PCL/Sodium-Alginate Based 3D-Printed Dual Drug Delivery System with Antibacterial Activity for Osteomyelitis Therapy
Abstract
:1. Introduction
2. Results
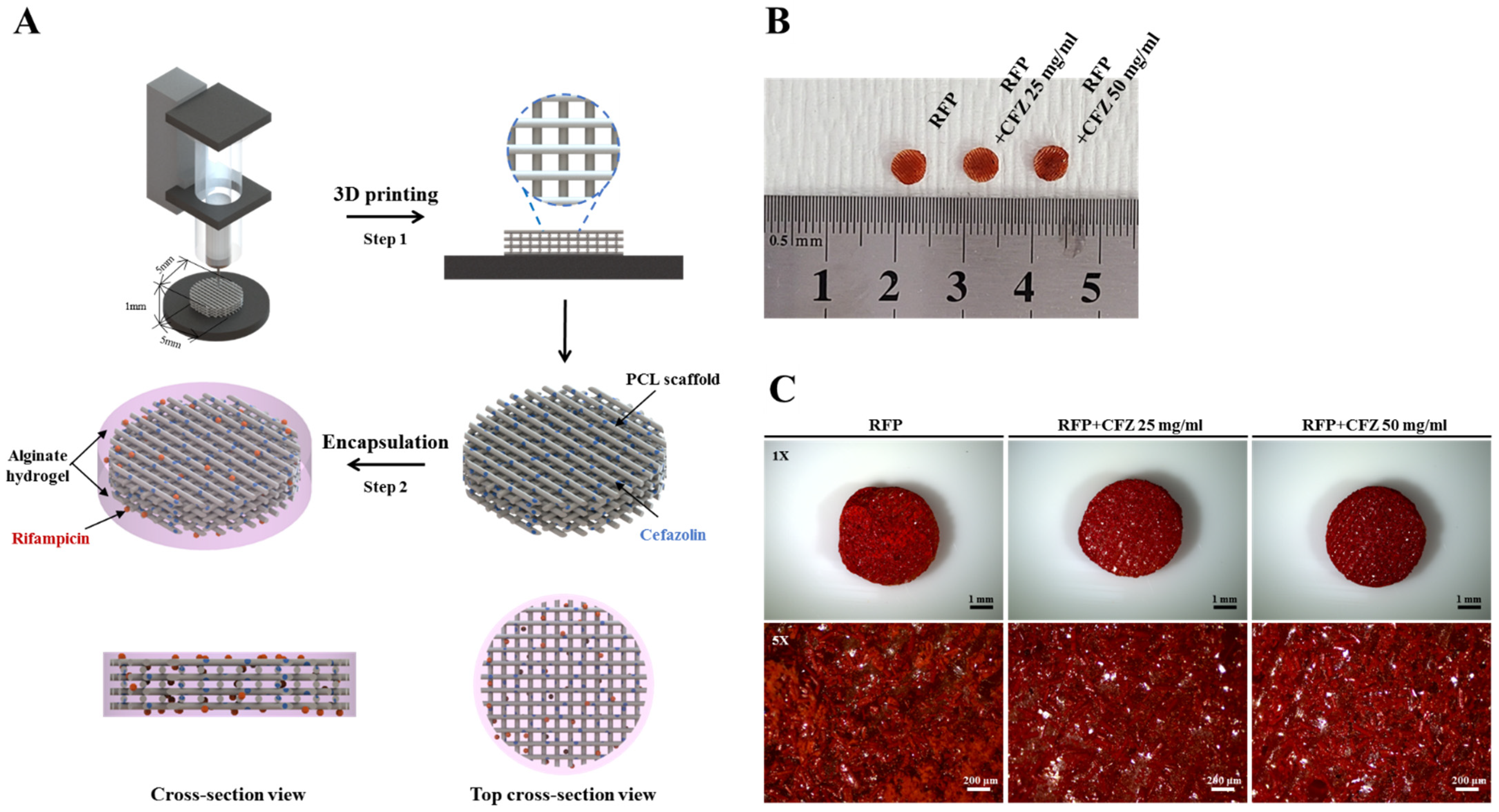
2.1. Characterization of the Dual-Drug-Based Scaffolds
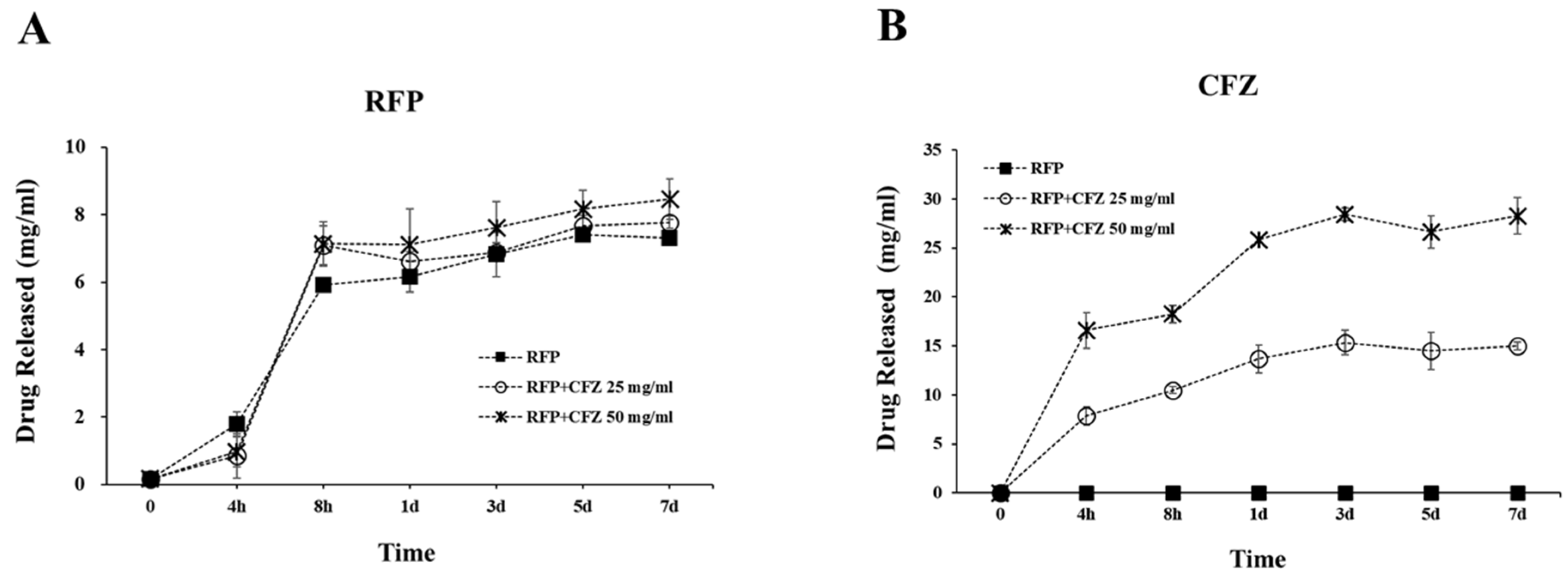
2.2. Release of RFP and CFZ from the Dual-Drug-Based Scaffolds
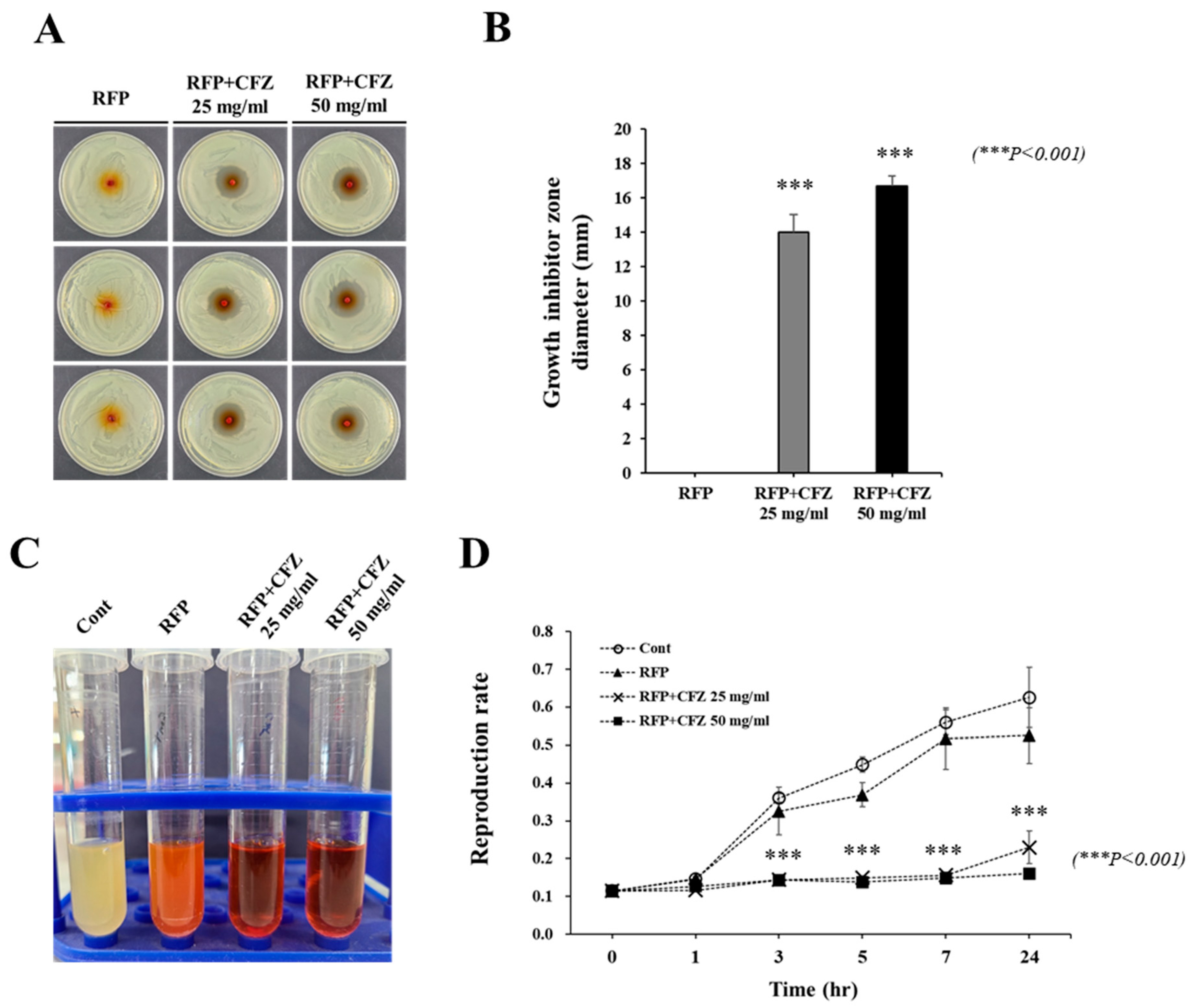
2.3. In Vitro Antibacterial Activity against S. Aureus
2.4. Anti-Biofilm Effect of Dual-Drug-Based Scaffolds
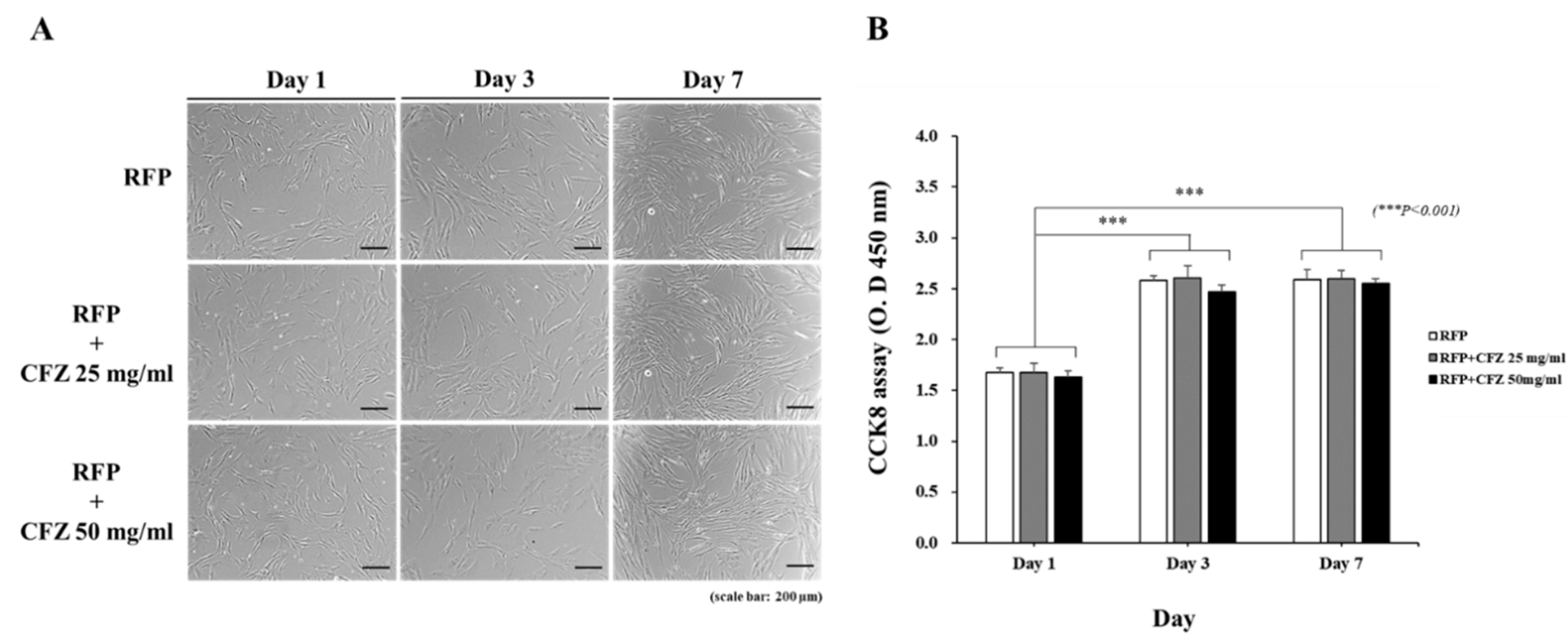
2.5. Analysis of Cell Viability
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Fabrication of Rifampicin- and Cefazolin-Loaded Dual-Drug Scaffolds
5.2. In Vitro Dual Drug Release
5.3. Antibacterial Activity Assay
5.4. Biofilm Formation Assay
5.5. Cell Proliferation Assay
5.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [Green Version]
- Rao, N.; Ziran, B.H.; Lipsky, B.A. Treating osteomyelitis: Antibiotics and surgery. Plast. Reconstr. Surg. 2011, 127 (Suppl. S1), 177s–187s. [Google Scholar] [CrossRef] [PubMed]
- Norden, C.W.; Bryant, R.; Palmer, D.; Montgomerie, J.Z.; Wheat, J. Chronic osteomyelitis caused by Staphylococcus aureus: Controlled clinical trial of nafcillin therapy and nafcillin-rifampin therapy. South. Med. J. 1986, 79, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Norden, C.W.; Fierer, J.; Bryant, R.E. Chronic staphylococcal osteomyelitis: Treatment with regimens containing rifampin. Rev. Infect. Dis. 1983, 5 (Suppl. S3), S495–S501. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.A.; Leid, J.G.; Calhoun, J.H.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 2008, 52, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [Green Version]
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Torigoe, R.; Arata, J. Interaction of Staphylococcus aureus cells and silk threads in vitro and in mouse skin. J. Dermatol. Sci. 1993, 6, 247–257. [Google Scholar] [CrossRef]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Baik, J.M.; Yu, Y.S.; Kim, J.H.; Ahn, C.B.; Son, K.H.; Kim, J.H.; Choi, E.S.; Lee, J.W. Development of a heat labile antibiotic eluting 3D printed scaffold for the treatment of osteomyelitis. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yeo, M.; Ahn, S.; Kang, D.O.; Jang, C.H.; Lee, H.; Park, G.M.; Kim, G.H. Designed hybrid scaffolds consisting of polycaprolactone microstrands and electrospun collagen-nanofibers for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, S.J.; Seok, J.M.; Lee, J.H.; Kim, W.D.; Kwon, I.K. Fabrication of 3D Printed PCL/PEG Polyblend Scaffold Using Rapid Prototyping System for Bone Tissue Engineering Application. J. Bionic Eng. 2018, 15, 435–442. [Google Scholar] [CrossRef]
- Mader, J.T.; Adams, K.; Morrison, L. Comparative evaluation of cefazolin and clindamycin in the treatment of experimental Staphylococcus aureus osteomyelitis in rabbits. Antimicrob. Agents Chemother. 1989, 33, 1760–1764. [Google Scholar] [CrossRef] [Green Version]
- Traub, W.H.; Leonhard, B. Heat stability of the antimicrobial activity of sixty-two antibacterial agents. J. Antimicrob. Chemother. 1995, 35, 149–154. [Google Scholar] [CrossRef]
- Barbu, A.; Neamtu, B.; Zăhan, M.; Iancu, G.M.; Bacila, C.; Mireșan, V. Current Trends in Advanced Alginate-Based Wound Dressings for Chronic Wounds. J. Pers. Med. 2021, 11, 890. [Google Scholar] [CrossRef]
- Liestyo, I. Recent Trends in Alginate, Chitosan and Alginate-Chitosan Antimicrobial Systems. Chem. J. Mold. 2016, 11, 17–25. [Google Scholar] [CrossRef]
- Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef]
- Barbu, A.; Bogdan, N.; Zăhan, M.; Vioara, M. Trends in alginate-based films and membranes for wound healing. Rom. Biotechnol. Lett. 2020, 25, 1683–1689. [Google Scholar] [CrossRef]
- Kim, B.N.; Kim, E.S.; Oh, M.D. Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J. Antimicrob. Chemother. 2014, 69, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.; Piper, K.E.; Rouse, M.S.; Steckelberg, J.M. Linezolid therapy of Staphylococcus aureus experimental osteomyelitis. Antimicrob. Agents Chemother. 2000, 44, 3438–3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monzón, M.; García-Alvarez, F.; Laclériga, A.; Gracia, E.; Leiva, J.; Oteiza, C.; Amorena, B. A simple infection model using pre-colonized implants to reproduce rat chronic Staphylococcus aureus osteomyelitis and study antibiotic treatment. J. Orthop. Res. 2001, 19, 820–826. [Google Scholar] [CrossRef]
- Curtin, J.J.; Donlan, R.M. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2006, 50, 1268–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaneau, M.; Chaby, G.; Guillot, B.; Martel, P.; Senet, P.; Téot, L.; Chosidow, O. Consensus panel recommendations for chronic and acute wound dressings. Arch. Dermatol. 2007, 143, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Sim, C.H.; Ng, S.F. Hydrogels Containing Antibiofilm and Antimicrobial Agents Beneficial for Biofilm-Associated Wound Infection: Formulation Characterizations and In vitro Study. AAPS PharmSciTech 2018, 19, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Madhusudana Rao, K.; Mallikarjuna, B.; Krishna Rao, K.S.; Siraj, S.; Chowdoji Rao, K.; Subha, M.C. Novel thermo/pH sensitive nanogels composed from poly(N-vinylcaprolactam) for controlled release of an anticancer drug. Colloids Surf. B Biointerfaces 2013, 102, 891–897. [Google Scholar] [CrossRef]
- Li, R.W.; Kirkland, N.T.; Truong, J.; Wang, J.; Smith, P.N.; Birbilis, N.; Nisbet, D.R. The influence of biodegradable magnesium alloys on the osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. A 2014, 102, 4346–4357. [Google Scholar] [CrossRef]
- Sivagangi Reddy, N.; Krishna Rao, K.S.V.; Eswaramma, S.; Madhusudana Rao, K. Development of temperature-responsive semi-IPN hydrogels from PVA-PNVC-PAm for controlled release of anti-cancer agent. Soft Mater. 2016, 14, 96–106. [Google Scholar] [CrossRef]
- Rao, K.M.; Sudhakar, P.P.; Rao, K.C.; Subha, M.C.S. Synthesis and Characterization of biodegradable Poly (Vinyl caprolactam) grafted on to sodium alginate and its microgels for controlled release studies of an anticancer drug. J. Appl. Pharm. Sci. 2013, 3, 61–69. [Google Scholar]
- Singh, S.; Alrobaian, M.M.; Molugulu, N.; Agrawal, N.; Numan, A.; Kesharwani, P. Pyramid-Shaped PEG-PCL-PEG Polymeric-Based Model Systems for Site-Specific Drug Delivery of Vancomycin with Enhance Antibacterial Efficacy. ACS Omega 2020, 5, 11935–11945. [Google Scholar] [CrossRef]
- Rao, K.M.; Rao, K.; Ha, C.S. Stimuli Responsive Poly(Vinyl Caprolactam) Gels for Biomedical Applications. Gels 2016, 2, 6. [Google Scholar] [CrossRef]
- Wang, C.; Huang, X.; Deng, W.; Chang, C.; Hang, R.; Tang, B. A nano-silver composite based on the ion-exchange response for the intelligent antibacterial applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 41, 134–141. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- García-Alvarez, R.; Izquierdo-Barba, I.; Vallet-Regí, M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 2017, 49, 113–126. [Google Scholar] [CrossRef]
- Hammer, G.S.; Ribner, B.S.; Meyers, B.R.; Hirschman, S.Z. Clinical studies with cefazolin: A new cephalosporin antibiotic. Mt. Sinai J. Med. 1975, 42, 142–149. [Google Scholar]
- Duewelhenke, N.; Krut, O.; Eysel, P. Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines. Antimicrob. Agents Chemother. 2007, 51, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Bae, Y.M.; Lee, S.Y.; Lee, S.Y. Biofilm Formation of Staphylococcus aureus on Various Surfaces and Their Resistance to Chlorine Sanitizer. J. Food Sci. 2015, 80, M2279–M2286. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Park, J.-K.; Son, K.-H.; Lee, J.-W. PCL/Sodium-Alginate Based 3D-Printed Dual Drug Delivery System with Antibacterial Activity for Osteomyelitis Therapy. Gels 2022, 8, 163. https://doi.org/10.3390/gels8030163
Lee J-H, Park J-K, Son K-H, Lee J-W. PCL/Sodium-Alginate Based 3D-Printed Dual Drug Delivery System with Antibacterial Activity for Osteomyelitis Therapy. Gels. 2022; 8(3):163. https://doi.org/10.3390/gels8030163
Chicago/Turabian StyleLee, Ji-Hyun, Jung-Kyu Park, Kuk-Hui Son, and Jin-Woo Lee. 2022. "PCL/Sodium-Alginate Based 3D-Printed Dual Drug Delivery System with Antibacterial Activity for Osteomyelitis Therapy" Gels 8, no. 3: 163. https://doi.org/10.3390/gels8030163
APA StyleLee, J.-H., Park, J.-K., Son, K.-H., & Lee, J.-W. (2022). PCL/Sodium-Alginate Based 3D-Printed Dual Drug Delivery System with Antibacterial Activity for Osteomyelitis Therapy. Gels, 8(3), 163. https://doi.org/10.3390/gels8030163







