol. Chem. 1997, 272, 23559–23564. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.; van de Weert, M.; Jiskoot, W.; Kasimova, M.R. Thermal and acid denaturation of bovine lens alpha-crystallin. Proteins 2011, 79, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Privalov, P.L.; Makhatadze, G.I. Heat capacity of proteins. II. Partial molar heat capacity of the unfolded polypeptide chain of proteins: Protein unfolding effects. J. Mol. Biol. 1990, 213, 385–391. [Google Scholar] [CrossRef]
- Farnsworth, P.N.; Groth-Vasselli, B.; Greenfield, N.J.; Singh, K. Effects of temperature and concentration on bovine lens alpha-crystallin secondary structure: A circular dichroism spectroscopic study. Int. J. Biol. Macromol. 1997, 20, 283–291. [Google Scholar] [CrossRef]
- Maiti, M.; Kono, M.; Chakrabarti, B. Heat-induced changes in the conformation of alpha- and beta-crystallins: Unique thermal stability of alpha-crystallin. FEBS Lett. 1988, 236, 109–114. [Google Scholar] [CrossRef]
- Khan, J.M.; Malik, A.; Ahmed, A.; Rehman, M.T.; AlAjmi, M.F.; Khan, R.H.; Fatima, S.; Alamery, S.F.; Abdullah, E.M. Effect of cetyltrimethylammonium bromide (CTAB) on the conformation of a hen egg white lysozyme: A spectroscopic and molecular docking study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 219, 313–318. [Google Scholar] [CrossRef]
- Malik, A.; Albogami, S.; Alsenaidy, A.M.; Aldbass, A.M.; Alsenaidy, M.A.; Khan, S.T. Spectral and thermal properties of novel eye lens zeta-crystallin. Int. J. Biol. Macromol. 2017, 102, 1052–1058. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, Y.; Ye, T.; Jiang, S.; Lin, L.; Lu, J. The Effect of Salt on the Gelling Properties and Protein Phosphorylation of Surimi-Crabmeat Mixed Gels. Gels 2021, 8, 10. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Khan, J.M.; Malik, A.; Sen, P.; Alsenaidy, M.A.; Husain, F.M.; Alsenaidy, A.M.; Khan, R.H.; Choudhry, H.; Zamzami, M.A.; et al. A quercetin-based flavanoid (rutin) reverses amyloid fibrillation in beta-lactoglobulin at pH 2.0 and 358 K. Spectrochim. Acta A 2019, 214, 40–48. [Google Scholar] [CrossRef]
- Khan, J.M.; Ahmed, A.; Alamery, S.F.; Farah, M.A.; Hussain, T.; Khan, M.I.; Khan, R.H.; Malik, A.; Fatima, S.; Sen, P. Millimolar concentration of sodium dodecyl sulfate inhibit thermal aggregation in hen egg white lysozyme via increased alpha-helicity. Colloid Surf. A 2019, 572, 167–173. [Google Scholar] [CrossRef]
- Khan, J.M.; Khan, M.R.; Sen, P.; Malik, A.; Irfan, M.; Khan, R.H. An intermittent amyloid phase found in gemini (G5 and G6) surfactant induced beta-sheet to alpha-helix transition in concanavalin A protein. J. Mol. Liq. 2018, 269, 796–804. [Google Scholar] [CrossRef]
- Park, S.J.; Borin, B.N.; Martinez-Yamout, M.A.; Dyson, H.J. The client protein p53 adopts a molten globule-like state in the presence of Hsp90. Nat. Struct. Mol. Biol. 2011, 18, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Haroon, A.; Jagirdar, H.; Alsenaidy, A.M.; Elrobh, M.; Khan, W.; Alanazi, M.S.; Bazzi, M.D. Spectroscopic and thermodynamic properties of recombinant heat shock protein A6 from Camelus dromedarius. Eur. Biophys. J. 2015, 44, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ouajd, S.; Kamel, B. Physiological Particularities of Dromedary (Camelus dromedarius) and Experimental Implications. Scand. J. Lab. Anim. Sci. 2009, 36, 19–29. [Google Scholar]
- Warda, M.; Prince, A.; Kim, H.K.; Khafaga, N.; Scholkamy, T.; Linhardt, R.J.; Jin, H. Proteomics of old world camelid (Camelus dromedarius): Better understanding the interplay between homeostasis and desert environment. J. Adv. Res. 2014, 5, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Kadim, I.T.; Mahgoub, O.; Purchas, R.W. A review of the growth, and of the carcass and meat quality characteristics of the one-humped camel (Camelus dromedaries). Meat Sci. 2008, 80, 555–569. [Google Scholar] [CrossRef]
- Wu, H.; Guang, X.; Al-Fageeh, M.B.; Cao, J.; Pan, S.; Zhou, H.; Zhang, L.; Abutarboush, M.H.; Xing, Y.; Xie, Z.; et al. Camelid genomes reveal evolution and adaptation to desert environments. Nat. Commun. 2014, 5, 5188. [Google Scholar] [CrossRef]
- Khalkhali-Evrigh, R.; Hafezian, S.H.; Hedayat-Evrigh, N.; Farhadi, A.; Bakhtiarizadeh, M.R. Genetic variants analysis of three dromedary camels using whole genome sequencing data. PLoS ONE 2018, 13, e0204028. [Google Scholar] [CrossRef]
- Duhaiman, A.S.; Rabbani, N.; AlJafari, A.A.; Alhomida, A.S. Purification and characterization of zeta-crystallin from the camel lens. Biochem. Biophys. Res. Commun. 1995, 215, 632–640. [Google Scholar] [CrossRef]
- Braun, N.; Zacharias, M.; Peschek, J.; Kastenmuller, A.; Zou, J.; Hanzlik, M.; Haslbeck, M.; Rappsilber, J.; Buchner, J.; Weinkauf, S. Multiple molecular architectures of the eye lens chaperone alphaB-crystallin elucidated by a triple hybrid approach. Proc. Natl. Acad. Sci. USA 2011, 108, 20491–20496. [Google Scholar] [CrossRef]
- Ryazantsev, S.N.; Poliansky, N.B.; Chebotareva, N.A.; Muranov, K.O. 3D structure of the native alpha-crystallin from bovine eye lens. Int. J. Biol. Macromol. 2018, 117, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Fouad, D.; Labrou, N.E.; Al-Senaidy, A.M.; Ismael, M.A.; Saeed, H.M.; Ataya, F.S. Structural and thermodynamic properties of kappa class glutathione transferase from Camelus dromedarius. Int. J. Biol. Macromol. 2016, 88, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Siezen, R.J.; Bindels, J.G. Stepwise Dissociation Denaturation and Reassociation Renaturation of Bovine Alpha-Crystallin in Urea and Guanidine-Hydrochloride—Sedimentation, Fluorescence, near-Ultraviolet and Far Ultraviolet Circular-Dichroism Studies. Exp. Eye Res. 1982, 34, 969–983. [Google Scholar] [CrossRef]
- Das, B.K.; Liang, J.J.; Chakrabarti, B. Heat-induced conformational change and increased chaperone activity of lens alpha-crystallin. Curr. Eye Res. 1997, 16, 303–309. [Google Scholar] [CrossRef]
- Robertson, A.D.; Murphy, K.P. Protein Structure and the Energetics of Protein Stability. Chem. Rev. 1997, 97, 1251–1268. [Google Scholar] [CrossRef]
- Neet, K.E.; Timm, D.E. Conformational stability of dimeric proteins: Quantitative studies by equilibrium denaturation. Protein Sci. 1994, 3, 2167–2174. [Google Scholar] [CrossRef]
- Steif, C.; Weber, P.; Hinz, H.J.; Flossdorf, J.; Cesareni, G.; Kokkinidis, M. Subunit interactions provide a significant contribution to the stability of the dimeric four-alpha-helical-bundle protein ROP. Biochemistry 1993, 32, 3867–3876. [Google Scholar] [CrossRef]
- Augusteyn, R.C. Alpha-Crystallin polymers and polymerization: The view from down under. Int. J. Biol. Macromol. 1998, 22, 253–262. [Google Scholar] [CrossRef]
- Robinson, C.R.; Rentzeperis, D.; Silva, J.L.; Sauer, R.T. Formation of a denatured dimer limits the thermal stability of Arc repressor. J. Mol. Biol. 1997, 273, 692–700. [Google Scholar] [CrossRef]
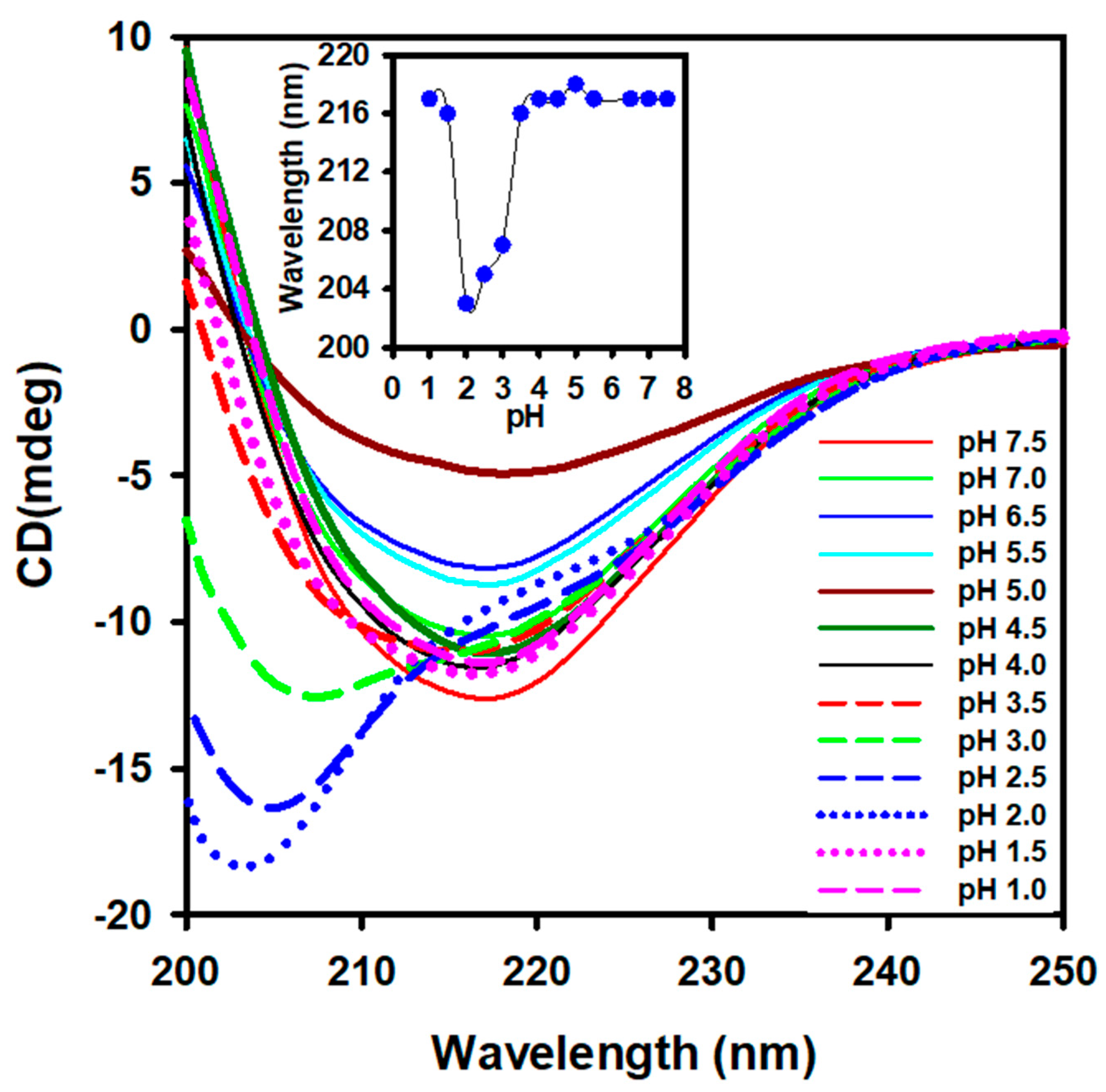
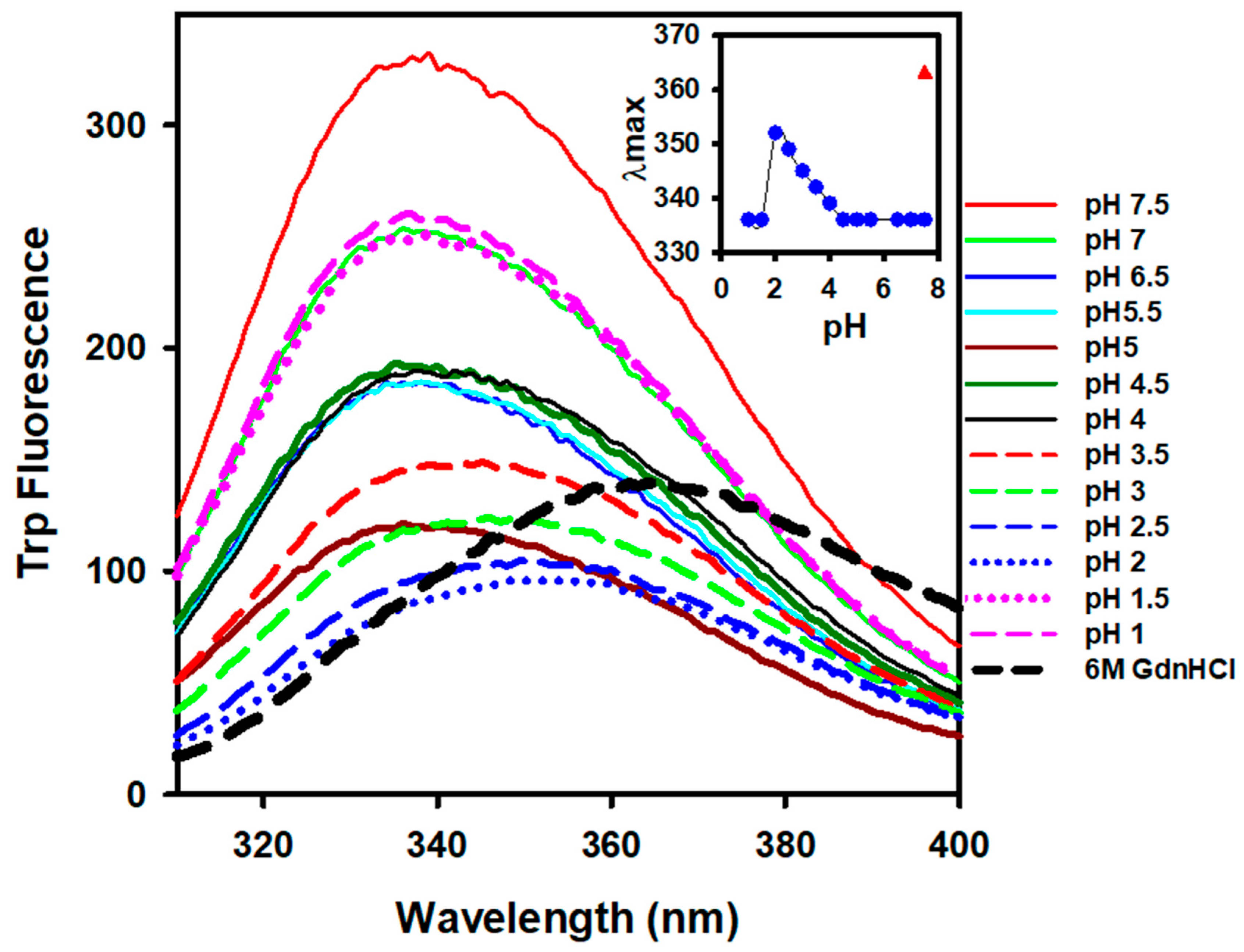
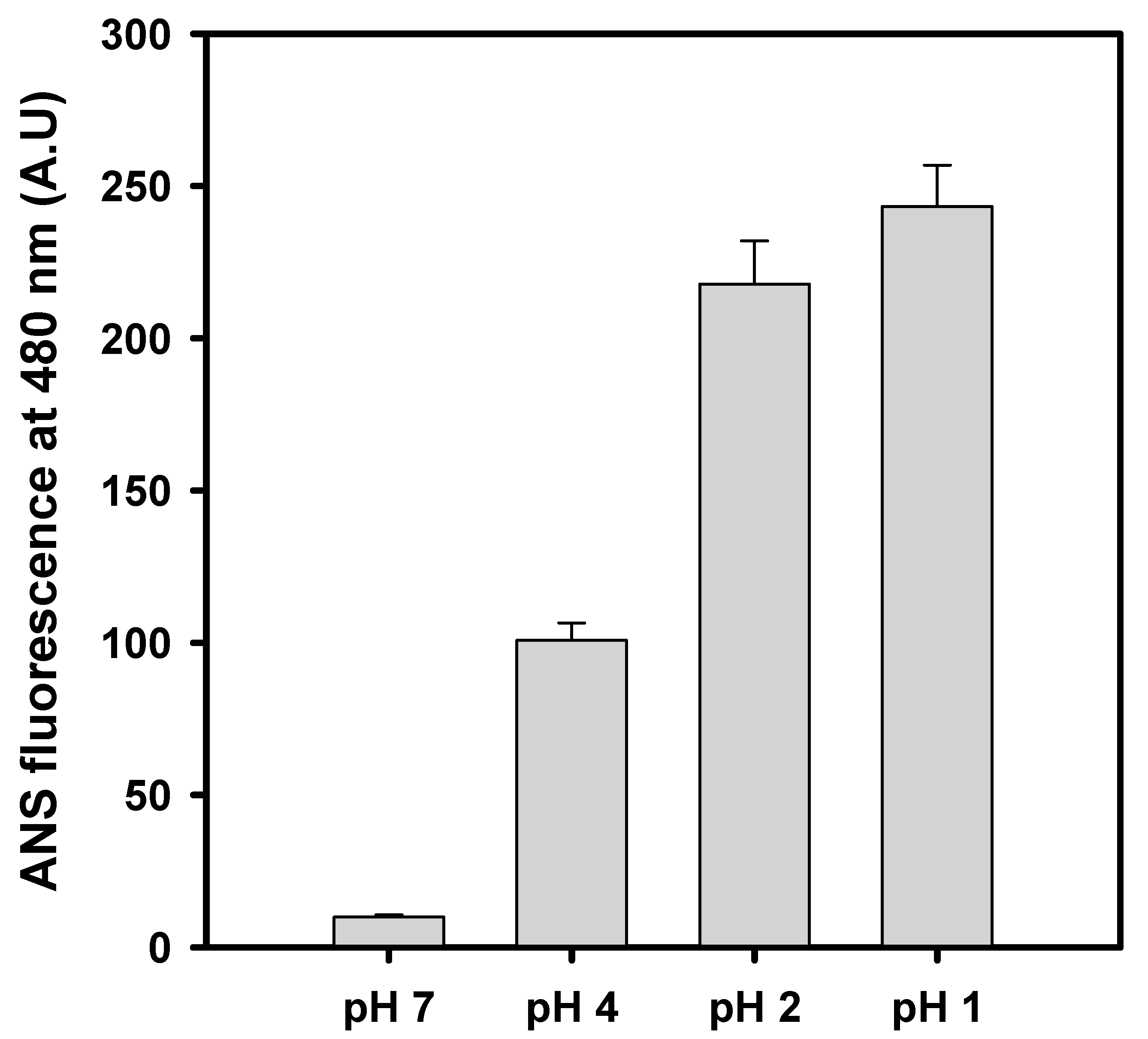
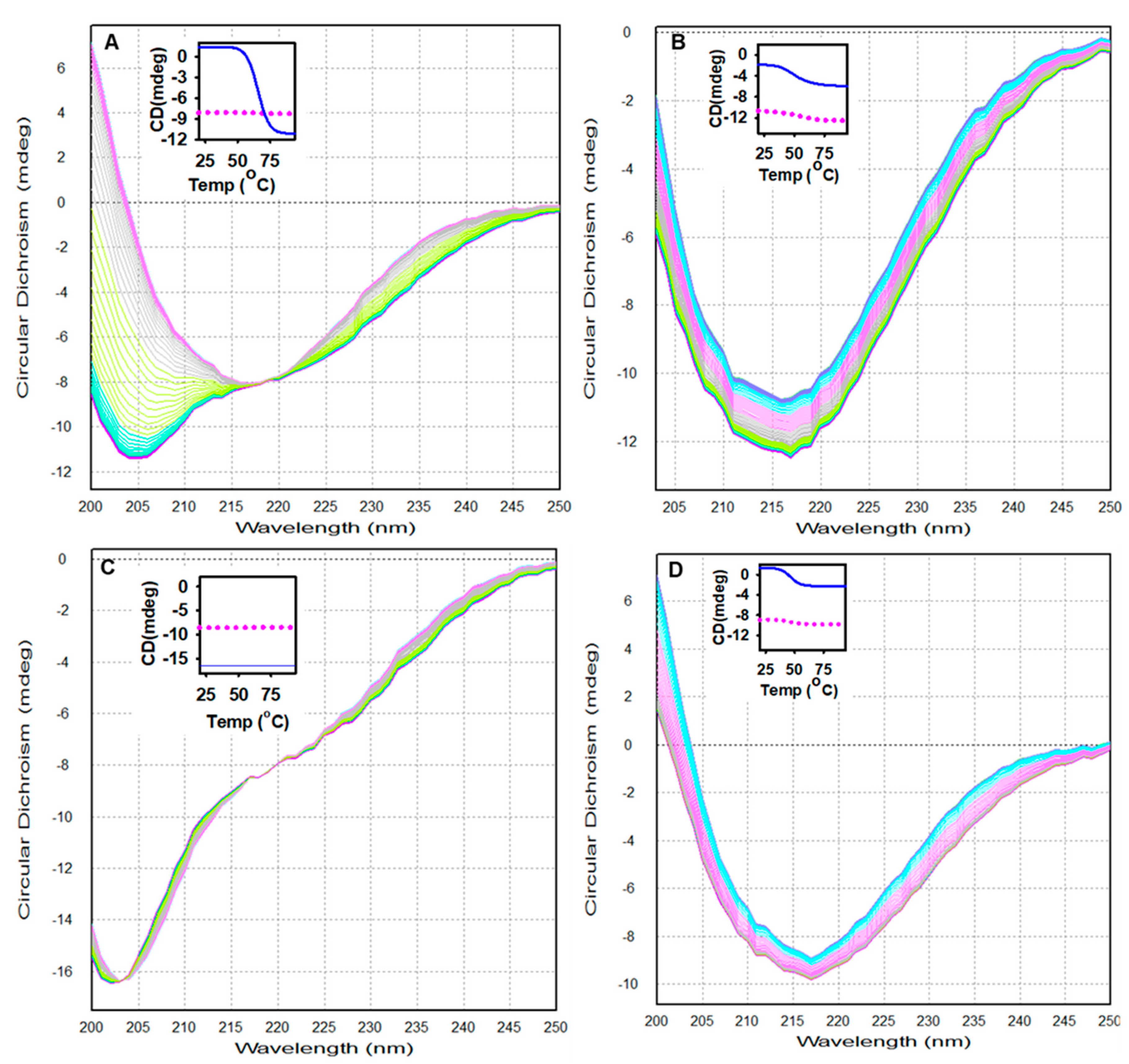

| pH | Van’t Hoff Enthalpy (kJ/mol) | Transition Temperature (°C) |
|---|---|---|
| 7.5 | 237.0 ± 1.9 | 60.9 ± 0.1 |
| 4.0 | 108.9 ± 4.7 | 48.1 ± 0.4 |
| 2.0 | 177.3 ± 9.2 | 59.1 ± 0.4 |
| 1.0 | 211.3 ± 10.2 | 43.5 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, A.; Khan, J.M.; Alhomida, A.S.; Ola, M.S. Modulation of the Structure and Stability of Novel Camel Lens Alpha-Crystallin by pH and Thermal Stress. Gels 2022, 8, 273. https://doi.org/10.3390/gels8050273
Malik A, Khan JM, Alhomida AS, Ola MS. Modulation of the Structure and Stability of Novel Camel Lens Alpha-Crystallin by pH and Thermal Stress. Gels. 2022; 8(5):273. https://doi.org/10.3390/gels8050273
Chicago/Turabian StyleMalik, Ajamaluddin, Javed Masood Khan, Abdullah S. Alhomida, and Mohammad Shamsul Ola. 2022. "Modulation of the Structure and Stability of Novel Camel Lens Alpha-Crystallin by pH and Thermal Stress" Gels 8, no. 5: 273. https://doi.org/10.3390/gels8050273
APA StyleMalik, A., Khan, J. M., Alhomida, A. S., & Ola, M. S. (2022). Modulation of the Structure and Stability of Novel Camel Lens Alpha-Crystallin by pH and Thermal Stress. Gels, 8(5), 273. https://doi.org/10.3390/gels8050273







