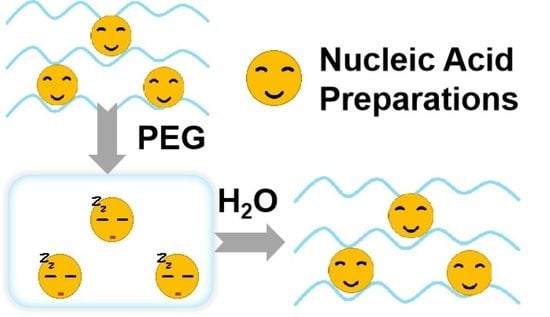
PEG Gels Significantly Improve the Storage Stability of Nucleic Acid Preparations
Abstract
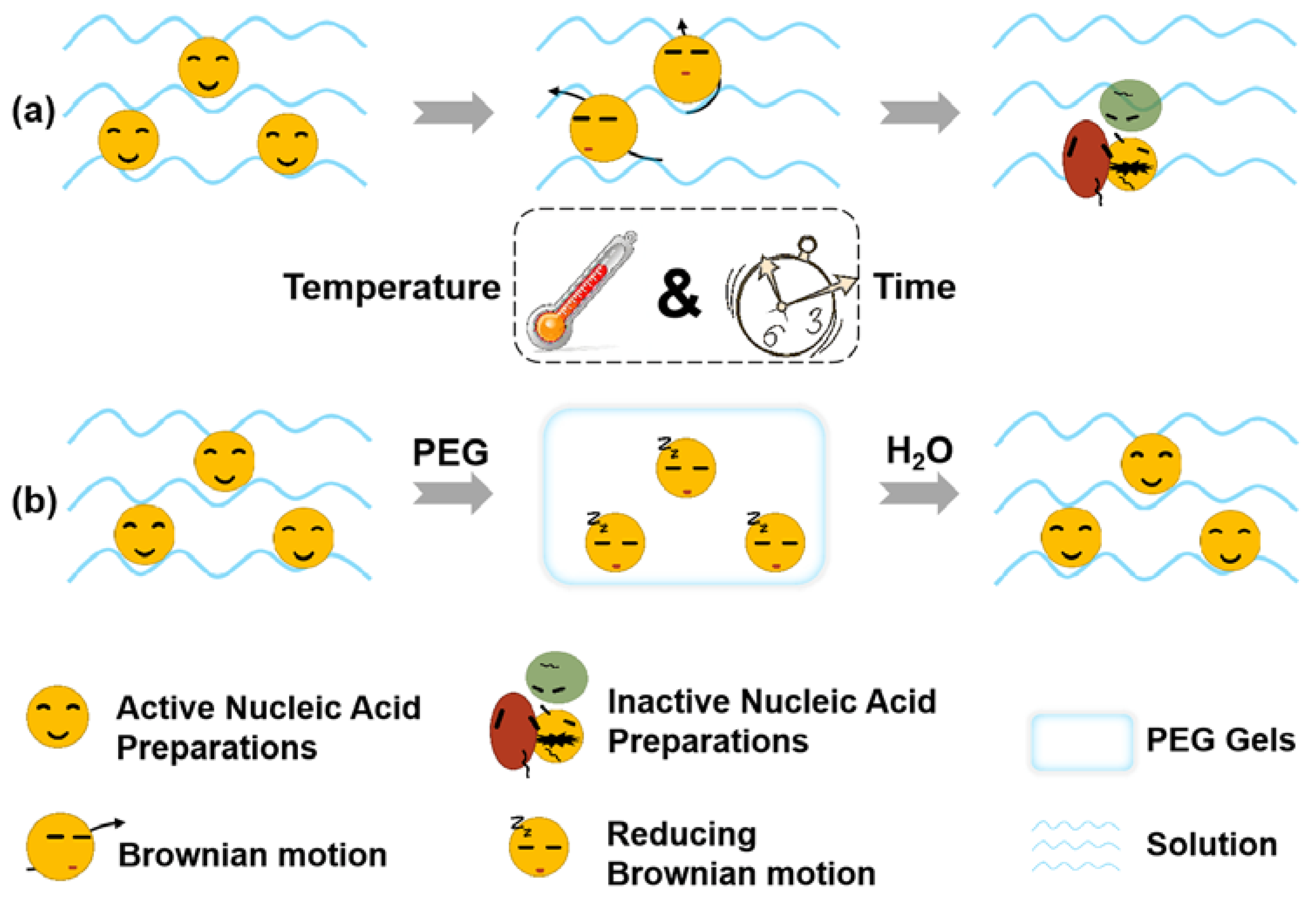
1. Introduction
2. Results and Discussion
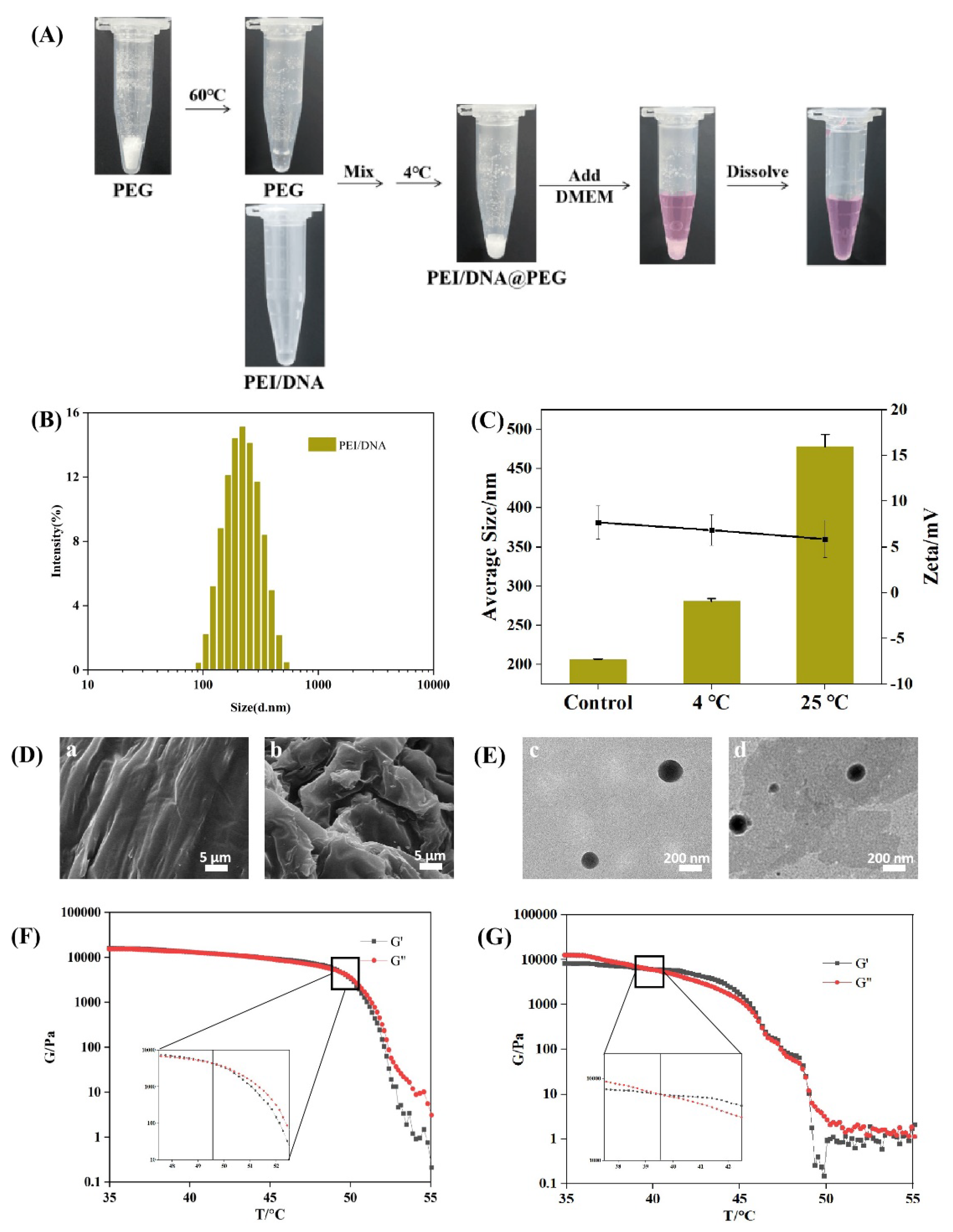
2.1. Synthesis of PEI/DNA@PEG Systems
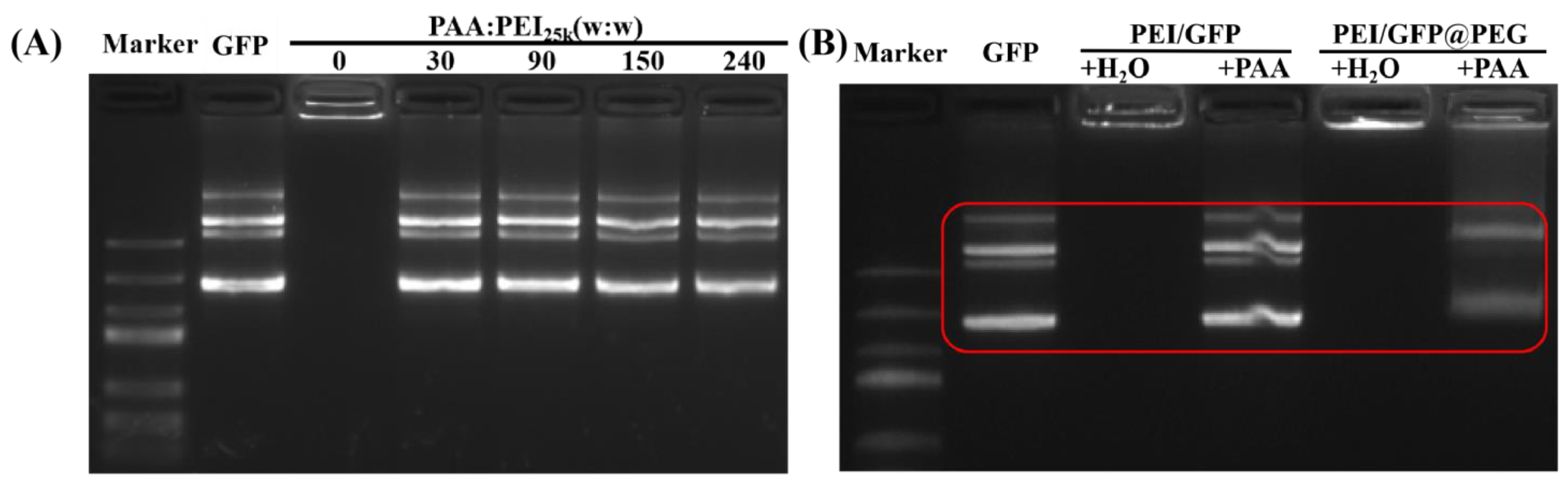
2.2. Combination of PAA and PEI in PEG System
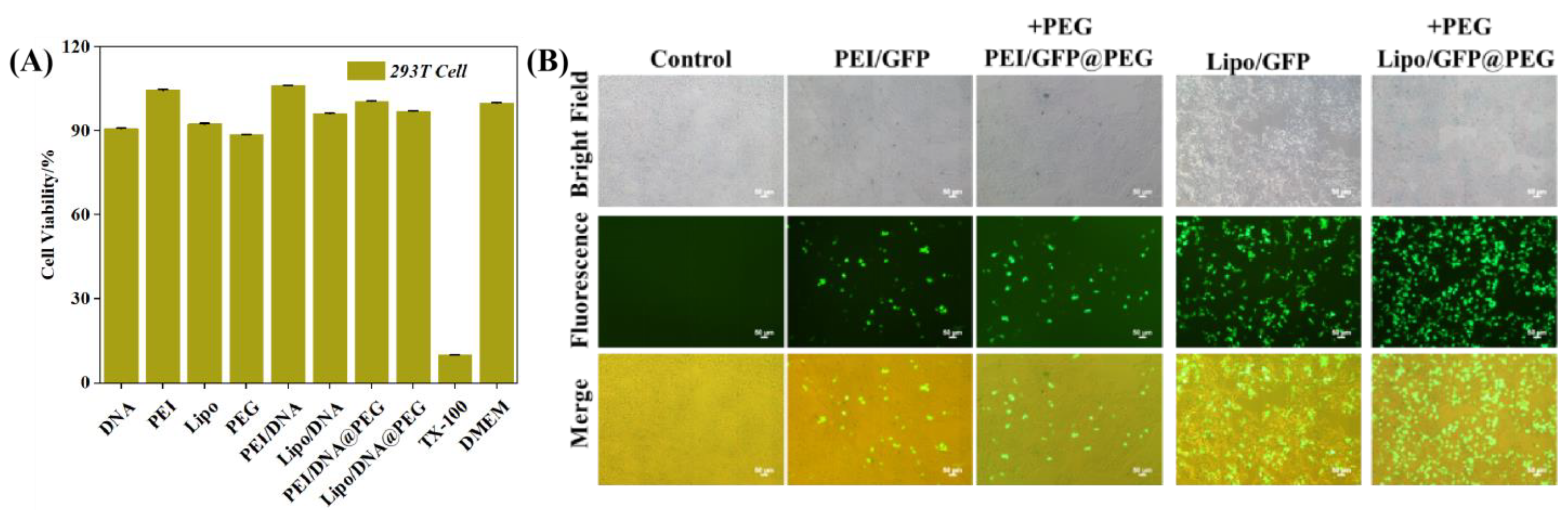
2.3. In Vitro Safety Assay of PEI/DNA@PEG and Lipo/DNA@PEG
2.4. Transfection Experiments of the System
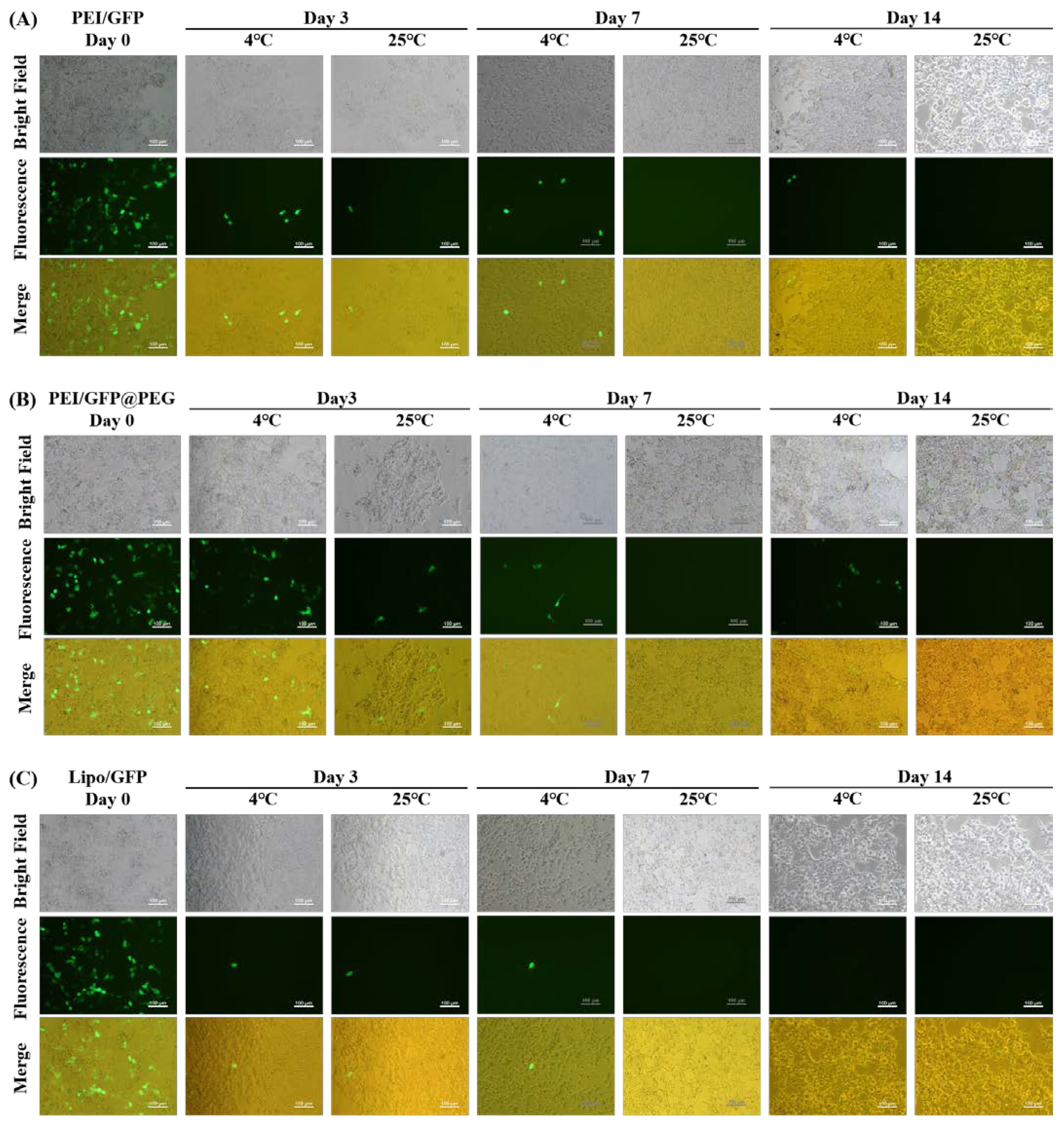
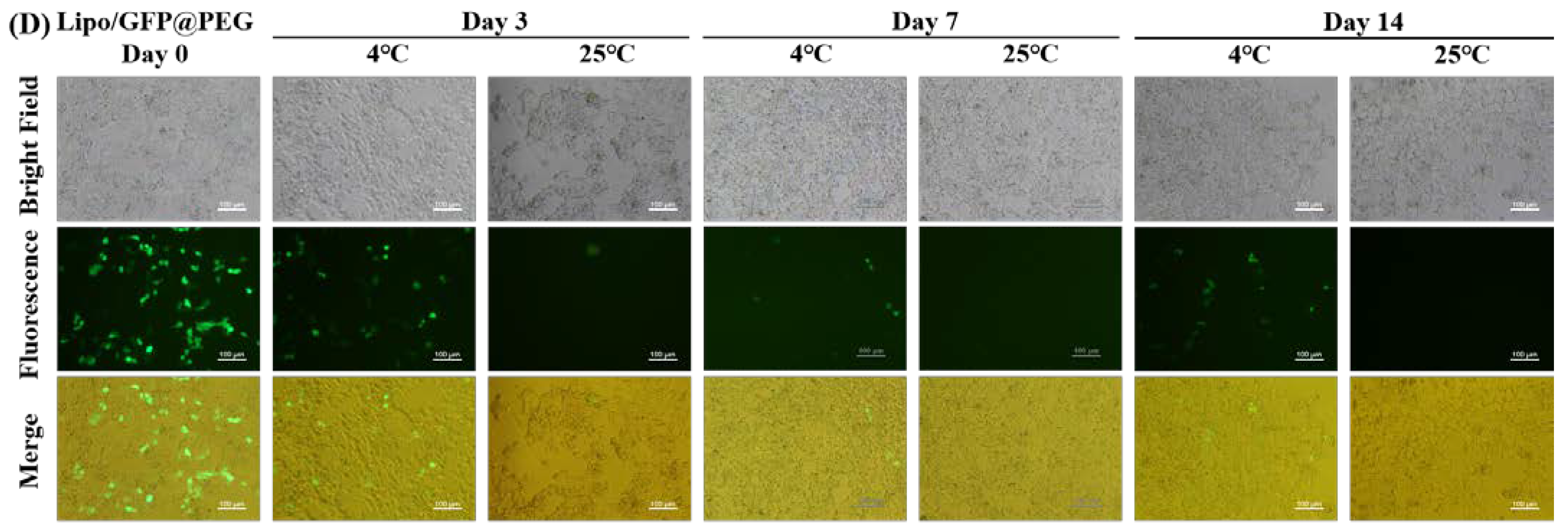
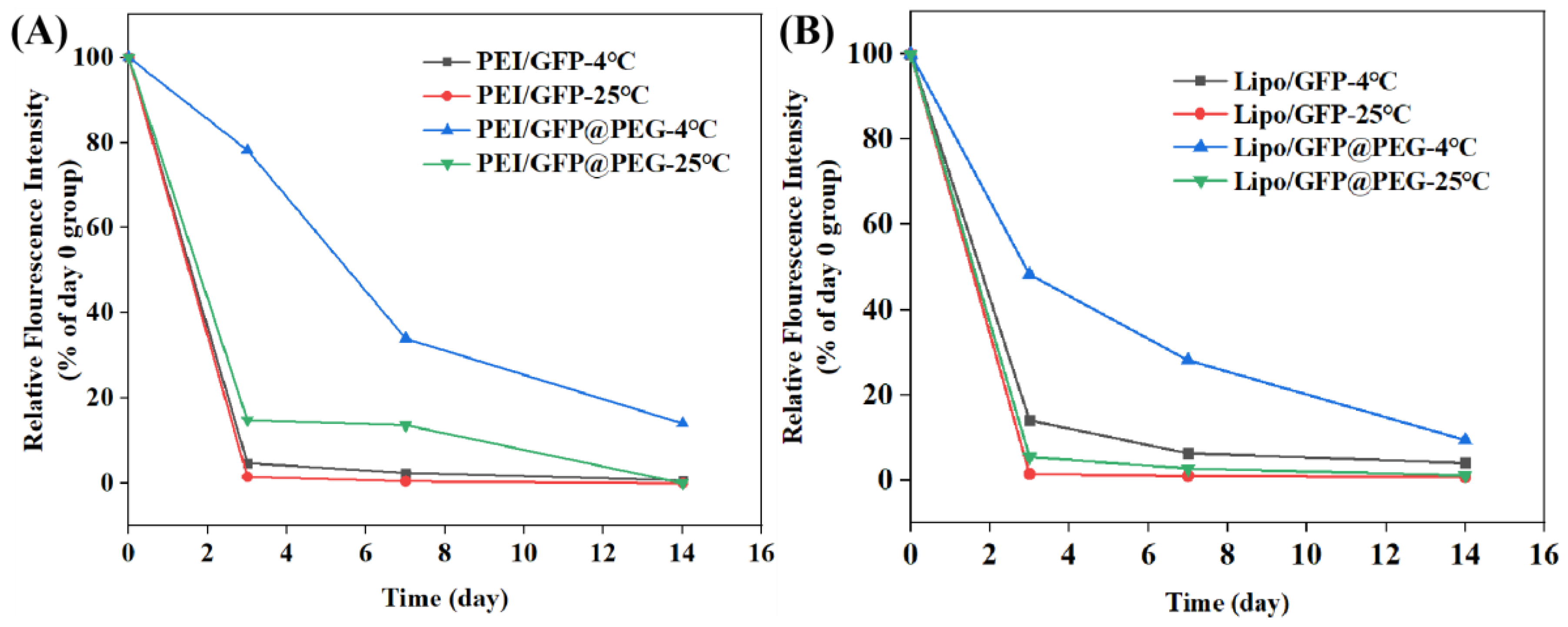
2.5. Long—Term Transfection Experiments
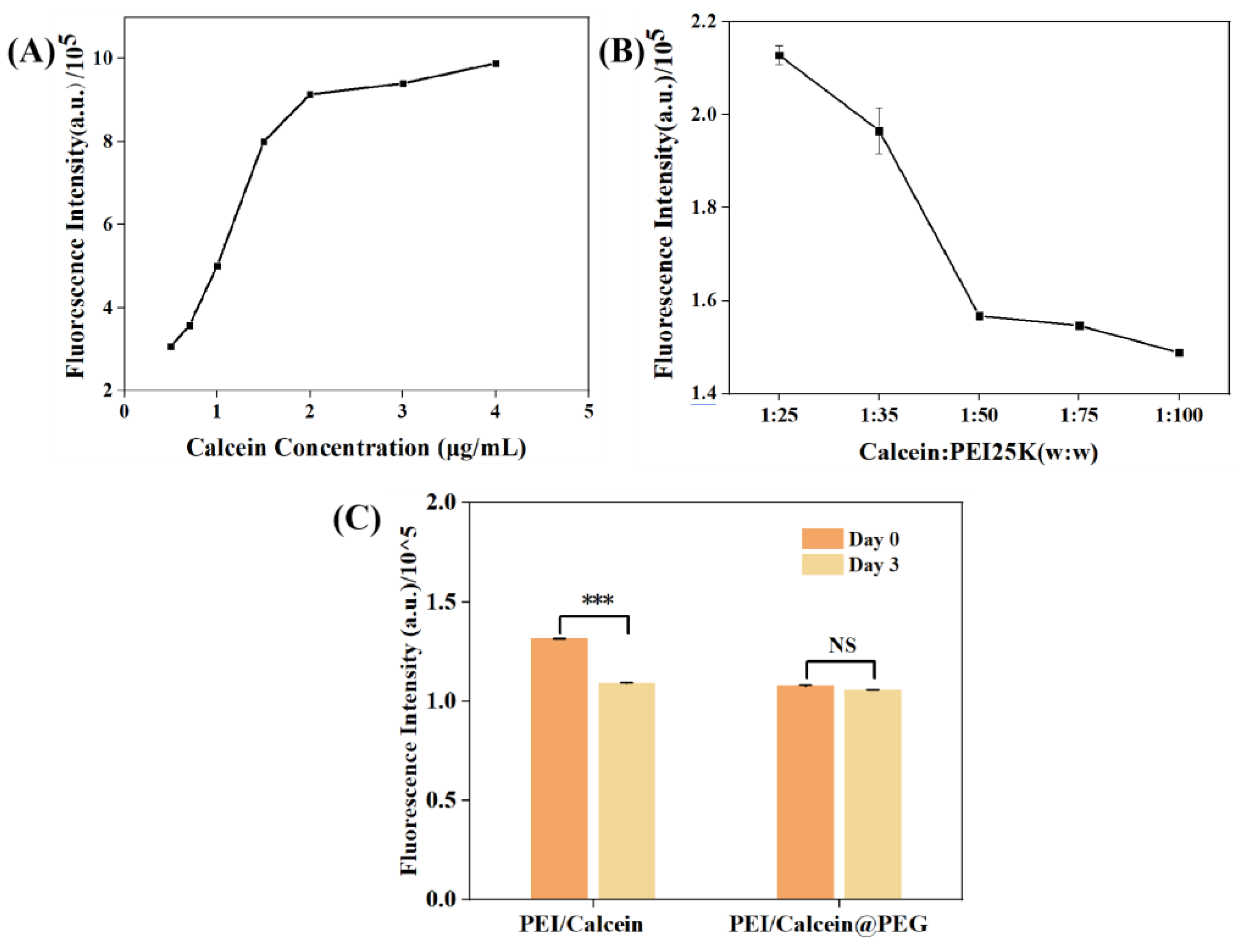
2.6. Combination of Calcein and PEI in PEG System
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of PEI/DNA@PEG
4.3. Combination of PAA and PEI in PEG System
4.4. In Vitro Safety Assay of PEI/DNA@PEG and Lipo/DNA@PEG
4.5. Transfection Experiments of the System
4.6. Long−Term Transfection Experiments
4.7. Combination of Calcein and PEI in PEG System
Author Contributions
Funding
Conflicts of Interest
References
- Hanna, E.; Remuzat, C.; Auquier, P.; Toumi, M. Gene therapies development: Slow progress and promising prospect. J. Mark. Access Health Policy 2017, 5, 1265293. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, H.; Patel, M.A.; Gupta, V.; Bansal, T.; Upadhyay, S.; Shaheen, N.; Jain, R. COVID−19 Vaccine Challenges in Developing and Developed Countries. Cureus 2022, 14, e23951. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid−19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Ladak, R.J.; He, A.J.; Huang, Y.H.; Ding, Y. The Current Landscape of mRNA Vaccines Against Viruses and Cancer−A Mini Review. Front. Immunol. 2022, 13, 885371. [Google Scholar] [CrossRef]
- Li, W.J.; Wu, L.L.; Huang, C.; Liu, R.X.; Li, Z.; Liu, L.H.; Shan, B.E. Challenges and strategies of clinical application of CAR−T therapy in the treatment of tumors−a narrative review. Ann. Transl. Med. 2020, 8, 1093. [Google Scholar] [CrossRef]
- Sutherland, A.R.; Owens, M.N.; Geyer, C.R. Modular Chimeric Antigen Receptor Systems for Universal CAR T Cell Retargeting. Int. J. Mol. Sci. 2020, 21, 7222. [Google Scholar] [CrossRef]
- Meng, Y.; Deng, B.P.; Rong, L.; Li, C.; Song, W.L.; Ling, Z.J.; Xu, J.L.; Duan, J.J.; Wang, Z.L.; Chang, A.H.; et al. Short−Interval Sequential CAR−T Cell Infusion May Enhance Prior CAR−T Cell Expansion to Augment Anti−Lymphoma Response in B−NHL. Front. Oncol. 2021, 11, 640166. [Google Scholar] [CrossRef]
- Larson, R.C.; Maus, M.V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 2021, 21, 145–161. [Google Scholar] [CrossRef]
- Yap, T.A.; Parkes, E.E.; Peng, W.Y.; Moyers, J.T.; Curran, M.A.; Tawbi, H.A. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov. 2021, 11, 1368–1397. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, M.K.; Gehl, A.; Fricke, B.; Nawaz, K.; Gluesenkamp, K.; Shen, B.; Munk, J.; Hagerman, J.; Lapsa, M. COVID 19 vaccine distribution solution to the last mile challenge: Experimental and simulation studies of ultra−low temperature refrigeration system. Int. J. Refrig. 2022, 133, 313–325. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of mRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Drane, D.; Gowans, E.J. Long−term storage of DNA−free RNA for use in vaccine studies. Biotechniques 2007, 43, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Joseph, N.; Crivera, C.; Jackson, C.C.; Valluri, S.; Cost, P.; Phelps, H.; Slowik, R.; Klein, T.; Yu, X.T.; et al. Total Car−T Cost of Care Beyond the Price of Car−T Cell Therapy in Patients with Multiple Myeloma. Blood 2021, 138, 4964. [Google Scholar] [CrossRef]
- Guha, P.; Katz, S.C. Strategies for manufacturing cell therapy products aligned with patient needs. Methods Cell Biol. 2022, 167, 203–226. [Google Scholar] [PubMed]
- Du, L.; Nai, Y.R.; Shen, M.Y.; Li, T.T.; Huang, J.J.; Han, X.J.; Wang, W.; Pang, D.; Jin, A.S. IL−21 Optimizes the CAR−T Cell Preparation Through Improving Lentivirus Mediated Transfection Efficiency of T Cells and Enhancing CAR−T Cell Cytotoxic Activities. Front. Mol. Biosci. 2021, 8, 675179. [Google Scholar] [CrossRef]
- Qin, F.R.; Xia, F.; Chen, H.L.; Cui, B.M.; Feng, Y.; Zhang, P.; Chen, J.; Luo, M. A Guide to Nucleic Acid Vaccines in the Prevention and Treatment of Infectious Diseases and Cancers: From Basic Principles to Current Applications. Front. Cell Dev. Biol. 2021, 9, 633776. [Google Scholar] [CrossRef]
- Bian, X.; Kim, C.; Karniadakis, G.E. 111 years of Brownian motion. Soft Matter 2016, 12, 6331–6346. [Google Scholar] [CrossRef]
- Shah, N.; Hussain, M.; Rehan, T.; Khan, A.; Khan, Z.U. Overview of Polyethylene Glycol−based Materials with a Special Focus on Core−Shell Particles for Drug Delivery Application. Curr. Pharm. Des. 2022, 28, 352–367. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, Y.; Zhang, L.; Zhang, Y.; Liu, J.; Yu, P. Poly ethylene glycol (PEG)−Related controllable and sustainable antidiabetic drug delivery systems. Eur. J. Med. Chem. 2021, 217, 113372. [Google Scholar] [CrossRef]
- Phelps, E.A.; Enemchukwu, N.O.; Fiore, V.F.; Sy, J.C.; Murthy, N.; Sulchek, T.A.; Barker, T.H.; Garcia, A.J. Maleimide Cross−Linked Bioactive PEG Hydrogel Exhibits Improved Reaction Kinetics and Cross−Linking for Cell Encapsulation and In Situ Delivery. Adv. Mater. 2012, 24, 64–70. [Google Scholar] [CrossRef]
- Fatmi, S.; Taouzinet, L.; Lahiani−Skiba, M.; Skiba, M.; Iguer−Ouada, M. New Formulation and Evaluation of Camptothecin Encapsulated and/or Dispersed Suppository. Anti−Cancer Agents Med. Chem. 2021, 21, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Fallahi, G.H.; Motamed, F.; Farahmand, F.; Khodadad, A.; Ghajarzadeh, M.; Rezaei, N.; Mehrabani, S. Comparison of one and two−day bowel preparation with polyethylene glycol in pediatric colonoscopy. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2015, 26, 232–235. [Google Scholar] [CrossRef]
- Lim, C.; Shin, Y.; Lee, S.; Lee, S.; Lee, M.-Y.; Shin, B.S.; Oh, K.T. Dynamic drug release state and PEG length in PEGylated liposomal formulations define the distribution and pharmacological performance of drug. J. Drug Deliv. Sci. Technol. 2022, 76, 103825. [Google Scholar] [CrossRef]
- Jia, H.-Z.; Luo, X.-H.; Cheng, H.; Yang, J.; Li, C.; Liu, C.-W.; Feng, J.; Zhang, X.-Z.; Zhuo, R.-X. Extraordinarily enhanced gene transfection and cellular uptake by aromatic hydrophobicization to PEI25K. J. Mater. Chem. 2012, 22, 24092–24101. [Google Scholar] [CrossRef]
- Duan, H.X.; Liu, Y.H.; Gao, Z.G.; Huang, W. Recent advances in drug delivery systems for targeting cancer stem cells. Acta Pharm. Sin. B 2021, 11, 55–70. [Google Scholar] [CrossRef]
- Sultan, T.; Hamid, S.; Hassan, S.; Hussain, K.; Ahmed, A.; Bashir, L.; Naz, S.; Maqbool, T. Development and evaluation of immediate release diclofenac sodium suppositories. Pak. J. Pharm. Sci. 2018, 31, 1791–1795. [Google Scholar]
- Ghazwani, M.; Hani, U.; Osmani, R.A.M.; Rahamathulla, M.; Begum, M.Y.; Wahab, S.; Gowda, D.V.; Ravikumar, A.A.; Kumar, H.Y.; Ather, H.; et al. An Efficient Herbal Approach for Treating Fungal Infection in Cervical Cancer Patients by Developing and Optimizing a Vaginal Suppository. Int. J. Polym. Sci. 2021, 2021, 9198387. [Google Scholar] [CrossRef]
- Cui, P.F.; Qi, L.Y.; Wang, Y.; Yu, R.Y.; He, Y.J.; Xing, L.; Jiang, H.L. Dex−Aco coating simultaneously increase the biocompatibility and transfection efficiency of cationic polymeric gene vectors. J. Control. Release 2019, 303, 253–262. [Google Scholar] [CrossRef]
- Burke, R.S.; Pun, S.H. Extracellular barriers to in Vivo PEI and PEGylated PEI polyplex−mediated gene delivery to the liver. Bioconjugate Chem. 2008, 19, 693–704. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Choi, C.H.; Han, H.; Davis, M.E. Polycation−siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3137–3142. [Google Scholar] [CrossRef]
- Cui, P.F.; Zhuang, W.R.; Qiao, J.B.; Zhang, J.L.; He, Y.J.; Luo, C.Q.; Jin, Q.R.; Xing, L.; Jiang, H.L. Histone−inspired biomimetic polymeric gene vehicles with excellent biocompatibility and enhanced transfection efficacy. Polym. Chem. 2016, 7, 7416–7426. [Google Scholar] [CrossRef]
- Choi, J.; Kim, N.; Oh, J.W.; Kim, F.S. Bandgap engineering of nanosized carbon dots through electron−accepting functionalization. J. Ind. Eng. Chem. 2018, 65, 104–111. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Friess, W. Freeze−drying of nanoparticles: How to overcome colloidal instability by formulation and process optimization. Eur. J. Pharm. Biopharm. 2021, 165, 345–360. [Google Scholar] [CrossRef]
- Casa, D.M.; Karam, T.K.; Alves, A.D.S.; Zgoda, A.A.; Khalil, N.M.; Mainardes, R.M. Bovine Serum Albumin Nanoparticles Containing Amphotericin B: Characterization, Cytotoxicity and In Vitro Antifungal Evaluation. J. Nanosci. Nanotechnol. 2015, 15, 10183–10188. [Google Scholar] [CrossRef] [PubMed]
- Maurstad, G.; Stokke, B.T.; Varum, K.M.; Strand, S.P. PEGylated chitosan complexes DNA while improving polyplex colloidal stability and gene transfection efficiency. Carbohydr. Polym. 2013, 94, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Jiang, H.; Wang, X.; Liu, J. Orthogonal Adsorption of Carbon Dots and DNA on Nanoceria. Langmuir 2020, 36, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, B.; Fu, T. Dual−emission fluorescence detection of histidine using carbon dots and calcein/Ni2+ complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 286, 121951. [Google Scholar] [CrossRef]
- Salem, J.K.; El−Nahhal, I.M.; Salama, S.F. Determination of the critical micelle concentration by absorbance and fluorescence techniques using fluorescein probe. Chem. Phys. Lett. 2019, 730, 445–450. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, L.; Liu, L.; Liu, F.; Maruyama, A.; Tian, H.; Chen, X. Ionic−crosslinked polysaccharide/PEI/DNA nanoparticles for stabilized gene delivery. Carbohydr. Polym. 2018, 201, 246–256. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, P.; Ma, L.; Jiang, P.; Wang, C.; Wang, J. PEG Gels Significantly Improve the Storage Stability of Nucleic Acid Preparations. Gels 2022, 8, 819. https://doi.org/10.3390/gels8120819
Cui P, Ma L, Jiang P, Wang C, Wang J. PEG Gels Significantly Improve the Storage Stability of Nucleic Acid Preparations. Gels. 2022; 8(12):819. https://doi.org/10.3390/gels8120819
Chicago/Turabian StyleCui, Pengfei, Luping Ma, Pengju Jiang, Cheng Wang, and Jianhao Wang. 2022. "PEG Gels Significantly Improve the Storage Stability of Nucleic Acid Preparations" Gels 8, no. 12: 819. https://doi.org/10.3390/gels8120819
APA StyleCui, P., Ma, L., Jiang, P., Wang, C., & Wang, J. (2022). PEG Gels Significantly Improve the Storage Stability of Nucleic Acid Preparations. Gels, 8(12), 819. https://doi.org/10.3390/gels8120819