Induced Radionuclides and Their Activity Concentration in Gel Dosimeters Irradiated by Carbon Ion Beam
Abstract
1. Introduction
2. Materials and Methods
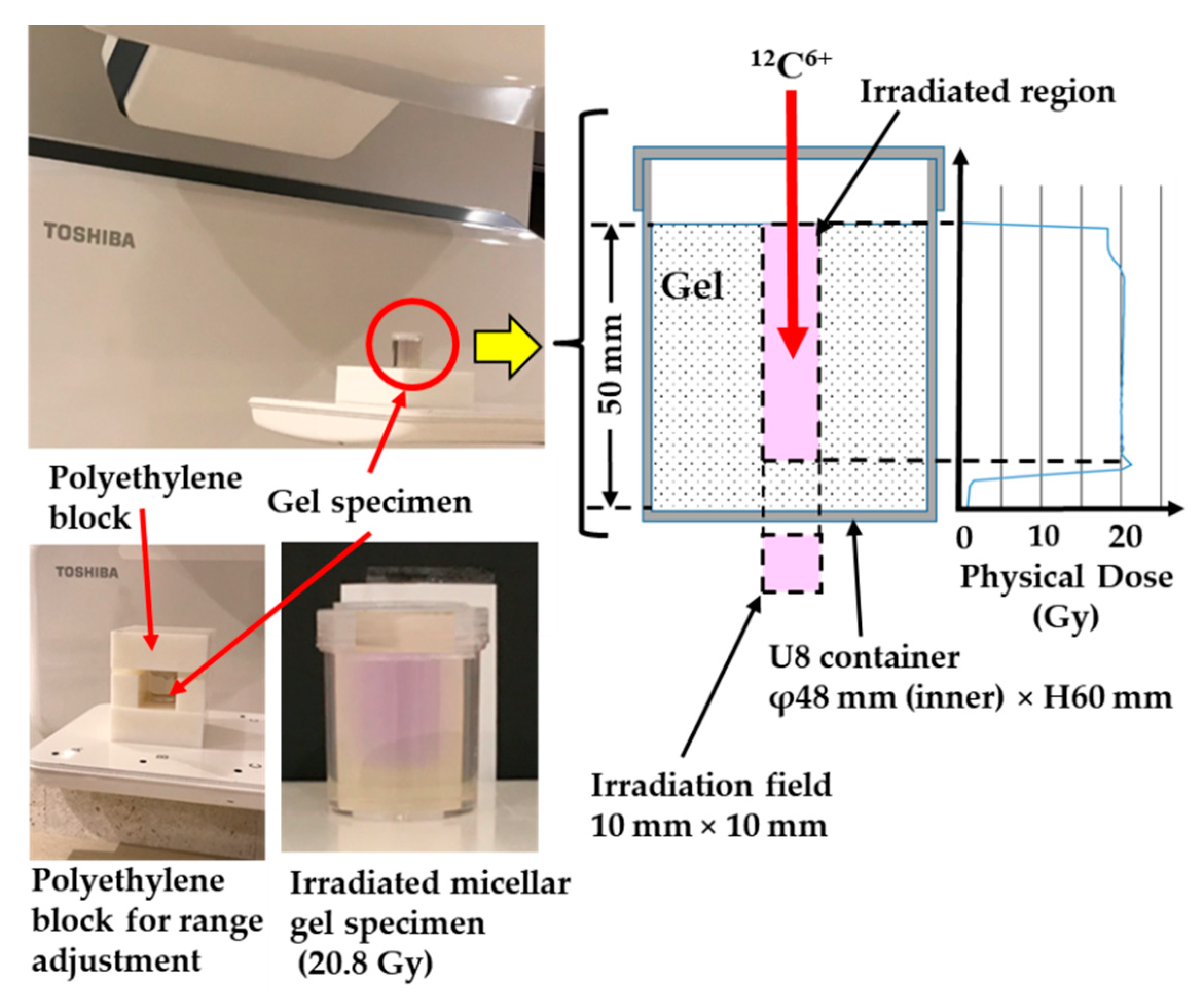
2.1. Specimens
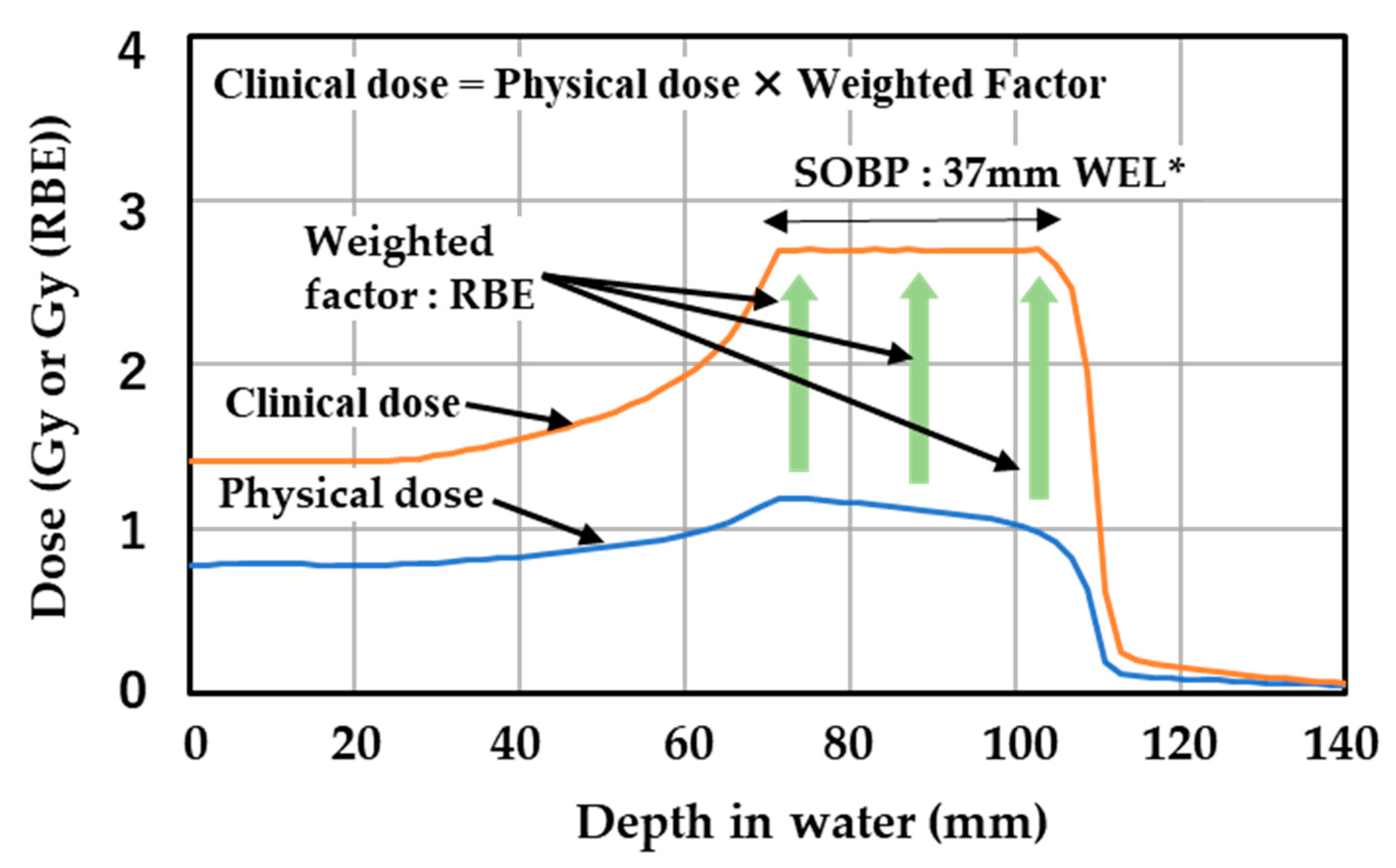
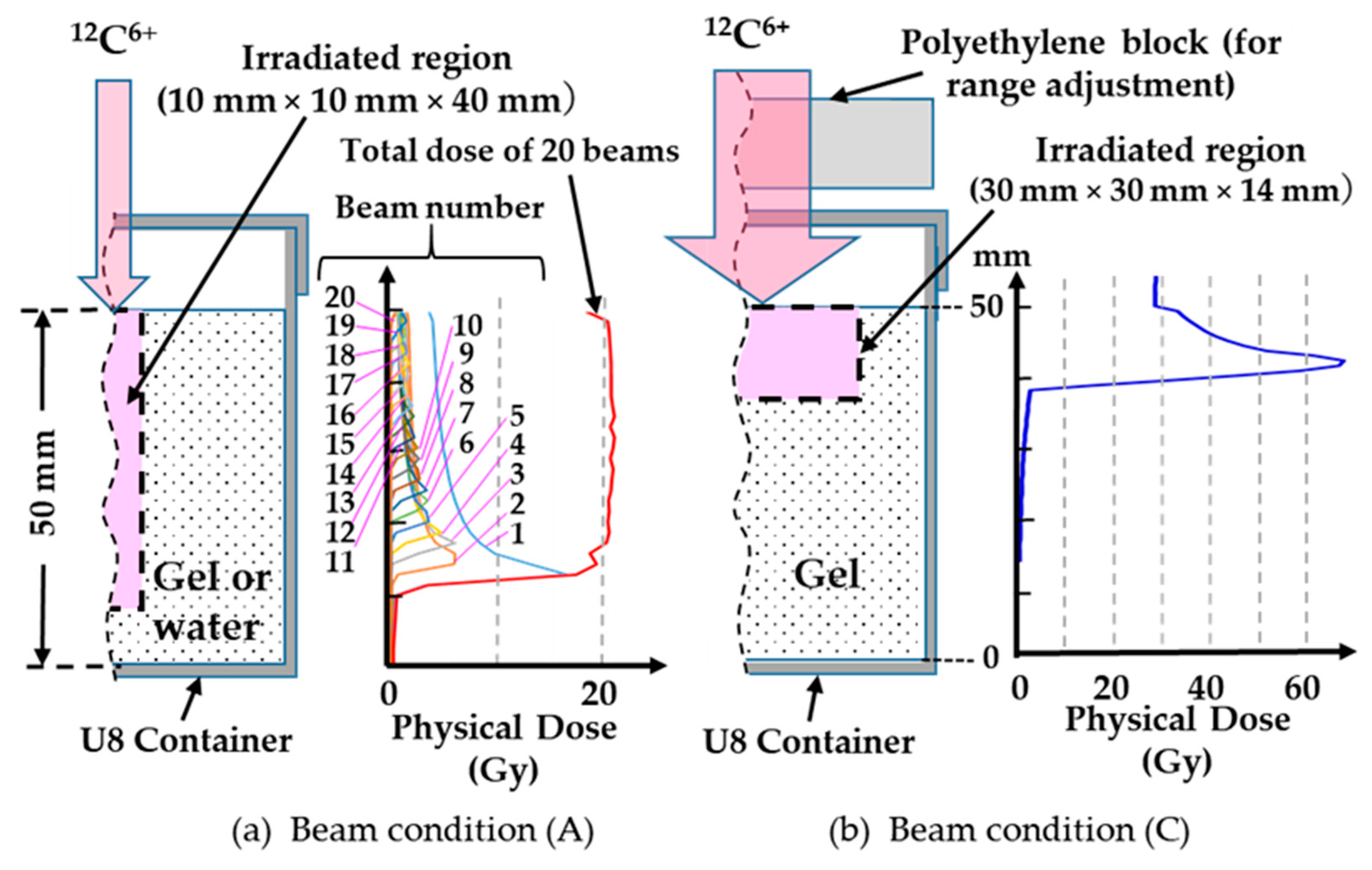
2.2. Carbon Ion Beam Irradiation
2.3. Measurement of Gamma-Emitting Radionuclides and Their Activity
2.4. Measurement of Beta-Emitting Nuclides and Their Activity
2.5. Simulation Configuration
3. Results and Discussion
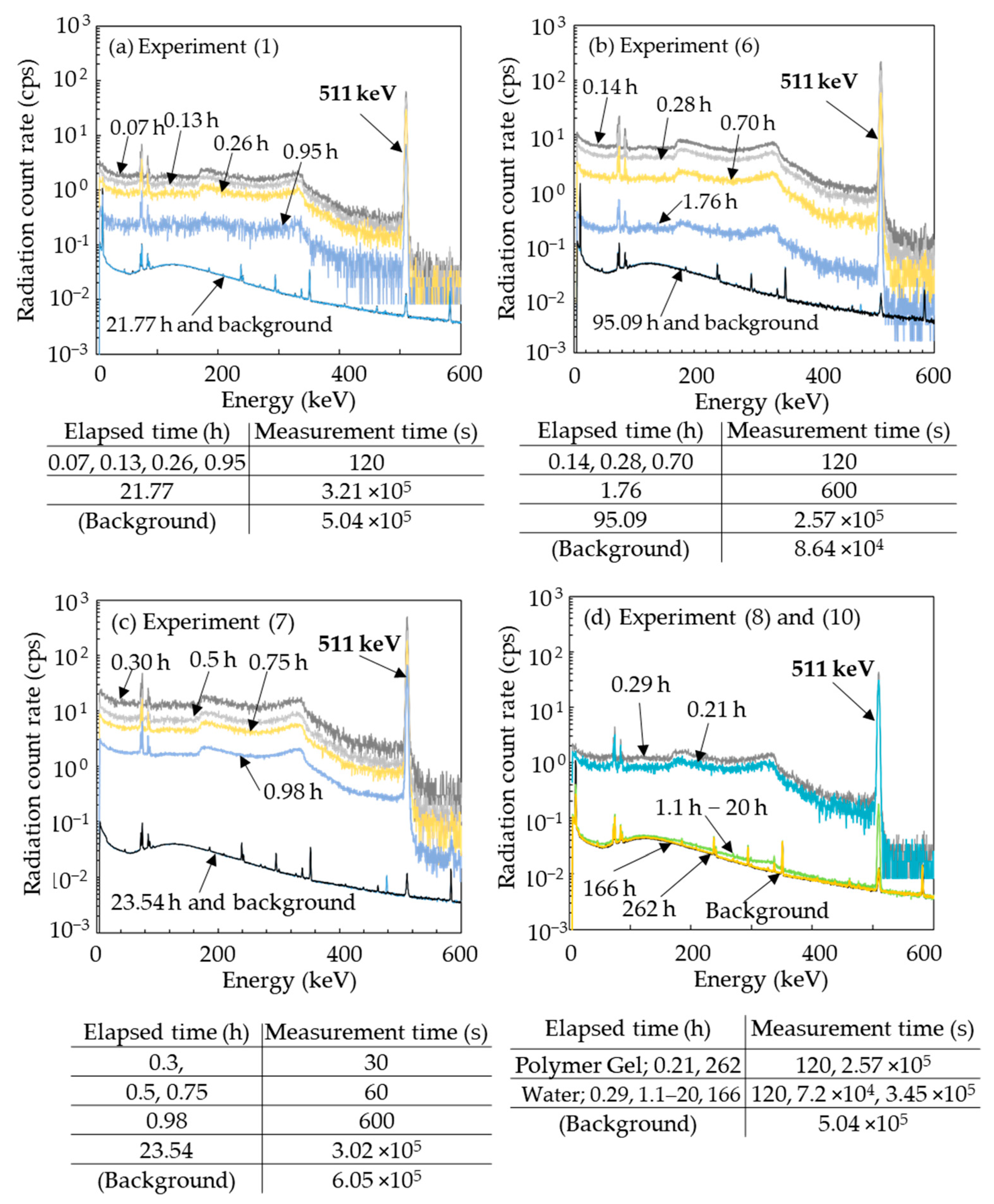
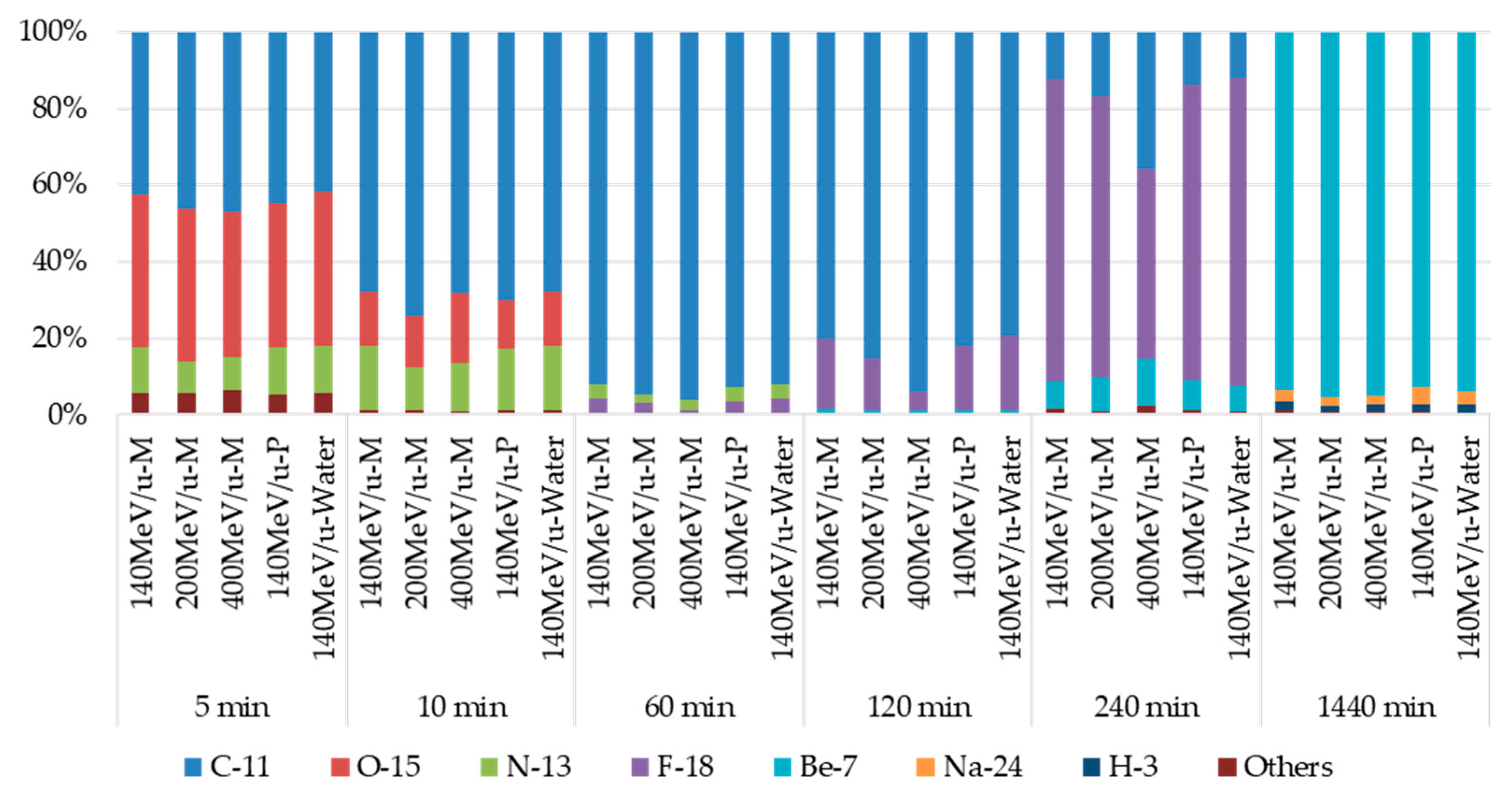
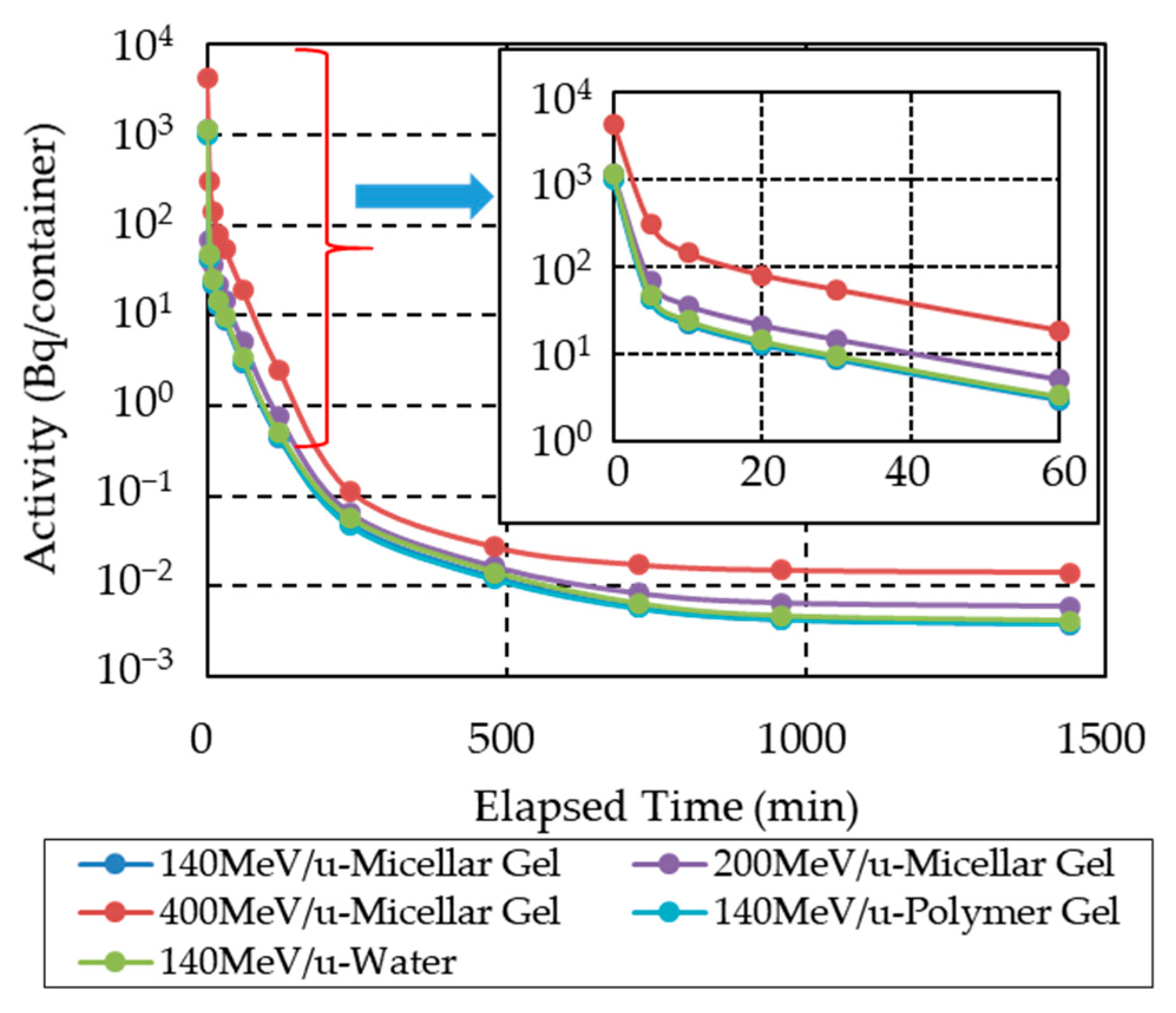
3.1. Radionuclides Identified a Short Time after Irradiation
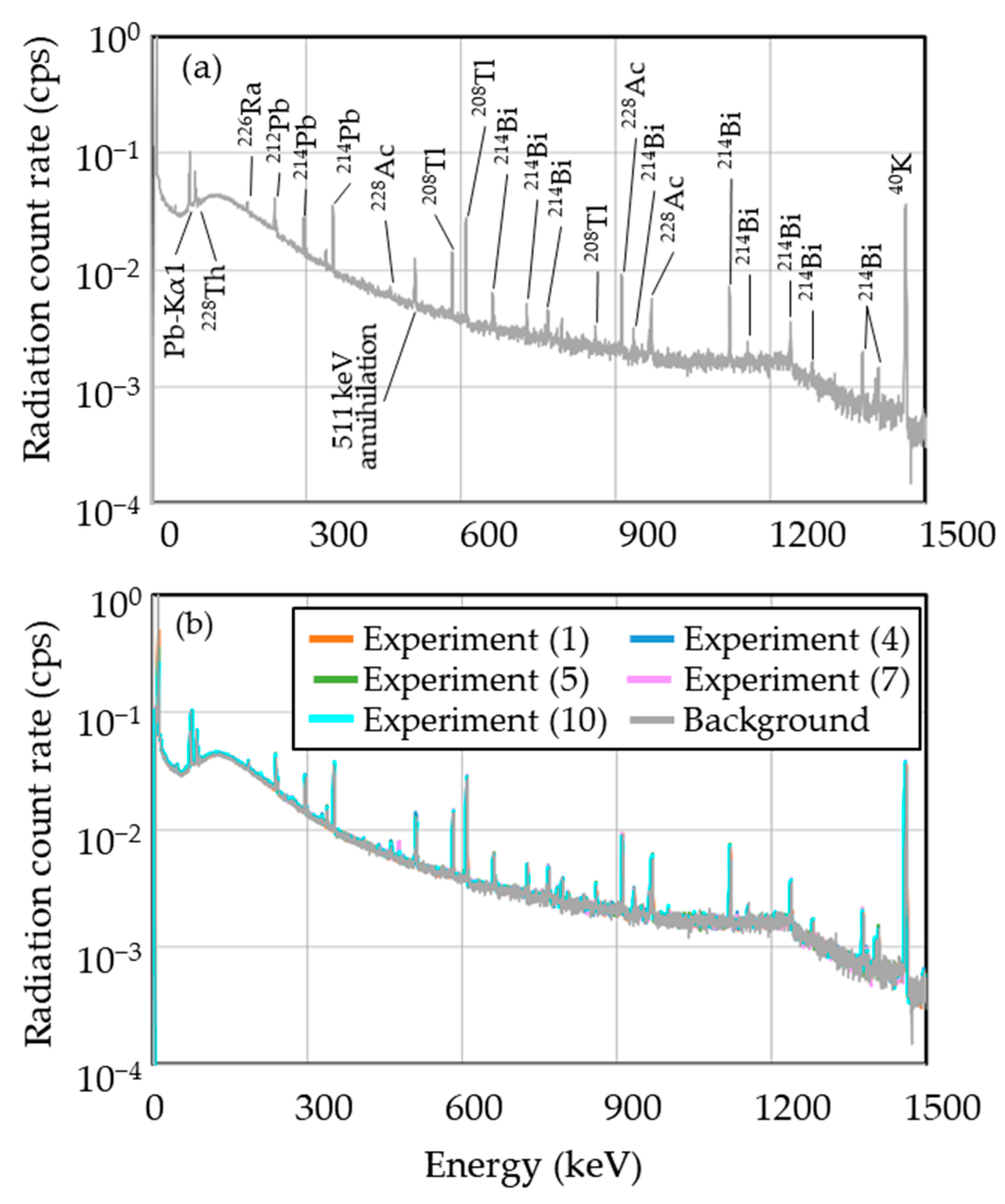
3.2. Radionuclides Identified Approximately 24 h after Irradiation
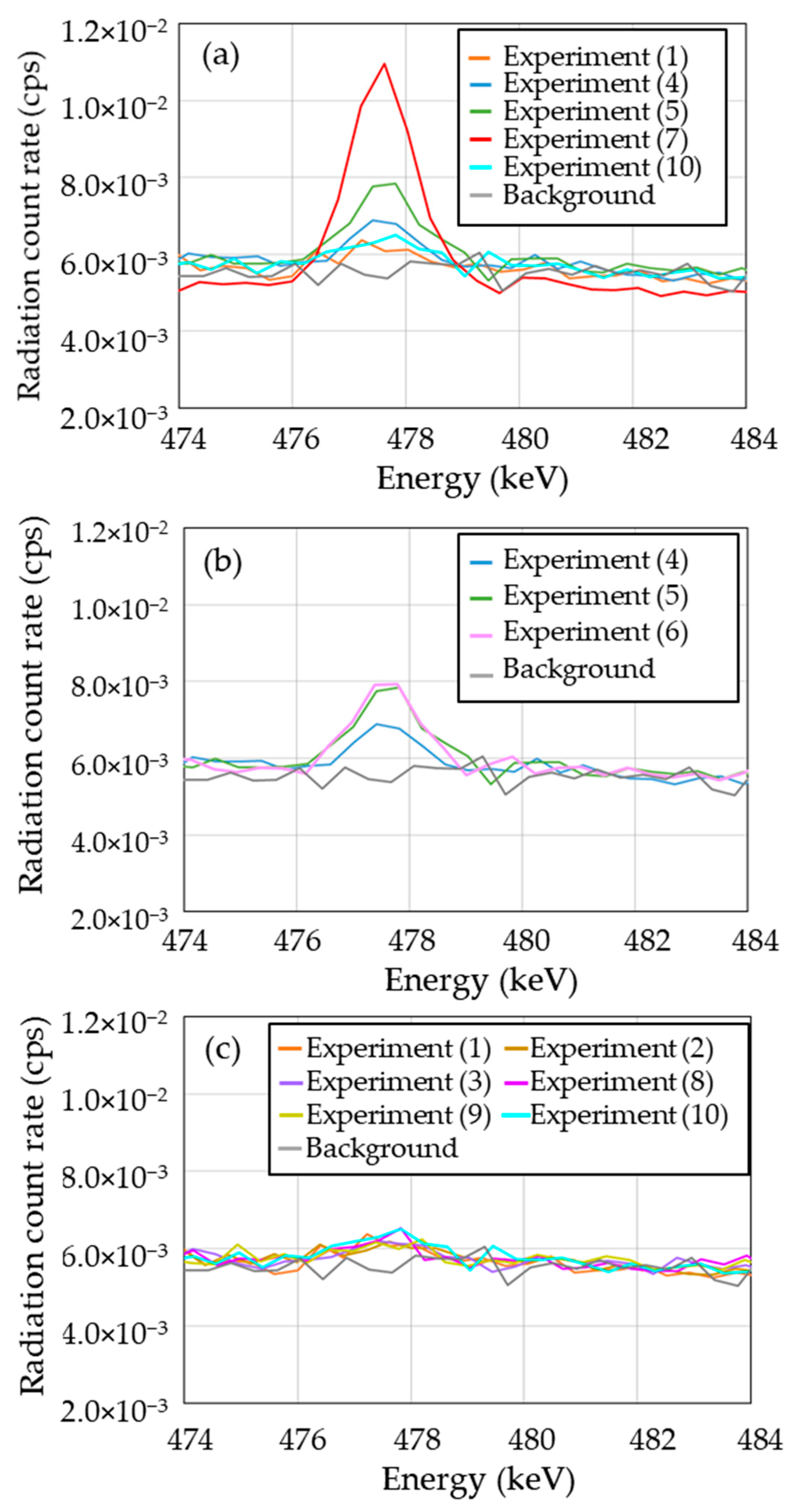
3.3. Identification of 7Be
3.4. Identification of 24Na and 3H
3.5. Effect on Induced Radioactivity
3.6. Consideration of Radioactive Waste in Spent Gel Dosimeters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nezhad, Z.A.; Geraily, G. A review study on application of gel dosimeters in low energy radiation dosimetry. Appl. Radiat. Isot. 2022, 179, 110015. [Google Scholar] [CrossRef] [PubMed]
- Marrale, M.; D’Errico, F. Hydrogels for Three-Dimensional Ionizing-Radiation Dosimetry. Gels 2021, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Ibbott, G.S. Applications of gel dosimetry. J. Phys. Conf. Ser. 2004, 3, 58–77. [Google Scholar] [CrossRef]
- Lepage, M.; Jordan, K. 3D dosimetry fundamentals: Gels and plastics. J. Phys. Conf. Ser. 2010, 250, 012055. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef]
- McAuley, K. Fundamentals of Polymer Gel Dosimeters. J. Phys. Conf. Ser. 2006, 56, 35–44. [Google Scholar] [CrossRef]
- Yoshioka, M.; Tominaga, T.; Usui, S.; Hayashi, S.; Haneda, K.; Tsunei, Y.; Katahira, K.; Suga, D.; Hishikawa, Y.; Teshima, T. Examination of fundamental characteristics of a polymer gel detector in a proton beam irradiation. Radiat. Meas. 2011, 46, 64–71. [Google Scholar] [CrossRef]
- Schreiner, L.J. Review of Fricke gel dosimeters. J. Phys. Conf. Ser. 2004, 3, 9–21. [Google Scholar] [CrossRef]
- de Almeida, W.D.S.; Alves, A.V.S.; Oliveira, W.F.; da Silveira, M.A.L.; de Souza, S.O.; d’Errico, F.; Sussuchi, E.M. Radiochromic Fricke gels with eriochrome cyanine R for radiotherapy dosimetry. Radia. Phys. Chem. 2022, 191, 109830. [Google Scholar] [CrossRef]
- Maeyama, T.; Fukunishi, N.; Ishikawa, K.L.; Fukasaku, K.; Fukuda, S. Organic-Gelatin-Free Nanocomposite Fricke Gel Dosimeter. J. Phys. Chem. B 2017, 121, 4238–4246. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Hailat, T.F.; Eyadeh, M.M.; Shorman, M.Y.A.; Aldweri, F.M.; Alheet, S.M.; Madas, B.G.; Awad, S.I. Dosimetric properties of sulfosalicylic acid-ferrous-polyvinyl alcohol-glutaraldehyde hydrogel dosimeters using magnetic and optical techniques. Radiat. Phys. Chem. 2020, 177, 109106. [Google Scholar] [CrossRef]
- Gallo, S.; Cremonesi, L.; Gambarini, G.; Ianni, L.; Lenardi, C.; Argentiere, S.; Bettega, D.; Gargano, M.; Ludwig, N.; Veronese, I. Study of the effect of laponite on Fricke xylenol orange gel dosimeter by optical techniques. Sens. Actuators B Chem. 2018, 272, 618–625. [Google Scholar] [CrossRef]
- Gambarini, G.; Veronese, I.; Bettinelli, L.; Felisi, M.; Gargano, M.; Ludwig, N.; Lenardi, C.; Carrara, M.; Collura, G.; Gallo, S.; et al. Study of optical absorbance and MR relaxation of Fricke xylenol orange gel dosimeters. Radiat. Meas. 2017, 106, 622–627. [Google Scholar] [CrossRef]
- Sunagawa, T.; Harvel, G.; Aoki, Y.; Umeda, M.; Hayami, J.; Sakakibara, K.; Goto, H.; Ebina, T.; Taguchi, M.; Nagasawa, N.; et al. Development of the gel indicator using PVA and KI. Mem. Fukui Univ. Technol. 2017, 47, 105–110. [Google Scholar]
- Aoki, Y.; Harvel, G.; Sakura, T.; Sunagawa, T. Development of a gel type dosimeter for X-ray fields. In Proceedings of the 25th International Conference on Nuclear Engineering, Shanghai, China, 2–6 July 2017. [Google Scholar] [CrossRef]
- Fujino, K.; Ono, K.; Hayashi, S.-I.; Yasuda, H.; Akagi, Y.; Hirokawa, Y. Influence of the components of a radiochromic PVA-Iodide gel dosimeter on the thermal and spatial stability. Radiat. Meas. 2020, 135, 106338. [Google Scholar] [CrossRef]
- Hayashi, S.-I.; Ono, K.; Fujino, K.; Ikeda, S.; Tanaka, K. Novel radiochromic gel dosimeter based on a polyvinyl alcohol – Iodide complex. Radiat. Meas. 2020, 131, 106226. [Google Scholar] [CrossRef]
- Jordan, K.; Avvakumov, N. Radiochromic leuco dye micelle hydrogels: I. Initial investigation. Phys. Med. Biol. 2009, 54, 6773–6789. [Google Scholar] [CrossRef]
- Gore, J.C.; Kang, Y.S. Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys. Med. Biol. 1984, 29, 1189–1197. [Google Scholar] [CrossRef]
- Babic, S.; Battista, J.; Jordan, K. Radiochromic leuco dye micelle hydrogels: II. Low diffusion rate leuco crystal violet gel. Phys. Med. Biol. 2009, 54, 6791–6808. [Google Scholar] [CrossRef]
- Colnot, J.; Huet, C.; Gschwind, R.; Clairand, I. Characterisation of two new radiochromic gel dosimeters TruView™ and ClearView™ in combination with the vista™ optical CT scanner: A feasibility study. Phys. Med. 2018, 52, 154–164. [Google Scholar] [CrossRef]
- Jordan, K.; Sekimoto, M. Non-diffusing radiochromic micelle gel. J. Phys. Conf. Ser. 2010, 250, 012031. [Google Scholar] [CrossRef]
- Hayashi, K.; Nemoto, M.; Takanashi, T.; Kang, Y.; Togo, H.; Kotoku, J.; Kobayashi, T.; Mihashi, M.; Hayashi, S.; Gotoh, H. Clear micelle gel dosimeter with nanoclay. J. Phys. Conf. Ser. 2019, 1305. [Google Scholar] [CrossRef]
- Hayashi, K.; Toyohara, M.; Kusano, Y.; Minohara, S.; Shimono, Y.; Gotoh, H. Behaviour and mechanism of micelle gel dosimeter for carbon-ion-beam irradiation. Radiat. Phys. Chem. 2021, 179, 109191. [Google Scholar] [CrossRef]
- Murshed, H. Fundamentals of Radiation Oncology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Tsujii, H.; Kamada, T. A Review of Update Clinical Results of Carbon Ion Radiotherapy. Jpn. J. Clin. Oncol. 2012, 42, 670–685. [Google Scholar] [CrossRef]
- Ohno, T. Particle radiotherapy with carbon ion beams. EPMA J. 2013, 4, 9. [Google Scholar] [CrossRef]
- Minohara, S.; Fukuda, S.; Kanematsu, N.; Takei, Y.; Furukawa, T.; Inaniwa, T.; Matsufuji, N.; Mori, S.; Noda, K. Recent Innovations in Carbon-Ion Radiotherapy. J. Radiat. Res. 2012, 51, 385–392. [Google Scholar] [CrossRef]
- Chu, W.T.T. Overview of light-ion beam therapy. In Proceedings of the Meeting Organized Jointly by the International Atomic Energy Agency and the International Commission on Radiation Units and Measurements, Columbus, OH, USA, 18–20 March 2006. pp. 5–28. Available online: https://www.iaea.org/publications/7767/dose-reporting-in-ion-beam-therapy (accessed on 15 December 2021).
- Kusano, Y.; Kanai, T.; Yonai, S.; Komori, M.; Ikeda, N.; Tachikawa, Y.; Ito, A.; Uchida, H. Field-size dependence of doses of therapeutic carbon beams. Med. Phys. 2007, 34, 4016–4022. [Google Scholar] [CrossRef]
- Lin, L.; Ainsley, C.G.; Mertens, T.; De Wilde, O.; Talla, P.T.; McDonough, J.E. A novel technique for measuring the low-dose envelope of pencil-beam scanning spot profiles. Phys. Med. Biol. 2013, 58, N171–N180. [Google Scholar] [CrossRef]
- Sawakuchi, G.O.; Zhu, X.R.; Poenisch, F.; Suzuki, K.; Ciangaru, G.; Titt, U.; Anand, A.; Mohan, R.; Gillin, M.T.; Sahoo, N. Experimental characterization of the low-dose envelope of spot scanning proton beams. Phys. Med. Biol. 2010, 55, 3467–3478. [Google Scholar] [CrossRef]
- Tadano, K.; Hayashi, K.; Toyohara, M.; Yamamoto, H.; Kusano, Y.; Minohara, S.; Shimono, Y.; Gotoh, H. Elucidation of PVA-KI gel dosimeter char-acteristics by predicting changes in radical concentrations and measuring responsiveness to heavy ion beams. Radiation. Phys. Chem. 2022; manuscript in preparation, to be submitted. [Google Scholar]
- Hartmann, G.H.; Jäkel, O.; Heeg, P.; Karger, C.P.; Kriessbach, A. Determination of water absorbed dose in a carbon ion beam using thimble ionization chambers. Phys. Med. Biol. 1999, 44, 1193–1206. [Google Scholar] [CrossRef]
- Matsufuji, N.; Tomura, H.; Futami, Y.; Yamashita, H.; Fukumura, A.; Kanai, T.; Higashi, A.; Akagi, T.; Komami, H.; Kohno, T. Energy distribution of projectile fragment particles in heavy ion therapeutic beams. Proc. 1997 Symp. Nucl. Data 1997, 101–106. Available online: https://jopss.jaea.go.jp/pdfdata/JAERI-Conf-98-003.pdf (accessed on 15 December 2021).
- Ying, C.K.; Bolst, D.; Tran, L.; Guatelli, S.; Rosenfeld, A.B.; Kamil, W.A. Contributions of secondary fragmentation by carbon ion beams in water phantom: Monte Carlo simulation. J. Phys. Conf. Ser. 2017, 851, 012033. [Google Scholar] [CrossRef]
- Gudowska, I.; Sobolevsky, N. Simulation of secondary particle production and absorbed dose to tissue in light ion beams. Radiat. Prot. Dosim. 2005, 116, 301–306. [Google Scholar] [CrossRef]
- Gudowska, I.; Kempe, J.; Sobolevsky, N. Low and high LET dose components in carbon beam. Radiat. Prot. Dosim. 2006, 122, 483–484. [Google Scholar] [CrossRef]
- Hultqvist, M.; Gudowska, I. Secondary doses delivered to an anthropomorphic male phantom under prostate irradiation with proton and carbon ion beams. Radiat. Meas. 2010, 45, 1410–1413. [Google Scholar] [CrossRef]
- International Atomic Energy Agency, Decommissioning of Particle Accelerators, IAEA Nuclear Energy Series, No. NW-T-2.9, 2020. International Atomic Energy Agency, Vinna, Austria. Available online: https://www.iaea.org/publications/12371/decommissioning-of-particle-accelerators (accessed on 15 December 2021).
- Yashima, H.; Uwamino, Y.; Sugita, H.; Nakamura, T.; Ito, S.; Fukumura, A. Projectile dependence of radioactive spallation products induced in copper by high-energy heavy ions. Phys. Rev. C 2002, 66, 044607. [Google Scholar] [CrossRef]
- Yashima, H.; Uwamino, Y.; Iwase, H.; Sugita, H.; Nakamura, T.; Ito, S.; Fukumura, A. Cross sections for the production of residual nuclides by high-energy heavy ions. Nucl. Instrum. Methods Phys. Res. B 2004, 226, 243–263. [Google Scholar] [CrossRef]
- Toshito, T.; Kodama, K.; Sihver, L.; Yusa, K.; Ozaki, M.; Amako, K.; Kameoka, S.; Murakami, K.; Sasaki, T.; Aoki, S.; et al. Measurements of total and partial charge-changing cross sections for 200- to 400-MeV/nucleonC12on water and polycarbonate. Phys. Rev. C 2007, 75, 054606. [Google Scholar] [CrossRef]
- Toshito, T.; Kodama, K.; Sihver, L.; Yusa, K.; Ozaki, M.; Amako, K.; Kameoka, S.; Murakami, K.; Sasaki, T.; Aoki, S.; et al. Measurements of projectile-likeBe8andB9production in 200–400 MeV/nucleonC12on water. Phys. Rev. C 2008, 78, 067602. [Google Scholar] [CrossRef]
- Toshito, T.; Kubota, H.; Ota, S.; Kanematsu, N.; Kodaira, S.; Komori, M.; Yasuda, N.; Kuge, K. Recent progress in emulsion technology to study fragmentation reactions of high energetic ion beams. Nucl. Instrum. Methods Phys. Res. B 2009, 267, 997–1000. [Google Scholar] [CrossRef][Green Version]
- Soltani-Nabipour, J.; Popovici, M.A.; Cata-Danil, G.H. Residual nuclides produced by 290 MeV/u 12C ion beam in a liquid water target. Rom. Rep. Phys. 2010, 62, 37–46. [Google Scholar]
- Goldhaber, A.S.; Heckman, H.H. High Energy Interactions of Nuclei. Annu. Rev. Nucl. Part. Sci. 1978, 28, 161–205. [Google Scholar] [CrossRef]
- Venning, A.J.; Hill, B.; Brindha, S.; Healy, B.J.; Baldock, C. Investigation of the PAGAT polymer gel dosimeter using magnetic resonance imaging. Phys. Med. Biol. 2005, 50, 3875–3888. [Google Scholar] [CrossRef]
- Nakayama, Y.; Minohara, S.; Nonaka, T.; Nomiya, T.; Kusano, Y.; Takeshita, E.; Mizoguchi, N.; Hagiwara, Y. The Ion-Beam Radiation Oncology Center in Kanagawa (i-ROCK) Carbon Ion Facility at the Kanagawa Cancer Center. Int. J. Part. Ther. 2015, 2, 478–480. [Google Scholar] [CrossRef]
- Furukawa, T.; Inaniwa, T.; Sato, S.; Shirai, T.; Takei, Y.; Takeshita, E.; Mizushima, K.; Iwata, Y.; Himukai, T.; Mori, S.; et al. Performance of the NIRS fast scanning system for heavy-ion radiotherapy. Med. Phys. 2010, 37, 5672. [Google Scholar] [CrossRef]
- Matsufuji, N.; Kanai, T.; Kanematsu, N.; Miyamoto, T.; Baba, M.; Kamada, T.; Kato, H.; Yamada, S.; Mizoe, J.; Tsujii, H. Treatment planning and dosimetric requirement for prescribing and reporting ion-beam therapy: The current CHIBA approach. In Proceedings of the Meeting Organized Jointly by the International Atomic Energy Agency and the International Commission on Radiation Units and Measurements, Columbus, OH, USA, 18–20 March 2006. [Google Scholar]
- Inaniwa, T.; Kanematsu, N.; Matsufuji, N.; Kanai, T.; Shirai, T.; Noda, K.; Tsuji, H.; Kamada, T.; Tsujii, H. Reformulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the National Institute of Radiological Sciences, Japan. Phys. Med. Biol. 2015, 60, 3271–3286. [Google Scholar] [CrossRef]
- Nomiya, T.; Tsuji, H.; Maruyama, K.; Toyama, S.; Suzuki, H.; Akakura, K.; Simazaki, J.; Nemoto, K.; Kamada, T.; Tsujii, H. Phase I/II trial of definitive carbon ion radiotherapy for prostate cancer: Evaluation of shortening of treatment period to 3 weeks. Br. J. Cancer 2014, 110, 2389–2395. [Google Scholar] [CrossRef]
- Sato, T.; Iwamoto, Y.; Hashimoto, S.; Ogawa, T.; Furuta, T.; Abe, S.-I.; Kai, T.; Tsai, P.-E.; Matsuda, N.; Iwase, H.; et al. Features of Particle and Heavy Ion Transport code System (PHITS) version 3.02. J. Nucl. Sci. Technol. 2018, 55, 684–690. [Google Scholar] [CrossRef]
- Ratliff, H.N.; Matsuda, N.; Abe, S.-I.; Miura, T.; Furuta, T.; Iwamoto, Y.; Sato, T. Modernization of the DCHAIN-PHITS activation code with new features and updated data libraries. Nucl. Instrum. Methods Phys. Res. B 2020, 484, 29–41. [Google Scholar] [CrossRef]
- Hamad, M.K. Bragg-curve simulation of carbon-ion beams for particle-therapy applications: A study with the GEANT4 toolkit. Nucl. Eng. Technol. 2021, 53, 2767–2773. [Google Scholar] [CrossRef]
- Chu, S.Y.F.; Ekstrom, L.P.; Firestone, R.B. The Lund/LBNL nuclear data search. 1999 Version 2.0. Available online: http://nucleardata.nuclear.lu.se/toi/index.asp (accessed on 26 November 2021).
- Tilley, D.; Cheves, C.; Godwin, J.; Hale, G.; Hofmann, H.; Kelley, J.; Sheu, C.; Weller, H. Energy levels of light nuclei A=5, 6, 7. Nucl. Phys. A 2002, 708, 3–163. [Google Scholar] [CrossRef]
- Doering, C. Measurement of the Distribution and Behaviour of Beryllium-7 in the Natural Environment. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2007. [Google Scholar]
- Japan Nuclear Regulation Authority. Gamma-Ray Spectral Analysis Using Germanium Detector in Emergencies. The Series of Environmental Radioactivity Measuring Methods No. 29. 2018. Available online: https://radioactivity.nsr.go.jp/en/list/332/list-1.html (accessed on 26 November 2021).
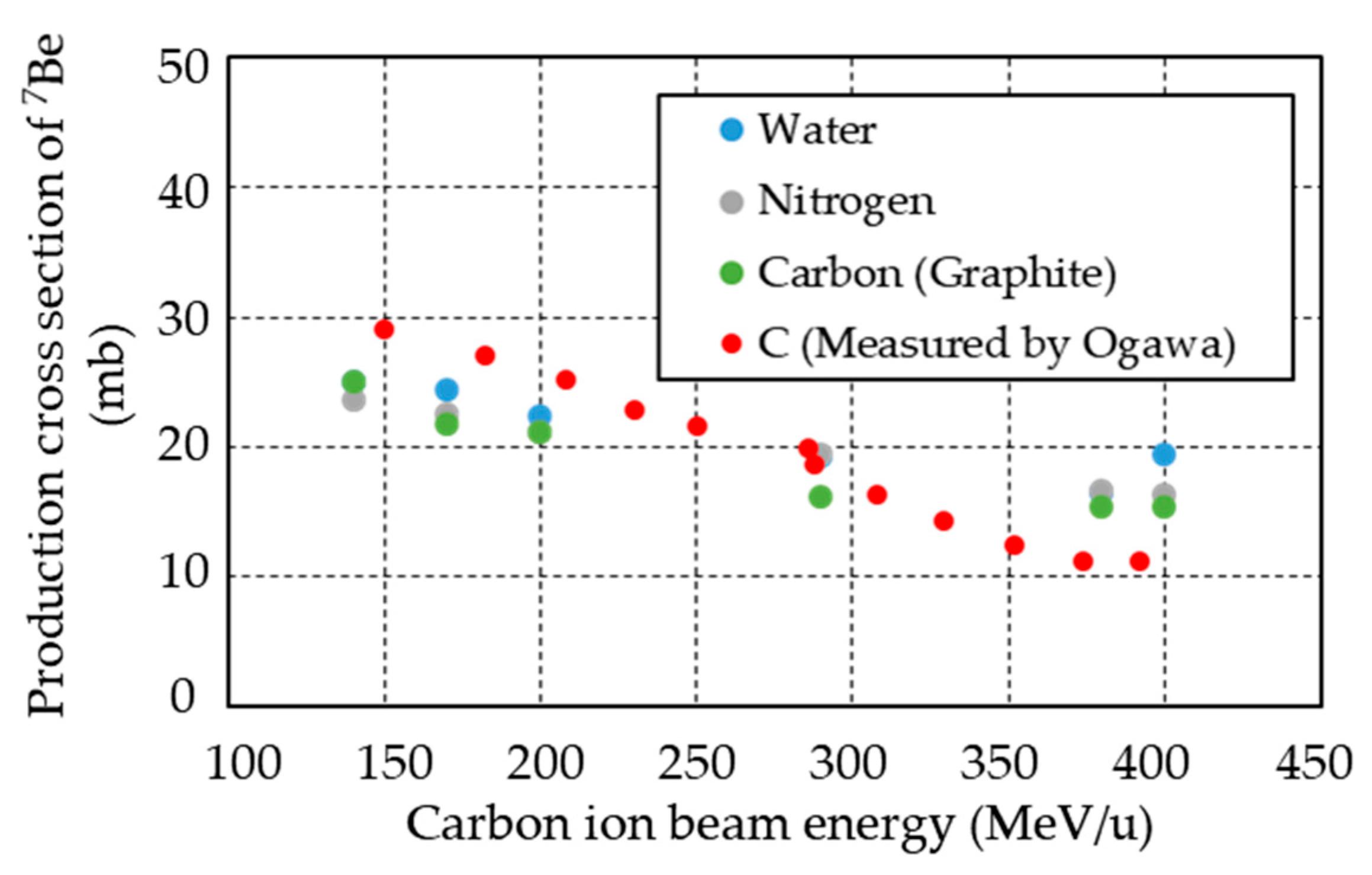
- Ogawa, T.; Sato, T.; Hashimoto, S.; Satoh, D.; Tsuda, S.; Niita, K. Energy-dependent fragmentation cross sections of relativistic 12C. Phys. Rev. C 2015, 92, 024614. [Google Scholar] [CrossRef]
- Geithner, O.; Andreo, P.; Sobolevsky, N.; Hartmann, G.; Jäkel, O. Calculation of stopping power ratios for carbon ion dosimetry. Phys. Med. Biol. 2006, 51, 2279–2292. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Application of the Concepts of Exclusion, Exemption and Clearance. IAEA Safety Standards Series, Safety Guide No. RS-G-1.7, 2004. International Atomic Energy Agency, Vinna, Austria. Available online: https://www-pub.iaea.org/mtcd/publications/pdf/pub1202_web.pdf (accessed on 15 December 2021).

| (a) Micellar gel dosimeter * | ||
| Component | Weight percent (%) | Supplier/Grade |
| Water | 91.6 | Ion exchange water |
| Gelatin | 4.95 | Nacalai Tesque, Inc. 16631-05 |
| “TritonX-100” | 2.86 | Alfa Aesar, 9002-93-1 |
| CH2Cl2 | 4.06 × 10−1 | Wako Co. Ltd., 044-28305 |
| Nano-clay | 1.53 × 10−1 | BYK Japan KK |
| LCV | 6.60 × 10−3 | Wako Co. Ltd., 321-56072 |
| (b) Polymer gel (PAGAT Gel) dosimeter ** | ||
| Component | Weight percent (%) | Supplier/Grade |
| Water | 89 | Ion exchange water |
| Gelatin | 5.0 | Sigma Aldrich G2500-500G, PREMIUM |
| Acrylamide | 3.0 | TCI A0139 |
| N,N-Methylene Bisacrylamide | 3.0 | Sigma Aldrich 146072-500G |
| THPC | 1.3 × 10−2 | TCI T0607 |
| No. | Specimen | Physical Dose | Beam Condition | Averaged Beam Energy | Remark |
|---|---|---|---|---|---|
| (1) | Micellar gel | 10.4 Gy at SOBP | (A) | 120 MeV/u * | |
| (2) | 10.4 Gy at SOBP | ||||
| (3) | 10.4 Gy at SOBP | Dissolve by heating after irradiation | |||
| (4) | 20.8 Gy at SOBP | ||||
| (5) | 31.2 Gy at SOBP | ||||
| (6) | 20.8 Gy at SOBP | (B) | 389 MeV/u * | ||
| (7) | 69 Gy at Bragg peak | (C) | 200 MeV/u | Mono- energy | |
| (8) | Polymer gel | 10.4 Gy at SOBP | (A) | 120 MeV/u * | |
| (9) | 10.4 Gy at SOBP | ||||
| (10) | Water | 10.4 Gy at SOBP |
| Beam Number | Beam Condition (A) | Beam Condition (B) | Beam Condition (C) | |||
|---|---|---|---|---|---|---|
| Energy (MeV/u) | RSF (mm WEL) * | Energy (MeV/u) | RSF (mm WEL) * | Energy (MeV/u) | RSF (mm WEL) * | |
| 1 | 170 | 18.1 | 400 | 0 | 200 | 0 |
| 2 | 140 | 1.16 | 400 | 2.09 | — | |
| 3 | 140 | 3.25 | 400 | 3.94 | ||
| 4 | 140 | 5.10 | 400 | 6.03 | ||
| 5 | 140 | 7.19 | 400 | 7.88 | ||
| 6 | 140 | 9.04 | 400 | 9.97 | ||
| 7 | 140 | 11.13 | 400 | 12.06 | ||
| 8 | 140 | 13.22 | 400 | 15.15 | ||
| 9 | 140 | 15.08 | 400 | 16.01 | ||
| 10 | 140 | 17.17 | 400 | 18.10 | ||
| 11 | 140 | 19.02 | 400 | 19.95 | ||
| 12 | 140 | 21.11 | 400 | 22.04 | ||
| 13 | 140 | 22.96 | 380 | 2.09 | ||
| 14 | 140 | 25.29 | 380 | 3.94 | ||
| 15 | 140 | 27.14 | 380 | 6.03 | ||
| 16 | 140 | 29.23 | 380 | 7.88 | ||
| 17 | 140 | 31.09 | 380 | 9.97 | ||
| 18 | 140 | 33.18 | 380 | 11.82 | ||
| 19 | 140 | 35.03 | 380 | 13.92 | ||
| 20 | 140 | 37.12 | 380 | 16.01 | ||
| Specimen | H | Li | C | N | O | Na | Mg | Si | P | S | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Micellar gel | 10.9 | 4.00 × 10−4 | 3.98 | 7.49 × 10−1 | 84.0 | 4.60 × 10−3 | 2.65 × 10−2 | 4.50 × 10−2 | - | 5.80 × 10−3 | 3.39 × 10−1 |
| Polymer gel | 12.3 | - | 3.50 | 1.68 | 82.5 | - | - | - | 3.13 × 10−2 | 5.51 × 10−3 | 3.54 × 10−2 |
| Water | 11.1 | - | - | - | 88.9 | - | - | - | - | - | - |
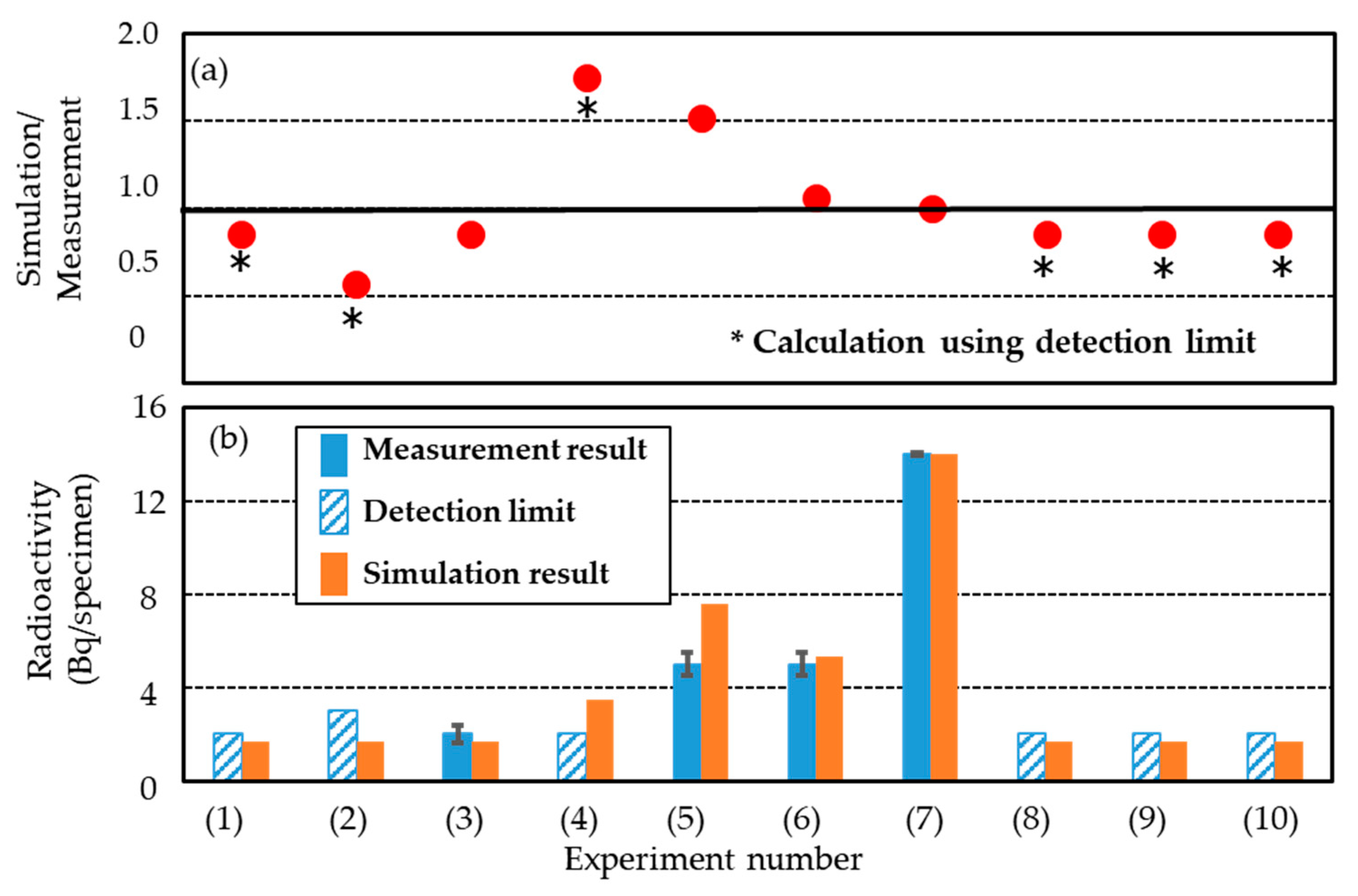
| Experiment | Specimen | Physical Dose (Gy) | Averaged Beam Energy * (MeV/u) | Radio-nuclide ** | Bq/specimen | Radioactivity Concentration (Bq/g) *** | Remark |
|---|---|---|---|---|---|---|---|
| (1) | Micellar gel | 10.4 | 120 * | 7Be | N.D. (<2) | < 5 × 10−1 | IAEA Safety Guide No. RS-G-1.7 [39] 7Be; 10 Bq/g 3H; 100 Bq/g |
| (2) | 10.4 | 7Be | N.D. (<3) | < 1 | |||
| (3) | 10.4 | 7Be | 2 ± 4 × 10−1 | 5 × 10−1 | |||
| (4) | 20.8 | 7Be | N.D. (<2) | < 5 × 10−1 | |||
| (5) | 31.2 | 7Be | 5 ± 5 × 10−1 | 1 | |||
| (6) | 20.8 | 389 * | 7Be | 5 ± 5 × 10−1 | 1 | ||
| (7) | 52 (in specimen) | 200 | 7Be | 14 ± 5 × 10−1 | 1 | ||
| 3H | N.D. (<1 × 10−1) | < 1 × 10−2 | |||||
| (8) | Polymer gel | 10.4 | 120 * | 7Be | N.D. (<2) | < 5 × 10−1 | |
| (9) | 10.4 | 7Be | N.D. (<2) | < 5 × 10−1 | |||
| (10) | Water | 10.4 | 7Be | N.D. (<2) | < 5 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyohara, M.; Minohara, S.; Kusano, Y.; Gotoh, H.; Tanaka, Y.; Yuhara, M.; Yamashita, Y.; Shimono, Y. Induced Radionuclides and Their Activity Concentration in Gel Dosimeters Irradiated by Carbon Ion Beam. Gels 2022, 8, 203. https://doi.org/10.3390/gels8040203
Toyohara M, Minohara S, Kusano Y, Gotoh H, Tanaka Y, Yuhara M, Yamashita Y, Shimono Y. Induced Radionuclides and Their Activity Concentration in Gel Dosimeters Irradiated by Carbon Ion Beam. Gels. 2022; 8(4):203. https://doi.org/10.3390/gels8040203
Chicago/Turabian StyleToyohara, Masumitsu, Shinichi Minohara, Yohsuke Kusano, Hiroaki Gotoh, Yoichiro Tanaka, Masaru Yuhara, Yu Yamashita, and Yoshiaki Shimono. 2022. "Induced Radionuclides and Their Activity Concentration in Gel Dosimeters Irradiated by Carbon Ion Beam" Gels 8, no. 4: 203. https://doi.org/10.3390/gels8040203
APA StyleToyohara, M., Minohara, S., Kusano, Y., Gotoh, H., Tanaka, Y., Yuhara, M., Yamashita, Y., & Shimono, Y. (2022). Induced Radionuclides and Their Activity Concentration in Gel Dosimeters Irradiated by Carbon Ion Beam. Gels, 8(4), 203. https://doi.org/10.3390/gels8040203






