Improved Dose Response of N-(hydroxymethyl)acrylamide Gel Dosimeter with Calcium Chloride for Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Irradiation
2.3. Nuclear Magnetic Resonance (NMR) Measurements
3. Results and Discussion
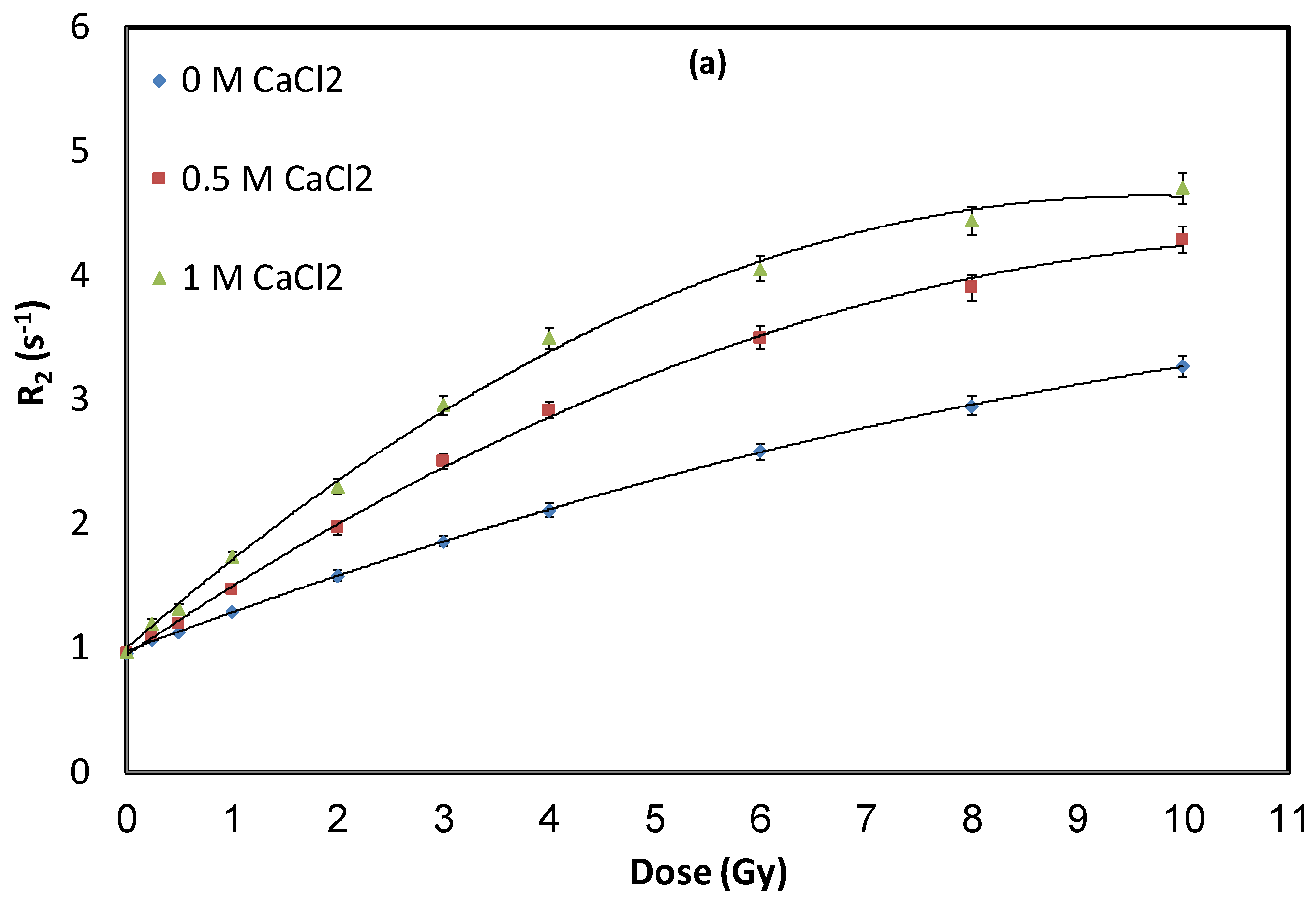
3.1. Impact of Calcium Chloride (CaCl2) Concentration
3.2. Stability of NHMAGA Dosimeters
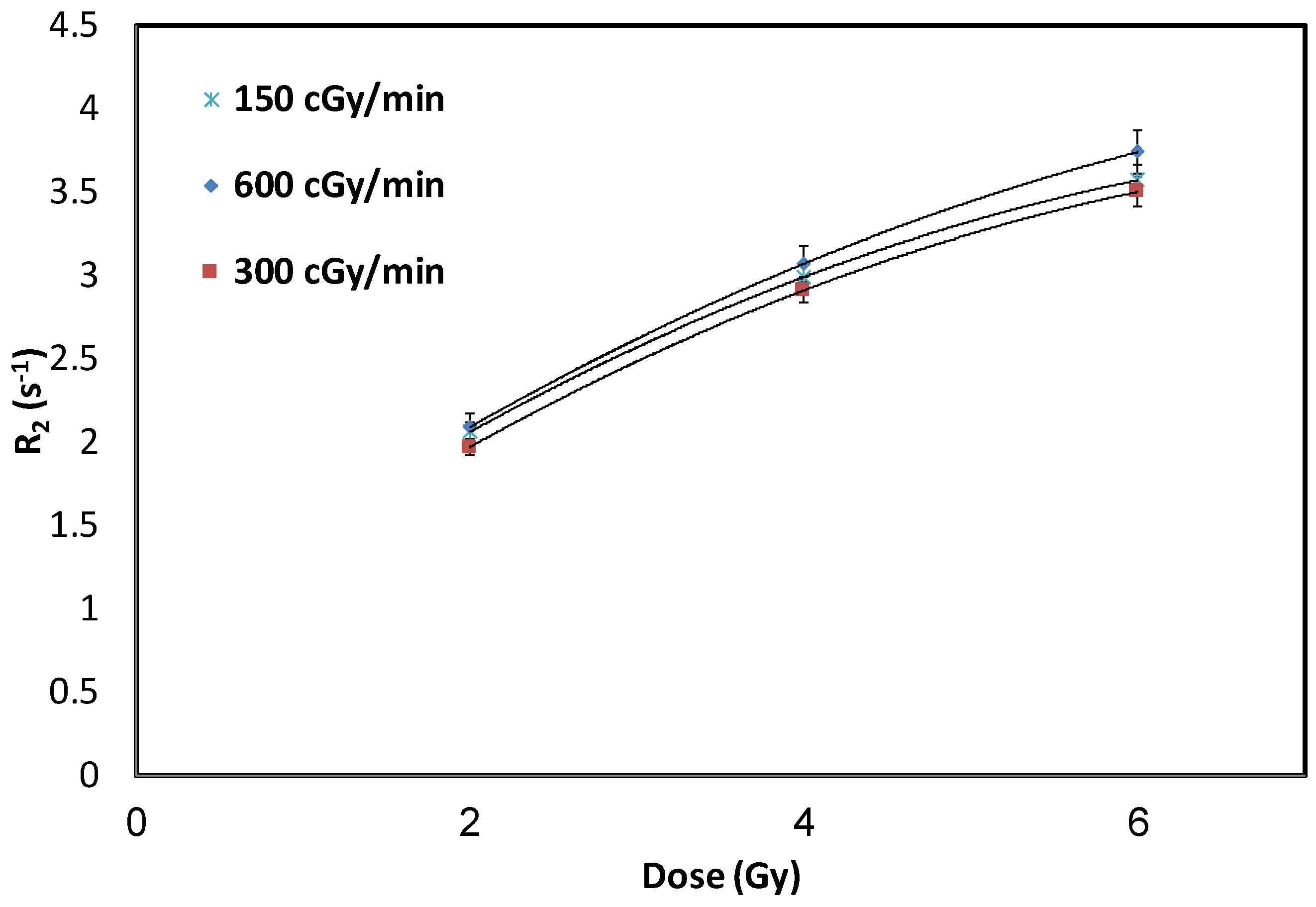
3.3. Influence of Dose-Rates
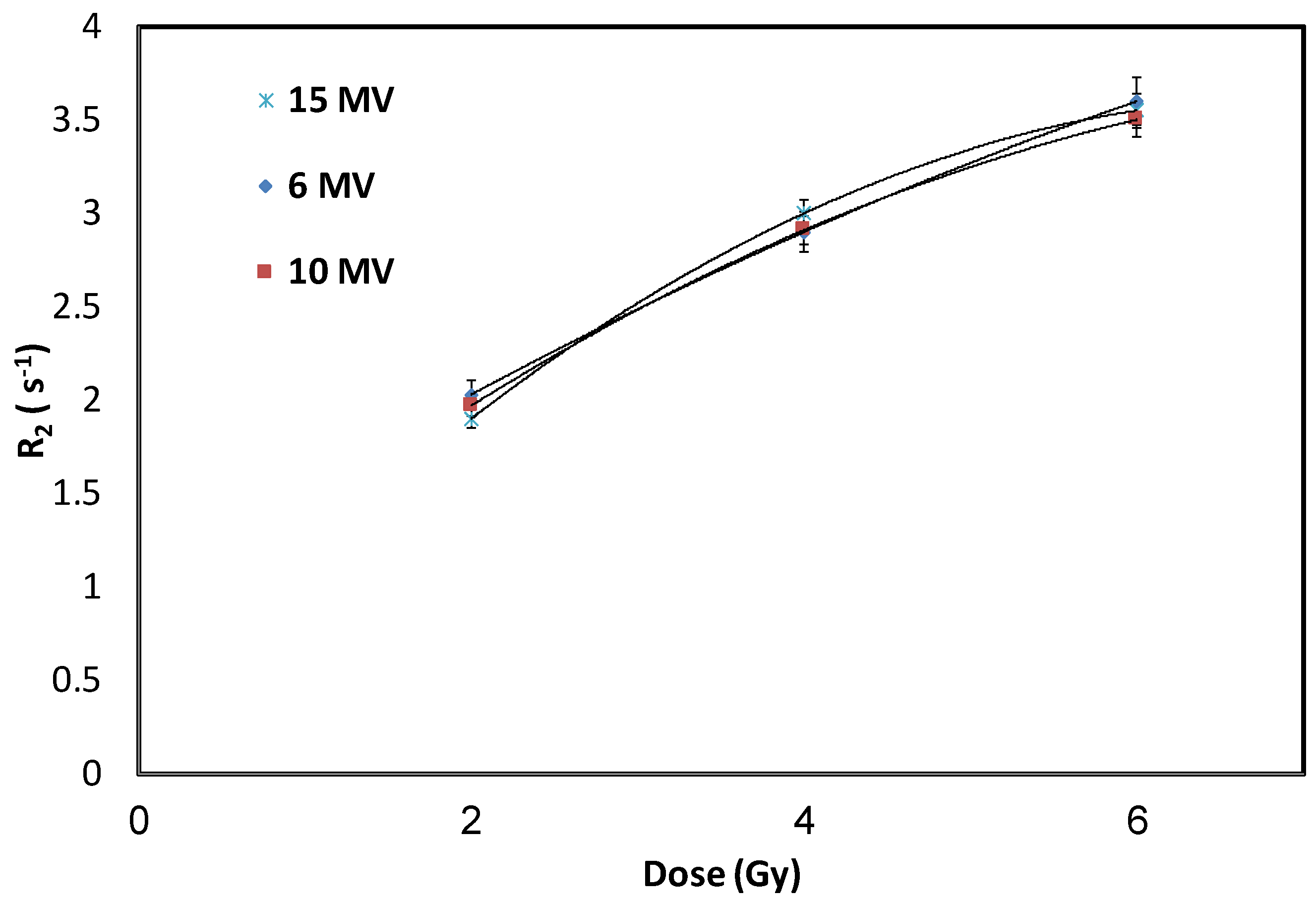
3.4. Effect of Radiation-Energies
3.5. Effect of Scanning Temperature
3.6. Uncertainty Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabaeh, K.A.; Basfar, A.A.; Moussa, A.A.; Msalam, R.I. Novel radio-chromic solution dosimeter for radiotherapy treatment planning. Phys. Med. 2013, 29, 374–378. [Google Scholar] [CrossRef] [Green Version]
- Soliman, Y.S. Gamma-radiation induced synthesis of silver nanoparticles in gelatin and its application for radiotherapy dose measurements. Radiat. Phys. Chem. 2014, 102, 60–67. [Google Scholar] [CrossRef]
- Eyadeh, M.M.; Rabaeh, K.A.; Hailat, T.F.; Aldweri, F.M. Evaluation of ferrous Methylthymol blue gelatin gel dosimeters using nuclear magnetic resonance and optical techniques. Radiat. Meas. 2018, 108, 26–33. [Google Scholar] [CrossRef]
- Soliman, Y.S.; Beshir, W.B.; Abdelgawad, M.H.; Brauer-Krisch, E.; Abdel-Fattah, A.A. Pergascript orange-based polymeric solution as a dosimeter for radiotherapy dosimetric validation. Phys. Med. 2019, 57, 169–176. [Google Scholar] [CrossRef]
- Watanabe, Y.; Mizukami, S.; Eguchi, K.; Maeyama, T.; Hayashi, S.-I.; Muraishi, H. Dose distribution verification in high-dose-rate brachytherapy using a highly sensitive normoxic N-vinylpyrrolidone polymer-gel dosimeter. Phys. Med. 2009, 57, 72–79. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Eyadeh, M.M.; Hailat, T.F.; Aldweri, F.M.; Alheet, S.M.; Eid, R.M. Characterization of ferrous-methylthymol blue-polyvinyl alcohol gel dosimeters using nuclear magnetic resonance and optical techniques. Radiat. Phys. Chem. 2018, 148, 25–32. [Google Scholar] [CrossRef]
- Adliene, D.; Jakstas, K.; Vaiciunaite, N. Application of optical methods for dose evaluation in normoxic polyacrylamide gels irradiated at two different geometries. Nucl. Instrum. Methods Phys. A 2014, 741, 88–94. [Google Scholar] [CrossRef]
- Basfar, A.A.; Moftah, B.; Rabaeh, K.A.; Almousa, A. Novel composition of polymer-gel dosimeters based on N-(Hydro-xymethyl) acrylamide for radiation therapy. Radiat. Phys. Chem. 2015, 112, 112–120. [Google Scholar] [CrossRef]
- Maeyama, T.; Ishida, Y.; Kudo, Y.; Fukasaku, K.; Ishikawa, K.L.; Fukunishi, N. Polymer-gel dosimeter with AQUAJOINT® as hydrogel matrix. Radiat. Phys. Chem. 2018, 146, 121–125. [Google Scholar] [CrossRef]
- Awad, S.I.; Moftah, B.; Basfer, A.; Almousa, A.A.; Al Kafi, M.A.; Eyadeh, M.M.; Rabaeh, K.A. 3-D Quality Assurance in CyberKnife Radiotherapy Using a Novel N-(3-methoxypropyl) Acrylamide Polymer-gel Dosimeter and Optical CT. Radiat. Phys. Chem. 2019, 161, 34–41. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Al-Ajaleen, M.S.; Abuzayed, M.H.; Aldweri, F.M.; Eyadeh, M.M. High dose sensitivity of N-(isobutoxymethyl)acrylamide polymer-gel dosimeters with improved monomer solubility using acetone co-solvent. Nucl. Instrum. Methods Phys. Res. Sect. B 2019, 442, 67–72. [Google Scholar] [CrossRef]
- De Deene, Y.; Vergote, K.; Claeys, C.; De Wagter, C. The fundamental radiation properties of normoxic polymer-gel dosimeters: A comparison between a methacrylic acid-based gel and acrylamide-based gels. Phys. Med. Biol. 2006, 51, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer-gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Watanabe, Y.; Mizukami, S.; Maeyama, T.; Terazaki, T.; Uehara, R.; Akimoto, T. End-to-end delivery quality assurance of computed tomography–based high-dose-rate brachytherapy using a gel dosimeter. Brachytherapy 2020, 19, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Maryanski, M.J.; Gore, J.C.; Kennan, R.P.; Schulz, R.J. NMR relaxation enhancement in gels polymerized and cross-linked by ionizing radiation: A new approach to 3D dosimetry by MRI. Magn. Reson. Imaging 1993, 11, 253–258. [Google Scholar] [CrossRef]
- Mizukami, S.; Watanabe, Y.; Mizoguchi, T.; Gomi, T.; Hara, H.; Takei, H.; Fukunishi, N.; Ishikawa, K.L.; Fukuda, S.; Maeyama, T. Whole Three-Dimensional Dosimetry of Carbon Ion Beams with an MRI-Based Nanocomposite Fricke Gel Dosimeter Using Rapid T1 Mapping Method. Gels 2021, 7, 233. [Google Scholar] [CrossRef]
- De Deene, Y. Gel dosimetry for the dose verification of Intensity Modulated Radiotherapy Treatments. Z. Med. Phys. 2002, 12, 77–88. [Google Scholar] [CrossRef]
- Gore, J.C.; Ranade, M.; Maryanski, M.J.; Schulz, R.J. Radiation dose distributions in three dimensions from tomographic optical density scanning of polymer-gels: I. Development of an optical scanner. Phys. Med. Biol. 1996, 41, 2695–2704. [Google Scholar] [CrossRef]
- Oldham, M.; Siewerdsen, J.H.; Shetty, A.; Jaffray, D.A. High resolution gel-dosimetry by optical-CT and MR scanning. Med. Phys. 2001, 28, 1436–1445. [Google Scholar] [CrossRef] [Green Version]
- Rabaeh, K.A.; Saion, E.; Omer, M.; Shahrim, I.; Alrahman, A.A.; Hussain, M. Enhancements in 3D Dosimetry Measurement using Polymer-gel and MRI. Radiat. Meas. 2008, 43, 1377–1382. [Google Scholar] [CrossRef]
- Ibbott, G.S.; Maryanski, M.J.; Eastman, P.; Holcomb, S.D.; Zhang, Y.; Avison, R.G.; Sanders, M.; Gore, J.C. Three-dimensional visualization and measurement of conformal dose distributions using magnetic resonance imaging of BANG polymer-gel dosimeters. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 1097–1103. [Google Scholar] [CrossRef]
- De Deene, Y.; De Wagter, C.; Van Duyse, B.; Derycke, S.; Mersseman, B.; De Gersem, W.; Voet, T.; Achten, E.; De Neve, W. Validation of MR-based polymer-gel dosimetry as a preclinical three-dimensional verification tool in conformal radiotherapy. Magn. Reson. Med. 2000, 43, 116–125. [Google Scholar] [CrossRef]
- Pappas, E.; Maris, T. Polymer-gel 3D dosimetry in radiotherapy. Z. Med. Phys. 2020, 30, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Rabaeh, K.A.; Issra’ME, H.; Oglat, A.A.; Eyadeh, M.M.; Ala’J, A.Q.; Aldweri, F.M.; Awad, S.I. Polymer-gel containing N, N′-methylene-bis-acrylamide (BIS) as a single monomer for radiotherapy dosimetry. Radiat. Phys. Chem. 2021, 187, 109522. [Google Scholar] [CrossRef]
- El-Khayatt, A.M. Water equivalence of some 3D dosimeters: A theoretical study based on the effective atomic number and effective fast neutron removal cross section. Nucl. Sci. Tech. 2017, 28, 170. [Google Scholar] [CrossRef]
- De Deene, Y. Gel-based Radiation Dosimetry Using Quantitative MRI. NMR MRI Gels 2020, 9, 275–357. [Google Scholar]
- Koeva, V.; Olding, T.; Jirasek, A.; Schreiner, L.; McAuley, K. Preliminary investigation of the NMR, optical and x-ray CT dose–response of polymer-gel dosimeters incorporating co solvents to improve dose sensitivity. Phys. Med. Biol. 2009, 54, 2779. [Google Scholar] [CrossRef]
- Trapp, J.V.; Partridge, M.; Hansen, V.N.; Childs, P.; Bedford, J.; Warrington, A.P.; Leach, M.O.; Webb, S. The use of gel dosimetry for verification of electron and photon treatment plants in carcinoma of the scalp. Phys. Med. Biol. 2004, 49, 1625–1635. [Google Scholar] [CrossRef]
- Gopishankar, N.; Vivekanandhan, S.; Kale, S.S.; Rath, G.K.; Senthilkumaran, S.; Thulkar, S.; Subramani, V.; Laviraj, M.A.; Bisht, R.K.; Mahapatra, A.K. MAGAT gel and EBT2 film-based dosimetry for evaluating source plugging-based treatment plan in Gamma Knife stereotactic radiosurgery. J. Appl. Clin. Med. Phys. 2012, 13, 46–61. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Saion, E.; Omer, M.A.A.; Rahman, A.A.; Hussain, M.Y.; Iskandar, S.; Ali, N.M. Rate of Elapsed Polymerization of Hydroxyethylacrylate Gel Induced by Gamma Radiation. Nucl. Sci. Tech. 2008, 19, 218–222. [Google Scholar] [CrossRef]
- Pappas, E.; Maris, T.; Angelopoulos, A.; Paparigopoulou, M.; Sakelliou, L.; Sandilos, P.; Voyiatzi, S.; Vlachos, L. A new polymer-gel for magnetic resonance imaging (MRI) radiation dosimetry. Phys. Med. Biol. 1999, 44, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Maras, P.; Rybka, K.; Biegański, T. VIPARnd—GeVero® tool in planning of TPS scheduled brain tumour radiotherapy. J. Phys. Conf. Ser. 2009, 164, 012061. [Google Scholar] [CrossRef]
- Kozicki, M.; Berg, A.; Maras, P.; Jaszczak, M.; Dudek, M. Clinical radiotherapy application of N-vinylpyrrolidone-containing 3D polymer-gel dosimeters with remote external MR-reading. Phys. Med. 2020, 69, 134–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattea, F.; Chacón, D.; Vedelago, J.; Valente, M.; Strumia, M.C. Polymer-gel dosimeter based on itaconic acid. Appl. Radiat. Isot. 2015, 105, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Abtahi, S.M.; Geraily, G.; Ghorbani, M.; Mahdavi, S.R.; Zahmatkesh, M.H. Dosimetric characteristics of PASSAG as a new polymer-gel dosimeter with negligible toxicity. Radiat. Phys. Chem. 2018, 147, 91–100. [Google Scholar] [CrossRef]
- Moftah, B.; Basfar, A.; Almousa, A.; Al Kafi, A.; Rabaeh, K. Novel 3D polymer-gel dosimeters based on N-(3-Methoxypropyl)acrylamide (NMPAGAT) for quality assurance in radiation oncology. Radiat. Meas. 2020, 135, 106372. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Basfar, A.A.; Almousa, A.A.; Devic, S.; Moftah, B. New normoxic N-(hydroxymethyl)acrylamide based polymer-gel for 3D dosimetry in radiation therapy. Phys. Med. 2017, 35, 121–126. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Salman, N.M.B.; Aldweri, F.M.; Saleh, H.H.; Eyadeh, M.M.; Awad, S.I.; Oglat, A.A. Substantial influence of magnesium chloride inorganic salt (MgCl2) on the polymer dosimeter containing N-(hydroxymethyl)acrylamide for radiation therapy. Results Phys. 2021, 22, 103862. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Issra’ME, H.; Eyadeh, M.M.; Aldweri, F.M.; Awad, S.I.; Oglat, A.A.; Shatnawi, M.T. Improved performance of N-(hydroxymethyl)acrylamide gel dosimeter using potassium chloride for radiotherapy. Radiat. Meas. 2021, 142, 106542. [Google Scholar] [CrossRef]
- Hayashi, S.I.; Fujiwara, F.; Usui, S.; Tominaga, T. Effect of inorganic salt on the dose sensitivity of polymer-gel dosimeter. Radiat. Phys. Chem. 2012, 81, 884–888. [Google Scholar] [CrossRef]
- Hayashi, S.I.; Kawamura, H.; Usui, S.; Tominaga, T. Comparison of the influence of inorganic salts on the NMR dose sensitivity of polyacrylamide-based gel dosimeter. J. Phys. Conf. Ser. 2013, 444, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.I.; Kawamura, H.; Usui, S.; Tominaga, T. Influence of magnesium chloride on the dose–response of polyacrylamide-type gel dosimeters. Radiol. Phys. Technol. 2018, 11, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Chacón, D.; Strumia, M.; Valente, M.; Mattea, F. Effect of inorganic salts and matrix crosslinking on the dose response of polymer-gel dosimeters based on acrylamide. Radiat. Meas. 2018, 117, 7–18. [Google Scholar] [CrossRef]
- De Deene, Y.; van de Walle, R.; Achten, E.; de Wagter, C. Mathematical analysis and experimental investigation of noise in quantitative magnetic resonance imaging applied in polymer-gel dosimetry. Signal Process. 1998, 70, 85–101. [Google Scholar] [CrossRef]
- Baldock, C.; Lepage, M.; Bäck, S.Å.J.; Murry, P.J.; Jayasekera, P.M.; Porter, D.; Kron, T. Dose resolution in radiotherapy polymer-gel dosimetry: Effect of echo spacing in MRI pulse sequence. Phys. Med. Biol. 2001, 46, 449–460. [Google Scholar] [CrossRef]
- Gochberg, D.F.; Fong, P.M.; Gore, J.C. Studies of magnetization transfer and relaxation in irradiated polymer-gels-interpretation of MRI-based dosimetry. Phys. Med. Biol. 2001, 46, 799–811. [Google Scholar] [CrossRef]
- Lepage, M.; Jayasakera, P.M.; Bäck, S.Å.J.; Baldock, C. Dose resolution optimization of polymer-gel dosimeters using different monomers. Phys. Med. Biol. 2001, 46, 2665–2680. [Google Scholar] [CrossRef]
- Eyadeh, M.M.; Smadi, S.A.; Rabaeh, K.A.; Oglat, A.A.; Diamond, K.R. Effect of lithium chloride inorganic salt on the performance of N-(hydroxymethyl)acrylamide polymer-gel dosimeter in radiation therapy. J. Radioanal. Nucl. Chem. 2021, 330, 1255–1261. [Google Scholar] [CrossRef]
- ISO/ASTM 51707. Guide for Estimating Uncertainties in Dosimetry for Radiation Processing. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
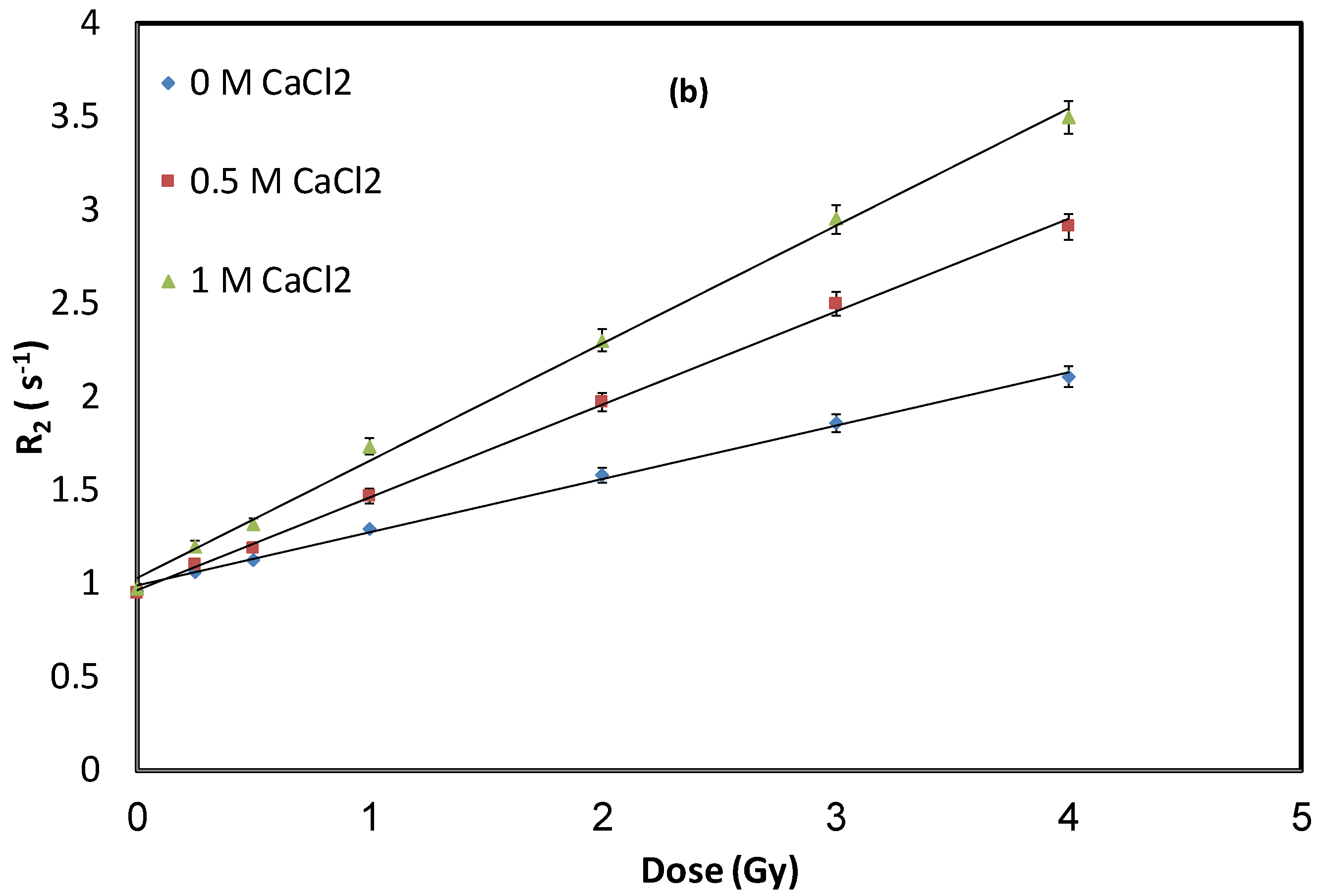



| Linear Equations | Sensitivity (Gy−1·s−1) | Recipe |
|---|---|---|
| R2 = 0.29D + 0.99 | 0.29 | NHMAGAT (1) |
| R2 = 0.50D + 0.96 | 0.50 | NHMAGAT (2) |
| R2 = 0.63D + 1.03 | 0.63 | NHMAGAT (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabaeh, K.A.; Al-Tarawneh, R.E.; Eyadeh, M.M.; Hammoudeh, I.M.E.; Shatnawi, M.T.M. Improved Dose Response of N-(hydroxymethyl)acrylamide Gel Dosimeter with Calcium Chloride for Radiotherapy. Gels 2022, 8, 78. https://doi.org/10.3390/gels8020078
Rabaeh KA, Al-Tarawneh RE, Eyadeh MM, Hammoudeh IME, Shatnawi MTM. Improved Dose Response of N-(hydroxymethyl)acrylamide Gel Dosimeter with Calcium Chloride for Radiotherapy. Gels. 2022; 8(2):78. https://doi.org/10.3390/gels8020078
Chicago/Turabian StyleRabaeh, Khalid A., Rawan E. Al-Tarawneh, Molham M. Eyadeh, Issra’ M. E. Hammoudeh, and Moneeb T. M. Shatnawi. 2022. "Improved Dose Response of N-(hydroxymethyl)acrylamide Gel Dosimeter with Calcium Chloride for Radiotherapy" Gels 8, no. 2: 78. https://doi.org/10.3390/gels8020078
APA StyleRabaeh, K. A., Al-Tarawneh, R. E., Eyadeh, M. M., Hammoudeh, I. M. E., & Shatnawi, M. T. M. (2022). Improved Dose Response of N-(hydroxymethyl)acrylamide Gel Dosimeter with Calcium Chloride for Radiotherapy. Gels, 8(2), 78. https://doi.org/10.3390/gels8020078






