Experimental Study on Physicochemical Properties of a Shear Thixotropic Polymer Gel for Lost Circulation Control
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dilution Performance of Shear Thixotropic Polymer Gel
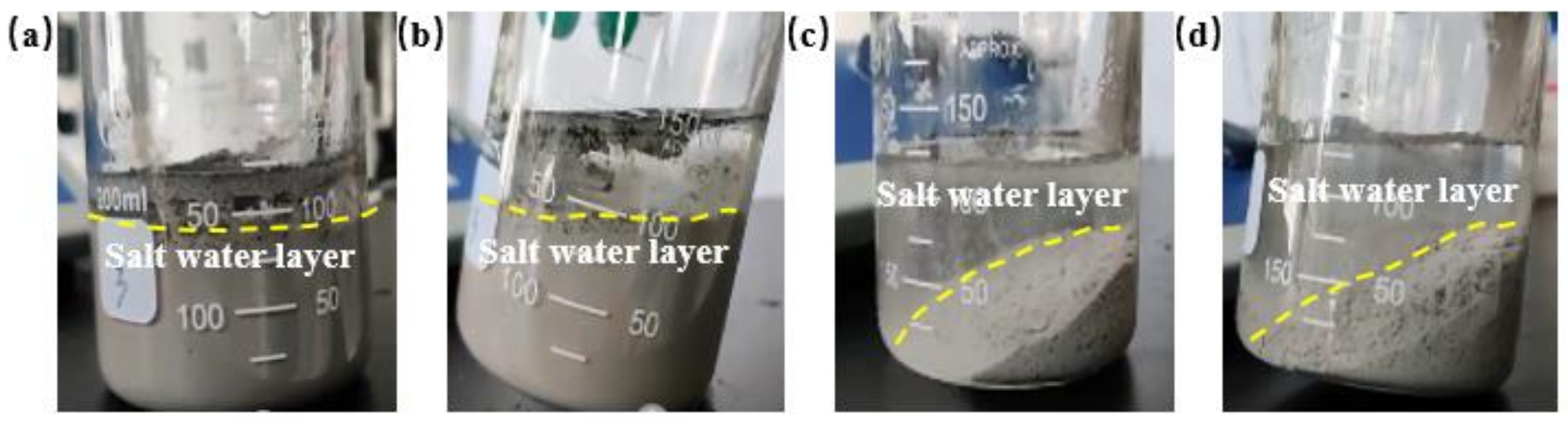
2.1.1. Anti-Dilution Performance of Salt Water
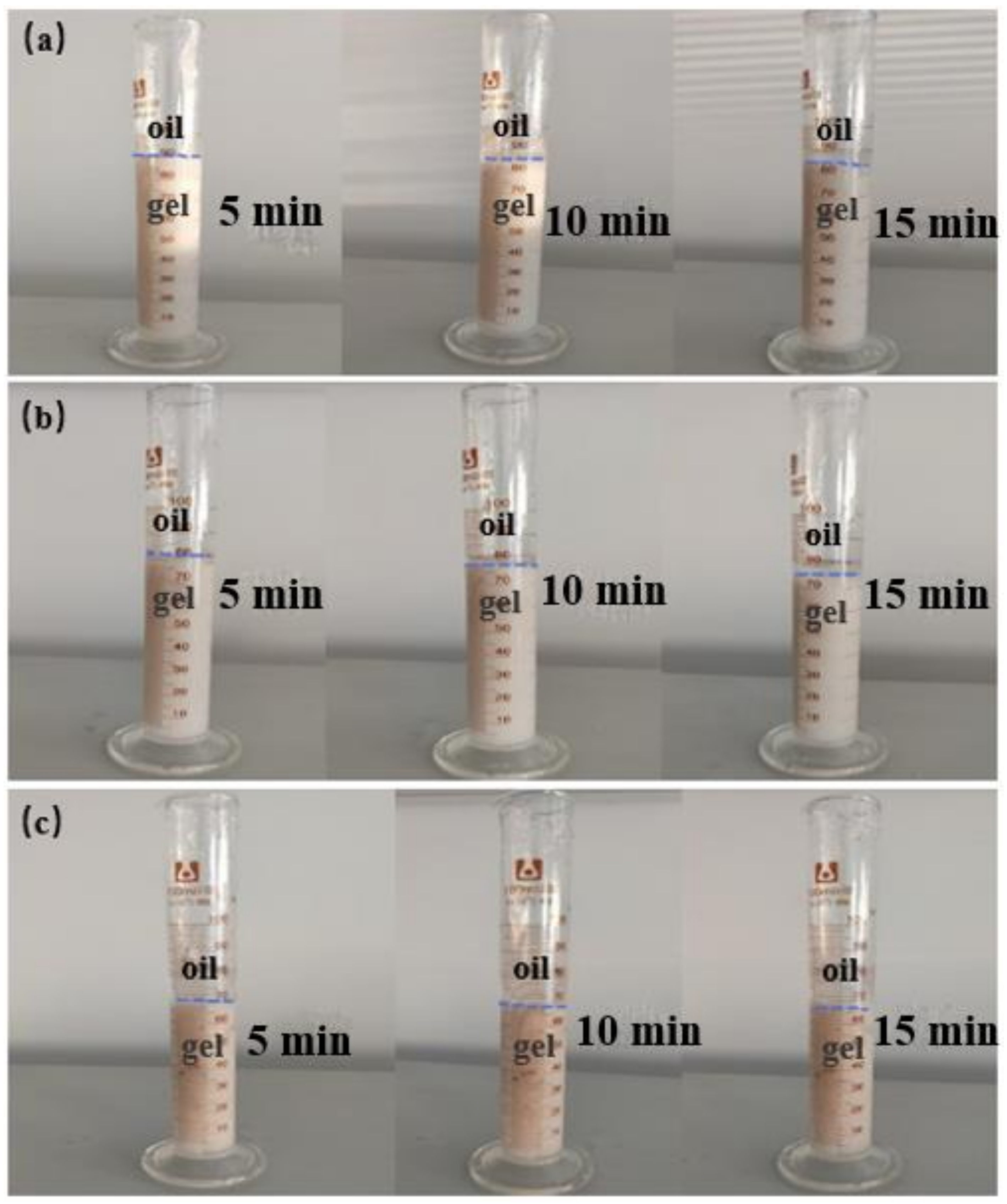
2.1.2. Anti-Dilution Performance of White Oil
2.2. Gelation Time under Different Initiator Concentrations
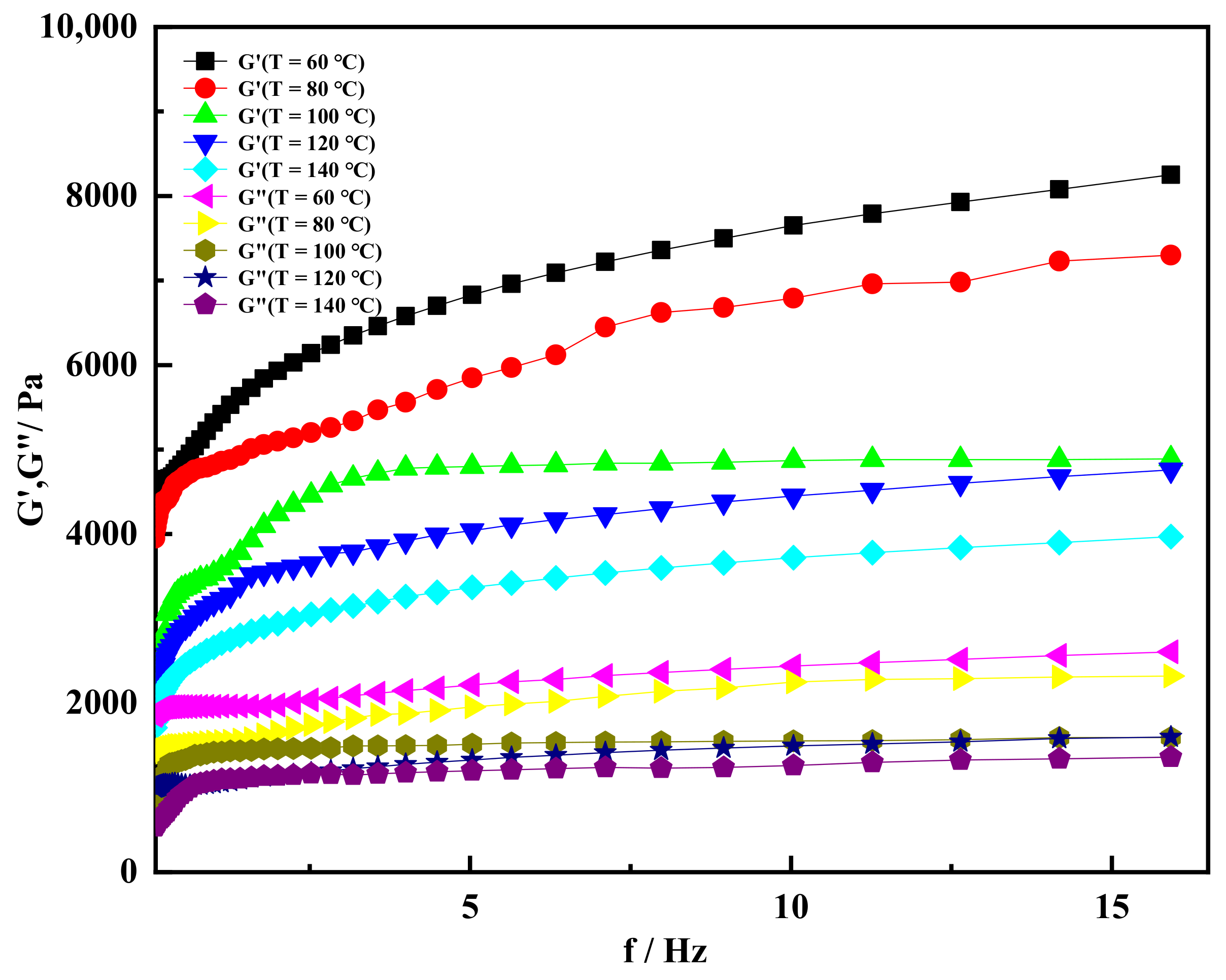
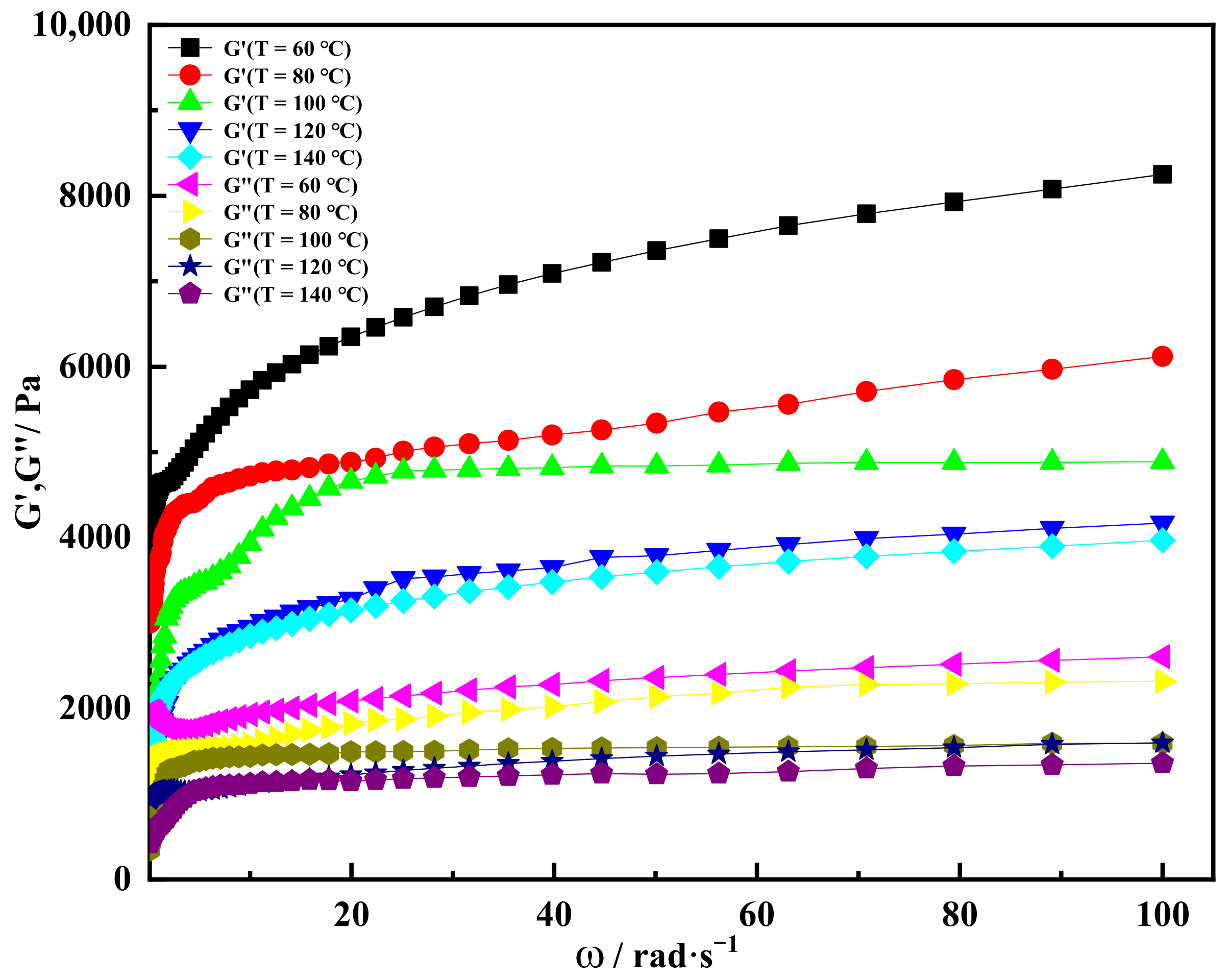
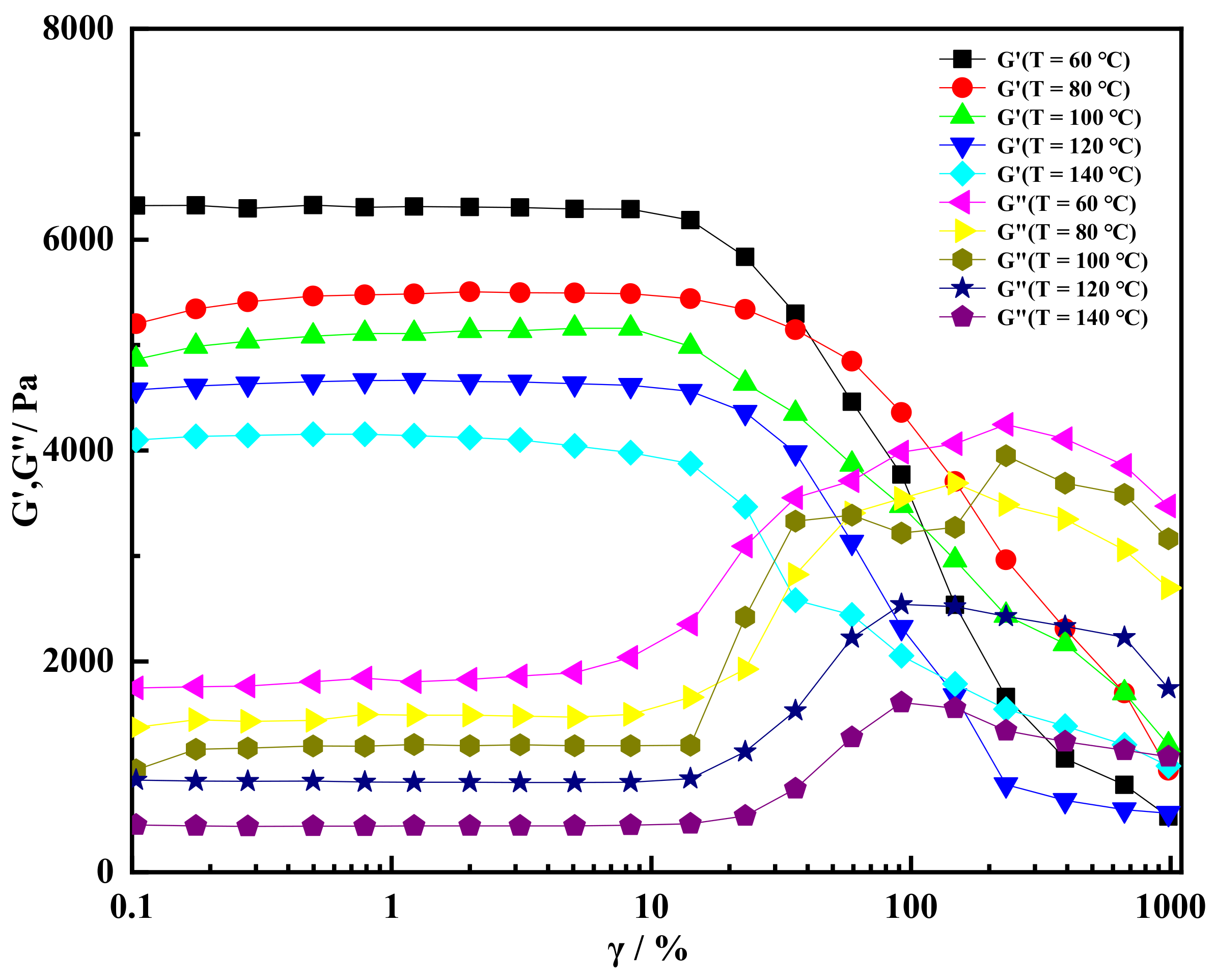
2.3. High Temperature Resistance of Shear Thixotropic Gel
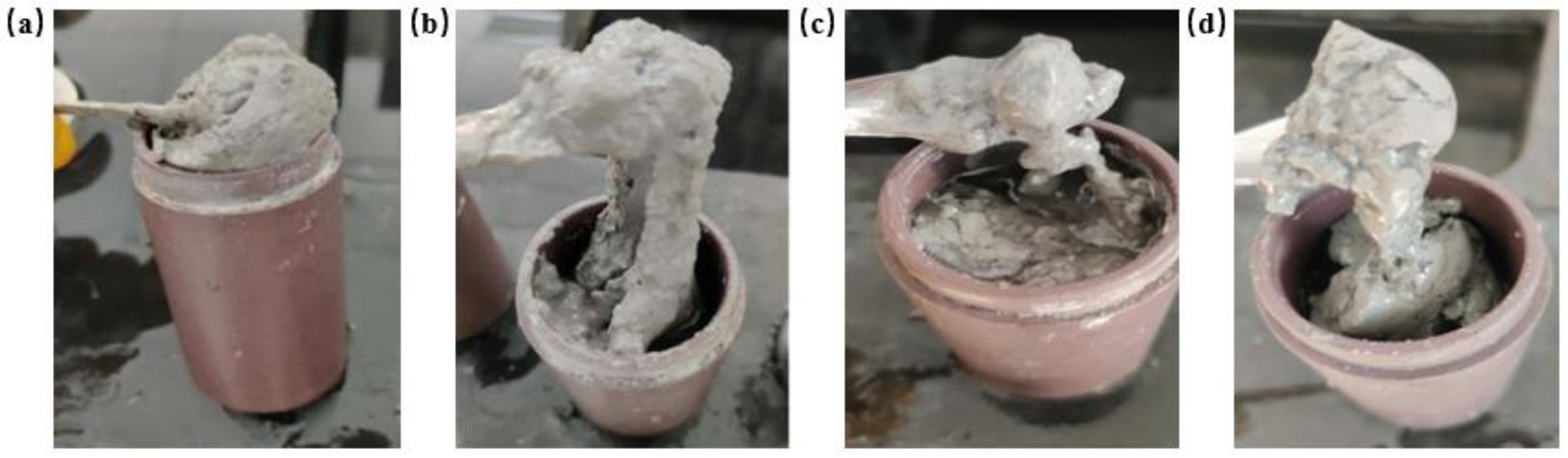
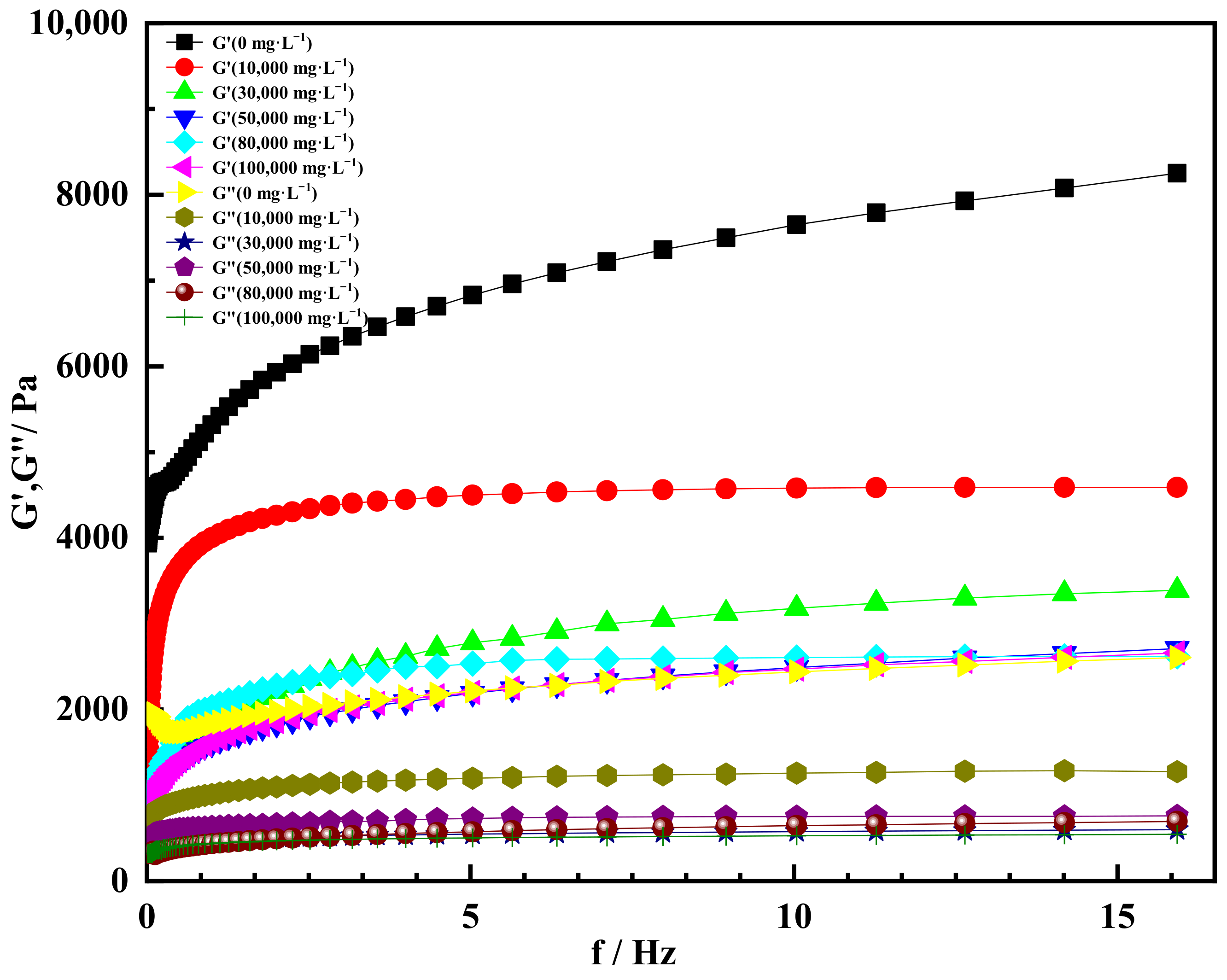
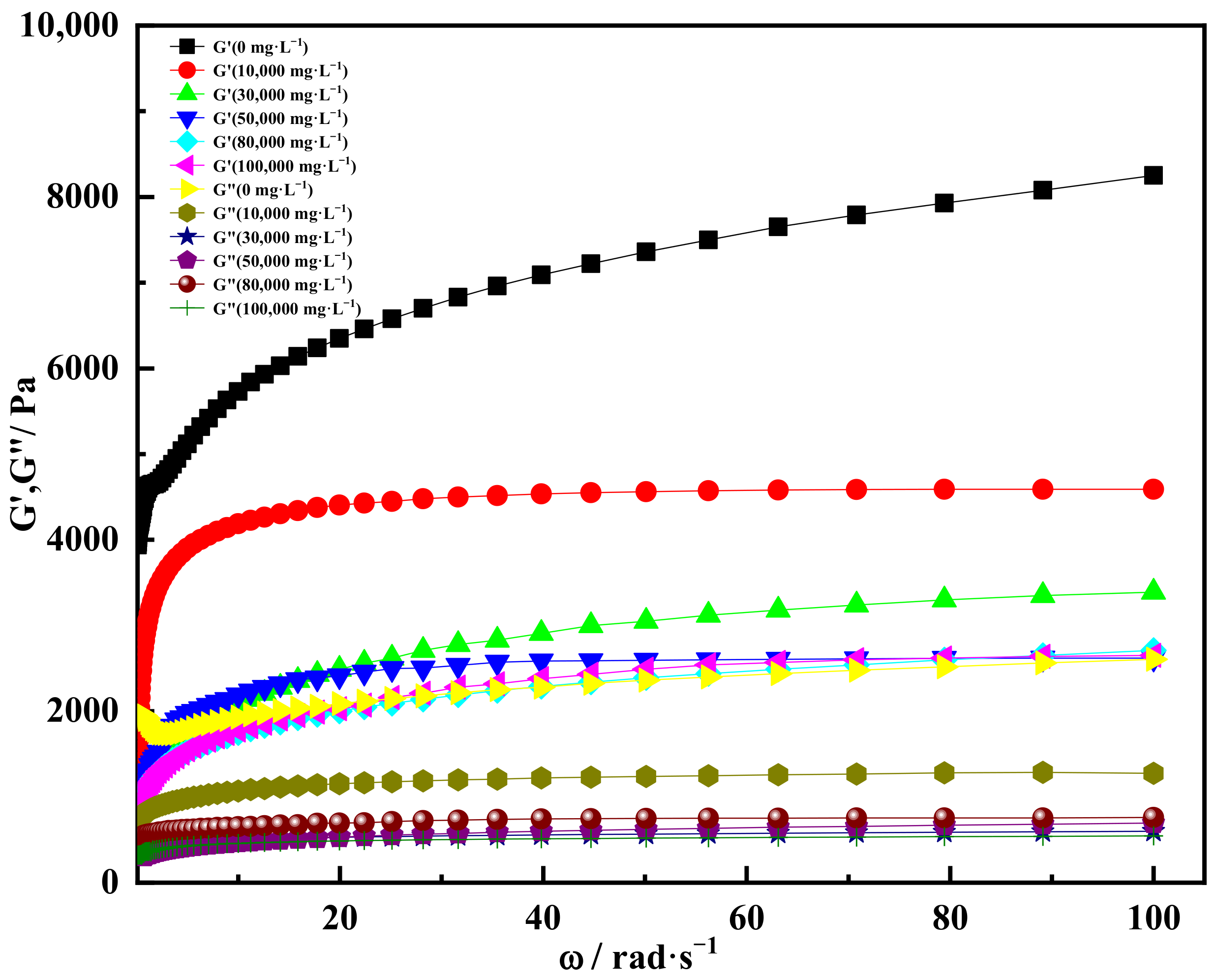
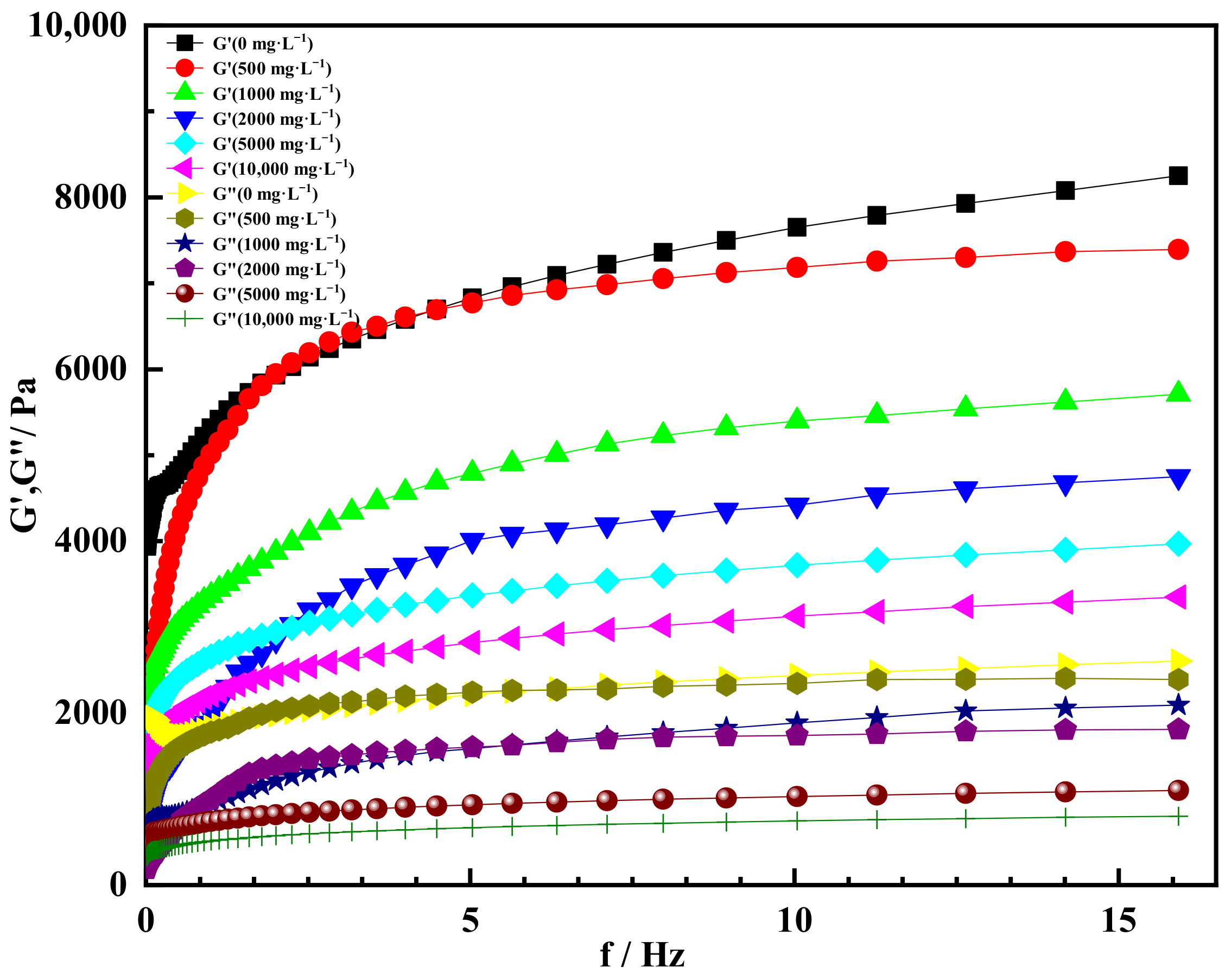
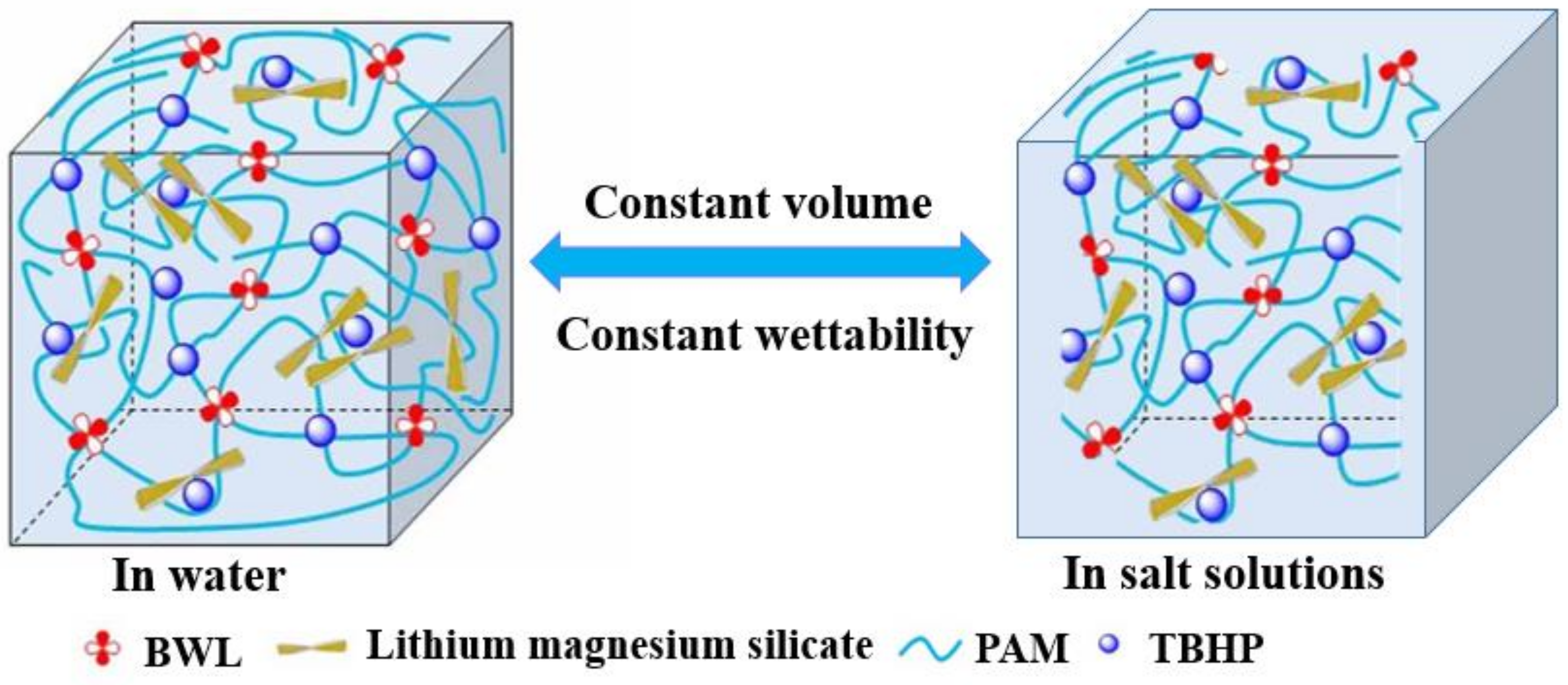
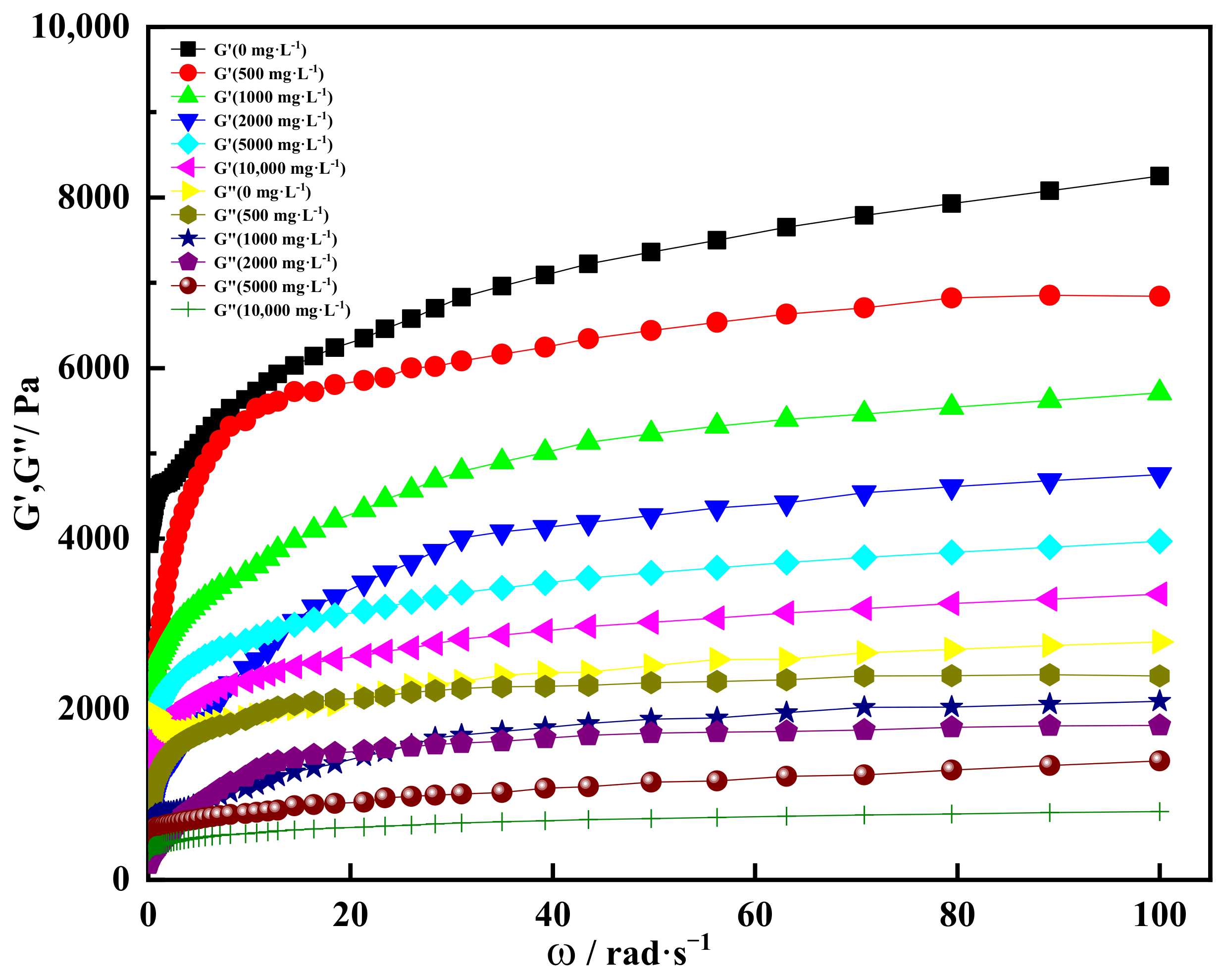
2.4. High Salt Resistance of Shear Thixotropic Polymer Gel
2.4.1. Effect of Salt Concentration on Gelation
2.4.2. Effect of Univalent Salt Ion Concentration on Gelation
2.4.3. Effect of Divalent Salt Ion Concentration on Gelation
3. Conclusions
- (1)
- The shear thixotropic polymer gel system had the ability of anti-dilution. The gel could be formed under the condition of mixing 3 times volume of heavy salt water and 3/7 times volume of white oil, and it could maintain the stability of structure and morphology. Meanwhile, the gelation time of the shear thixotropic polymer gel system could be controlled in 2–6 h under the condition of 140 °C and different initiator concentrations.
- (2)
- The shear thixotropic polymer gel had the ability to resist high temperature, and the storage modulus of the gel was greater than the loss modulus at 140 °C When the temperature was 140 °C and the scanning frequency was 16 Hz, the storage modulus of the gel still reached 3800 Pa. Shear thixotropic gels could maintain a good three-dimensional network structure at high temperature and have certain high temperature stability.
- (3)
- The shear thixotropic polymer gel system had stable three-dimensional reticular structure and excellent salt resistance under the condition of high salt. It was found that the gel had good gelation effect and strong resistance to high salt in 100,000 mg/L sodium chloride and 10,000 mg/L calcium chloride. When the scanning frequency was 4 Hz, the storage modulus of the gel was 4700 Pa. The gel was dominated by elasticity and had excellent mechanical properties.
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Methods
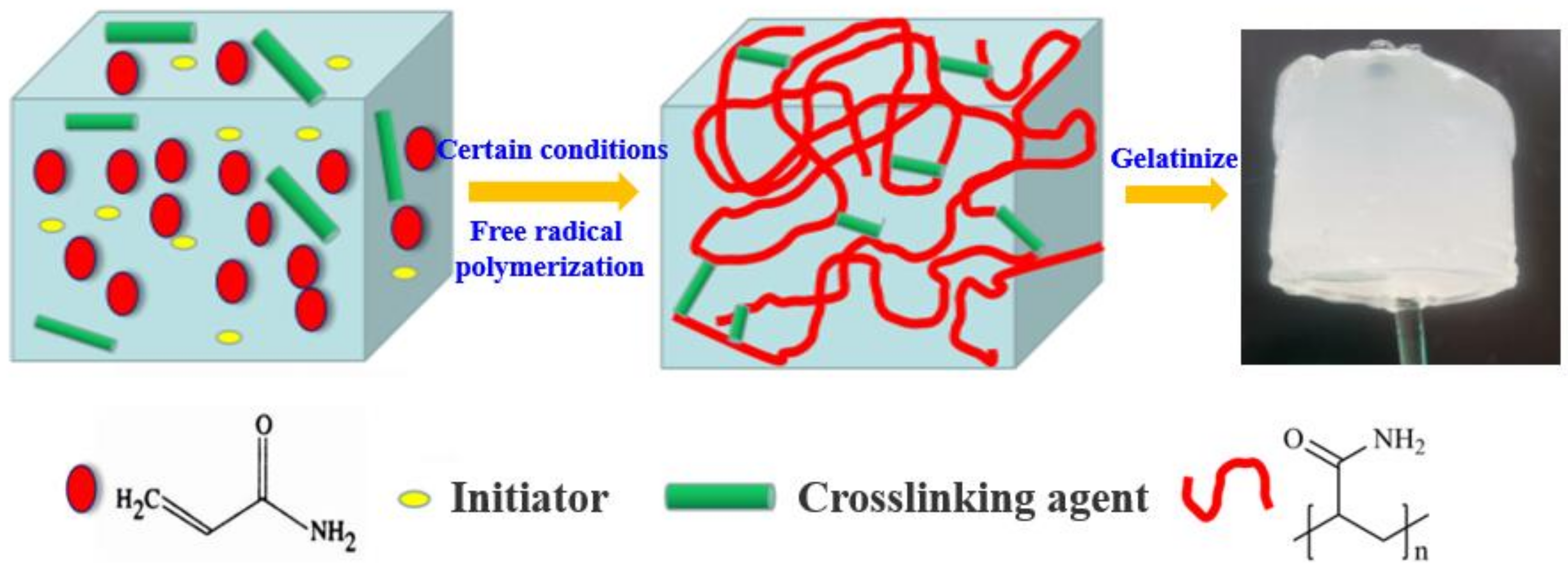
4.2.1. Gel Preparation
4.2.2. Dilution Performance
- (1)
- The shear thixotropic polymer gel system was prepared by weighting water with salt water instead of deionized water. The weighted shear thixotropic polymer gel system was diluted with salt water with a high concentration of sodium chloride, and the dispersion of the gel system and salt water was observed at different dilution levels. Dilution was carried out in the following four ways:
- (a)
- Pour 1/3 volume of the salt water into the gel system.
- (b)
- Pour 2/3 volume of the salt water into the gel system.
- (c)
- Pour 1/3 volume of the shear thixotropic polymer gel into the salt water.
- (d)
- Pour 2/3 volume of the shear thixotropic polymer gel into the salt water.
- (2)
- In the dilution experiment of white oil, the method of mutual dilution between white oil and the gel system was used and the gel formation test was carried out. The dispersion of gel and white oil was observed at different dilution levels. The specific experimental steps were as follows:
- (a)
- The 90 mL gel system and 10 mL white oil were mixed and stirred, and placed in a measuring cylinder to observe the stratification state of the gel and white oil, respectively. The experimental recording time was 5 min, 10 min and 15 min, respectively. The gel solution was tested after being stirred evenly.
- (b)
- The 80 mL gel system and 20 mL white oil were mixed and stirred, and placed in a measuring cylinder to observe the stratification state of the gel and white oil, respectively. The experimental recording time was 5 min, 10 min and 15 min, respectively. The gel solution was tested after being stirred evenly.
- (c)
- The 70 mL gel system and 30 mL white oil were mixed and stirred, and placed in a measuring cylinder to observe the stratification state of the gel and white oil, respectively. The experimental recording time was 5 min, 10 min and 15 min, respectively. The gel solution was tested after being stirred evenly.
4.2.3. Microstructure Characterization
4.2.4. Rheological Property
4.2.5. Salt Resistance
4.2.6. Temperature Resistance
4.2.7. Gelation Time
4.2.8. Tensile Strength
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Bai, Y.; Cheng, R.; Lyu, K.; Liu, F.; Feng, J.; Lei, S.; Zhang, J.; Hao, H. Research progress and prospect of plugging technologies for fractured formation with severe lost circulation. Pet. Explor. Dev. 2021, 48, 732–743. [Google Scholar] [CrossRef]
- Jiang, G.; Sun, J.; He, Y.; Cui, K.; Dong, T.; Yang, L.; Yang, X.; Wang, X. Novel water-based drilling and completion fluid technology to improve wellbore quality during drilling and protect unconventional reservoirs. Engineering 2021, in press. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, R.; Zhou, J.; Chen, H.; Tan, Y. A novel hyper-cross-linked polymer for high-efficient fluid-loss control in oil-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127004. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Zhu, M.; Kang, Y.; Yan, X.; Zhang, J.; Bai, Y. Experimental study on the controlling factors of frictional coefficient for lost circulation control and formation damage prevention in deep fractured tight reservoir. Petroleum 2021, in press. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, Y.; Pu, H.; Peng, G.; Wang, J. Borehole temperature distribution when drilling fluid loss occurs in the two-dimensional area at the bottom-hole during drilling. J. Nat. Gas Sci. Eng. 2020, 83, 103523. [Google Scholar] [CrossRef]
- Pang, H.; Meng, H.; Wang, H.; Fan, Y.; Nie, Z.; Jin, Y. Lost circulation prediction based on machine learning. J. Pet. Sci. Eng. 2021, 208, 109364. [Google Scholar] [CrossRef]
- Cai, W.; Deng, J.; Feng, Y.; Lin, H.; Tanko, M.O.; Ma, C. Developing a geomechanics-modeling based method for lost circulation risk assessment: A case study in Bohai Bay, China. J. Pet. Sci. Eng. 2021, 210, 110045. [Google Scholar] [CrossRef]
- Xu, C.; Xie, Z.; Kang, Y.; Yu, G.; You, Z.; You, L.; Zhang, J.; Yan, X. A novel material evaluation method for lost circulation control and formation damage prevention in deep fractured tight reservoir. Energy 2020, 210, 118574. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Ouyang, Y.; Han, Q. Synthesis of hydrophobically modified polyampholyte based on epoxidized soybean oil as shale inhibitor in water-based drilling fluid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126664. [Google Scholar] [CrossRef]
- Mohamed, A.; Salehi, S.; Ahmed, R.; Li, G. Experimental study on rheological and settling properties of shape memory polymer for fracture sealing in geothermal formations. J. Pet. Sci. Eng. 2021, 208, 109535. [Google Scholar] [CrossRef]
- Bai, B.; Elgmati, M.; Sun, Y.; Liu, L. Petrophysical properties characterization of Ordovician Utica gas shale in Quebec, Canada. Pet. Explor. Dev. 2016, 43, 74–81. [Google Scholar] [CrossRef]
- Bai, B.; Leng, J.; Wei, M. A comprehensive review of in-situ polymer gel simulation for conformance control. Pet. Sci. 2021, 19, 189–202. [Google Scholar] [CrossRef]
- Pereira, F.C.; Clinckspoor, K.J.; Moreno, R.B.Z.L. Optimization of an in-situ polymerized and crosslinked hydrogel formulation for lost circulation control. J. Pet. Sci. Eng. 2021, 208, 109687. [Google Scholar] [CrossRef]
- Hamza, A.; Shamlooh, M.; Hussein, I.A.; Nasser, M.; Salehi, S. Polymeric formulations used for loss circulation materials and wellbore strengthening applications in oil and gas wells: A review. J. Pet. Sci. Eng. 2019, 180, 197–214. [Google Scholar] [CrossRef]
- Xie, B.; Ma, J.; Wang, Y.; Tchameni, A.P.; Luo, M.; Wen, J. Enhanced hydrophobically modified polyacrylamide gel for lost circulation treatment in high temperature drilling. J. Mol. Liq. 2020, 325, 115155. [Google Scholar] [CrossRef]
- Zhu, Z.-X.; Li, L.; Liu, J.-W.; Chen, J.; Xu, Z.-Z.; Wu, Y.-N.; Dai, C.-L. Probing the effect of Young’s modulus on the plugging performance of micro-nano-scale dispersed particle gels. Pet. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Xu, Z.; Sun, Y.; Wu, Y.; Dai, C. Biomimetic functional hydrogel particles with enhanced adhesion characteristics for applications in fracture conformance control. J. Ind. Eng. Chem. 2021, 106, 482–491. [Google Scholar] [CrossRef]
- Zhu, D.; Xu, Z.; Sun, R.; Fang, X.; Gao, D.; Jia, X.; Hu, J.; Weng, J. Laboratory evaluation on temporary plugging performance of degradable preformed particle gels (DPPGs). Fuel 2020, 289, 119743. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, D.; Bai, B. Experimental study of degradable preformed particle gel (DPPG) as temporary plugging agent for carbonate reservoir matrix acidizing to improve oil recovery. J. Pet. Sci. Eng. 2021, 205, 108760. [Google Scholar] [CrossRef]
- Yang, M.; Chen, Y. Investigation of LCM soaking process on fracture plugging for fluid loss remediation and formation damage control. J. Nat. Gas Sci. Eng. 2020, 81, 103444. [Google Scholar] [CrossRef]
- Esfahlan, M.S.; Khodapanah, E.; Tabatabaei-Nezhad, S.A. Comprehensive review on the research and field application of preformed particle gel conformance control technology. J. Pet. Sci. Eng. 2021, 202, 108440. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.; Sun, J.; Shang, X.; Lv, K.; Zhu, Y.; Wang, F. High temperature resistant polymer gel as lost circulation material for fractured formation during drilling. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128244. [Google Scholar] [CrossRef]
- Jiang, G.; Deng, Z.; He, Y.; Li, Z.; Ni, X. Cross-linked polyacrylamide gel as loss circulation materials for combating lost circulation in high temperature well drilling operation. J. Pet. Sci. Eng. 2019, 181, 106250. [Google Scholar] [CrossRef]
- Nutan, B.; Chandel, A.K.S.; Biswas, A.; Kumar, A.; Yadav, A.; Maiti, P.; Jewrajka, S.K. Gold Nanoparticle Promoted Formation and Biological Properties of Injectable Hydrogels. Biomacromolecules 2020, 21, 3782–3794. [Google Scholar] [CrossRef]
- Jia, H.; Yang, X.; Li, S.; Yu, P.; Zhang, J. Nanocomposite gel of high-strength and degradability for temporary plugging in ultralow-pressure fracture reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2019, 585, 124108. [Google Scholar] [CrossRef]
- Jia, H.; Niu, C.-C.; Yang, X.-Y. Improved understanding nanocomposite gel working mechanisms: From laboratory investigation to wellbore plugging application. J. Pet. Sci. Eng. 2020, 191, 107214. [Google Scholar] [CrossRef]
- Wu, Q.; Ge, J.; Ding, L.; Guo, H.; Wang, W.; Fan, J. Insights into the key aspects influencing the rheological properties of polymer gel for water shutoff in fractured reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2021, 634, 127963. [Google Scholar] [CrossRef]
- Yamagata, Y.; Miyamoto, K. Gel formation and its relaxation mechanism of shear-induced aqueous suspensions comprised of bentonite and heptaethylene oleyl ether. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126786. [Google Scholar] [CrossRef]
- Kawasaki, S.; Kobayashi, M. Affirmation of the effect of pH on shake-gel and shear thickening of a mixed suspension of polyethylene oxide and silica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Pozzo, D.C.; Walker, L.M. Reversible shear gelation of polymer–clay dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 187–198. [Google Scholar] [CrossRef]
- Zhou, B.; Kang, W.; Yang, H.; Li, Z.; Zhang, H.; Zhang, M.; Xie, A.; Sun, Z.; Sarsenbekuly, B. The shear stability mechanism of cyclodextrin polymer and amphiphilic polymer inclusion gels. J. Mol. Liq. 2021, 328, 115399. [Google Scholar] [CrossRef]
- Collini, H.; Mohr, M.; Luckham, P.; Shan, J.; Russell, A. The effects of polymer concentration, shear rate and temperature on the gelation time of aqueous Silica-Poly(ethylene-oxide) “Shake-gels”. J. Colloid Interface Sci. 2018, 517, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Poggi, G.; Chelazzi, D.; Laurati, M. Mechanical response and yielding transition of silk-fibroin and silk-fibroin/cellulose nanocrystals composite gels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 636, 128121. [Google Scholar] [CrossRef]
- Ge, J.; Wu, Q.; Ding, L.; Guo, H.; Zhao, A. Preparation and rheological Evaluation of a thixotropic polymer gel for water shutoff in fractured tight reservoirs. J. Pet. Sci. Eng. 2021, 208, 109542. [Google Scholar] [CrossRef]
- Linsha, V.; Mohamed, A.P.; Ananthakumar, S. Nanoassembling of thixotropically reversible alumino-siloxane hybrid gels to hierarchically porous aerogel framework. Chem. Eng. J. 2015, 259, 313–322. [Google Scholar] [CrossRef]
- Varadan, P.; Solomon, M.J. Shear-Induced Microstructural Evolution of a Thermoreversible Colloidal Gel. Langmuir 2001, 17, 2918–2929. [Google Scholar] [CrossRef]
- Ghaderi, S.; Ahmad Ramazani, S.A.; Haddadi, S.A. Applications of highly salt and highly temperature resistance terpolymer of acrylamide/styrene/maleic anhydride monomers as a rheological modifier: Rheological and corrosion protection properties studies. J. Mol. Liq. 2019, 294, 111635. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, P.; Chen, L.; Zhao, L.; Feng, J. Antifouling poly(N-(2-hydroxyethyl) acrylamide)/sodium alginate double network hydrogels with eminent mechanical properties. Polym. Test. 2021, 95, 107087. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, J.; Jin, H.; Gao, C.; Liu, H.; Cai, N.; Liu, Y.; Zhang, P.; Wang, M. In situ initiator-free gelation of highly concentrated lithium bis(fluorosulfonyl)imide-1,3-dioxolane solid polymer electrolyte for high performance lithium-metal batteries. Mater. Today Energy 2020, 20, 100623. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, J.; Zhang, Z.; Yang, J.; Xin, K.; Zhao, Z.; An, L.; Kong, D. Effect of potassium ferrate treatment on adhesive gelatinous biopolymer structure and erosion resistance of sewer sediments: Promotion or inhibition? Chem. Eng. J. 2021, 431, 134025. [Google Scholar] [CrossRef]
- Wang, K.; Liu, G.; Guo, Y.; Yang, H.; Chen, Z.; Su, G.; Wang, Y.; Wei, B.; Yu, X. Preparation and properties of degradable hydrogels as a temporary plugging agent used for acidizing treatments to enhance oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2021, 637, 128218. [Google Scholar] [CrossRef]
- Ning, J.; Li, G.; Haraguchi, K. Synthesis of Highly Stretchable, Mechanically Tough, Zwitterionic Sulfobetaine Nanocomposite Gels with Controlled Thermosensitivities. Macromolecules 2013, 46, 5317–5328. [Google Scholar] [CrossRef]
- Ay, A.; Gucuyener, I.H.; Kök, M.V. An experimental study of silicate–polymer gel systems to seal shallow water flow and lost circulation zones in top hole drilling. J. Pet. Sci. Eng. 2014, 122, 690–699. [Google Scholar] [CrossRef]
- McCarvill, W.T.; Fitch, R.M. The surface chemistry of polystyrene latices initiated by the persulfate/bisulfite/iron system. J. Colloid Interface Sci. 1978, 67, 204–212. [Google Scholar] [CrossRef]
- Oyama, R.; Abe, M. Reactivity and Product Analysis of a Pair of Cumyloxyl and tert-Butoxyl Radicals Generated in Photolysis of tert-Butyl Cumyl Peroxide. J. Org. Chem. 2020, 85, 8627–8638. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Q.; Sun, J.; Shang, X.; Lv, K.; Wang, F. Disproportionate filtration behaviors of polymer/chromium gel used for fracture plugging. J. Mol. Liq. 2021, 343, 117567. [Google Scholar] [CrossRef]
- Zong, Y.; Xu, P.; Yue, H.; Li, X. Synthesis and gelation properties of HPMC-g-poly(AM/AA/APEG2400) quaternary copolymer. J. Mol. Liq. 2022, 353, 118789. [Google Scholar] [CrossRef]
- Bai, Y.; Lian, Y.; Ban, C.; Wang, Z.; Zhao, J.; Zhang, H. Facile synthesis of temperature-resistant hydroxylated carbon black/polyacrylamide nanocomposite gel based on chemical crosslinking and its application in oilfield. J. Mol. Liq. 2021, 329, 115578. [Google Scholar] [CrossRef]
- Shen, J.; Pan, X.; Bhatia, S.R. Self-assembly and thermoreversible rheology of perfluorocarbon nanoemulsion-based gels with amphiphilic copolymers. Colloids Surf. B Biointerfaces 2021, 202, 111641. [Google Scholar] [CrossRef]
- Yang, H.; Shao, S.; Zhu, T.; Chen, C.; Liu, S.; Zhou, B.; Hou, X.; Zhang, Y.; Kang, W. Shear resistance performance of low elastic polymer microspheres used for conformance control treatment. J. Ind. Eng. Chem. 2019, 79, 295–306. [Google Scholar] [CrossRef]
- Hosono, N.; Furukawa, H.; Masubuchi, Y.; Watanabe, T.; Horie, K. Photochemical control of network structure in gels and photo-induced changes in their viscoelastic properties. Colloids Surf. B Biointerfaces 2007, 56, 285–289. [Google Scholar] [CrossRef]
- Lipatova, I.; Yusova, A.; Losev, N.; Indeikin, E. Gelation in solutions of low deacetylated chitosan initiated by high shear stresses. Int. J. Biol. Macromol. 2019, 139, 550–557. [Google Scholar] [CrossRef]
- Ningtyas, D.W.; Tam, B.; Bhandari, B.; Prakash, S. Effect of different types and concentrations of fat on the physico-chemical properties of soy protein isolate gel. Food Hydrocoll. 2020, 111, 106226. [Google Scholar] [CrossRef]
- Maiti, S.; Khillar, P.S.; Mishra, D.; Nambiraj, N.A.; Jaiswal, A.K. Physical and self–crosslinking mechanism and characterization of chitosan-gelatin-oxidized guar gum hydrogel. Polym. Test. 2021, 97, 107155. [Google Scholar] [CrossRef]
- Bai, S.; Wang, Y.; Liu, B.; Zhu, Y.; Guo, R. Dispersion copolymerization of acrylamide and sodium 2-acrylamido-2-methylpropanesulfonate in aqueous salt solution stabilized with a macro-RAFT agent. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 446–455. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, N.; Zhang, Y.; Hou, J.; Gao, Y.; Tian, L.; Jin, Z.; Shen, Y.; Guo, S. The gelling behavior of gellan in the presence of different sodium salts. Int. J. Biol. Macromol. 2021, 193, 768–777. [Google Scholar] [CrossRef]
- Lu, Q.; Dong, L.; Chen, L.; Fu, J.; Shi, L.; Li, M.; Zeng, X.; Lei, H.; Zheng, F. Inorganic-organic gel electrolytes with 3D cross-linking star-shaped structured networks for lithium ion batteries. Chem. Eng. J. 2020, 393, 124708. [Google Scholar] [CrossRef]
- Yang, H.; Iqbal, M.W.; Lashari, Z.A.; Cao, C.; Tang, X.; Kang, W. Experimental research on amphiphilic polymer/organic chromium gel for high salinity reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123900. [Google Scholar] [CrossRef]
- Pilathottathil, S.; Kottummal, T.K.; Thayyil, M.S.; Perumal, P.M.; Purakakath, J.A. Inorganic salt grafted ionic liquid gel electrolytes for efficient solid state supercapacitors: Electrochemical and dielectric studies. J. Mol. Liq. 2018, 264, 72–79. [Google Scholar] [CrossRef]
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties. Chem. Mater. 2018, 30, 3110–3121. [Google Scholar] [CrossRef]
- Sögaard, C.; Kolman, K.; Christensson, M.; Otyakmaz, A.B.; Abbas, Z. Hofmeister effects in the gelling of silica nanoparticles in mixed salt solutions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 611, 125872. [Google Scholar] [CrossRef]
- Li, R.; Li, G.; Feng, Y.; Yang, X.; Teng, Y.; Hu, Y. Innovative experimental method for particle bridging behaviors in natural fractures. J. Nat. Gas Sci. Eng. 2021, 97, 104379. [Google Scholar] [CrossRef]
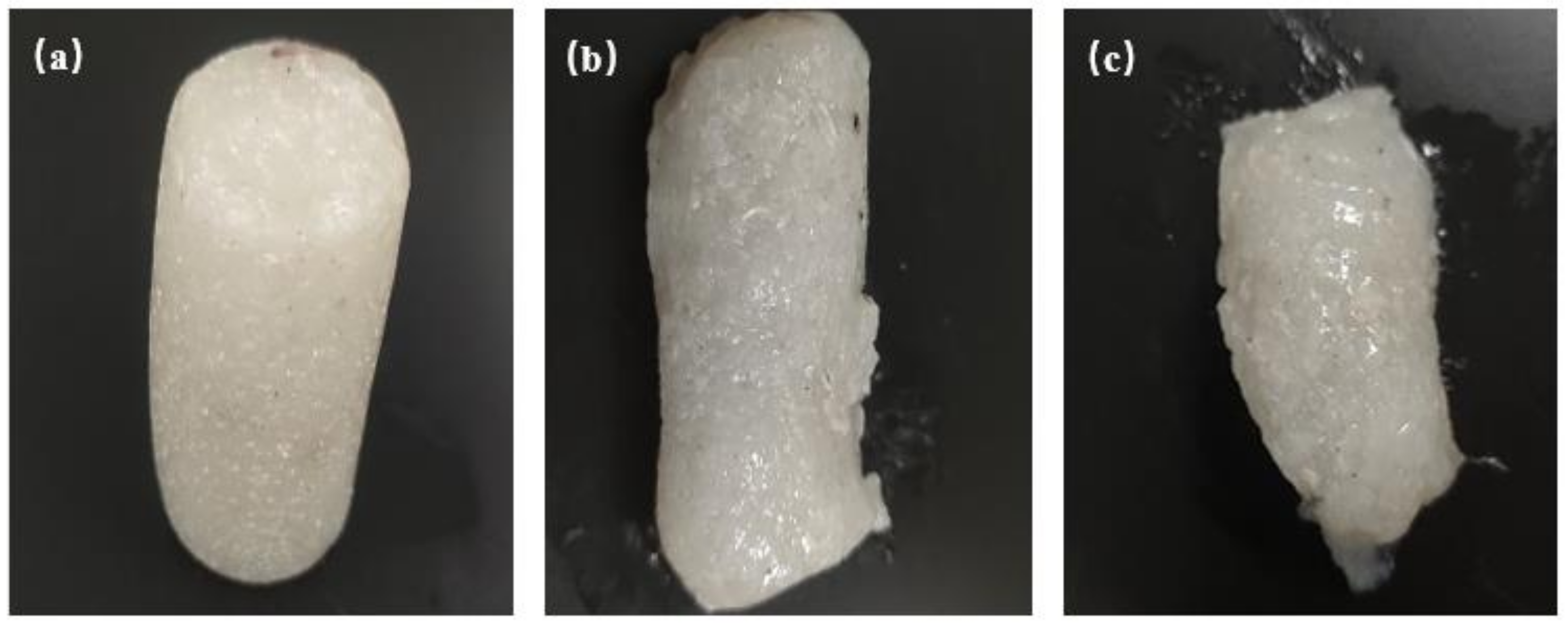


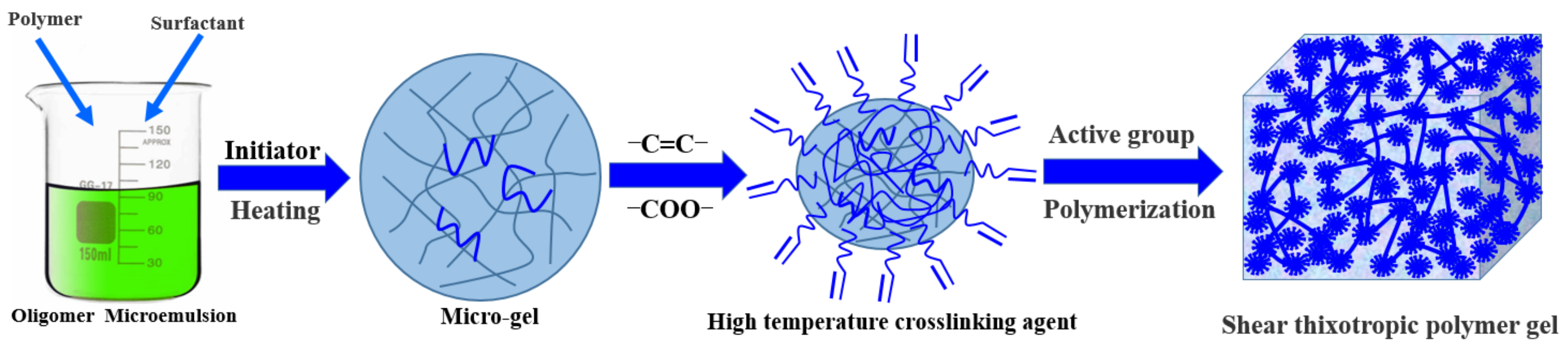
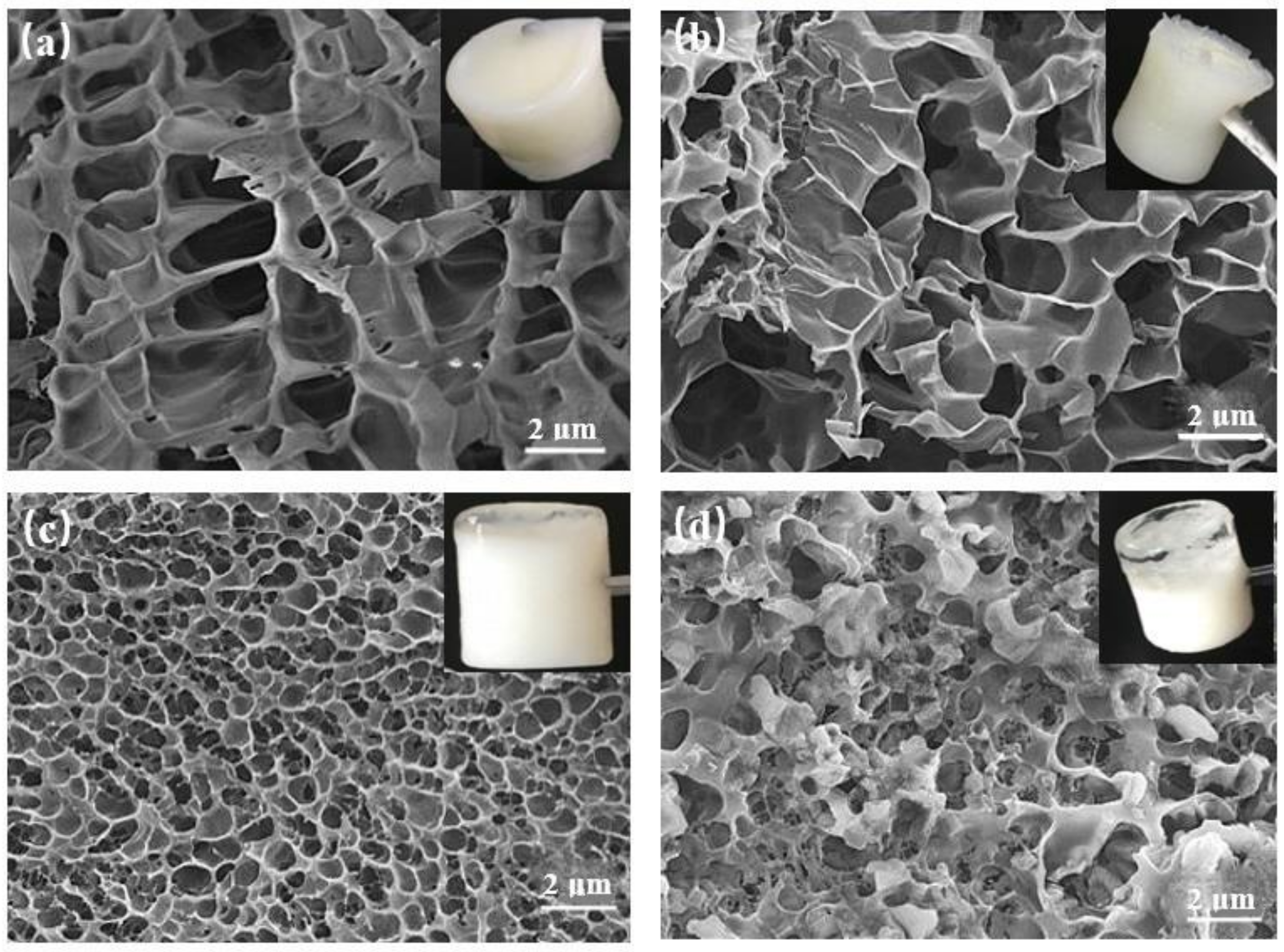
| Serial Number | 1 | 2 | 3 |
|---|---|---|---|
| Tensile strength σ/MPa | 4.46 | 4.21 | 3.66 |
| Elongation ε/% | 473 | 396 | 326 |
| Temperature | Initiator Concentration | Gelation Time |
|---|---|---|
| 140 °C | 0.1% | 6 h |
| 0.2% | 5 h | |
| 0.3% | 4 h | |
| 0.5% | 2 h |
| Component | AM/% | Active Polymer/% | Initiator/% | Deionized Water/% | Rheological Regulator/% | Crosslinking Agent/% | Toughening Agent /% |
|---|---|---|---|---|---|---|---|
| Content | 15 | 2 | 0.2 | 76.3 | 4 | 1 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Bai, Y.; Sun, J.; Lv, K.; Han, J.; Dai, L. Experimental Study on Physicochemical Properties of a Shear Thixotropic Polymer Gel for Lost Circulation Control. Gels 2022, 8, 229. https://doi.org/10.3390/gels8040229
Yang J, Bai Y, Sun J, Lv K, Han J, Dai L. Experimental Study on Physicochemical Properties of a Shear Thixotropic Polymer Gel for Lost Circulation Control. Gels. 2022; 8(4):229. https://doi.org/10.3390/gels8040229
Chicago/Turabian StyleYang, Jingbin, Yingrui Bai, Jinsheng Sun, Kaihe Lv, Jinliang Han, and Liyao Dai. 2022. "Experimental Study on Physicochemical Properties of a Shear Thixotropic Polymer Gel for Lost Circulation Control" Gels 8, no. 4: 229. https://doi.org/10.3390/gels8040229
APA StyleYang, J., Bai, Y., Sun, J., Lv, K., Han, J., & Dai, L. (2022). Experimental Study on Physicochemical Properties of a Shear Thixotropic Polymer Gel for Lost Circulation Control. Gels, 8(4), 229. https://doi.org/10.3390/gels8040229







