Effect of NaCl on the Rheological, Structural, and Gelling Properties of Walnut Protein Isolate-κ-Carrageenan Composite Gels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Na+ Concentration on the Rheological and Texture Properties of WNPI-KC Composite Gel
2.1.1. Rheological Properties
2.1.2. Textural Properties
2.2. Effect of Na+ on the Apparent Viscosity, Bond Strength, Water-Holding Capacity, and Thermal Stability of WNPI-KC Composite Gel
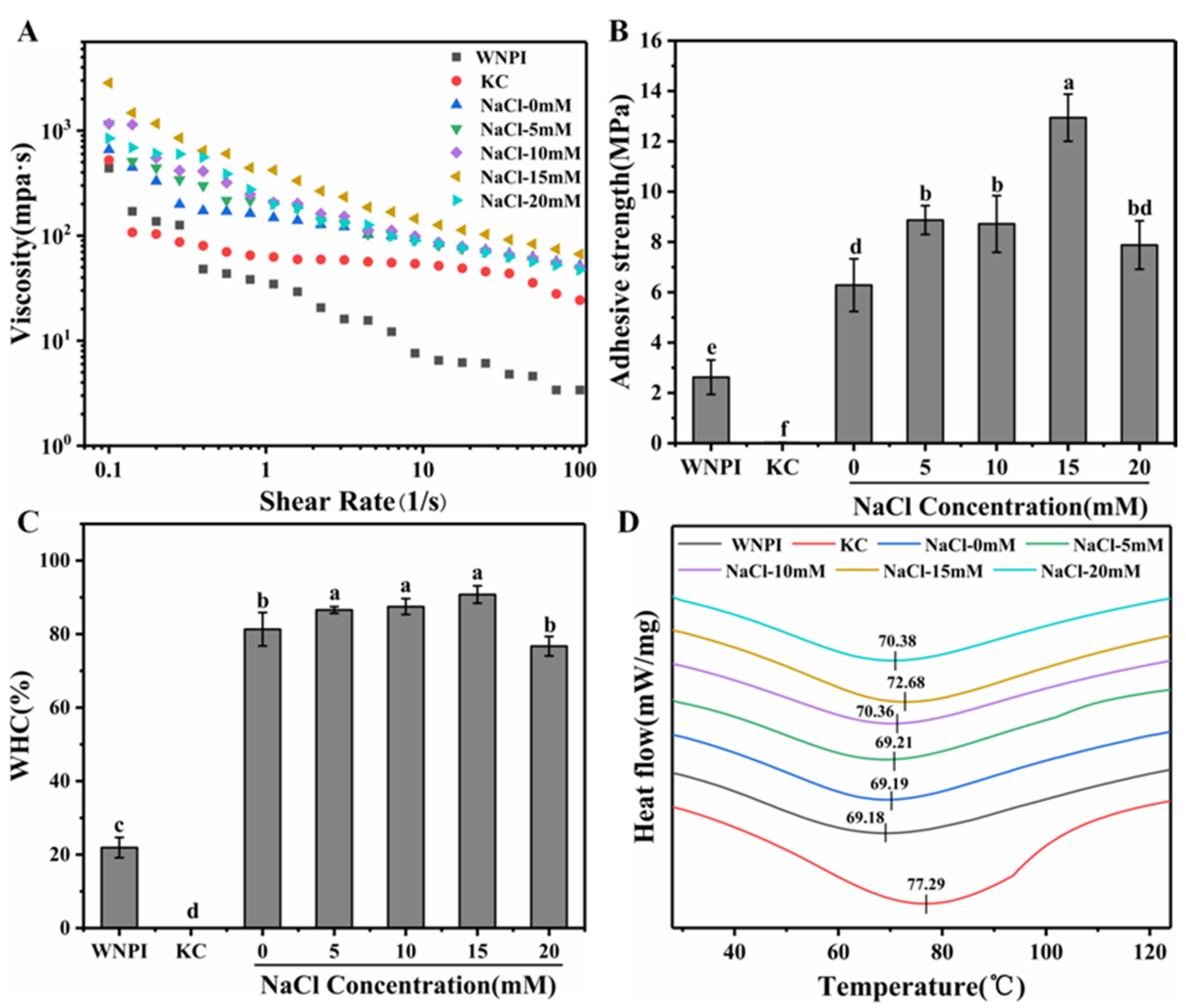
2.2.1. Apparent Viscosity
2.2.2. Bond Strength
2.2.3. Water-Holding Capacity (WHC)
2.2.4. Thermal Stability
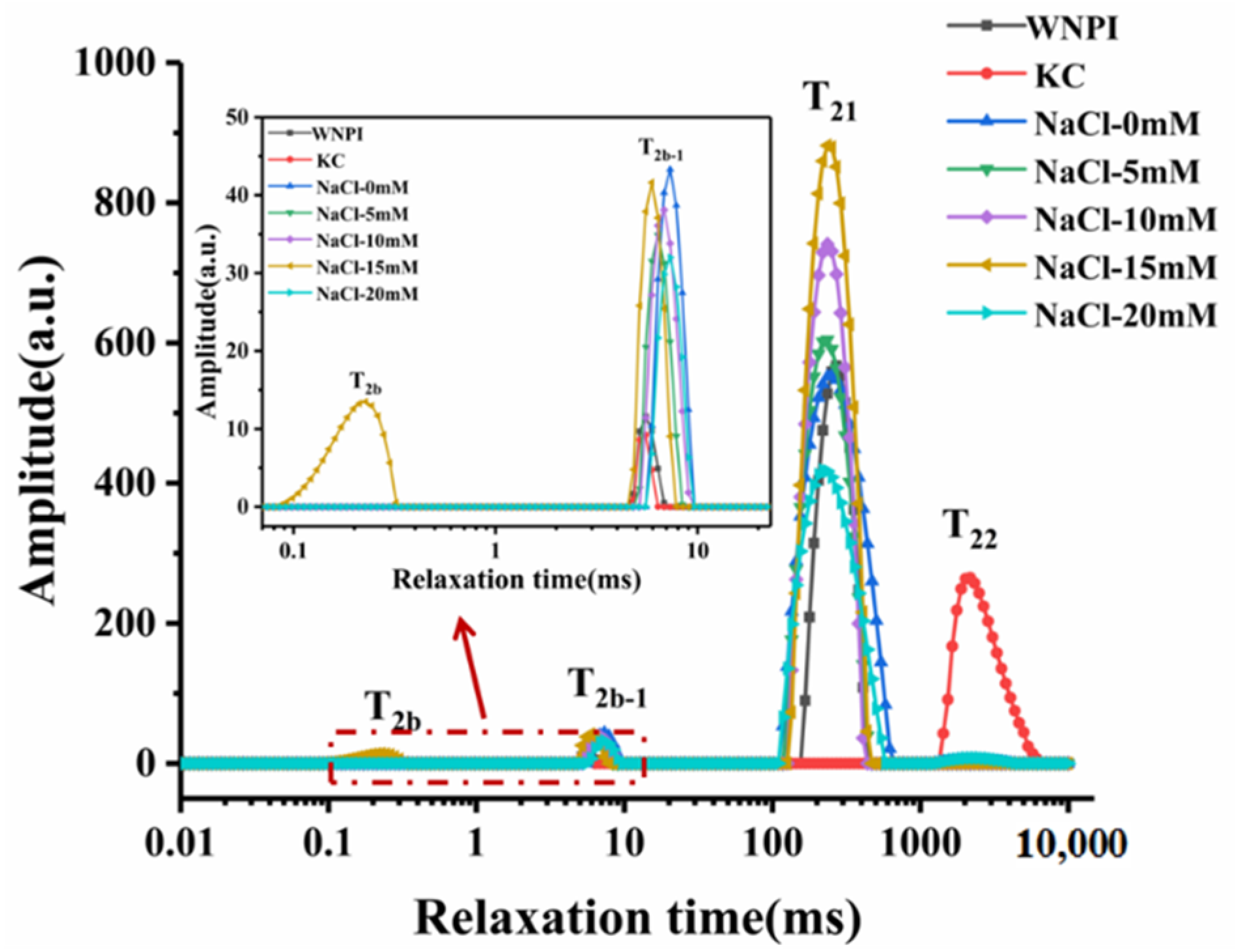
2.3. Effect of Na+ Concentration on the Moisture Distribution of WNPI-KC Composite Gel
2.4. Effect of Na+ on the Particle Size and Zeta Potential of WNPI-KC Composite Gel
2.5. Effect of Na+ on the Structural Properties of WNPI-KC Composite Gel
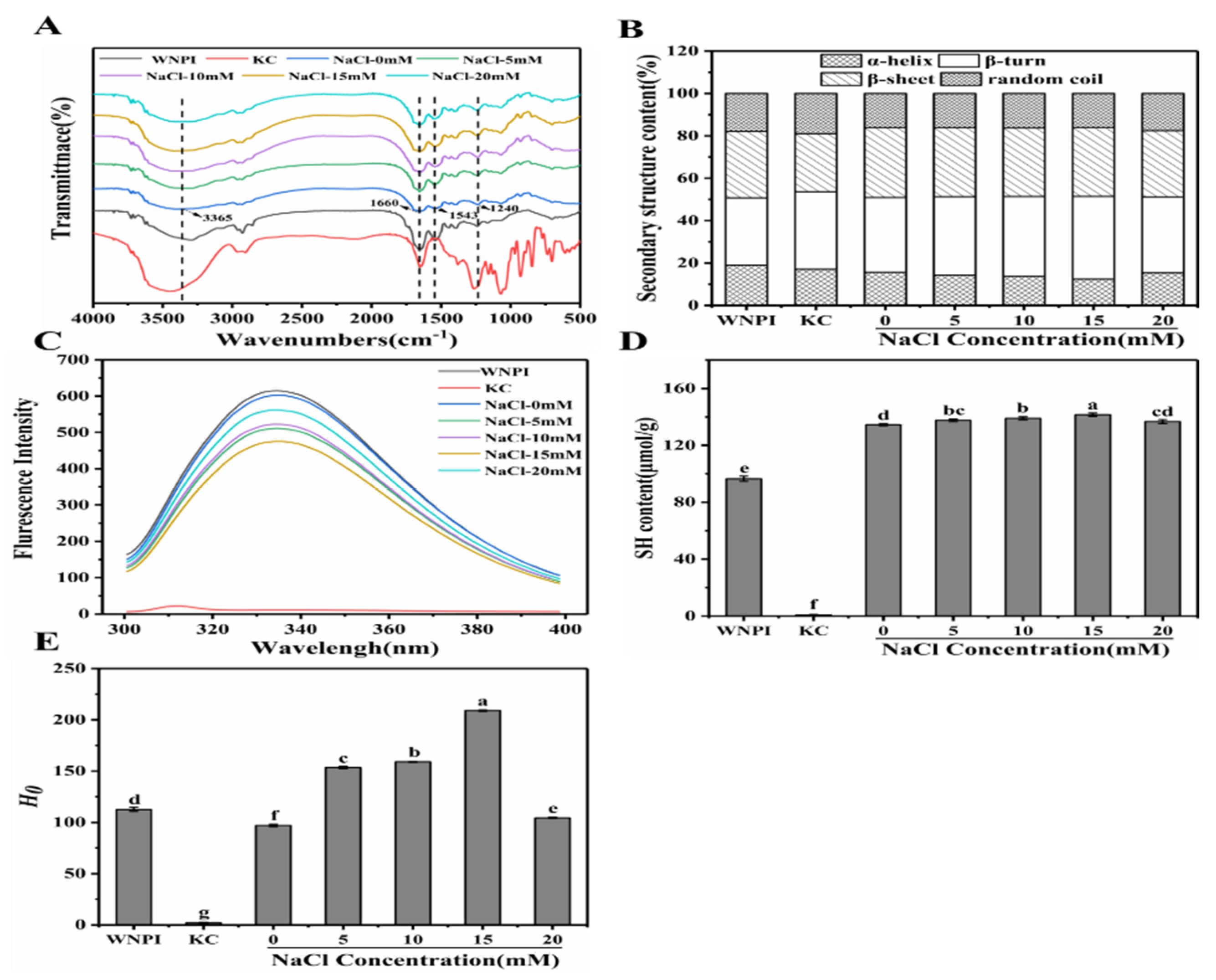
2.5.1. FTIR
2.5.2. Fluorescence Spectroscopy
2.5.3. Sulfhydryl Content
2.5.4. Surface Hydrophobicity
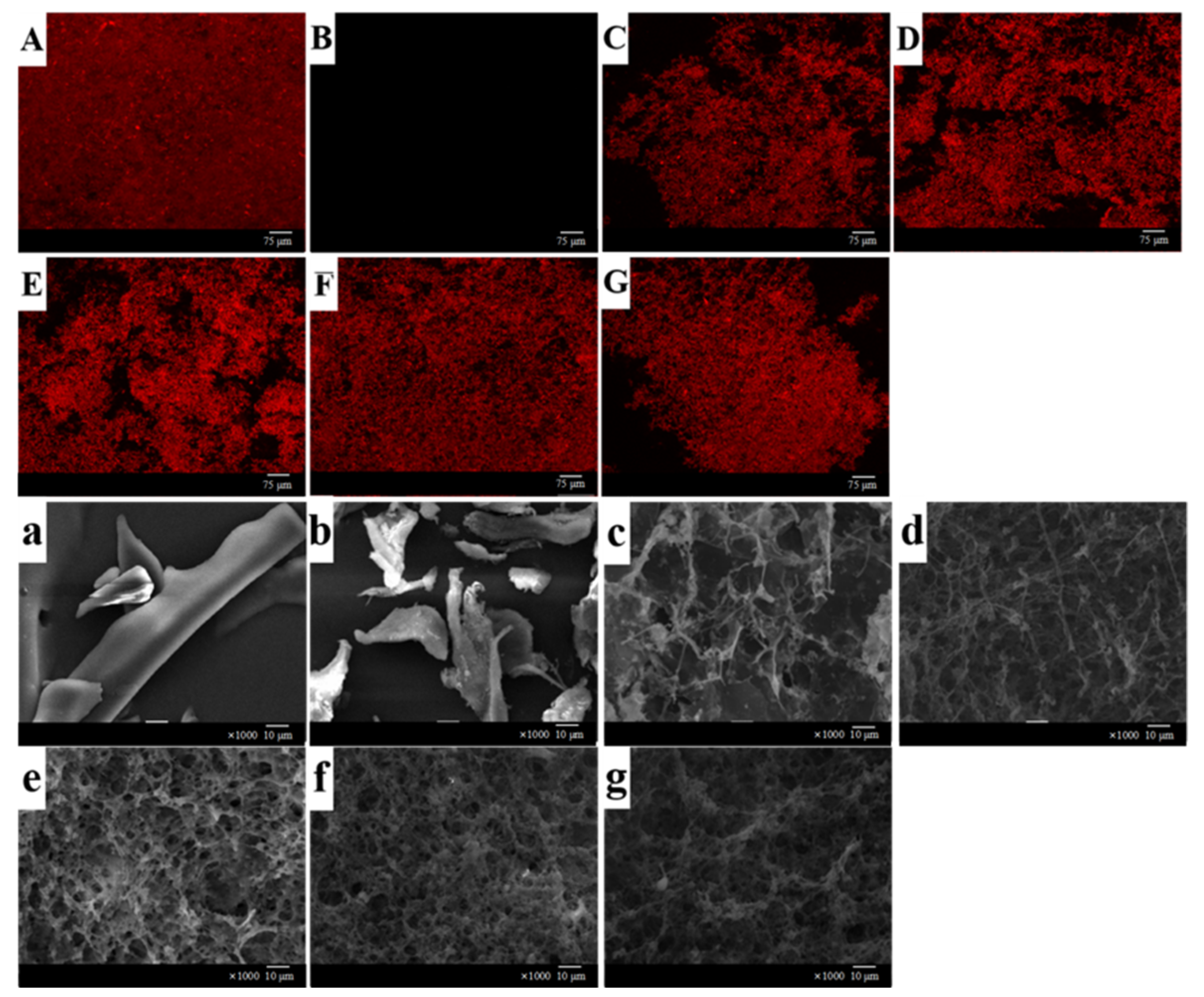
2.5.5. Microstructure
2.6. Effect of Na+ on the Intermolecular Force of WNPI-KC Composite Gel
2.7. Effect of Na+ on the Schematic Mechanism of WNPI-KC Composite Gel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of WNPI and WNPI-KC Composite Gels
4.3. Rheological Measurement
4.4. Texture Analysis
4.5. Determination of Bond Strength
4.6. Water-Holding Capacity (WHC)
4.7. Thermal Stability
4.8. Low-Field Nuclear Magnetic Resonance (LF-NMR)
4.9. Particle Size and Potential
4.10. Fourier Transform Infrared (FTIR) Spectroscopy
4.11. Fluorescence Spectroscopy
4.12. SH Content
- A412 represents the absorbance at 412 nm;
- D represents the dilution factor; and
- Cpr represents the sample concentration (mg/mL).
4.13. Surface Hydrophobicity
4.14. Microstructure
4.15. Molecular Interaction Forces
4.16. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Pei, H.; Dai, Q.; Zhang, C.; Kong, X.; Hua, Y. Raw walnut kernel: A natural source for dietary proteases and bioactive proteins. Food Chem. 2021, 369, 130961. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Salvadó, J.-S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Núñez, I.; Pérez-Heras, A.; Serra, M.; Gilabert, R.; Casals, E.; Deulofeu, R. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation 2004, 109, 1609–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Decker, E.A.; McClements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Emam-Djomeh, Z.; Jahanbani, R.; Moosavi-Movahedi, A.A. Physicochemical and bio-functional properties of walnut proteins as affected by trypsin-mediated hydrolysis. Food Biosci. 2020, 36, 100611. [Google Scholar] [CrossRef]
- Sze-Tao, K.; Sathe, S. Walnuts (Juglans regia L.): Proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J. Sci. Food Agric. 2000, 80, 1393–1401. [Google Scholar] [CrossRef]
- Yan, C.; Zhou, Z. Solubility and emulsifying properties of phosphorylated walnut protein isolate extracted by sodium trimetaphosphate. LWT 2021, 143, 111117. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, L.; Song, W.; Zhang, C.; Li, X. Separation, identification and molecular binding mechanism of dipeptidyl peptidase IV inhibitory peptides derived from walnut (Juglans regia l.) protein. Food Chem. 2021, 347, 129062. [Google Scholar] [CrossRef]
- Shi, A.; Jiao, B.; Liu, H.; Zhu, S.; Shen, M.; Feng, X.; Hu, H.; Liu, L.; Faisal, S.; Wang, Q.; et al. Effects of proteolysis and transglutaminase crosslinking on physicochemical characteristics of walnut protein isolate. LWT 2018, 97, 662–667. [Google Scholar] [CrossRef]
- Cai, Y.; Deng, X.; Liu, T.; Zhao, M.; Zhao, Q.; Chen, S. Effect of xanthan gum on walnut protein/xanthan gum mixtures, interfacial adsorption, and emulsion properties. Food Hydrocoll. 2018, 79, 391–398. [Google Scholar] [CrossRef]
- Zheng, H.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Effect of κ-carrageenan on gelation and gel characteristics of Antarctic krill (Euphausia superba) protein isolated with isoelectric solubilization/precipitation. Food Chem. 2019, 278, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Le, X.T.; Rioux, L.E.; Turgeon, S.L. Formation and functional properties of protein-polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, J.; Hemar, Y.; Cui, B. Improvement of the rheological and textural properties of calcium sulfate-induced soy protein isolate gels by the incorporation of different polysaccharides. Food Chem. 2020, 310, 125983. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, L.; Jiang, X.; Chen, Y.; Zhou, G. Insight into the mechanism of myofibrillar protein gel influenced by konjac glucomannan: Moisture stability and phase separation behavior. Food Chem. 2021, 339, 127941. [Google Scholar] [CrossRef] [PubMed]
- Yemenicioğlu, A.; Farris, S.; Turkyilmaz, M.; Gulec, S. A review of current and future food applications of natural hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1389–1406. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Cao, C.; Feng, Y.; Kong, B.; Xia, X.; Liu, M.; Chen, J.; Liu, Q. Textural and gel properties of frankfurters as influenced by various κ-carrageenan incorporation methods. Meat Sci. 2021, 176, 108483. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Yarmand, M.S.; Salami, M.; Momen, S.; Moosavi-Movahedi, A.A. Cold gelation of curcumin loaded whey protein aggregates mixed with k-carrageenan: Impact of gel microstructure on the gastrointestinal fate of curcumin. Food Hydrocoll. 2018, 85, 267–280. [Google Scholar] [CrossRef]
- Xia, W.; Ma, L.; Chen, X.; Li, X.; Zhang, Y. Physicochemical and structural properties of composite gels prepared with myofibrillar protein and lecithin at various ionic strengths. Food Hydrocoll. 2018, 82, 135–143. [Google Scholar] [CrossRef]
- Xiao, Y.; Kang, S.; Liu, Y.; Guo, X.; Li, M.; Xu, H. Effect and mechanism of calcium ions on the gelation properties of cellulose nanocrystals-whey protein isolate composite gels. Food Hydrocoll. 2021, 111, 106401. [Google Scholar] [CrossRef]
- Yang, Z.; Campo, L.; Gilbert, E.P.; Knott, R.; Cheng, L.; Storer, B.; Lin, X.; Luo, L.; Patole, S.; Hemar, Y. Effect of Na+ and CaCl2 concentration on the rheological and structural characteristics of thermally-induced quinoa protein gels. Food Hydrocoll. 2022, 124, 107350. [Google Scholar] [CrossRef]
- Savadkoohi, S.; Farahnaky, A. Dynamic rheological and thermal study of the heat-induced gelation of tomato-seed proteins. J. Food Eng. 2012, 113, 479–485. [Google Scholar] [CrossRef]
- Zhou, F.; Pan, M.; Liu, Y.; Guo, N.; Zhang, Q.; Wang, J. Effects on Na+ on the cold gelation between a low-methoxyl pectin extracted from Premna microphylla turcz and soy protein isolate. Food Hydrocoll. 2020, 104, 105762. [Google Scholar] [CrossRef]
- Beck, M.; Jekle, M.; Becker, T. Impact of sodium chloride on wheat flour dough for yeast-leavened products. I. Rheological attributes. J. Sci. Food Agric. 2012, 92, 585–592. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Li-Sha, Y.; Chen, H. Structural, gelation properties and microstructure of rice glutelin/sugar beet pectin composite gels: Effects of ionic strengths. Food Chem. 2020, 346, 128956. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, C.; Wen, Q.; Yang, X.; Li, L.; Deng, W. Thermal aggregation and gelation of kidney bean (Phaseolus vulgaris L.) protein isolate at pH 2.0: Influence of ionic strength. Food Hydrocoll. 2010, 24, 266–274. [Google Scholar] [CrossRef]
- Wang, L.; Fogliano, V.; Heising, J.; Meulenbroeks, E.; Dekker, M. Volatile antimicrobial absorption in food gel depends on the food matrix characteristics. Food Hydrocoll. 2020, 107, 105933. [Google Scholar] [CrossRef]
- Jin, H.; Chen, J.; Zhang, J.; Sheng, L. Impact of phosphates on heat-induced egg white gel properties: Texture, water state, micro-rheology and microstructure. Food Hydrocoll. 2021, 110, 106200. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Huang, Q.; Geng, F. Microwave pretreatment enhanced the properties of ovalbumin-inulin-oil emulsion gels and improved the storage stability of pomegranate seed oil. Food Hydrocoll. 2021, 113, 106548. [Google Scholar] [CrossRef]
- Huang, W.; Sun, X. Adhesive properties of soy proteins modified by urea and guanidine hydrochloride. J. Am. Oil Chem. Soc. 2000, 77, 101–104. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Liu, X.; Liu, W.; Liu, Q.; Huang, J.; Zhang, L.; Hu, H. Effect of salt ions on mixed gels of wheat gluten protein and potato isolate protein. LWT 2022, 154, 112564. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A.; Labbafi, M. Modulating the textural characteristics of whey protein nanofibril gels with different concentrations of calcium chloride. J. Dairy Res. 2016, 83, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Panchal, B.; Truong, T.; Prakash, S.; Bansal, N.; Bhandari, B. Effect of water content, droplet size, and gelation on fat phase transition and water mobility in water-in-milk fat emulsions. Food Chem. 2020, 333, 127538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Zhong, M.; Qi, B.; Li, Y. Soy and whey protein isolate mixture/calcium chloride thermally induced emulsion gels: Rheological properties and digestive characteristics. Food Chem. 2022, 380, 132212. [Google Scholar] [CrossRef] [PubMed]
- Griffin, K.; Khouryieh, H. Influence of electrostatic interactions on the formation and stability of multilayer fish oil-in-water emulsions stabilized by whey protein-xanthan-locust bean complexes. J. Food Eng. 2020, 277, 109893. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit- and vegetable-based matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, Q.; Guo, L.; Ho, H.; Wang, B.; Sun, J.; Xu, X.; Huang, M. Effects of ultrafine comminution treatment on gelling properties of myofibrillar proteins from chicken breast. Food Hydrocoll. 2019, 97, 105199. [Google Scholar] [CrossRef]
- Zhao, Z.; Mu, T.; Zhang, M.; Richel, A. Effect of salts combined with high hydrostatic pressure on structure and gelation properties of sweet potato protein. LWT Food Sci. Technol. 2018, 93, 36–44. [Google Scholar] [CrossRef]
- Sheng, L.; Su, P.; Han, K.; Chen, J.; Cao, A.; Zhang, Z.; Jin, Y.; Ma, M. Synthesis and structural characterization of lysozyme-pullulan conjugates obtained by the Maillard reaction. Food Hydrocoll. 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Xiong, W.F.; Wang, Y.T.; Zhang, C.L. High intensity ultrasound modified ovalbumin: Structure, interface and gelation properties. Ultrason. Sonochem. 2016, 31, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Pallarès, I.; Vendrell, J.; Avilés, F.X.; Ventura, S. Amyloid fibril formation by a partially structured intermediate state of α-chymotrypsin. J. Mol. Biol. 2004, 342, 321–331. [Google Scholar] [CrossRef]
- Liu, K.; Li, Q.; Pan, L.; Qian, X.; Zhang, H.; Zha, X.; Luo, J. The effects of lotus root amylopectin on the formation of whey protein isolate gels. Carbohydr. Polym. 2017, 175, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li-Chan, E.C.Y.; Wan, L.; Tian, M.; Pan, S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013, 32, 303–311. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Li-Sha, Y.; Chen, H. Physicochemical, structural and gelation properties of arachin-basil seed gum composite gels: Effects of salt types and concentrations. Food Hydrocoll. 2021, 113, 106545. [Google Scholar] [CrossRef]
- Wang, W.; Shen, M.; Liu, S.; Jiang, L.; Song, Q.; Xie, J. Gel properties and interactions of Mesona blumes polysaccharide-soy protein isolates mixed gel: The effect of salt addition. Carbohydr. Polym. 2018, 192, 193–201. [Google Scholar] [CrossRef]
- Diao, X.; Guan, H.; Zhao, X.; Diao, X.; Kong, B. Physicochemical and structural properties of composite gels prepared with myofibrillar protein and lard diacylglycerols. Meat Sci. 2016, 121, 333–341. [Google Scholar] [CrossRef]
- Xia, Q.; Gu, M.; Liu, J.; Niu, Y.; Yu, L. Novel composite gels of gelatin and soluble dietary fiber from black bean coats with interpenetrating polymer networks. Food Hydrocoll. 2018, 83, 72–78. [Google Scholar] [CrossRef]
- Sun, C.; Chen, S.; Dai, L.; Gao, Y. Structural characterization and formation mechanism of zein-propylene glycol alginate binary complex induced by calcium ions. Food Res. Int. 2017, 100, 57–68. [Google Scholar] [CrossRef]
- Lei, Y.; Gao, S.; Xiang, X.; Li, X.; Yu, X.; Li, S. Physicochemical, structural and adhesion properties of walnut protein isolate-xanthan gum composite adhesives using walnut protein modified by ethanol. Int. J. Biol. Macromol. 2021, 192, 644–653. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, M.; Qin, F.; Adhikari, B.; He, Z.; Chen, J. Enhanced CaSO4-induced gelation properties of soy protein isolate emulsion by pre-aggregation. Food Chem. 2018, 242, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Wang, Y.; Wang, Y.; Wang, X.; Gao, G.; Chen, G.; Liu, A. Effects of nanofiber cellulose on functional properties of heat-induced chicken salt-soluble protein gel enhanced with microbial transglutaminase. Food Hydrocoll. 2018, 84, 1–8. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Mo, J.; Lyu, Y.; Shen, X. Effect of guar gum on adhesion properties of soybean protein isolate onto porcine bones. Int. J. Adhes. Adhes. 2017, 74, 124–131. [Google Scholar] [CrossRef]
- Tang, C.H.; Chen, L.; Foegeding, E.A. Mechanical and water-holding properties and microstructures of soy protein isolate emulsion gels induced by CaCl2, glucono-δ-lactone (GDL), and transglutaminase: Influence of thermal Treatments before and/or after emulsification. J. Agric. Food Chem. 2011, 59, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, H.; Qi, H.; Ying, X. Cryoprotective characteristics of different sugar alcohols on peeled Pacific white shrimp (Litopenaeus vannamei) during frozen storage and their possible mechanisms of action. Int. J. Food Prop. 2020, 23, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Paraskevi, Z.; Spyridon, M.; Anastasia, B.; Sophia, G.A. Preparation, physicochemical properties, and in vitro toxicity towards cancer cells of novel types of arsonoliposomes. Pharmaceutics 2020, 12, 327. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Sun, Z.; Ma, M.; Jin, Y.; Sheng, L. Effect of microwave-assisted phosphorylation modification on the structural and foaming properties of egg white powder. LWT Food Sci. Technol. 2018, 97, 151–156. [Google Scholar] [CrossRef]
- Liu, K.; Li, Q.; Zha, X.; Pan, L.; Bao, L.; Zhang, H.; Luo, J. Effects of calcium or sodium ions on the properties of whey protein isolate-lotus root amylopectin composite gel. Food Hydrocoll. 2019, 87, 629–636. [Google Scholar] [CrossRef]
- Wang, K.; Luo, S.; Zhong, X.; Cai, J.; Jiang, S.; Zheng, Z. Effects of partial hydrolysis and subsequent cross-linking on wheat gluten physicochemical properties and structure. Food Chem. 2016, 197, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Dou, W.; Alaxi, S.; Niu, Y.; Yu, L. Modified soluble dietary fiber from black bean coats with its rheological and bile acid binding properties. Food Hydrocoll. 2017, 62, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.D.; Arntfield, S.D. Molecular forces involved in heat-induced pea protein gelation: Effects of various reagents on the rheological properties of salt extracted pea protein gels. Food Hydrocoll. 2012, 28, 325–332. [Google Scholar] [CrossRef]




| Hardness | Adhesiveness | Springiness | Cohesiveness | Gumminess | Chewiness | Resilience | |
|---|---|---|---|---|---|---|---|
| WNPI | 9.05 ± 1.06 e | −3.71 ± 0.16 d | 0.55 ± 0.05 b | 0.38 ± 0.03 c | 3.48 ± 0.70 e | 1.92 ± 0.55 e | 0.32 ± 0.01 b |
| NaCl-0 mM | 47.94 ± 2.06 d | −5.80 ± 0.69 c | 0.86 ± 0.05 a | 0.52 ± 0.15 bc | 24.07 ± 1.58 d | 21.60 ± 2.12 d | 0.51 ± 0.08 a |
| NaCl-5 mM | 61.91 ± 0.14 c | −7.82 ± 0.90 b | 0.91 ± 0.04 a | 0.65 ± 0.11 ab | 41.19 ± 2.65 c | 37.71 ± 2.44 c | 0.53 ± 0.09 a |
| NaCl-10 mM | 70.83 ± 0.60 b | −7.92 ± 1.01 b | 0.91 ± 0.00 a | 0.76 ± 0.03 a | 53.65 ± 2.21 b | 48.54 ± 2.1 b | 0.51 ± 0.04 a |
| NaCl-15 mM | 96.18 ± 3.85 a | −10.79 ± 1.85 a | 0.92 ± 0.01 a | 0.76 ± 0.05 a | 70.38 ± 2.72 a | 64.86 ± 1.89 a | 0.50 ± 0.03 a |
| NaCl-20 mM | 76.29 ± 1.07 b | −6.99 ± 0.52b c | 0.92 ± 0.04 a | 0.69 ± 0.16 ab | 52.30 ± 2.97 b | 48.47 ± 2.24 b | 0.52 ± 0.07 a |
| T2b(ms) | T2b-1(ms) | T21(ms) | T22(ms) | P2b(%) | P2b-1(%) | P21(%) | P22(%) | |
|---|---|---|---|---|---|---|---|---|
| WNPI | -- | 5.29 ± 0.21 e | 264.45 ± 10.47 a | -- | -- | 0.71 ± 0.07 d | 99.29 ± 0.07 a | -- |
| KC | -- | 5.18 ± 0.36 e | -- | 2025.50 ± 0.00 c | -- | 0.41 ± 0.02 e | -- | 99.59 ± 0.02 a |
| NaCl-0 mM | -- | 7.32 ± 0.00 a | 241.07 ± 9.77 b | 2171.12 ± 0.00 b | -- | 2.21 ± 0.00 a | 97.18 ± 0.16 c | 0.62 ± 0.16 b |
| NaCl-5 mM | -- | 6.37 ± 0.00 c | 224.90 ± 9.12 c | -- | -- | 1.92 ± 0.07 b | 98.08 ± 0.07 b | -- |
| NaCl-10 mM | -- | 6.83 ± 0.00 b | 235.43 ± 0.00 bc | -- | -- | 2.13 ± 0.05 a | 97.87 ± 0.05 b | -- |
| NaCl-15 mM | 0.22 ± 0.01 a | 5.94 ± 0.00 d | 235.43 ± 0.00 bc | -- | 1.46 ± 0.18 a | 1.69 ± 0.06 c | 96.85 ± 0.12 c | -- |
| NaCl-20 mM | -- | 6.99 ± 0.28 ab | 230.17 ± 9.12 bc | 2327.20 ± 0.00 a | -- | 2.17 ± 0.08 a | 96.75 ± 0.50 c | 1.07 ± 0.43 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Ouyang, H.; Peng, W.; Yu, X.; Jin, L.; Li, S. Effect of NaCl on the Rheological, Structural, and Gelling Properties of Walnut Protein Isolate-κ-Carrageenan Composite Gels. Gels 2022, 8, 259. https://doi.org/10.3390/gels8050259
Lei Y, Ouyang H, Peng W, Yu X, Jin L, Li S. Effect of NaCl on the Rheological, Structural, and Gelling Properties of Walnut Protein Isolate-κ-Carrageenan Composite Gels. Gels. 2022; 8(5):259. https://doi.org/10.3390/gels8050259
Chicago/Turabian StyleLei, Yuqing, Hui Ouyang, Wu Peng, Xiongwei Yu, Long Jin, and Shugang Li. 2022. "Effect of NaCl on the Rheological, Structural, and Gelling Properties of Walnut Protein Isolate-κ-Carrageenan Composite Gels" Gels 8, no. 5: 259. https://doi.org/10.3390/gels8050259
APA StyleLei, Y., Ouyang, H., Peng, W., Yu, X., Jin, L., & Li, S. (2022). Effect of NaCl on the Rheological, Structural, and Gelling Properties of Walnut Protein Isolate-κ-Carrageenan Composite Gels. Gels, 8(5), 259. https://doi.org/10.3390/gels8050259