An Alternative Radiation Shielding Material Based on Barium-Sulphate (BaSO4)-Modified Fly Ash Geopolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. OPC Preparation
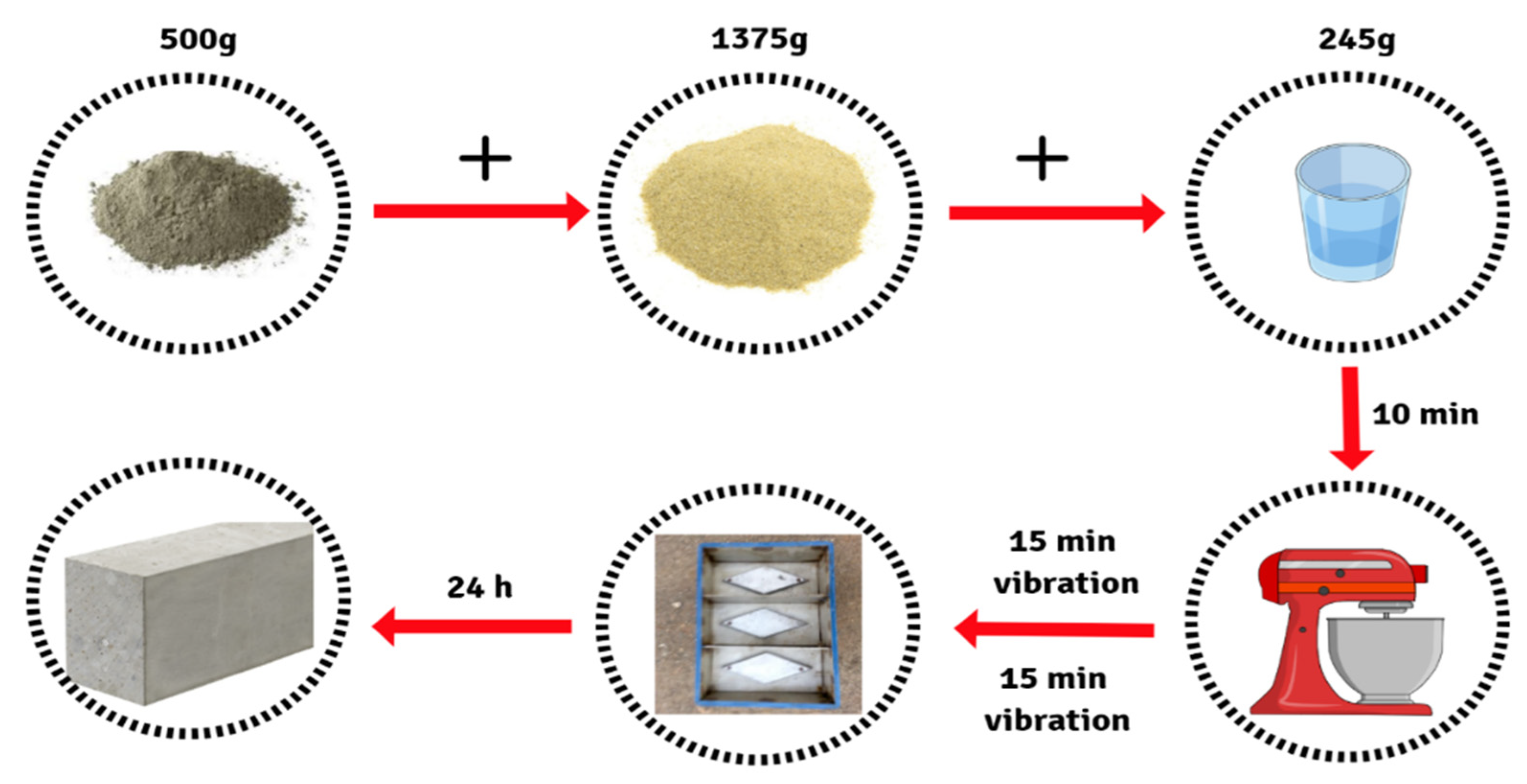
2.2. FAGP Preparation
2.3. Leaching Text on Synthesized Samples
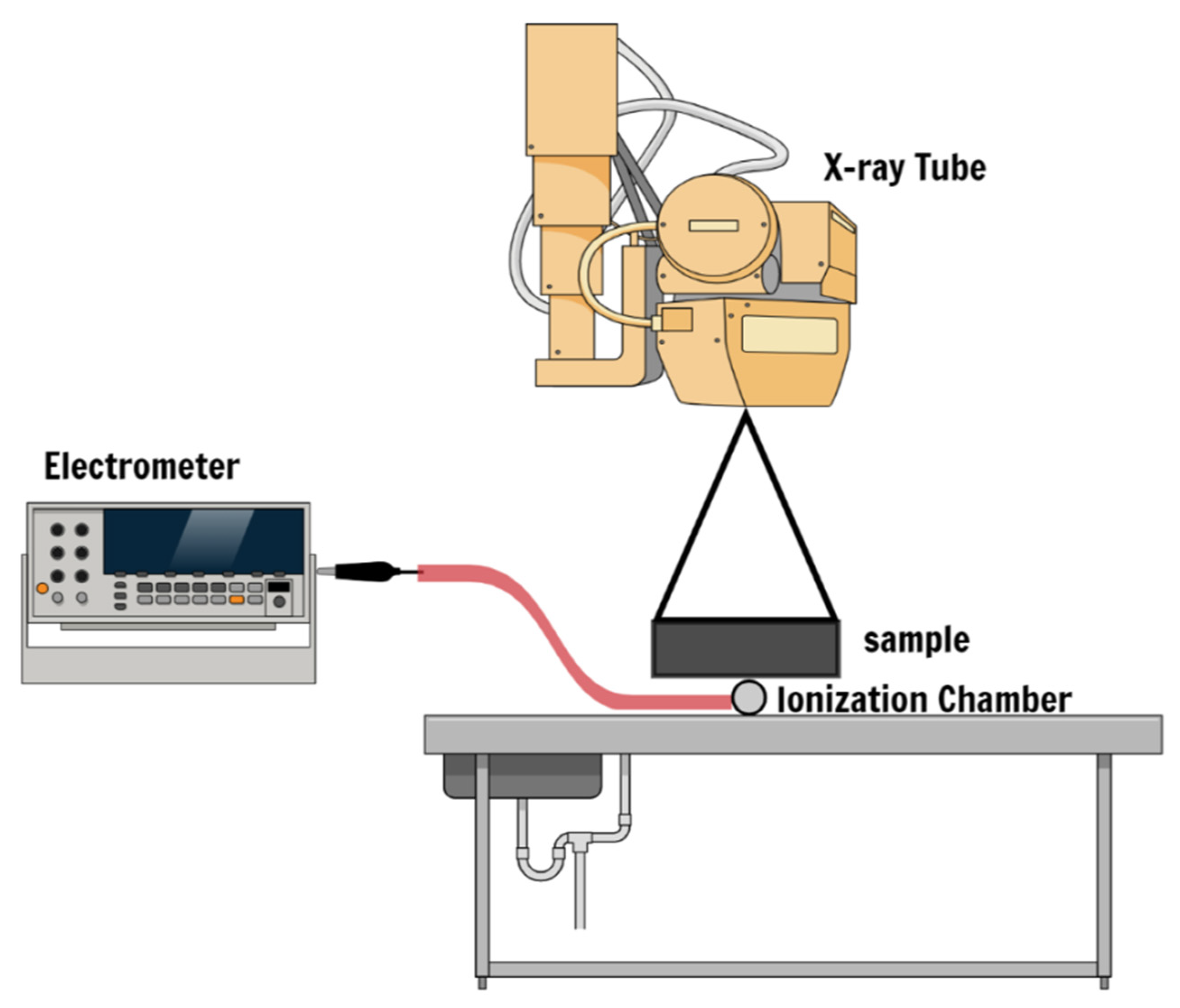
X-ray Dose Measurement Setup
3. Results
3.1. EDX and Effective Atomic Number (Zeff)
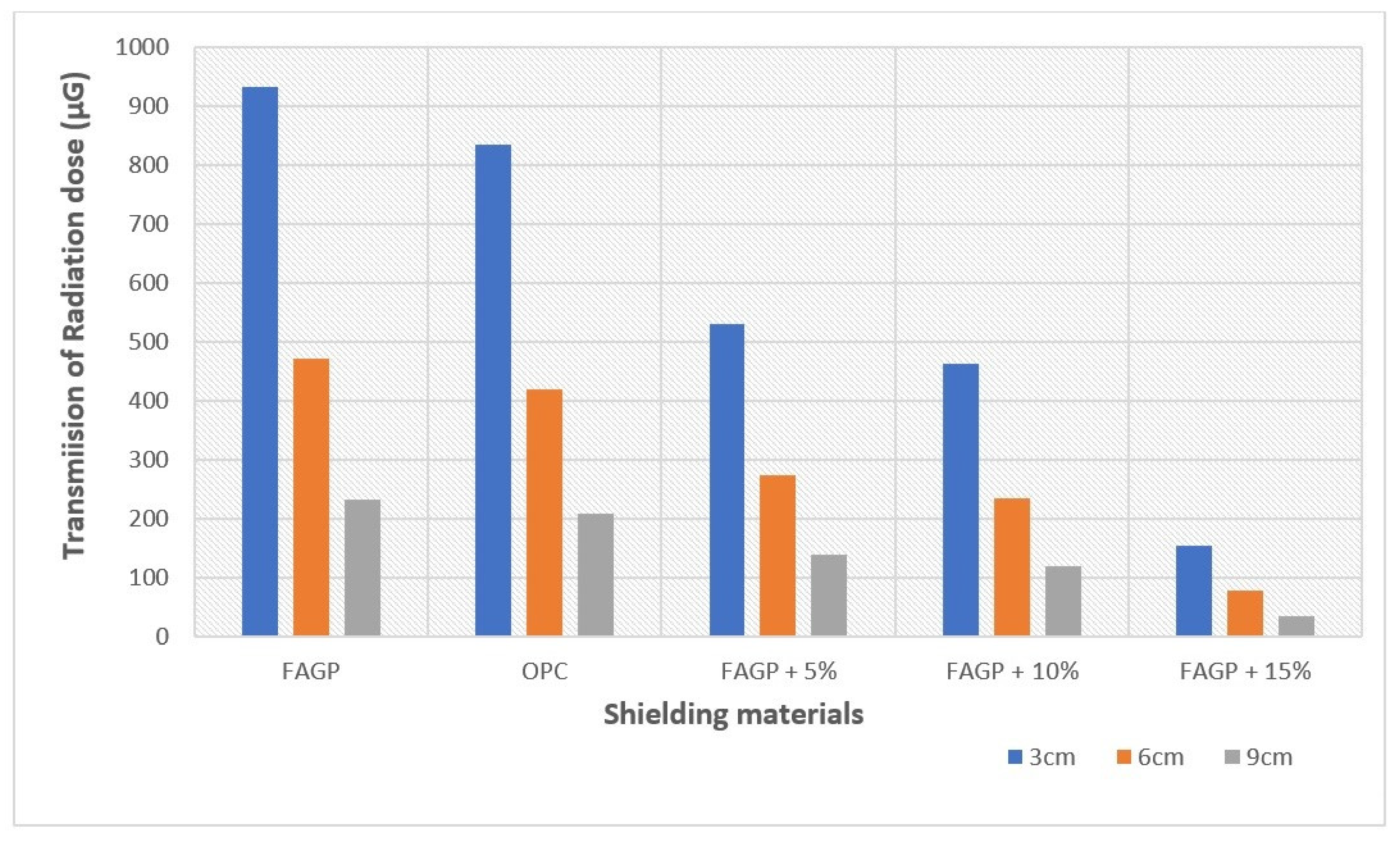
3.2. Effect of Thickness on the Radiation Shielding Capacities of OPC and FAGP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A. Gamma ray shielding properties of PbO-Li2O-B2O3 glasses. Radiat. Phys. Chem. 2017, 136, 50–53. [Google Scholar] [CrossRef]
- Basu, P.; Sarangapani, R.; Venkatraman, B. Gamma ray buildup factors for conventional shielding materials and buildup factors computed for tungsten with a thickness beyond 40 mean free paths. Appl. Radiat. Isot. 2019, 154, 108864. [Google Scholar] [CrossRef] [PubMed]
- Oglat, A.A. Acceptance experimentation and quality monitor of X-ray radiography units. Radiat. Phys. Chem. 2020, 172, 108810. [Google Scholar] [CrossRef]
- Kalita, J.; Chithambo, M. Thermoluminescence and infrared light stimulated luminescence of limestone (CaCO3) and its dosimetric features. Appl. Radiat. Isot. 2019, 154, 108888. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; El-Mallawany, R.; Sayyed, M.; Tekin, H. Shielding properties of 80TeO2–5TiO2–(15 − x) WO3–xAnOm glasses using WinXCom and MCNP5 code. Radiat. Phys. Chem. 2017, 141, 172–178. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, K.; Anand, V. Structural properties of Bi2O3–B2O3–SiO2–Na2O glasses for gamma ray shielding applications. Radiat. Phys. Chem. 2016, 120, 63–72. [Google Scholar] [CrossRef]
- Oglat, A.A. Chemistry. Studying the radiation absorption and scattering of gamma rays by using different absorbers. Radiat. Phys. Chem. 2020, 176, 109072. [Google Scholar] [CrossRef]
- Tashlykov, O.; Shcheklein, S.Y.; Lukyanenko, V.Y.; Mikhaylova, A.; Russkikh, I.; Seleznev, Y.N.; Kozlov, A. The optimization of radiation protection composition. Nucl. Energy Technol. 2016, 2, 42–44. [Google Scholar] [CrossRef]
- Boeykens, S.; Redondo, N.; Obeso, R.A.; Caracciolo, N.; Vázquez, C. Chromium and Lead adsorption by avocado seed biomass study through the use of Total Reflection X-ray Fluorescence analysis. Appl. Radiat. Isot. 2019, 153, 108809. [Google Scholar] [CrossRef]
- Davidovits, J. High-alkali cements for 21st century concretes. Spec. Publ. 1994, 144, 383–398. [Google Scholar]
- Rehan, R.; Nehdi, M. Carbon dioxide emissions and climate change: Policy implications for the cement industry. Environ. Sci. Policy 2005, 8, 105–114. [Google Scholar] [CrossRef]
- Kong, D.L.; Sanjayan, J.G. Damage behavior of geopolymer composites exposed to elevated temperatures. Cem. Concr. Compos. 2008, 30, 986–991. [Google Scholar] [CrossRef]
- Gunasekara, M.; Law, D.; Setunge, S. Effect of composition of fly ash on compressive strength of fly ash based geopolymer mortar. In Proceedings of the 23rd Australasian Conference on the Mechanics of Structures and Materials, Byron Bay, Australia, 9–12 December 2014. [Google Scholar]
- Davidovits, J. Global warming impact on the cement and aggregates industries. World Resour. Rev. 1994, 6, 263–278. [Google Scholar]
- BrahimiMoussa, S.; Benamar, M.E.A.; LounisMokrani, Z. Characterization of the chemical and structural modifications induced by gamma rays on the MAGIC polymer gel. Radiat. Phys. Chem. 2019, 108451. [Google Scholar] [CrossRef]
- Hu, S.; Wang, H.; Zhang, G.; Ding, Q. Bonding and abrasion resistance of geopolymeric repair material made with steel slag. Cem. Concr. Compos. 2008, 30, 239–244. [Google Scholar] [CrossRef]
- Avcıbaşı, U.; Ateş, B.; Ünak, P.; Gümüşer, F.G.; Gülcemal, S.; Ol, K.K.; Akgöl, S.; Tekin, V. A novel radiolabeled graft polymer: Investigation of the radiopharmaceutical potential using Albino Wistar rats. Appl. Radiat. Isot. 2019, 154, 108872. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.; Rangan, B.V. On the development of fly ash-based geopolymer concrete. ACI Mater. J.-Am. Concr. Inst. 2004, 101, 467–472. [Google Scholar]
- Cong, P.; Cheng, Y.; Engineering, T. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Kaze, C.R.; Lecomte-Nana, G.L.; Kamseu, E.; Camacho, P.S.; Provis, J.L.; Duttine, M.; Wattiaux, A.; Melo, U.C.; Research, C. Mechanical and physical properties of inorganic polymer cement made of iron-rich laterite and lateritic clay: A comparative study. Cem. Concr. Res. 2021, 140, 106320. [Google Scholar] [CrossRef]
- Kaze, C.R.; Lecomte-Nana, G.L.; Adesina, A.; Nemaleu, J.G.D.; Kamseu, E.; Melo, U.C.; Composites, C. Influence of mineralogy and activator type on the rheology behaviour and setting time of laterite based geopolymer paste. Cem. Concr. Res. 2022, 126, 104345. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, S.; Yang, J.; Chen, Y.; Ye, N.; Ke, Y.; Tao, S.; Xiao, K.; Hu, J.; Hou, H.; et al. Role of Fe species in geopolymer synthesized from alkali-thermal pretreated Fe-rich Bayer red mud. Constr. Build. Mater. 2019, 200, 398–407. [Google Scholar]
- Yang, X.; Yan, Z.; Yin, S.; Gao, Q.; Li, W. The Ratio Optimization and Strength Mechanism of Composite Cementitious Material with Low-Quality Fly Ash. Gels 2022, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Mirković, M.; Kljajević, L.; Dolenec, S.; Nenadović, M.; Pavlović, V.; Rajačić, M.; Nenadović, S.J.G. Potential Usage of Hybrid Polymers Binders Based on Fly Ash with the Addition of PVA with Satisfying Mechanical and Radiological Properties. Gels 2021, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Curtin University of Technology: Perth, Australia, 2005. [Google Scholar]
- Palomo, A.; Grutzeck, M.; Blanco, M. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Hardjito, D. Studies of Fly Ash-Based Geopolymer Concrete; Curtin University of Technology: Perth, Australia, 2005. [Google Scholar]
- Nawy, E.G. Concrete Construction Engineering Handbook; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Temuujin, J.; Van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Lee, W.; Van Deventer, J. The interface between natural siliceous aggregates and geopolymers. Cem. Concr. Res. 2004, 34, 195–206. [Google Scholar] [CrossRef]
- Wallah, S.; Rangan, B.V. Low-Calcium Fly Ash-Based Geopolymer Concrete: Long-Term Properties; Curtin University of Technology: Perth, Australia, 2006. [Google Scholar]
- Palomo, A.; Macias, A.; Blanco, M.; Puertas, F. Physical, chemical and mechanical characterization of geopolymers. In Proceedings of the 9th International Congress on the Chemistry of Cement, New Delhi, India, 23–28 November 1992; pp. 505–511. [Google Scholar]
- Luna-Galiano, Y.; Cornejo, A.; Leiva, C.; Vilches, L.; Fernández-Pereira, C. Properties of fly ash and metakaolín based geopolymer panels under fire resistance tests. Mater. Construc. 2015, 65, 059. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S. Thermal evolution of metakaolin geopolymers: Part 1–Physical evolution. J. Non-Cryst. Solids 2006, 352, 5541–5555. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, T. Production geopolymer materials by coal fly ash. In Proceedings of the 7th International Symposium on East Asian Resources Recycling Technology, Tainan, Taiwan, 10–14 November 2003; pp. 263–266. [Google Scholar]
- Naji, A.T.; Jaafar, M.S.; Ali, E. X-ray Protection Using Mixture of Cement Shielding with Barium Sulfate. J. Sci. Technol. 2015, 20, 34–42. [Google Scholar] [CrossRef][Green Version]
- ASTM Standart 109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM: West Conshohocken, PA, USA, 1993; Volume 4.
- Mijarsh, M.J.A.; Megat Johari, M.A.; Ahmad, Z.A. Synthesis of geopolymer from large amounts of treated palm oil fuel ash: Application of the Taguchi method in investigating the main parameters affecting compressive strength. Constr. Build. Mater. 2014, 52, 473–481. [Google Scholar] [CrossRef]
- Granizo, M.L.; Alonso, S.; Blanco-Varela, M.T.; Palomo, A. Alkaline activation of metakaolin: Effect of calcium hydroxide in the products of reaction. J. Am. Ceram. Soc. 2002, 85, 225–231. [Google Scholar] [CrossRef]
- Oglat, A.A.; Alshipli, M.; Sayah, M.A.; Farhat, O.; Ahmad, M.S.; Abuelsamen, A. Fabrication and characterization of epoxy resin-added Rhizophora spp. particleboards as phantom materials for computer tomography (CT) applications. J. Adhes. 2021, 1–18. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, X.; Chang, C.; Liu, S.; Luo, X. X-ray shielding structural and properties design for the porous transparent BaSO4/cellulose nanocomposite membranes. Int. J. Biol. Macromol. 2019, 139, 793–800. [Google Scholar] [CrossRef] [PubMed]

| Element | OPC W (%) | FAGP W (%) | FAGP + 5% BaSO4 W (%) | FAGP + 10% BaSO4 W (%) | FAGP + 15% BaSO4 W (%) |
|---|---|---|---|---|---|
| C | 12.94 | 10.98 | 11.19 | 10.82 | 10.11 |
| O | 56.47 | 44.04 | 47.18 | 46.01 | 45.47 |
| Na | 14.47 | 13.54 | 14.03 | 14.53 | |
| Mg | 11.41 | 1.67 | 0.22 | 0.22 | 0.21 |
| Al | 0.23 | 6.51 | 7.78 | 7.22 | 6.55 |
| Si | 1.11 | 10.2 | 7.64 | 8.61 | 9.55 |
| P | 0.37 | 0.19 | 0.11 | 0.17 | |
| S | 0.32 | 0.4 | 0.25 | 0.71 | 1.22 |
| K | 0.07 | 0.12 | 0.37 | 0.56 | |
| Ca | 17.52 | 8.95 | 7.04 | 6.34 | 5.85 |
| Ti | 0.29 | 0.94 | 0.83 | 0.75 | |
| Fe | 2.05 | 2.8 | 2.13 | 1.6 | |
| Ba | 1.11 | 2.25 | 3.43 | ||
| Total | 100 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oglat, A.A.; Shalbi, S.M. An Alternative Radiation Shielding Material Based on Barium-Sulphate (BaSO4)-Modified Fly Ash Geopolymers. Gels 2022, 8, 227. https://doi.org/10.3390/gels8040227
Oglat AA, Shalbi SM. An Alternative Radiation Shielding Material Based on Barium-Sulphate (BaSO4)-Modified Fly Ash Geopolymers. Gels. 2022; 8(4):227. https://doi.org/10.3390/gels8040227
Chicago/Turabian StyleOglat, Ammar A., and Sabri M. Shalbi. 2022. "An Alternative Radiation Shielding Material Based on Barium-Sulphate (BaSO4)-Modified Fly Ash Geopolymers" Gels 8, no. 4: 227. https://doi.org/10.3390/gels8040227
APA StyleOglat, A. A., & Shalbi, S. M. (2022). An Alternative Radiation Shielding Material Based on Barium-Sulphate (BaSO4)-Modified Fly Ash Geopolymers. Gels, 8(4), 227. https://doi.org/10.3390/gels8040227






