Optimizing Delivery of Therapeutic Growth Factors for Bone and Cartilage Regeneration
Abstract
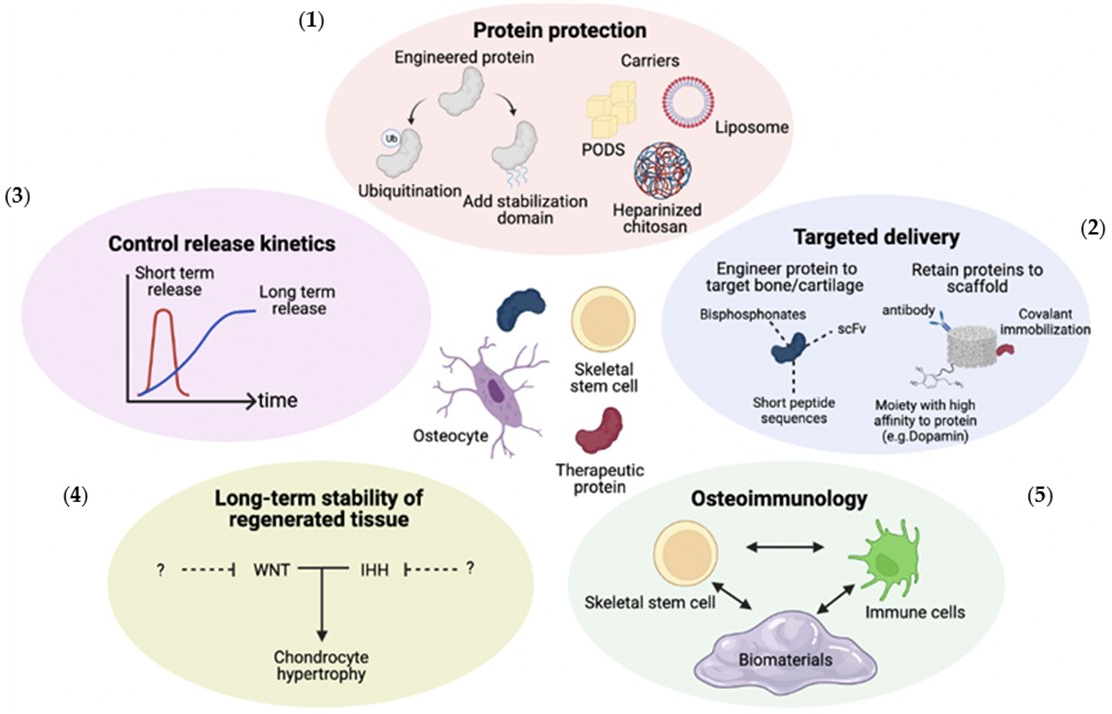
1. Introduction
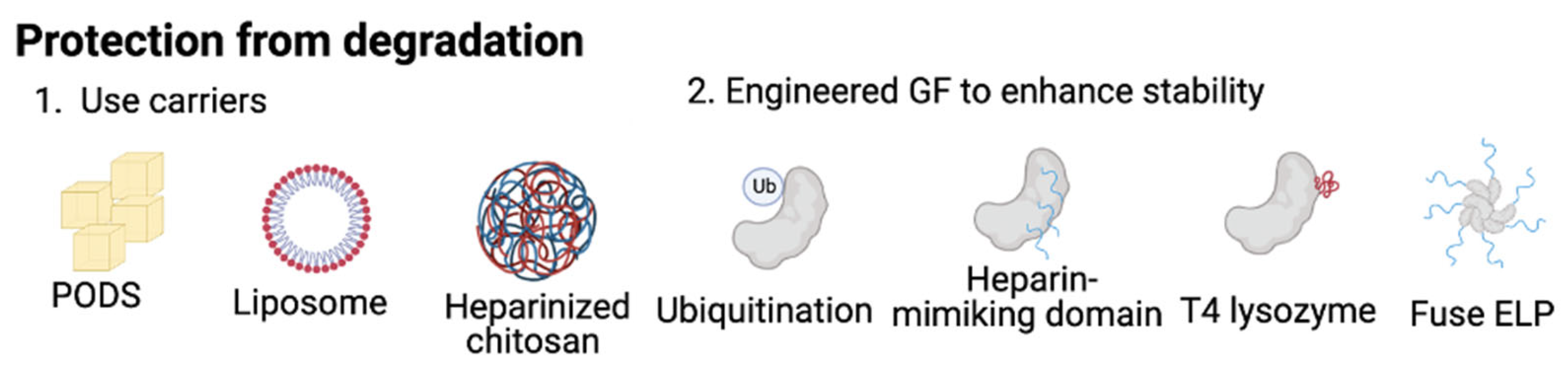
2. Protection from Physical and Enzymatic Degradation
2.1. Usage of Carriers to Protect Growth Factors
2.2. Engineering Proteins to Increase Stability of GFs
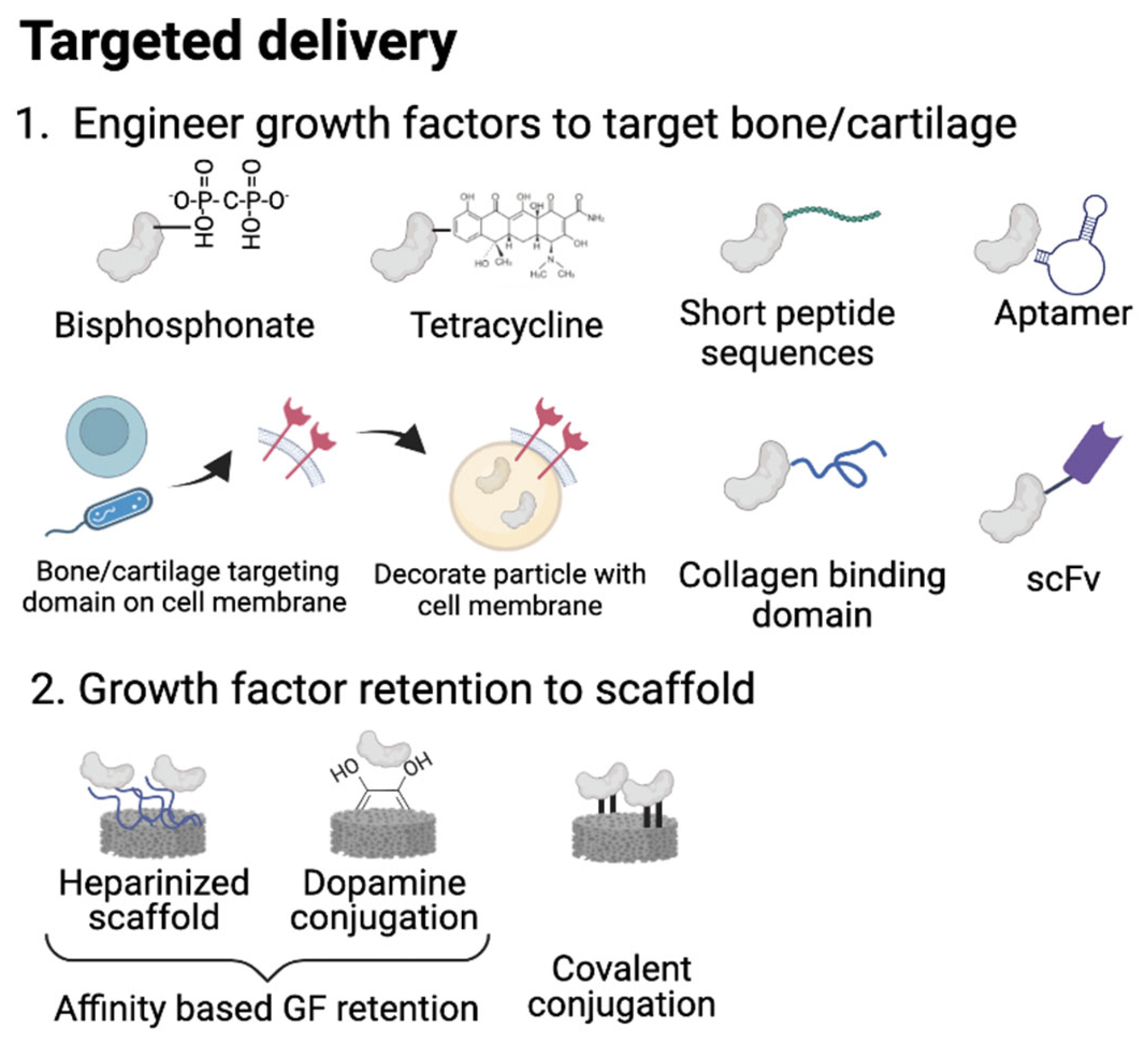
3. Targeted Delivery of Growth Factors
3.1. Active Delivery with Bone/Cartilage Targeting Motifs
3.2. Enhancing Growth Factor Retention in Delivery Scaffolds
4. Controlling GF Release Kinetics
4.1. Short-Term Release
4.2. Long-Term Release
4.3. Synergistic Delivery of Multiple GFs
4.4. Sequential Delivery
4.5. Spatial Control
5. Promoting the Long-term Stability of Regenerated Tissues
6. Osteoimmunomodulatory (OIM) Effects
7. Conclusions and Remarks for Future Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- a.I.S. Global Burden of Disease 2019. Disease. Available online: https://www.healthdata.org/results/gbd_summaries/2019/osteoarthritis-level-3-cause (accessed on 1 April 2023).
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Bryant, S.J.; Ahn, J.; Hankenson, K.D. Chapter 24—Bone Regeneration. In Translational Regenerative Medicine; Atala, A., Allickson, J.G., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 313–333. [Google Scholar] [CrossRef]
- Kuru, P.; Akyüz, G.; Cerşit, H.P.; Çelenlioğlu, A.E.; Cumhur, A.; Biricik, Ş.; Kozan, S.; Gökşen, A.; Özdemir, M.; Lüleci, E. Fracture history in osteoporosis: Risk factors and its effect on quality of life. Balkan Med. J. 2014, 31, 295–301. [Google Scholar] [CrossRef]
- Creamer, P.; Hochberg, M.C. Osteoarthritis. Lancet 1997, 350, 503–508. [Google Scholar] [CrossRef]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheumatol. 2008, 58, 26–35. [Google Scholar] [CrossRef]
- March, L.M.; Bachmeier, C.J. Economics of osteoarthritis: A global perspective. Baillieres Clin. Rheumatol. 1997, 11, 817–834. [Google Scholar] [CrossRef]
- Chen, J.S.; Sambrook, P.N. Antiresorptive therapies for osteoporosis: A clinical overview. Nat. Rev. Endocrinol. 2011, 8, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ponnapakkam, T.; Katikaneni, R.; Sakon, J.; Stratford, R.; Gensure, R.C. Treating osteoporosis by targeting parathyroid hormone to bone. Drug Discov. Today 2014, 19, 204–208. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Berger, A.J.; Meals, R.A. Management of osteoarthrosis of the thumb joints. J. Hand Surg. Am. 2015, 40, 843–850. [Google Scholar] [CrossRef]
- Shakoor, S.; Kibble, E.; El-Jawhari, J.J. Bioengineering Approaches for Delivering Growth Factors: A Focus on Bone and Cartilage Regeneration. Bioengineering 2022, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, M. Chapter 6—Biological therapeutic modalities. In Translational Biotechnology; Hasija, Y., Ed.; Academic Press: Boston, MA, USA, 2021; pp. 137–164. [Google Scholar] [CrossRef]
- Nuventra. Points to Consider in Drug Development of Biologics and Small Molecules. 2020. Available online: https://www.allucent.com/resources/blog/points-consider-drug-development-biologics-and-small-molecules (accessed on 1 April 2023).
- Sharma, A.R.; Kundu, S.K.; Nam, J.-S.; Sharma, G.; Priya Doss, C.G.; Lee, S.-S.; Chakraborty, C. Next generation delivery system for proteins and genes of therapeutic purpose: Why and how? BioMed Res. Int. 2014, 2014, 327950. [Google Scholar] [CrossRef] [PubMed]
- Luca, L.; Rougemont, A.L.; Walpoth, B.H.; Gurny, R.; Jordan, O. The effects of carrier nature and pH on rhBMP-2-induced ectopic bone formation. J. Control. Release 2010, 147, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Dorai, H.; Ganguly, S. Mammalian cell-produced therapeutic proteins: Heterogeneity derived from protein degradation. Curr. Opin. Biotechnol. 2014, 30, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Hamid Akash, M.S.; Akhtar, B.; Tariq, M.; Mahmood, A.; Ibrahim, M. Delivery of Therapeutic Proteins: Challenges and Strategies. Curr. Drug Targets 2016, 17, 1172–1188. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Niu, M.; Lu, Y.; Hovgaard, L.; Wu, W. Liposomes containing glycocholate as potential oral insulin delivery systems: Preparation, in vitro characterization, and improved protection against enzymatic degradation. Int. J. Nanomed. 2011, 6, 1155–1166. [Google Scholar] [CrossRef]
- Matsumoto, G.; Ueda, T.; Shimoyama, J.; Ijiri, H.; Omi, Y.; Yube, H.; Sugita, Y.; Kubo, K.; Maeda, H.; Kinoshita, Y.; et al. Bone regeneration by polyhedral microcrystals from silkworm virus. Sci. Rep. 2012, 2, 935. [Google Scholar] [CrossRef]
- Kim, S.; Fan, J.; Lee, C.-S.; Chen, C.; Bubukina, K.; Lee, M. Heparinized chitosan stabilizes the bioactivity of BMP-2 and potentiates the osteogenic efficacy of demineralized bone matrix. J. Biol. Eng. 2020, 14, 6. [Google Scholar] [CrossRef]
- Mathews, S.; Gupta, P.K.; Bhonde, R.; Totey, S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Prolif. 2011, 44, 537–549. [Google Scholar] [CrossRef]
- Khattar, V.; Lee, J.H.; Wang, H.; Bastola, S.; Ponnazhagan, S. Structural determinants and genetic modifications enhance BMP2 stability and extracellular secretion. FASEB bioAdv. 2019, 1, 180–190. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kim, S.-H.; Decker, C.G.; Wong, D.Y.; Loo, J.A.; Maynard, H.D. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nat. Chem. 2013, 5, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tan, B.; Bao, Z.; Wang, S.; Tang, R.; Wang, Z.; Chen, G.; Chen, S.; Lu, W.W.; Yang, D.; et al. Enhanced bone regeneration via spatiotemporal and controlled delivery of a genetically engineered BMP-2 in a composite Hydrogel. Biomaterials 2021, 277, 121117. [Google Scholar] [CrossRef] [PubMed]
- Hassouneh, W.; MacEwan, S.R.; Chilkoti, A. Chapter nine—Fusions of Elastin-like Polypeptides to Pharmaceutical Proteins. In Methods in Enzymology; Wittrup, K.D., Verdine, G.L., Eds.; Academic Press: Boston, MA, USA, 2012; Volume 502, pp. 215–237. [Google Scholar]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Gittens, S.A.; Matyas, J.R.; Zernicke, R.F.; Uludağ, H. Imparting Bone Affinity to Glycoproteins Through the Conjugation of Bisphosphonates. Pharm. Res. 2003, 20, 978–987. [Google Scholar] [CrossRef]
- Doschak, M.R.; Kucharski, C.M.; Wright, J.E.I.; Zernicke, R.F.; Uludaǧ, H. Improved Bone Delivery of Osteoprotegerin by Bisphosphonate Conjugation in a Rat Model of Osteoarthritis. Mol. Pharm. 2009, 6, 634–640. [Google Scholar] [CrossRef]
- Katsumi, H.; Sano, J.-i.; Nishikawa, M.; Hanzawa, K.; Sakane, T.; Yamamoto, A. Molecular Design of Bisphosphonate-Modified Proteins for Efficient Bone Targeting In Vivo. PLoS ONE 2015, 10, e0135966. [Google Scholar] [CrossRef]
- Bhandari, K.H.; Newa, M.; Chapman, J.; Doschak, M.R. Synthesis, characterization and evaluation of bone targeting salmon calcitonin analogs in normal and osteoporotic rats. J. Control. Release 2012, 158, 44–52. [Google Scholar] [CrossRef]
- Sibenaller, Z.A.; Welsh, J.L.; Du, C.; Witmer, J.R.; Schrock, H.E.; Du, J.; Buettner, G.R.; Goswami, P.C.; Cieslak, J.A., 3rd; Cullen, J.J. Extracellular superoxide dismutase suppresses hypoxia-inducible factor-1α in pancreatic cancer. Free Radic. Biol. Med. 2014, 69, 357–366. [Google Scholar] [CrossRef]
- Perrin, D.D. Binding of Tetracyclines to Bone. Nature 1965, 208, 787–788. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, C.; Huang, H.; Huang, J.; Deng, A.; Zou, P.; Tan, X. Bone-targeted delivery of simvastatin-loaded PEG-PLGA micelles conjugated with tetracycline for osteoporosis treatment. Drug Deliv. Transl. Res. 2018, 8, 1090–1102. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Ruoslahti, E. Molecular changes in the vasculature of injured tissues. Am. J. Pathol. 2007, 171, 702–711. [Google Scholar] [CrossRef]
- Wang, J.; Hu, L.; Huang, H.; Yu, Y.; Wang, J.; Yu, Y.; Li, K.; Li, Y.; Tian, T.; Chen, F. CAR (CARSKNKDC) Peptide Modified ReNcell-Derived Extracellular Vesicles as a Novel Therapeutic Agent for Targeted Pulmonary Hypertension Therapy. Hypertension 2020, 76, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yu, X.; Carbone, E.J.; Nelson, C.; Kan, H.M.; Lo, K.W.H. Poly aspartic acid peptide-linked PLGA based nanoscale particles: Potential for bone-targeting drug delivery applications. Int. J. Pharm. 2014, 475, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhou, K.; Liu, M.; Guo, X.; Qu, Y.; Cui, W.; Shao, Z.; Zhang, X.; Xu, S. Loading of BMP-2-related peptide onto three-dimensional nano-hydroxyapatite scaffolds accelerates mineralization in critical-sized cranial bone defects. J. Tissue Eng. Regen. Med. 2018, 12, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ye, X.; Cai, M.; Liu, X.; Xiao, J.; Zhang, C.; Wang, Y.; Yang, L.; Liu, J.; Li, S.; et al. Osteoblast-Targeting-Peptide Modified Nanoparticle for siRNA/microRNA Delivery. ACS Nano 2016, 10, 5759–5768. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Zhang, X.; Pi, Y.; Wang, X.; Jia, Z.; Zhu, J.; Dai, L.; Chen, W.; Yin, L.; Chen, H.; et al. Polycaprolactone electrospun mesh conjugated with an MSC affinity peptide for MSC homing in vivo. Biomaterials 2012, 33, 3375–3387. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Liu, W.; He, Y.; Huang, J.; Duan, L.; Xiong, J.; Liu, L.; Wang, D. Recent advances in kartogenin for cartilage regeneration. J. Drug Target. 2019, 27, 28–32. [Google Scholar] [CrossRef]
- Pi, Y.; Zhang, X.; Shi, J.; Zhu, J.; Chen, W.; Zhang, C.; Gao, W.; Zhou, C.; Ao, Y. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials 2011, 32, 6324–6332. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, X.; Li, X.; Xiong, J.; Li, B.; Duan, L.; Wang, D.; Xia, J. Chondrocyte-Targeted MicroRNA Delivery by Engineered Exosomes toward a Cell-Free Osteoarthritis Therapy. ACS Appl. Mater. Interfaces 2020, 12, 36938–36947. [Google Scholar] [CrossRef]
- Newman, M.R.; Benoit, D.S.W. Local and targeted drug delivery for bone regeneration. Curr. Opin. Biotechnol. 2016, 40, 125–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Liang, C.; Guo, B.; Wu, H.; Shao, N.; Li, D.; Liu, J.; Dang, L.; Wang, C.; Li, H.; Li, S.; et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference–based bone anabolic strategy. Nat. Med. 2015, 21, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.; Wiskirchen, J.; Guo, K.; Neumann, B.; Kehlbach, R.; Pintaske, J.; Voth, V.; Walker, T.; Scheule, A.M.; Greiner, T.O.; et al. Aptamer-based isolation and subsequent imaging of mesenchymal stem cells in ischemic myocard by magnetic resonance imaging. In RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der Bildgebenden Verfahren; Georg Thieme Verlag KG: Stutgard, Germany; New York, NY, USA, 2007; Volume 179, pp. 1009–1015. [Google Scholar] [CrossRef]
- Wang, M.; Wu, H.; Li, Q.; Yang, Y.; Che, F.; Wang, G.; Zhang, L. Novel Aptamer-Functionalized Nanoparticles Enhances Bone Defect Repair By Improving Stem Cell Recruitment. Int. J. Nanomed. 2019, 14, 8707–8724. [Google Scholar] [CrossRef] [PubMed]
- Ardjomandi, N.; Niederlaender, J.; Aicher, W.K.; Reinert, S.; Schweizer, E.; Wendel, H.-P.; Alexander, D. Identification of an Aptamer Binding to Human Osteogenic-Induced Progenitor Cells. Nucleic Acid Ther. 2013, 23, 44–61. [Google Scholar] [CrossRef]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T.; Hanson, D.; Bohmann, J.A. DNA Aptamer Beacon Assay for C-Telopeptide and Handheld Fluorometer to Monitor Bone Resorption. J. Fluoresc. 2011, 21, 2021. [Google Scholar] [CrossRef]
- Park, J.H.; Jiang, Y.; Zhou, J.; Gong, H.; Mohapatra, A.; Heo, J.; Gao, W.; Fang, R.H.; Zhang, L. Genetically engineered cell membrane–coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci. Adv. 2021, 7, eabf7820. [Google Scholar] [CrossRef]
- Su, N.; Villicana, C.; Barati, D.; Freeman, P.; Luo, Y.; Yang, F. Stem Cell Membrane-Coated Microribbon Scaffolds Induce Regenerative Innate and Adaptive Immune Responses in a Critical-Size Cranial Bone Defect Model. Adv. Mater. 2023, 35, 2208781. [Google Scholar] [CrossRef] [PubMed]
- Saito, W.; Uchida, K.; Ueno, M.; Matsushita, O.; Inoue, G.; Nishi, N.; Ogura, T.; Hattori, S.; Fujimaki, H.; Tanaka, K.; et al. Acceleration of bone formation during fracture healing by injectable collagen powder and human basic fibroblast growth factor containing a collagen-binding domain from Clostridium histolyticum collagenase. J. Biomed. Mater. Res. A 2014, 102, 3049–3055. [Google Scholar] [CrossRef]
- Addi, C.; Murschel, F.; De Crescenzo, G. Design and Use of Chimeric Proteins Containing a Collagen-Binding Domain for Wound Healing and Bone Regeneration. Tissue Eng. B Rev. 2017, 23, 163–182. [Google Scholar] [CrossRef]
- Lui, J.C.; Colbert, M.; Cheung, C.S.F.; Ad, M.; Lee, A.; Zhu, Z.; Barnes, K.M.; Dimitrov, D.S.; Baron, J. Cartilage-Targeted IGF-1 Treatment to Promote Longitudinal Bone Growth. Mol. Ther. 2019, 27, 673–680. [Google Scholar] [CrossRef]
- Ferrari, M.; Onuoha, S.C.; Fossati-Jimack, L.; Nerviani, A.; Alves, P.L.; Pagani, S.; Deantonio, C.; Colombo, F.; Santoro, C.; Sblattero, D.; et al. Novel Bispecific Antibody for Synovial-Specific Target Delivery of Anti-TNF Therapy in Rheumatoid Arthritis. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Ko, J.; Lee, H.R.; Do, S.H.; Park, K. Mesenchymal cells condensation-inducible mesh scaffolds for cartilage tissue engineering. Biomaterials 2016, 85, 18–29. [Google Scholar] [CrossRef]
- Mammadov, R.; Mammadov, B.; Guler, M.O.; Tekinay, A.B. Growth factor binding on heparin mimetic peptide nanofibers. Biomacromolecules 2012, 13, 3311–3319. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Krishnan, L.; Rouse, T.; Chou, C.; McDevitt, T.C.; Guldberg, R.E. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci. Adv. 2020, 6, eaay1240. [Google Scholar] [CrossRef]
- Ikegami, Y.; Mizumachi, H.; Yoshida, K.; Ijima, H. Heparin-conjugated collagen as a potent growth factor-localizing and stabilizing scaffold for regenerative medicine. Regen. Ther. 2020, 15, 236–242. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, S.H.; Min, H.K.; Lee, J.H. Dual growth factor-immobilized asymmetrically porous membrane for bone-to-tendon interface regeneration on rat patellar tendon avulsion model. J. Biomed. Mater. Res. A 2018, 106, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guo, X.; Zheng, Q.; Wu, Y.; Cui, F.; Wu, B. Improving osteogenesis of three-dimensional porous scaffold based on mineralized recombinant human-like collagen via mussel-inspired polydopamine and effective immobilization of BMP-2-derived peptide. Colloids Surf. B Biointerfaces 2017, 152, 124–132. [Google Scholar] [CrossRef]
- Enriquez-Ochoa, D.; Robles-Ovalle, P.; Mayolo-Deloisa, K.; Brunck, M.E.G. Immobilization of Growth Factors for Cell Therapy Manufacturing. Front. Bioeng. Biotechnol. 2020, 8, 620. [Google Scholar] [CrossRef]
- Karfeld-Sulzer, L.S.; Siegenthaler, B.; Ghayor, C.; Weber, F.E. Fibrin Hydrogel Based Bone Substitute Tethered with BMP-2 and BMP-2/7 Heterodimers. Materials 2015, 8, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Tao, H.; Wu, Y.; Hu, Y.; Yan, Y.; Luo, Z. TGF-β3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid hybrid scaffold for cartilage regeneration. J. Biomed. Mater. Res. A 2010, 95, 982–992. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Briquez, P.S.; Tsai, H.-M.; Watkins, E.A.; Hubbell, J.A. Engineered bridge protein with dual affinity for bone morphogenetic protein-2 and collagen enhances bone regeneration for spinal fusion. Sci. Adv. 2021, 7, eabh4302. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Freire, M.O.; Pang, E.-K.; Abdelhamid, A.I.; Almohaimeed, M.; Zadeh, H.H. Immobilization of Murine Anti-BMP-2 Monoclonal Antibody on Various Biomaterials for Bone Tissue Engineering. BioMed Res. Int. 2014, 2014, 940860. [Google Scholar] [CrossRef] [PubMed]
- Udomluck, N.; Lee, H.; Hong, S.; Lee, S.-H.; Park, H. Surface functionalization of dual growth factor on hydroxyapatite-coated nanofibers for bone tissue engineering. Appl. Surf. Sci. 2020, 520, 146311. [Google Scholar] [CrossRef]
- Cabanas-Danés, J.; Huskens, J.; Jonkheijm, P. Chemical strategies for the presentation and delivery of growth factors. J. Mater. Chem. B 2014, 2, 2381–2394. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, É.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in Growth Factor Delivery for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Takematsu, E.; Massidda, M.; Auster, J.; Chen, P.-C.; Im, B.; Srinath, S.; Canga, S.; Singh, A.; Majid, M.; Sherman, M.; et al. Transmembrane stem cell factor protein therapeutics enhance revascularization in ischemia without mast cell activation. Nat. Commun. 2022, 13, 2497. [Google Scholar] [CrossRef]
- Re’em, T.; Kaminer-Israeli, Y.; Ruvinov, E.; Cohen, S. Chondrogenesis of hMSC in affinity-bound TGF-beta scaffolds. Biomaterials 2012, 33, 751–761. [Google Scholar] [CrossRef]
- Freeman, I.; Kedem, A.; Cohen, S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials 2008, 29, 3260–3268. [Google Scholar] [CrossRef]
- Lee, S.S.; Santschi, M.; Ferguson, S.J. A Biomimetic Macroporous Hybrid Scaffold with Sustained Drug Delivery for Enhanced Bone Regeneration. Biomacromolecules 2021, 22, 2460–2471. [Google Scholar] [CrossRef]
- Hassan, A.H.; Hosny, K.M.; Murshid, Z.A.; Alhadlaq, A.; Yamani, A.; Naguib, G.; Alkhalidi, H.M.; Afify, A.R. Controlled release of injectable liposomal in situ gel loaded with recombinant human bone morphogenetic protein-2 for the repair of alveolar bone clefts in rabbits. J. Liposome Res. 2016, 26, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.-H.; Jeong, B.-C.; Kook, M.-S.; Kim, S.-H.; Koh, J.-T. Evaluation of a Thiolated Chitosan Scaffold for Local Delivery of BMP-2 for Osteogenic Differentiation and Ectopic Bone Formation. BioMed Res. Int. 2013, 2013, 878930. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.G.L.; Kempen, D.H.R.; Liu, X.; Dadsetan, M.; Tryfonidou, M.A.; Yaszemski, M.J.; Dhert, W.J.A.; Lu, L. Bone morphogenetic protein-2 release profile modulates bone formation in phosphorylated hydrogel. J. Tissue Eng. Regen. Med. 2018, 12, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Lee, S.; Bararpour, L.; Yang, F. Long-Term Controlled Protein Release from Poly(Ethylene Glycol) Hydrogels by Modulating Mesh Size and Degradation. Macromol. Biosci. 2015, 15, 1679–1686. [Google Scholar] [CrossRef]
- Sun, T.; Liu, M.; Yao, S.; Ji, Y.; Shi, L.; Tang, K.; Xiong, Z.; Yang, F.; Chen, K.; Guo, X. Guided osteoporotic bone regeneration with composite scaffolds of mineralized ECM/heparin membrane loaded with BMP2-related peptide. Int. J. Nanomed. 2018, 13, 791–804. [Google Scholar] [CrossRef]
- Chen, R.; Yu, J.; Gong, H.-L.; Jiang, Y.; Xue, M.; Xu, N.; Wei, D.-X.; Shi, C. An easy long-acting BMP7 release system based on biopolymer nanoparticles for inducing osteogenic differentiation of adipose mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2020, 14, 964–972. [Google Scholar] [CrossRef]
- Barati, D.; Gegg, C.; Yang, F. Nanoparticle-Mediated TGF-β Release from Microribbon-Based Hydrogels Accelerates Stem Cell-Based Cartilage Formation In Vivo. Ann. Biomed. Eng. 2020, 48, 1971–1981. [Google Scholar] [CrossRef]
- Suliman, S.; Xing, Z.; Wu, X.; Xue, Y.; Pedersen, T.O.; Sun, Y.; Døskeland, A.P.; Nickel, J.; Waag, T.; Lygre, H.; et al. Release and bioactivity of bone morphogenetic protein-2 are affected by scaffold binding techniques in vitro and in vivo. J. Control. Release 2015, 197, 148–157. [Google Scholar] [CrossRef]
- Minardi, S.; Fernandez-Moure, J.S.; Fan, D.; Murphy, M.B.; Yazdi, I.K.; Liu, X.; Weiner, B.K.; Tasciotti, E. Biocompatible PLGA-Mesoporous Silicon Microspheres for the Controlled Release of BMP-2 for Bone Augmentation. Pharmaceutics 2020, 12, 118. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Z.; Liu, M.; Yang, L.; Yao, S.; Chen, K.; Yu, K.; Qu, Y.; Sun, T.; Guo, X. Creation of Bony Microenvironment with Extracellular Matrix Doped-Bioactive Ceramics to Enhance Osteoblast Behavior and Delivery of Aspartic Acid-Modified BMP-2 Peptides. Int. J. Nanomed. 2020, 15, 8465–8478. [Google Scholar] [CrossRef]
- Filová, E.; Rampichová, M.; Litvinec, A.; Držík, M.; Míčková, A.; Buzgo, M.; Košťáková, E.; Martinová, L.; Usvald, D.; Prosecká, E.; et al. A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. Int. J. Pharm. 2013, 447, 139–149. [Google Scholar] [CrossRef]
- Li, X.; Yi, W.; Jin, A.; Duan, Y.; Min, S. Effects of sequentially released BMP-2 and BMP-7 from PELA microcapsule-based scaffolds on the bone regeneration. Am. J. Transl. Res. 2015, 7, 1417–1428. [Google Scholar] [PubMed]
- Crecente-Campo, J.; Borrajo, E.; Vidal, A.; Garcia-Fuentes, M. New scaffolds encapsulating TGF-β3/BMP-7 combinations driving strong chondrogenic differentiation. Eur. J. Pharm. Biopharm. 2017, 114, 69–78. [Google Scholar] [CrossRef]
- Murphy, M.P.; Koepke, L.S.; Lopez, M.T.; Tong, X.; Ambrosi, T.H.; Gulati, G.S.; Marecic, O.; Wang, Y.; Ransom, R.C.; Hoover, M.Y.; et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020, 26, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Nakade, O.; Takada, Y.; Kaku, T.; Lau, K.H.W. Melatonin at Pharmacologic Doses Increases Bone Mass by Suppressing Resorption Through Down-Regulation of the RANKL-Mediated Osteoclast Formation and Activation. J. Bone Miner. Res. 2002, 17, 1219–1229. [Google Scholar] [CrossRef]
- Liu, J.; Huang, F.; He, H.-W. Melatonin Effects on Hard Tissues: Bone and Tooth. Int. J. Mol. Sci. 2013, 14, 10063–10074. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, H.; Çetin Altındal, D.; Gümüşderelioğlu, M. Scaffold-based osteogenic dual delivery system with melatonin and BMP-2 releasing PLGA microparticles. Int. J. Pharm. 2021, 600, 120489. [Google Scholar] [CrossRef]
- Strobel, C.; Bormann, N.; Kadow-Romacker, A.; Schmidmaier, G.; Wildemann, B. Sequential release kinetics of two (gentamicin and BMP-2) or three (gentamicin, IGF-I and BMP-2) substances from a one-component polymeric coating on implants. J. Control. Release 2011, 156, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Cho, T.-J.; Aizawa, T.; Yamazaki, M.; Nooh, N.; Graves, D.; Gerstenfeld, L.C.; Einhorn, T.A. Expression of Osteoprotegerin, Receptor Activator of NF-κB Ligand (Osteoprotegerin Ligand) and Related Proinflammatory Cytokines During Fracture Healing. J. Bone Miner. Res. 2001, 16, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, G.; Wang, Y.; Yang, G.; Ding, S.; Zhou, S. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials 2015, 37, 218–229. [Google Scholar] [CrossRef]
- Ma, X.; Xu, Z.; Ding, S.; Yi, G.; Wang, Q. Alendronate promotes osteoblast differentiation and bone formation in ovariectomy-induced osteoporosis through interferon-β/signal transducer and activator of transcription 1 pathway. Exp. Ther. Med. 2018, 15, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Wufuer, M.; Kim, I.; Choi, T.H.; Kim, B.J.; Jung, H.G.; Jeon, B.; Lee, G.; Jeon, O.H.; Chang, H.; et al. Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci. Rep. 2021, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Raiche, A.T.; Puleo, D.A. In vitro effects of combined and sequential delivery of two bone growth factors. Biomaterials 2004, 25, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, Y.; Gu, Y.; Xu, Y.; Liu, Y.; Li, B.; Chen, L. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials 2016, 106, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.N.; Dwivedi, N.; Phillips, L.M.; Yu, X.; Herberg, S.; Bowerman, C.; Solorio, L.D.; Murphy, W.L.; Alsberg, E. Controlled Dual Growth Factor Delivery from Microparticles Incorporated within Human Bone Marrow-Derived Mesenchymal Stem Cell Aggregates for Enhanced Bone Tissue Engineering via Endochondral Ossification. Stem Cells Transl. Med. 2016, 5, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.A.; Bodde, E.W.; Cuijpers, V.M.; Baggett, L.S.; Tabata, Y.; Mikos, A.G.; Jansen, J.A. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthr. Cartil. 2007, 15, 187–197. [Google Scholar] [CrossRef]
- Lin, D.; Chai, Y.; Ma, Y.; Duan, B.; Yuan, Y.; Liu, C. Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials 2019, 196, 122–137. [Google Scholar] [CrossRef]
- Re’em, T.; Witte, F.; Willbold, E.; Ruvinov, E.; Cohen, S. Simultaneous regeneration of articular cartilage and subchondral bone induced by spatially presented TGF-beta and BMP-4 in a bilayer affinity binding system. Acta Biomater. 2012, 8, 3283–3293. [Google Scholar] [CrossRef]
- Dorcemus, D.L.; Kim, H.S.; Nukavarapu, S.P. Gradient scaffold with spatial growth factor profile for osteochondral interface engineering. Biomed. Mater. 2021, 16, 035021. [Google Scholar] [CrossRef]
- Longoni, A.; Li, J.; Lindberg, G.C.J.; Rnjak-Kovacina, J.; Wise, L.M.; Hooper, G.J.; Woodfield, T.B.F.; Kieser, D.C.; Lim, K.S. Strategies for inclusion of growth factors into 3D printed bone grafts. Essays Biochem. 2021, 65, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kwon, J.S.; Lee, B.S.; Park, J.H.; Lee, B.K.; Yun, J.-H.; Lee, B.Y.; Kim, J.H.; Min, B.H.; Yoo, T.H.; et al. BMP2-modified injectable hydrogel for osteogenic differentiation of human periodontal ligament stem cells. Sci. Rep. 2017, 7, 6603. [Google Scholar] [CrossRef] [PubMed]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Prantl, L.; Kujat, R.; Nerlich, M.; Tuan, R.S.; Angele, P. Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs 2010, 192, 158–166. [Google Scholar] [CrossRef]
- Bian, L.; Zhai, D.Y.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32, 6425–6434. [Google Scholar] [CrossRef]
- Van der Kraan, P.M.; Blaney Davidson, E.N.; van den Berg, W.B. Bone Morphogenetic Proteins and articular cartilage: To serve and protect or a wolf in sheep clothing’s? Osteoarthr. Cartil. 2010, 18, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Hallett, S.A.; Matsushita, Y.; Ono, W.; Sakagami, N.; Mizuhashi, K.; Tokavanich, N.; Nagata, M.; Zhou, A.; Hirai, T.; Kronenberg, H.M.; et al. Chondrocytes in the resting zone of the growth plate are maintained in a Wnt-inhibitory environment. eLife 2021, 10, e64513. [Google Scholar] [CrossRef] [PubMed]
- Usami, Y.; Gunawardena, A.T.; Iwamoto, M.; Enomoto-Iwamoto, M. Wnt signaling in cartilage development and diseases: Lessons from animal studies. Lab. Investig. 2016, 96, 186–196. [Google Scholar] [CrossRef]
- Miyatake, K.; Kumagai, K.; Imai, S.; Yamaguchi, Y.; Inaba, Y. Sclerostin inhibits interleukin-1β-induced late stage chondrogenic differentiation through downregulation of Wnt/β-catenin signaling pathway. PLoS ONE 2020, 15, e0239651. [Google Scholar] [CrossRef]
- Martin, T.J. Parathyroid Hormone-Related Protein, Its Regulation of Cartilage and Bone Development, and Role in Treating Bone Diseases. Physiol. Rev. 2016, 96, 831–871. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, X.; Wei, L. Indian Hedgehog, a critical modulator in osteoarthritis, could be a potential therapeutic target for attenuating cartilage degeneration disease. Connect. Tissue Res. 2014, 55, 257–261. [Google Scholar] [CrossRef]
- Mak, K.K.; Kronenberg, H.M.; Chuang, P.T.; Mackem, S.; Yang, Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development 2008, 135, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wei, X.; Zhang, Z.; Wang, X.; Wang, C.; Li, P.; Wang, C.; Wei, L. Ipriflavone attenuates the degeneration of cartilage by blocking the Indian hedgehog pathway. Arthritis Res. Ther. 2019, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, M.; Liu, Q.; Zhang, H.; Zhang, J.; Lu, L.; Xie, M.; Chen, D.; Wang, M. Inhibition of Ihh Reverses Temporomandibular Joint Osteoarthritis via a PTH1R Signaling Dependent Mechanism. Int. J. Mol. Sci. 2019, 20, 3797. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.-Y.; Cheng, C.-M.; Kao, S.-W.; Liu, J.-W.; Chang, C.-S.; Harhaus, L.; Huang, J.-J. The effect of bone inhibitors on periosteum-guided cartilage regeneration. Sci. Rep. 2020, 10, 8372. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Chen, Y.; Zhang, Z.; Saunders, L.; Schipani, E.; Chen, Q.; Ma, P.X. Suppressing mesenchymal stem cell hypertrophy and endochondral ossification in 3D cartilage regeneration with nanofibrous poly(l-lactic acid) scaffold and matrilin-3. Acta Biomater. 2018, 76, 29–38. [Google Scholar] [CrossRef]
- Wei, F.; Zhou, Y.; Wang, J.; Liu, C.; Xiao, Y. The Immunomodulatory Role of BMP-2 on Macrophages to Accelerate Osteogenesis. Tissue Eng. A 2017, 24, 584–594. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Fang, R.; Bu, X.; Cai, S.; Du, J. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, F.; Taylor, R.; Knowles, H.J.; Athanasou, N.A. RANKL-independent human osteoclast formation with APRIL, BAFF, NGF, IGF I and IGF II. Bone 2011, 48, 938–944. [Google Scholar] [CrossRef]
- Fasolino, I.; Raucci, M.G.; Soriente, A.; Demitri, C.; Madaghiele, M.; Sannino, A.; Ambrosio, L. Osteoinductive and anti-inflammatory properties of chitosan-based scaffolds for bone regeneration. Mater. Sci. Eng. C 2019, 105, 110046. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, L.; Cao, Y.; He, Y.; Lei, C.; Xiao, Y.; Sun, W.; Ahadian, S.; Zhou, X.; Khademhosseini, A.; et al. Mesoporous silica rods with cone shaped pores modulate inflammation and deliver BMP-2 for bone regeneration. Nano Res. 2020, 13, 2323–2331. [Google Scholar] [CrossRef]
- Fan, Q.; Bai, J.; Shan, H.; Fei, Z.; Chen, H.; Xu, J.; Ma, Q.; Zhou, X.; Wang, C. Implantable blood clot loaded with BMP-2 for regulation of osteoimmunology and enhancement of bone repair. Bioact. Mater. 2021, 6, 4014–4026. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Chen, X.; Huang, L.; Tan, J. Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen. Biomater. 2020, 7, 233–245. [Google Scholar] [CrossRef]
- Bordoni, V.; Reina, G.; Orecchioni, M.; Furesi, G.; Thiele, S.; Gardin, C.; Zavan, B.; Cuniberti, G.; Bianco, A.; Rauner, M.; et al. Stimulation of bone formation by monocyte-activator functionalized graphene oxide in vivo. Nanoscale 2019, 11, 19408–19421. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Lei, C. The Delivery and Activation of Growth Factors Using Nanomaterials for Bone Repair. Pharmaceutics 2023, 15, 1017. [Google Scholar] [CrossRef]
- Shin, R.L.-Y.; Lee, C.-W.; Shen, O.Y.-J.; Xu, H.; Lee, O.K.-S. The Crosstalk between Mesenchymal Stem Cells and Macrophages in Bone Regeneration: A Systematic Review. Stem Cells Int. 2021, 2021, 8835156. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Longaker, M.T.; Chan, C.K.F. A Revised Perspective of Skeletal Stem Cell Biology. Front. Cell Dev. Biol. 2019, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.F.; Seo, E.Y.; Chen, J.Y.; Lo, D.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T.; et al. Identification and Specification of the Mouse Skeletal Stem Cell. Cell 2015, 160, 285–298. [Google Scholar] [CrossRef]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56. [Google Scholar] [CrossRef]
- Tare, R.S.; Babister, J.C.; Kanczler, J.; Oreffo, R.O.C. Skeletal stem cells: Phenotype, biology and environmental niches informing tissue regeneration. Mol. Cell. Endocrinol. 2008, 288, 11–21. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Guan, M.; Zhou, T.; Duan, X.; Xiang, Z. Growth Factor and Its Polymer Scaffold-Based Delivery System for Cartilage Tissue Engineering. Int. J. Nanomed. 2020, 15, 6097–6111. [Google Scholar] [CrossRef] [PubMed]
- Josephson, A.M.; Bradaschia-Correa, V.; Lee, S.; Leclerc, K.; Patel, K.S.; Muinos Lopez, E.; Litwa, H.P.; Neibart, S.S.; Kadiyala, M.; Wong, M.Z.; et al. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 6995. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Marecic, O.; McArdle, A.; Sinha, R.; Gulati, G.S.; Tong, X.; Wang, Y.; Steininger, H.M.; Hoover, M.Y.; Koepke, L.S.; et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature 2021, 597, 256–262. [Google Scholar] [CrossRef]
| Bone/Cartilage Targeting Motif | Structure/Sequence | Target Tissue | Conjugated Protein/Nanoparticles | Result | Ref. |
|---|---|---|---|---|---|
| Bisphosphonates(BPs) | 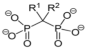 R = single atoms, alkyl chains, amino group etc. | Bone | Osteoprotegerin (OPG) | Four folds increase in targeting bone (tibia) in comparison to OPG only group. | [30] |
| Superoxide dismutase (SOD) | BP conjugation achieved 36% of delivery rate while SOD alone showed no accumulation to bone. | [31] | |||
| Salmon calcitonin | 4 folds increase in targeting bone mineral component compared to GF only control. | [32] | |||
| Tetracycline | 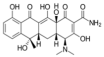 | Simvastatin | Preferential accumulation of simvastatin in bone tissue was observed in comparison to simvastatin only control. | [34] | |
| Peptide sequences | CARSKNKDC | Tendon | N/A | CAR sequence was accumulated at tendon and skin in vitro and in vivo. | [36] |
| DDDDDDDC (Poly-Asp) | Bone | P28 (BMP2 related peptide) | Conjugation of poly-asp with P28 to achieve targeted delivery. | [38,39] | |
| SDSSD | Osteoblast | anti-miR-214 in polyurethane nanomicelles | SDSSD peptide selectively bound to osteoblast via periostin in vitro, improving the delivery efficiency in vivo with mice osteoporosis model. | [40] | |
| EPLQLKM | Cartilage | Kartogenin | MSC-targeting sequence (EPLQLKM) improved the efficiency of KGN delivery to MSCs in vitro and enhanced cartilage regeneration in vivo. | [41] | |
| DWRVIIPPRPSA | mi-RNA 140 | Chondrocyte targeting sequence was encoded after exosome enriched protein, allowing targeted delivery of drug in exosome. | [43] | ||
| Aptamer | Bone | siRNA | Osteoblast-specific aptamer-decorated liposome was used to deliver siRNA to bone and promoted osteoblast function. | [47] | |
| Antibody | scFv | Cartilage | IGF1 | Conjugation of IGF1 with scFV targeting matrilin-3 showed enhanced IGF1 accumulation at cartilage and reduced off-target delivery at other organ. | [56] |
| Synovium | scFv-anti-TNFa (Adalimumab) and scFv-A7 | Bispecific antibody; one targeting to TNFa to suppress inflammation and other end targeting to synovium (scFV-A7). Results showed successful accumulation at xenografted human synovium in mice. | [57] | ||
| Protein | Scaffold | Method | Result | Ref. |
|---|---|---|---|---|
| BMP2 | Collagen | Affinity | Heparin was immobilized to collagen scaffold to achieve the spatial localization of BMP2, successfully reducing the heterotopic bone formation. | [60] |
| PDGF-BB BMP2 | PCL | GFs were immobilized on heparin coated PCL scaffold. Continuous release of initial loading amount after 5 weeks without an initial burst were observed, leading to better tendon regeneration. | [62] | |
| P24 | nHA/RHLC/PLA | Polydopamin was coated on nHA/RHLC/PLA to have an affinity to P24, resulting slower release/high retention. | [63] | |
| BMP2 BMP7 | Fibrin | Covalent | Covalent conjugation allowed slower release kinetics in vitro. Enhanced bone regeneration was observed in critical size calvarial defects model with rat. | [65] |
| TGFβ3 | PLGA-GCH | Prolonged release of TGFβ and improved cartilage regeneration were observed. | [66] | |
| BMP2 | Collagen | Engineered bridge | Dual affinity bridge protein connected collagen scaffold and BMP2. Lower BMP2 dosage was required to induce bone formation in vivo. | [68] |
| Collagen/alginate/Titanium | Antibody | BMP2 mAbs was immobilized to scaffold to capture endogenous BMP-2 for bone regeneration, improving bone formation in rat calvarial defects model. | [69] | |
| BMP2 FGF2 | Gelatin | Biotin-avidin | Biotinylated BMP2 and FGF2 were bound to avidin functionalized nanofiber, showing controlled release of BMP2 and FGF2. | [70] |
| Materials | Modification | Protein | Target | Release Duration | Result | Ref. | |
|---|---|---|---|---|---|---|---|
| Natural | Alginate sulfate | Sulfation | bFGF | Vascular | 5 days | Slower release with sulfate-conjugated alginate. Sulfated alginate released 50% of bFGF by day 5 while control alginate released 50% of bFGF at day 0. | [75] |
| TGFb | Cartilage | 7 days | The sulfate group exhibited an affinity for TGFb, resulting in a slower release rate compared to non-sulfated alginate. | [76] | |||
| Gelatin-PCL | N/A | BMP2 | Bone | 10–45 days | Gelatin/heparin gel enhanced cells viability and PCL enhanced mechanical property. Release kinetics was controllable by combining gelatin and PCL. | [77] | |
| Liposome | 100 hours | Achieved steady release of rh-BMP2 for 100 hours in vitro. | [78] | ||||
| Chitosan | Thiolation | BMP2 | Bone | 14 days | The thiolate modification contributed to the upregulation of ALP activity and better bone regeneration by prolonging the release of BMP2. | [79] | |
| Synthetic | PEG-based hydrogel | PLGA microparticle | BMP2 | Bone | 3 days | BMP-2 was encapsulated within PLGA microparticles, which were further enclosed within a PEG-based hydrogel. Initially, 75% of the BMP-2 was released within 3 days. | [80] |
| N/A | bFGF | Cartilage | 60 days (BSA) 35 days (FGF) | Hydrolytically degradable structures increased the hydrogel swelling ratio and mesh size, enabling sustained protein release over 2 months. | [81] | ||
| P34HB nanoparticles | Soybean coating | BMP7 | Bone | 20 days | Soybean coating on nanoparticle significantly slowed down BMP7 release kinetics. | [83] | |
| Mesoporous silica in Hydrogel | Dopamine coating | TGFb3 | Cartilage | 75 days | TGFb3 was loaded in mesoporous silica coated with DOPA. Thicker DOPA coating achieved slower release and achieved 75 days release duration in vitro. | [84] | |
| PLGA particle in poly(LLA-co-CL) | N/A | BMP2 | Bone | 70 days | BMP2 was incorporated in PLGA microsphere, which were further encapsulated in scaffold. Result showed better bone formation in rat calvarial model. | [85] | |
| Mesoporous silica in PLGA | BMP2 | Bone | 40 days | BMP2 was incorporated in Mesoporous silica, then mixed with PLGA to create microsphere. In vitro functional assay showed improved bone formation. | [86] | ||
| MBG/SIS scaffold | Heparin | P28 | Bone | 40 days | BMP2 was incorporated in MBG, which were further encapsulated in SIS. Enhanced bone regeneration was observed in rat calvarial defect model. | [87] | |
| Hybrid | hyaluronate/type I collagen/fibrin composite containing PVA nanofibers enriched with liposomes | N/A | bFGF Insulin | Cartilage | 19 days | Achieved steady release of both bFGF and insulin for 19 days in vitro. Nanofiber provided mechanical stiffness and elasticity closer to native cartilage. In vivo mini-pig experiment demonstrated cartilage regeneration. | [88] |
| System | Protein | Target | Mechanism | Release Kinetics/Spatial Release Strategy | Result | Ref. |
|---|---|---|---|---|---|---|
| Synergistic | BMP7 & BMP2 | Bone | BMP7 and BMP2 were loaded in PELA microparticle. | BMP7 and BMP2 showed steady release for 42 days in vitro. | In vivo rat femoral defect model demonstrated improved bone regeneration. | [89] |
| BMP7 & TGFb3 | Cartilage | BMP7 and TGFb were loaded in PLGA microsphere. | BMP7 and TGFb showed steady release for 30 days in vitro. | Synergistic effect of chondrogenic promotion in vitro. | [90] | |
| BMP2 & VEGFR | Cartilage | BMP2 and VEGFR were loaded in PEG based hydrogel. | BMP2 promoted osteogenic differentiation of SSCs, which was further directed to chondrocyte with VEGFR. | Implantation of hydrogel containing BMP2 and VEGFR at femoral defect promoted cartilage formation. | [91] | |
| BMP2 & Melatonin | Bone | BMP2 and Melatonin were loaded in PLGA microparticle, which is further encapsulated in Chitosan-Hap scaffold. | BMP2 and melatonin showed steady release for 20 days in vitro. | Improved osteogenic ability was confirmed by alizarin red and ALP-von Kossa staining using MC3T3-E1 cells. | [94] | |
| Sequential delivery | BMP2 & Dex | Bone | BMP2 is encapsulated in chitosan particle, which is further incorporated in PCE nanofiber with DEX. | Dex exhibited a burst release during the first 5 days, whereas BMP2 displayed a consistent release over 35 days. | Dual delivery demonstrated a better bone regeneration in rat calvarial bone defect. | [97] |
| BMP2 & ALN | Bone | ALN is encapsulated in PLGA microsphere, which is further incorporated in collagen hydroxyapatite. | The release profile of BMP2 exhibited a burst kinetics for the first 5 days, whereas ALN demonstrated a delayed release between 2 to 6 weeks. | Dual delivery demonstrated a better bone regeneration in rat calvarial bone defect. | [98] | |
| BMP2 & IGF | Bone | BMP2 was encapsulated in the 1st gelatin layer and BMP2 and IGF were loaded in the 2nd gelatin layer. | BMP2 in 1st layer was released in 2 days and 2nd layer in 6 days. | Increased AP activity and matrix calcium content compared to control. | [100] | |
| SDF1 & BMP2 | Bone | BMP2 is encapsulated in silk fibroin particle, which is further incorporated in Hap scaffold with SDF1. | SDF1 demonstrated a burst release for first 5 days while BMP2 showed steady release for 35 days. | Dual delivery showed a better bone regeneration in rat calvarial bone defect. | [101] | |
| TGFb & BMP2 | Cartilage | TGFb is encapsulated in gelatin microparticle and BMP2 in mineral-coated hydroxyapatite microparticles. | TGFb displayed an initial burst release for 10 days and sustained BMP2 release for 60 days. | Dual delivery resulted in an enhanced GAG and Col2 expression, as demonstrated by immunostaining. | [102] | |
| IGF1& TGFb1 | Cartilage | IGF1 is incorporated in gelatin microparticle, which is encapsulated in OPF with TGFb. | An initial burst release of TGFb was observed, followed by a slower release of IGF1. | The release kinetics were able to be adjusted by modifying the crosslinking amount. | [103] | |
| IL-8 & BMP2 | Bone | BMP2 is incorporated in mesoporous bioactive glass (MBG) which is coated by PEG with IL-8. | Initial burst release of IL-8 for 1 day and steady release of BMP2 for 7 days. | The recruitment of stem cells by IL-8 and the promotion of osteogenesis by BMP2 resulted in enhanced bone regeneration. | [104] | |
| Spatial control | bFGF & BMP4 | Bone& Cartilage | Use high affinity between sulfate and proteins to control spatial distribution. | Two layered alginate-sulfate: One layer with bFGF, another one with BMP. | bFGF induced chondrogenic differentiation. BMP4 induced endochondral ossification of endogenous cells. | [105] |
| BMP2 & TGFb | Bone& Cartilage | hyaluronic acid hydrogel was filled in porous PLGA scaffold. | BMP2 adsorbed to PLGA scaffold and TGFb incorporated in the hydrogel. Gradient was created by these 2 layers. | Cartilaginous regions were marked by increased GAG production, and osteogenesis was seen in the graft. | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takematsu, E.; Murphy, M.; Hou, S.; Steininger, H.; Alam, A.; Ambrosi, T.H.; Chan, C.K.F. Optimizing Delivery of Therapeutic Growth Factors for Bone and Cartilage Regeneration. Gels 2023, 9, 377. https://doi.org/10.3390/gels9050377
Takematsu E, Murphy M, Hou S, Steininger H, Alam A, Ambrosi TH, Chan CKF. Optimizing Delivery of Therapeutic Growth Factors for Bone and Cartilage Regeneration. Gels. 2023; 9(5):377. https://doi.org/10.3390/gels9050377
Chicago/Turabian StyleTakematsu, Eri, Matthew Murphy, Sophia Hou, Holly Steininger, Alina Alam, Thomas H. Ambrosi, and Charles K. F. Chan. 2023. "Optimizing Delivery of Therapeutic Growth Factors for Bone and Cartilage Regeneration" Gels 9, no. 5: 377. https://doi.org/10.3390/gels9050377
APA StyleTakematsu, E., Murphy, M., Hou, S., Steininger, H., Alam, A., Ambrosi, T. H., & Chan, C. K. F. (2023). Optimizing Delivery of Therapeutic Growth Factors for Bone and Cartilage Regeneration. Gels, 9(5), 377. https://doi.org/10.3390/gels9050377







