Mechanisms of Basement Membrane Micro-Perforation during Cancer Cell Invasion into a 3D Collagen Gel
Abstract
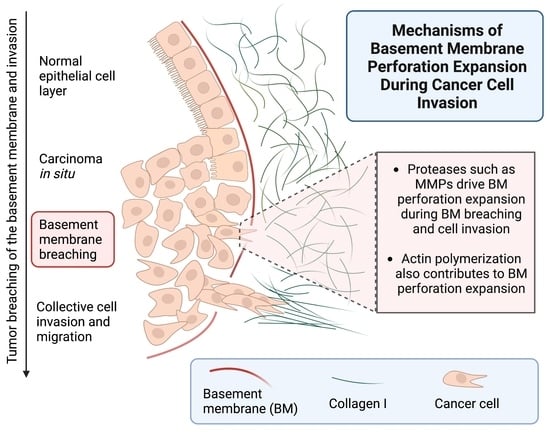
1. Introduction
2. Results and Discussion
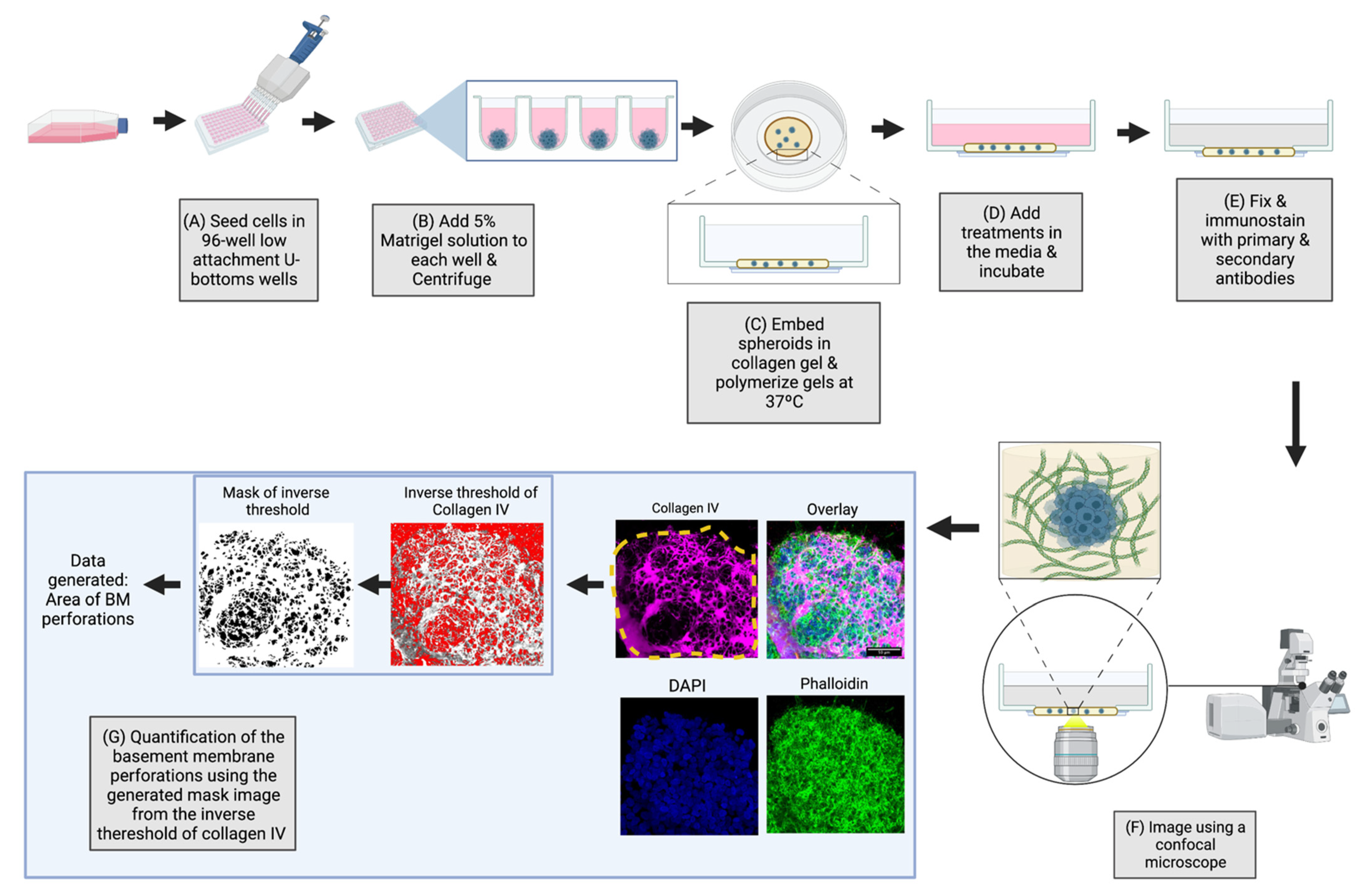
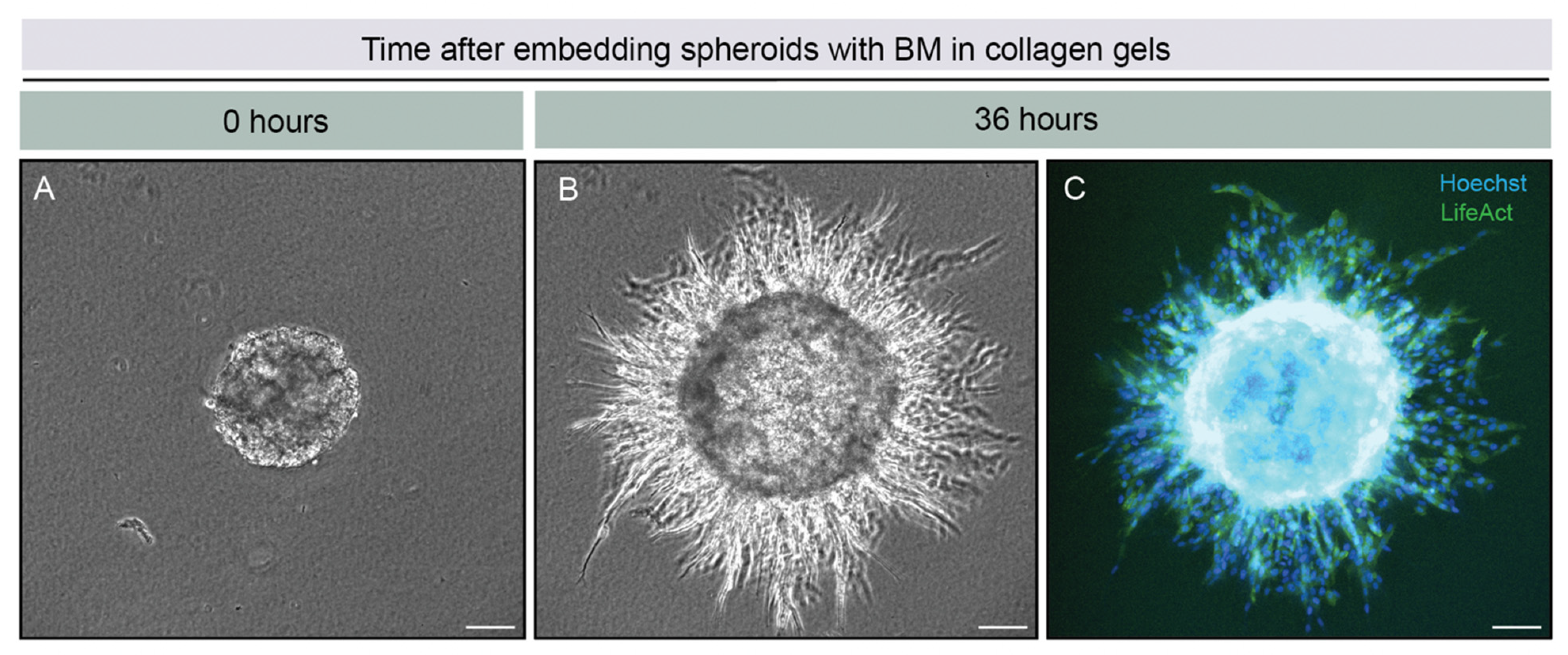
2.1. Overview of the 3D-Spheroid Model
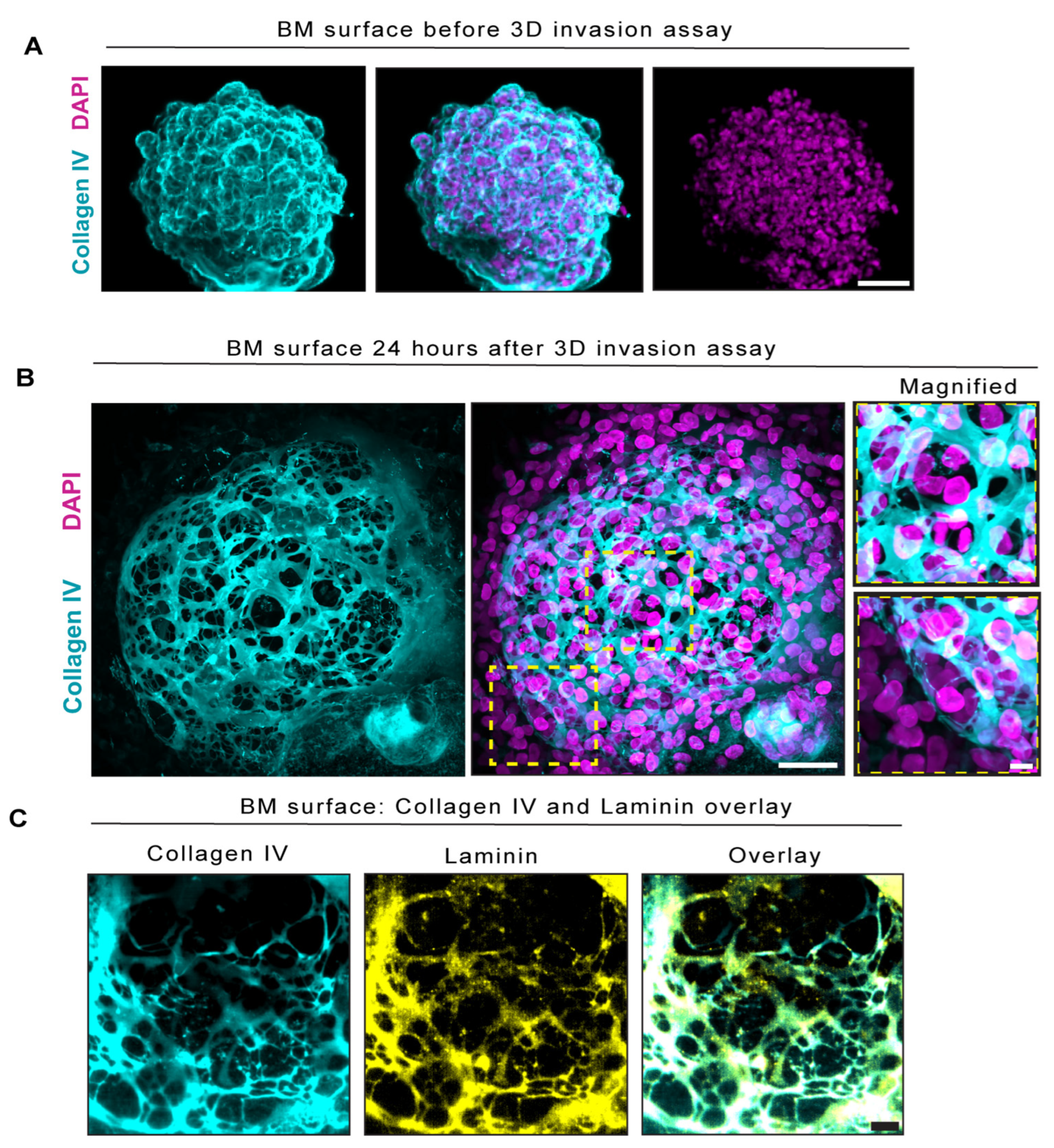
2.2. BM Encapsulated Spheroids before Invasion and Became Extensively Perforated in Our Three-Dimensional In Vitro Invasion Assay
2.3. BM Perforations Expanded over Time as Cancer Cells Initiated Invasion through the BM
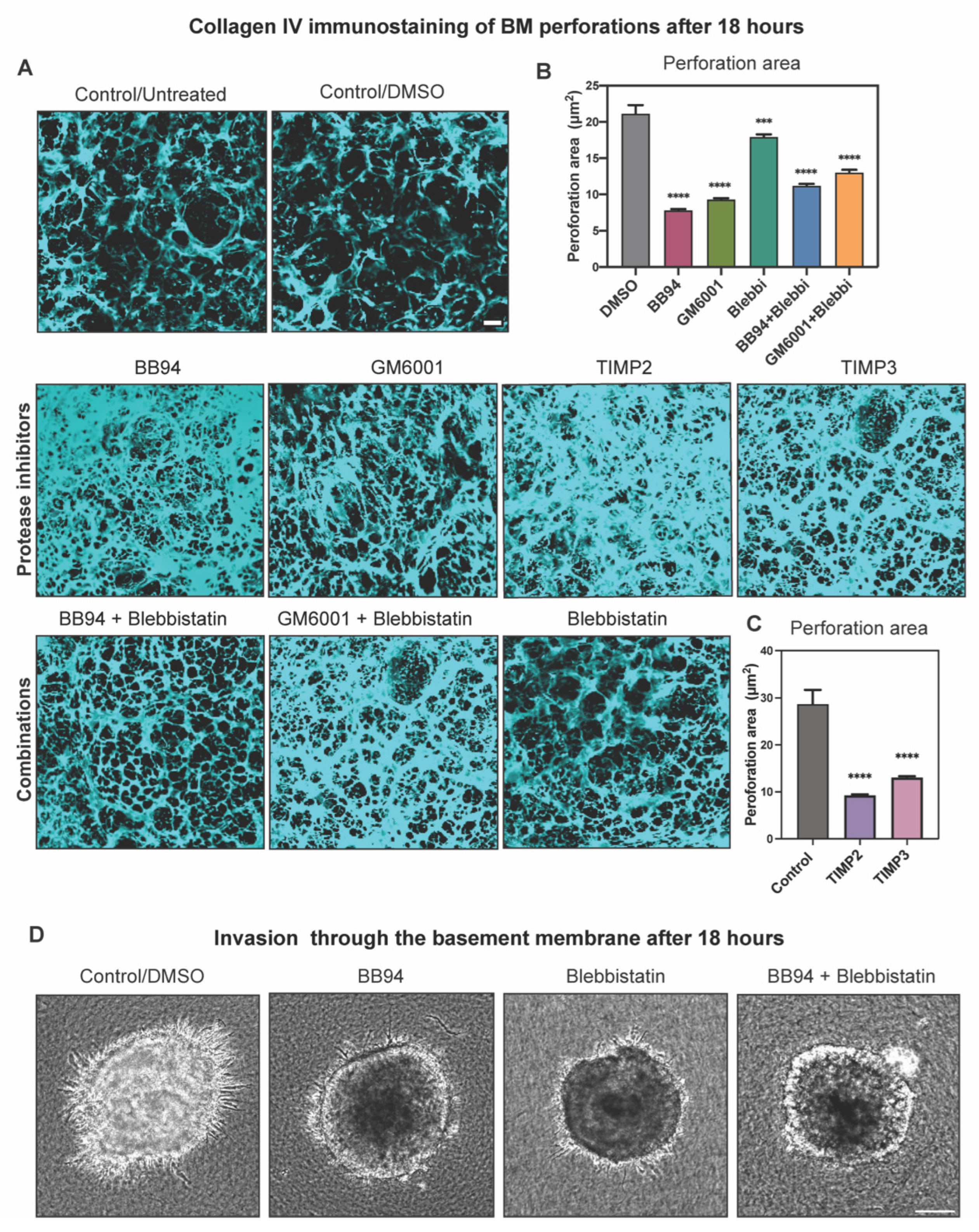
2.4. Protease Activity Played a More Important Role Than Actomyosin Contractility in Perforating the BM during Cancer Cell Invasion
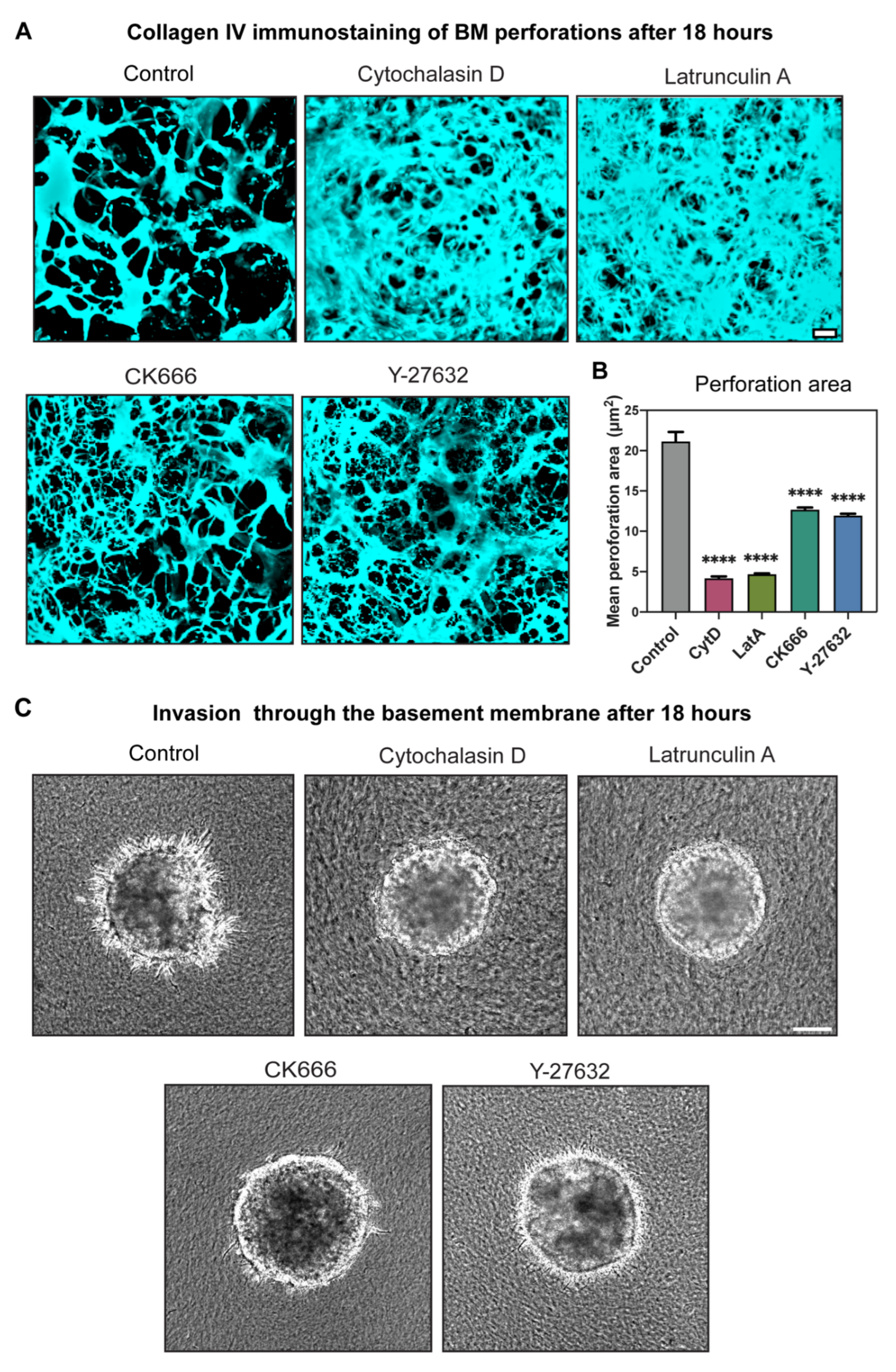
2.5. Actin Polymerization Dramatically Affected Hole Formation and Acted as a Third Mechanism Contributing to Perforation of the BM
2.6. Comparisons with Other Cell Types Confirmed Findings for Another Metastatic Cell but Not for a Non-Metastatic Cell Line
3. Conclusions
4. Materials and Methods
4.1. Cell Culture and Media
3-Dimensional Spheroid Cell Culture
4.2. Inhibitors
4.3. Immunostaining
4.4. Confocal Imaging
4.5. Live Cell Imaging
4.6. Perforation Area Analysis
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Jayadev, R.; Sherwood, D.R. Basement membranes. Curr. Biol. 2017, 27, R207–R211. [Google Scholar] [CrossRef]
- Chang, J.; Chaudhuri, O. Beyond proteases: Basement membrane mechanics and cancer invasion. J. Cell Biol. 2019, 218, 2456–2469. [Google Scholar] [CrossRef]
- Sekiguchi, R.; Yamada, K.M. Basement Membranes in Development and Disease. Curr. Top. Dev. Biol. 2018, 130, 143–191. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.C.; Chi, Q.; Caceres, R.; Hastie, E.; Schindler, A.J.; Jiang, Y.; Matus, D.Q.; Plastino, J.; Sherwood, D.R. Adaptive F-Actin Polymerization and Localized ATP Production Drive Basement Membrane Invasion in the Absence of MMPs. Dev. Cell 2019, 48, 313–328.e318. [Google Scholar] [CrossRef]
- Gaiko-Shcherbak, A.; Fabris, G.; Dreissen, G.; Merkel, R.; Hoffmann, B.; Noetzel, E. The Acinar Cage: Basement Membranes Determine Molecule Exchange and Mechanical Stability of Human Breast Cell Acini. PLoS ONE 2015, 10, e0145174. [Google Scholar] [CrossRef]
- Eddy, R.J.; Weidmann, M.D.; Sharma, V.P.; Condeelis, J.S. Tumor Cell Invadopodia: Invasive Protrusions that Orchestrate Metastasis. Trends Cell Biol. 2017, 27, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Martin, G.; Tagit, O.; Guichard, A.; Cambi, A.; Voituriez, R.; Vassilopoulos, S.; Chavrier, P. MT1-MMP directs force-producing proteolytic contacts that drive tumor cell invasion. Nat. Commun. 2019, 10, 4886. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Courtneidge, S.A. The ‘ins’ and ‘outs’ of podosomes and invadopodia: Characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 2011, 12, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Linder, S.; Wiesner, C.; Himmel, M. Degrading devices: Invadosomes in proteolytic cell invasion. Annu. Rev. Cell Dev. Biol. 2011, 27, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. J. Int. Soc. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef]
- Wisdom, K.M.; Indana, D.; Chou, P.E.; Desai, R.; Kim, T.; Chaudhuri, O. Covalent cross-linking of basement membrane-like matrices physically restricts invasive protrusions in breast cancer cells. Matrix Biol. J. Int. Soc. Matrix Biol. 2020, 85–86, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Havenith, M.; Cleutjens, J.P. Basement membranes in cancer. Ultrastruct. Pathol. 1985, 8, 291–304. [Google Scholar] [CrossRef]
- Nazari, S.S. Generation of 3D Tumor Spheroids with Encapsulating Basement Membranes for Invasion Studies. Curr. Protoc. Cell Biol. 2020, 87, e105. [Google Scholar] [CrossRef] [PubMed]
- Glentis, A.; Oertle, P.; Mariani, P.; Chikina, A.; El Marjou, F.; Attieh, Y.; Zaccarini, F.; Lae, M.; Loew, D.; Dingli, F.; et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 2017, 8, 924. [Google Scholar] [CrossRef]
- Sirka, O.K.; Shamir, E.R.; Ewald, A.J. Myoepithelial cells are a dynamic barrier to epithelial dissemination. J. Cell Biol. 2018, 217, 3368–3381. [Google Scholar] [CrossRef]
- Bahr, J.C.; Weiss, S.J. Macrophage-Dependent Trafficking and Remodeling of the Basement Membrane-Interstitial Matrix Interface. bioRxiv 2018. [Google Scholar] [CrossRef]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef]
- Harunaga, J.S.; Doyle, A.D.; Yamada, K.M. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev. Biol. 2014, 394, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Kyprianou, C.; Christodoulou, N.; Hamilton, R.S.; Nahaboo, W.; Boomgaard, D.S.; Amadei, G.; Migeotte, I.; Zernicka-Goetz, M. Basement membrane remodelling regulates mouse embryogenesis. Nature 2020, 582, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Kaufman, P.L. Comparisons of actin filament disruptors and Rho kinase inhibitors as potential antiglaucoma medications. Expert Rev. Ophthalmol. 2012, 7, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006, 16, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Williams, P.J.; Hiraga, T.; Niewolna, M.; Nishimura, R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Min. Res 2001, 16, 1486–1495. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazari, S.S.; Doyle, A.D.; Yamada, K.M. Mechanisms of Basement Membrane Micro-Perforation during Cancer Cell Invasion into a 3D Collagen Gel. Gels 2022, 8, 567. https://doi.org/10.3390/gels8090567
Nazari SS, Doyle AD, Yamada KM. Mechanisms of Basement Membrane Micro-Perforation during Cancer Cell Invasion into a 3D Collagen Gel. Gels. 2022; 8(9):567. https://doi.org/10.3390/gels8090567
Chicago/Turabian StyleNazari, Shayan S., Andrew D. Doyle, and Kenneth M. Yamada. 2022. "Mechanisms of Basement Membrane Micro-Perforation during Cancer Cell Invasion into a 3D Collagen Gel" Gels 8, no. 9: 567. https://doi.org/10.3390/gels8090567
APA StyleNazari, S. S., Doyle, A. D., & Yamada, K. M. (2022). Mechanisms of Basement Membrane Micro-Perforation during Cancer Cell Invasion into a 3D Collagen Gel. Gels, 8(9), 567. https://doi.org/10.3390/gels8090567