Identification of the Collagen Types Essential for Mammalian Breast Acinar Structures
Abstract
1. Introduction
2. Results
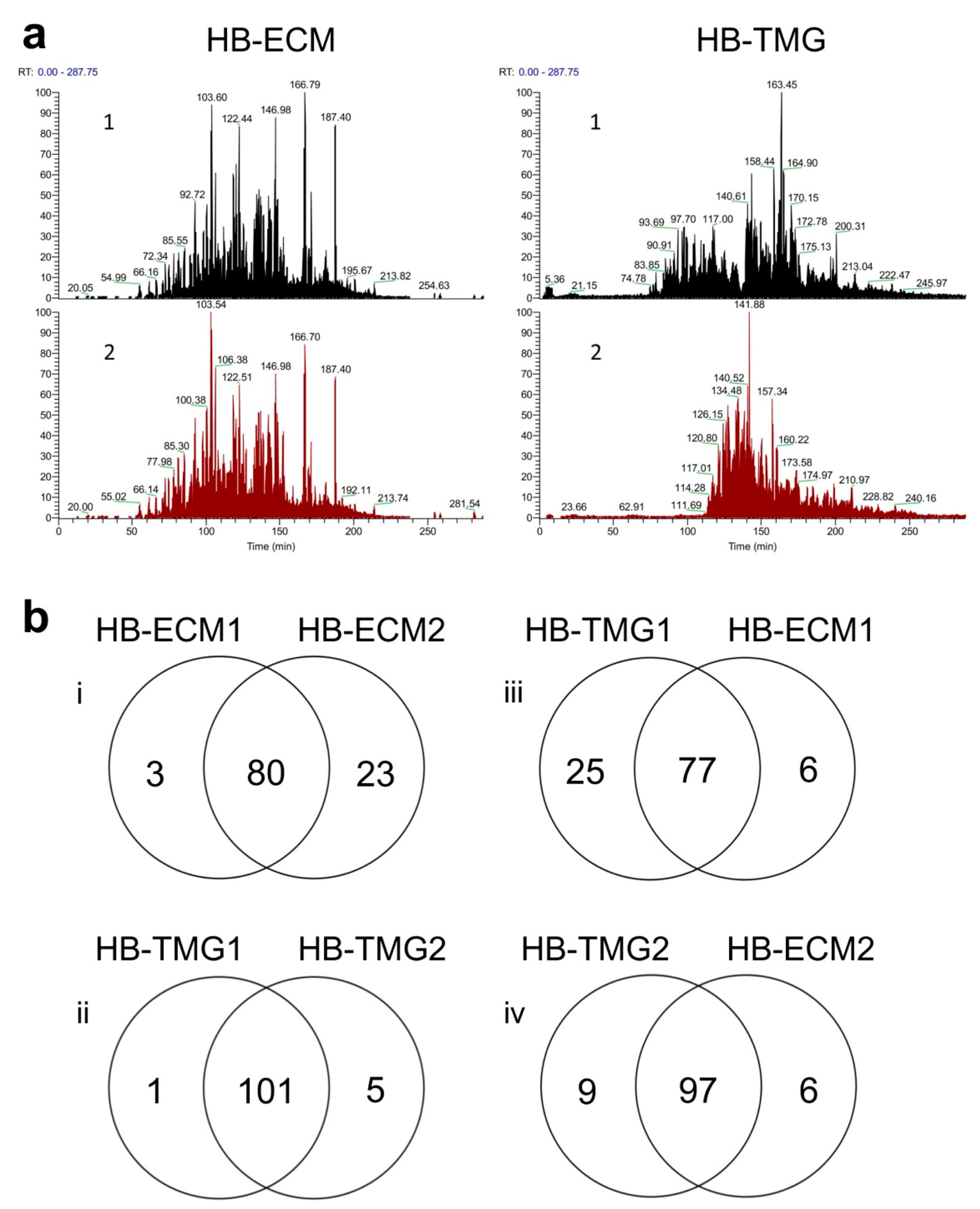
2.1. Proteomic Comparison of Human Breast Tissue ECM and ECM Hydrogel
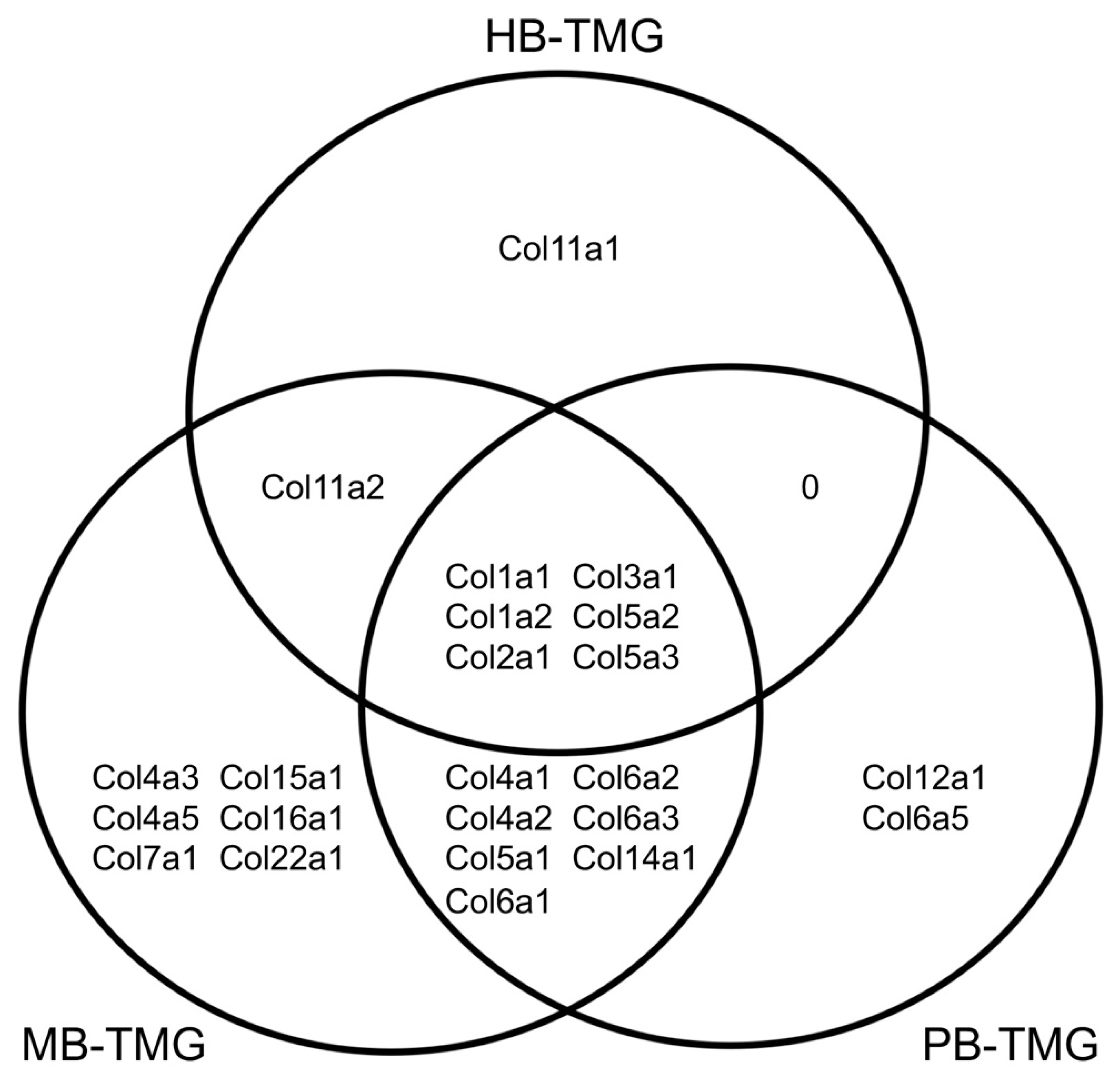
2.2. The Common Collagen Types in Human, Pig and Mouse Breast ECM
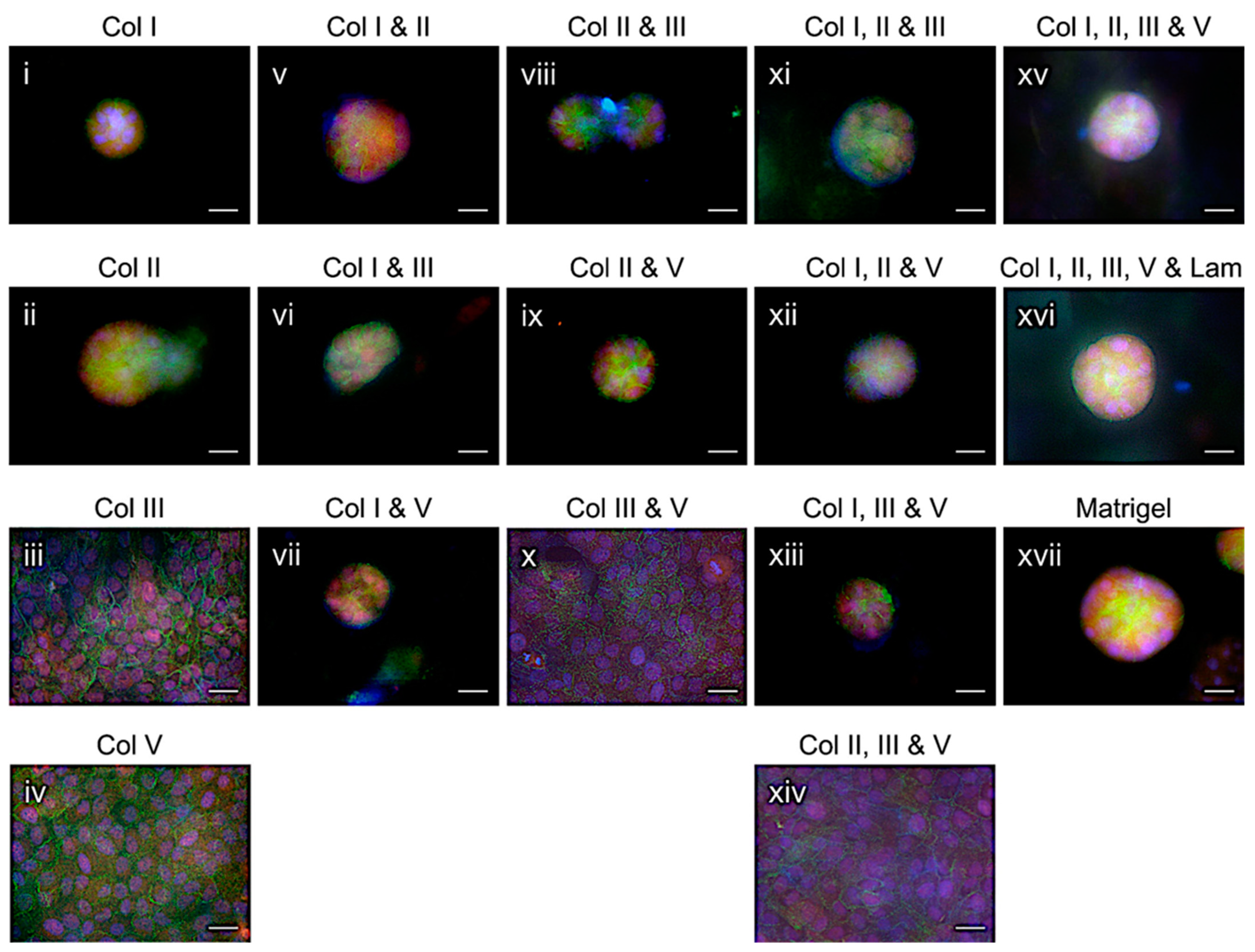
2.3. Mammary Epithelial Acini Formation in Formulated Collagens
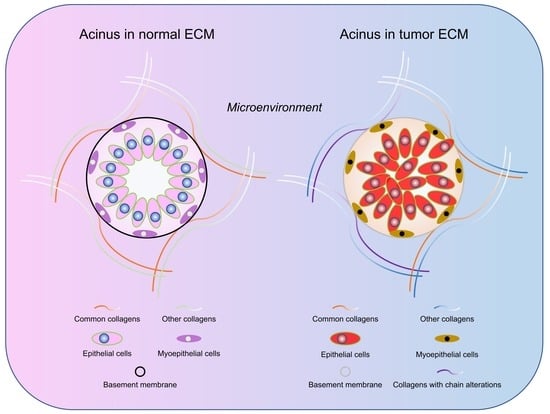
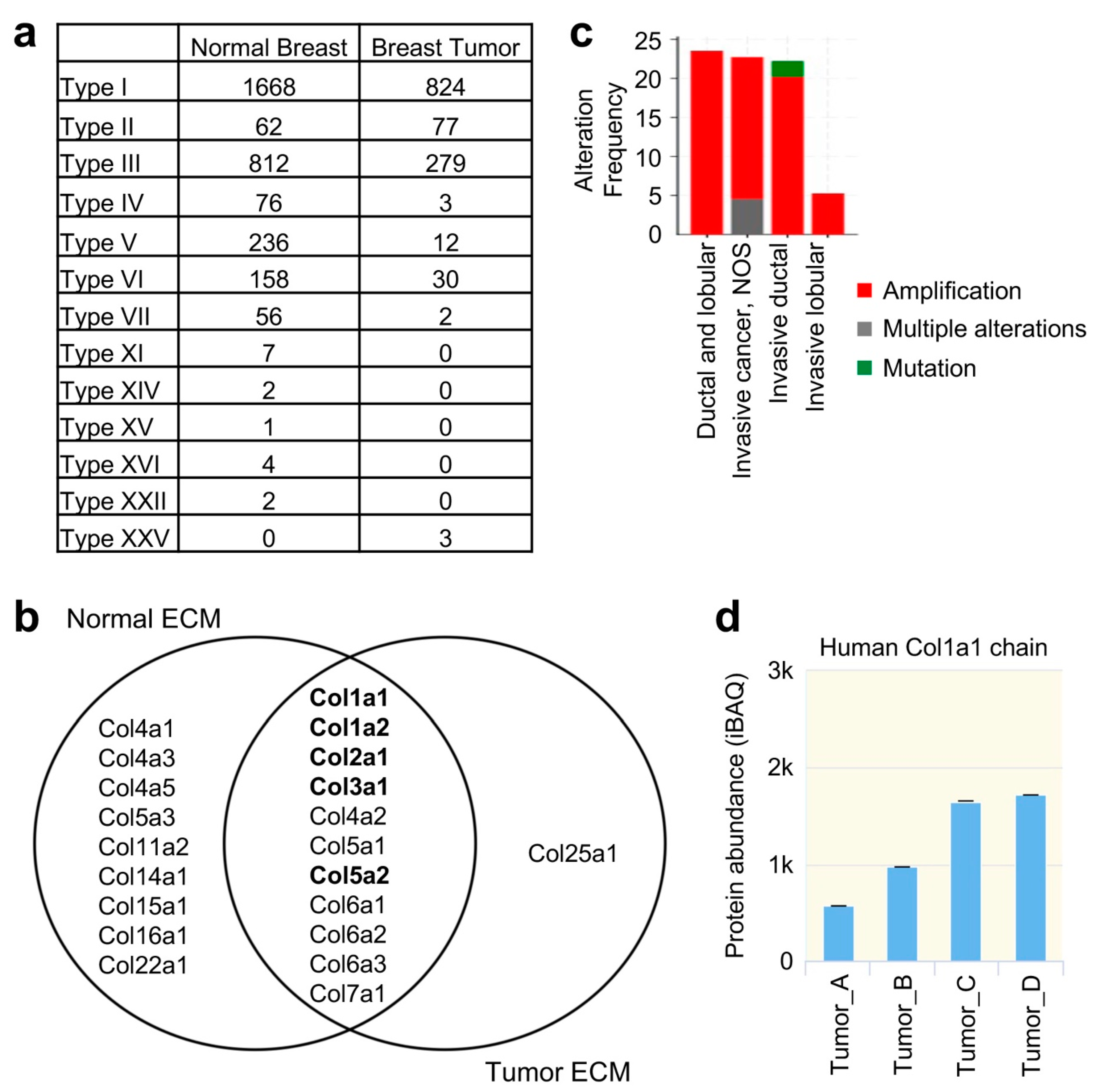
2.4. Differences of Collagen Types in Normal Breast and Breast Tumor Tissue ECM
3. Discussion
4. Materials and Methods
4.1. Patient Specimens
4.2. Human Breast Tissue ECM Extraction
4.3. Hydrogel Generation from Breast Tissue ECM
4.4. Identification of Human Breast Tissue ECM Proteins
4.5. Cell Culture
4.6. Acini Formation
4.7. Immunofluorescence (IF) Staining of FFPE Samples
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316 (Pt 1), 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Hulmes, D.J. Building Collagen Molecules, Fibrils, and Suprafibrillar Structures. J. Struct. Biol. 2002, 137, 2–10. [Google Scholar] [CrossRef]
- Giraud-Guille, M.-M. Twisted Liquid Crystalline Supramolecular Arrangements in Morphogenesis. Int. Rev. Cytol. 1996, 166, 59–101. [Google Scholar] [CrossRef] [PubMed]
- Ruud, K.F.; Hiscox, W.C.; Yu, I.; Chen, R.K.; Li, W. Distinct phenotypes of cancer cells on tissue matrix gel. Breast Cancer Res. 2020, 22, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.R.; Hu, Y.; Ruud, K.F.; VanDeen, A.E.; Martinez, S.R.; Kahn, B.T.; Zhang, Z.; Chen, R.K.; Li, W. Human breast ex-tracellular matrix microstructures and protein hydrogel 3d cultures of mammary epithelial cells. Cancers 2021, 13, 5857. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Wang, J.; Yu, I.; Gang, D.R.; Chen, R.K.; Li, W. Porcine Breast Extracellular Matrix Hydrogel for Spatial Tissue Culture. Int. J. Mol. Sci. 2018, 19, 2912. [Google Scholar] [CrossRef]
- Rijal, G.; Li, W. A versatile 3D tissue matrix scaffold system for tumor modeling and drug screening. Sci. Adv. 2017, 3, e1700764. [Google Scholar] [CrossRef]
- Shinsato, Y.; Doyle, A.D.; Li, W.; Yamada, K.M. Direct comparison of five different 3D extracellular matrix model systems for characterization of cancer cell migration. Cancer Rep. 2020, 3, e1257. [Google Scholar] [CrossRef]
- Orkin, R.W.; Gehron, P.; McGoodwin, E.B.; Martin, G.R.; Valentine, T.; Swarm, R. A murine tumor producing a matrix of basement membrane. J. Exp. Med. 1977, 145, 204–220. [Google Scholar] [CrossRef]
- Gudjonsson, T.; Ronnov-Jessen, L.; Villadsen, R.; Rank, F.; Bissell, M.J.; Petersen, O.W. Normal and tumor-derived myoepi-thelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J. Cell Sci. 2002, 115, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Lteif, A.; Javed, A. Development of the Human Breast. Semin. Plast. Surg. 2013, 27, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tu-mor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Pearce, O.M.T.; Del Rosario, A.; Ma, D.; Ding, H.; Rajeeve, V.; Cutillas, P.R.; Balkwill, F.R.; Hynes, R.O. Charac-terization of the extracellular matrix of normal and diseased tissues using proteomics. J. Proteome Res. 2017, 16, 3083–3091. [Google Scholar] [CrossRef]
- Little, C.D.; Church, R.L.; Miller, R.A.; Ruddle, F.H. Procollagen and collagen produced by a teratocarcinoma-derived cell line, TSD4: Evidence for a new molecular form of collagen. Cell 1977, 10, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Minafra, I.; Luparello, C.; Sciarrino, S.; Tomasino, R.; Minafra, S. Quantitative determination of collagen types present in the ductal infiltrating carcinoma of human mammary gland. Cell Biol. Int. Rep. 1985, 9, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Rupard, J.H.; Dimari, S.J.; Damjanov, I.; A Haralson, M. Synthesis of type I homotrimer collagen molecules by cultured human lung adenocarcinoma cells. Am. J. Pathol. 1988, 133, 316–326. [Google Scholar] [PubMed]
- Han, S.; Makareeva, E.; Kuznetsova, N.V.; DeRidder, A.M.; Sutter, M.B.; Losert, W.; Phillips, C.; Visse, R.; Nagase, H.; Leikin, S. Molecular Mechanism of Type I Collagen Homotrimer Resistance to Mammalian Collagenases. J. Biol. Chem. 2010, 285, 22276–22281. [Google Scholar] [CrossRef]
- Sharma, U.; Carrique, L.; Goff, S.V.-L.; Mariano, N.; Georges, R.-N.; Delolme, F.; Koivunen, P.; Myllyharju, J.; Moali, C.; Aghajari, N.; et al. Structural basis of homo- and heterotrimerization of collagen I. Nat. Commun. 2017, 8, 14671. [Google Scholar] [CrossRef]
- Minafra, S.; Luparello, C.; Rallo, F.; Pucciminafra, I. Collagen biosynthesis by a breast carcinoma cell strain and biopsy fragments of the primary tumour. Cell Biol. Int. Rep. 1988, 12, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Makareeva, E.; Han, S.; Vera, J.C.; Sackett, D.L.; Holmbeck, K.; Phillips, C.L.; Visse, R.; Nagase, H.; Leikin, S. Carcinomas Contain a Matrix Metalloproteinase–Resistant Isoform of Type I Collagen Exerting Selective Support to Invasion. Cancer Res 2010, 70, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Sheterline, P.; Pucci-Minafra, I.; Minafra, S. A comparison of spreading and motility behaviour of 8701-BC breast carcinoma cells on type I, I-trimer and type V collagen substrata. Evidence for a permissive effect of type I-trimer collagen on cell locomotion. J. Cell Sci. 1991, 100, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Li, W. 3D scaffolds in breast cancer research. Biomaterials 2016, 81, 135–156. [Google Scholar] [CrossRef]
- Bella, J.; Hulmes, D.J. Fibrillar collagens. Struct. Mech. 2017, 82, 457–490. [Google Scholar]
- Slepicka, P.F.; Somasundara, A.V.H.; dos Santos, C.O. The molecular basis of mammary gland development and epithelial differentiation. Semin. Cell Dev. Biol. 2020, 114, 93–112. [Google Scholar] [CrossRef]
- Swarm, R.L. Transplantation of a Murine Chondrosarcoma in Mice of Different Inbred Strains. J. Natl. Cancer Inst. 1963, 31, 953–975. [Google Scholar] [CrossRef]
- Futaki, S.; Hayashi, Y.; Yamashita, M.; Yagi, K.; Bono, H.; Hayashizaki, Y.; Okazaki, Y.; Sekiguchi, K. Molecular basis of con-stitutive production of basement membrane components. Gene expression profiles of engelbreth-holm-swarm tumor and f9 embryonal carcinoma cells. J. Biol. Chem. 2003, 278, 50691–50701. [Google Scholar] [CrossRef]
- Englund, J.I.; Ritchie, A.; Blaas, L.; Cojoc, H.; Pentinmikko, N.; Döhla, J.; Iqbal, S.; Patarroyo, M.; Katajisto, P. Laminin alpha 5 regulates mammary gland remodeling through luminal cell differentiation and Wnt4-mediated epithelial crosstalk. Development 2021, 148. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Insua-Rodríguez, J.; Oskarsson, T. The extracellular matrix in breast cancer. Adv. Drug Deliv. Rev. 2016, 97, 41–55. [Google Scholar] [CrossRef]
- Bodelon, C.; Mullooly, M.; Pfeiffer, R.M.; Fan, S.; Abubakar, M.; Lenz, P.; Vacek, P.M.; Weaver, D.L.; Herschorn, S.D.; Johnson, J.M.; et al. Mammary collagen architecture and its association with mammographic density and lesion severity among women undergoing image-guided breast biopsy. Breast Cancer Res. 2021, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.D.; Carvajal, N.; Jin, A.; Matsumoto, K.; Yamada, K.M. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 2015, 6, 8720. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Ng, M.; Brugge, J.S. A Stiff Blow from the Stroma: Collagen Crosslinking Drives Tumor Progression. Cancer Cell 2009, 16, 455–457. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Lyons, T.; Monks, J.; Lucia, M.S.; Wilson, R.S.; Hines, L.; Man, Y.-G.; Borges, V.; Schedin, P. Alternatively Activated Macrophages and Collagen Remodeling Characterize the Postpartum Involuting Mammary Gland across Species. Am. J. Pathol. 2010, 176, 1241–1255. [Google Scholar] [CrossRef]
- Liu, J.; Shen, J.-X.; Wu, H.-T.; Li, X.-L.; Wen, X.-F.; Du, C.-W.; Zhang, G.-J. Collagen 1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discov. Med. 2018, 25, 211–223. [Google Scholar]
- Ma, H.-P.; Chang, H.-L.; Bamodu, O.A.; Yadav, V.K.; Huang, T.-Y.; Wu, A.T.H.; Yeh, C.-T.; Tsai, S.-H.; Lee, W.-H. Collagen 1A1 (COL1A1) Is a Reliable Biomarker and Putative Therapeutic Target for Hepatocellular Carcinogenesis and Metastasis. Cancers 2019, 11, 786. [Google Scholar] [CrossRef]
- Bergamaschi, A.; Tagliabue, E.; Sørlie, T.; Naume, B.; Triulzi, T.; Orlandi, R.; Russnes, H.G.; Nesland, J.M.; Tammi, R.; Auvinen, P.; et al. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J. Pathol. 2007, 214, 357–367. [Google Scholar] [CrossRef]
- Inman, J.L.; Robertson, C.; Mott, J.D.; Bissell, M.J. Mammary gland development: Cell fate specification, stem cells and the microenvironment. Development 2015, 142, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Al Hrout, A.; Cervantes-Gracia, K.; Chahwan, R.; Amin, A. Modelling liver cancer microenvironment using a novel 3D culture system. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, C.R.; Ruud, K.F.; Martinez, S.R.; Li, W. Identification of the Collagen Types Essential for Mammalian Breast Acinar Structures. Gels 2022, 8, 837. https://doi.org/10.3390/gels8120837
Keller CR, Ruud KF, Martinez SR, Li W. Identification of the Collagen Types Essential for Mammalian Breast Acinar Structures. Gels. 2022; 8(12):837. https://doi.org/10.3390/gels8120837
Chicago/Turabian StyleKeller, Chandler R., Kelsey F. Ruud, Steve R. Martinez, and Weimin Li. 2022. "Identification of the Collagen Types Essential for Mammalian Breast Acinar Structures" Gels 8, no. 12: 837. https://doi.org/10.3390/gels8120837
APA StyleKeller, C. R., Ruud, K. F., Martinez, S. R., & Li, W. (2022). Identification of the Collagen Types Essential for Mammalian Breast Acinar Structures. Gels, 8(12), 837. https://doi.org/10.3390/gels8120837